Abstract
Asthma is characterized by the accumulation of eosinophils in the airways in most phenotypes. Eosinophils are inflammatory cells that require an external survival-prolonging stimulus such as granulocyte macrophage-colony-stimulating factor (GM-CSF), interleukin (IL)-5, or IL-3 for survival. In their absence, eosinophils are programmed to die by spontaneous apoptosis in a few days. Eosinophil apoptosis can be accelerated by Fas ligation or by pharmacological agents such as glucocorticoids. Evidence exists for the relevance of these survival-prolonging and pro-apoptotic agents in the regulation of eosinophilic inflammation in inflamed airways. Much less is known about the physiological significance and mechanisms of spontaneous eosinophil apoptosis even though it forms the basis of regulation of eosinophil longevity by pathophysiological factors and pharmacological agents. This review concentrates on discussing the mechanisms of spontaneous eosinophil apoptosis compared to those of glucocorticoid- and Fas-induced apoptosis. We aim to answer the question whether the external apoptotic stimuli only augment the ongoing pathway of spontaneous apoptosis or truly activate a specific pathway.
Keywords: asthma, eosinophils, spontaneous apoptosis, glucocorticoids, Fas
Introduction
Eosinophilic granulocytes account only for approximately 3% of blood leukocytes in healthy individuals. Similar to neutrophilic granulocytes, they are cells specialized to kill pathogens by the secretion of toxic mediators but also able to regulate function of other immune cells. Although evolutionary, the function of eosinophils is thought to be the innate immune response against parasitic helminthes;1 they are also critically involved in the pathogenesis of allergic, gastrointestinal, and hypereosinophilic disorders and in tumor immunity.2–5 Allergic asthma is characterized by the accumulation of eosinophils in the airways. The current evidence suggests that eosinophils are critical mediators of asthma exacerbations and airway remodelling.6–9
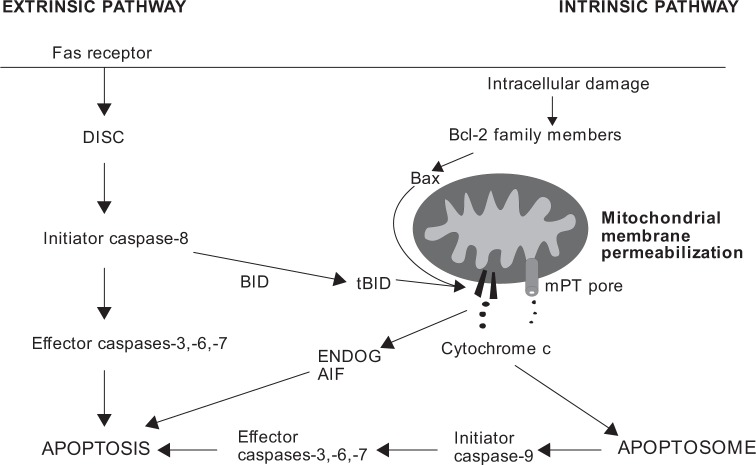
The biology of eosinophils differs from many other immune cells or malignant cell lines in their requirement for an external stimulus for continuation of survival. In the absence of any such stimulant (eg granulocyte macrophage-colony stimulating factor (GM-CSF), interleukin (IL)-5 or IL-3), they die by spontaneous (also termed as “passive”) apoptosis in a few days.10 Apoptosis is characterized by cell shrinkage, nuclear coalescence, chromatin condensation, and DNA fragmentation leading to the formation of apoptotic bodies and in vivo, to their ingestion by macrophages or other phagocytes. Generally, apoptosis can be induced via two different pathways, extrinsic (receptor mediated) or intrinsic (mitochondrial centered) (Fig. 1).11
Figure 1.
The main features of extrinsic and intrinsic apoptosis pathways. Fas receptor-mediated pathway is shown as an example of extrinsic apoptotic pathway. Extrinsic apoptosis is initiated by ligation of death receptor Fas leading to the formation of DISC and activation of caspases. Sometimes, BID cleavage into truncated BID (tBID) and mitochondrial route is required for caspase activation in extrinsic apoptosis. Intracellular stress conditions initiate intrinsic pathway of apoptosis, where Bcl-2 family members and MMP play major roles. MMP can be mediated by pore-forming activity of Bax and/or tBID or by mPT. If caspases are inhibited, apoptosis may be executed by apoptosis-inducing factor (AIF) and endonuclease G (ENDOG).
A majority of the studies on the regulation of eosinophil apoptosis have used allergic asthma as a starting point, ie they have focused on the significance and mechanisms of survival-prolonging cytokines.10,12,13 Given the importance of eosinophils in certain phenotypes of asthma,14,15 this approach is very sensible. However, eosinophils, albeit in low numbers, are also present in the blood and tissues in healthy individuals. It has been estimated that the mean turnover of eosinophils is approximately 2.2 × 108 cells/kg/day. Once in the circulation, eosinophils have a half-life of approximately 8–18 h and a mean blood transit time of approximately 25–26 h.10,16,17 In two recent studies, migration of radiolabeled eosinophils was followed in healthy individuals in real time. Eosinophils, after leaving the blood circulation, transited through the lungs and accumulated in the liver and spleen.16,18 Evidence was also attained regarding the re-entrance of eosinophils from the liver to the circulation.16 However, 48 h after injection of the radiolabeled eosinophils, approximately 50% of them resided in the liver, 30% in the spleen, and the rest in other organs. It is reasonable to hypothesize that the liver may be the primary site of eosinophil clearance through apoptosis, possibly spontaneous apoptosis. No direct evidence, however, exists about the occurrence of spontaneous (or passive) apoptosis in vivo. As studying the mechanisms of eosinophil survival and apoptosis in tissue samples is largely impossible, a vast majority of eosinophil studies have used eosinophils isolated from the peripheral blood by the CD16-negative selection. Indirect evidence of the appearance of spontaneous eosinophil apoptosis in blood was obtained recently in a study evaluating the aging of cells in blood samples, where eosinophils showed a decrease in forward side scatter (FSC) values indicative of smaller cell size typical to apoptosis.19 Furthermore, the occurrence of blood eosinophil apoptosis, even though not spontaneous, was demonstrated in mice in vivo after the administration of Siglec-F antibody.20
Eosinophil apoptosis can be induced by several agents facilitating clearance of eosinophilic inflammation. Eosinophil apoptosis can be induced eg by ligation of Fas or by ligation of tumor necrosis factor receptor (TNFR) family member CD30 and by many pharmacological agents, such as glucocorticoids, theophylline, and leukotriene modifiers.21–27 Plenty of evidence exists supporting the occurrence of steroid-induced eosinophil apoptosis in the airways of steroid-treated asthmatics.28–31 Many cell types of the airways, such as bronchial epithelial cells, bronchial smooth muscle cells, fibroblasts, T cells, and eosinophils express Fas ligand (FasL),29,32–34 and in T cells, the expression is reduced by Th2 cytokines GM-CSF, IL-5, and IL-4.35 Neutralization of FasL enhanced airway eosinophilia in a mouse model of allergic asthma providing evidence that FasL is a relevant pro-apoptotic agent for eosinophils in vivo.36 Treatment with anti-Fas mAb was shown to enhance apoptosis of airway tissue eosinophils in mice. However, this treatment resulted in aggravated airway inflammation due to cytolysis and progression of apoptosis into secondary necrosis.37 This emphasizes the importance of efficient phagocytic clearance of apoptotic cells.
To combat the eosinophilia associated with several disease conditions, understanding the signaling patterns related to eosinophil survival and apoptosis is extremely important. Ideally, a novel pharmacological agent aimed specifically to deplete eosinophils by inducing eosinophil apoptosis could be targeted to cover all the following options: (1) inhibit the action or signaling of survival-prolonging factors, (2) mimic the action and/or signaling of known external inducers of apoptosis such as FasL or glucocorticoids, and (3) enhance the intrinsic pro-apoptotic signaling pathway during spontaneous (passive) eosinophil death. This review focuses on the signaling of spontaneous eosinophil death, a phenomenon that is largely neglected, and compares it with the mechanisms of known inducers of eosinophil apoptosis, FasL, and glucocorticoids.
Inhibitory Signals for Spontaneous Apoptosis
Many eosinophil survival-prolonging inflammatory agents, such as GM-CSF, IL-5 and IL-3, are present in the lungs of asthmatics, and eosinophil apoptosis has been shown to be reduced in the airway submucosa of patients with steroid-untreated asthma when compared to healthy controls.29,38–40 Eosinophil survival may be prolonged up to 1–2 weeks in response to these cytokines, and IL-5 is the most potent.41 GM-CSF, however, seems to be the main eosinophil survival-prolonging cytokine in asthmatic airways.39,40 Pathways activated by IL-5/GM-CSF include Lyn/Syk–Ras–Raf-1–extracellular signal-regulated kinases (ERK) 1/2, Jak2-STAT1, and PI3K-Akt in eosinophils.42–49 Of these, the ERK pathway does not seem to be involved in the survival-prolonging action.50,51 In addition, inhibition of Bax translocation to mitochondria by IL-5 and GM-CSF has been shown in eosinophils.49,52 TGF-β, interestingly, abrogated IL-5/GM-CSF-induced eosinophil survival, and this mechanism involved inhibition of tyrosine phopshorylation of Jak2, Lyn, and ERK 1/2 as well as inhibition of phosphorylation of STAT1 and Akt.53–55
In addition, other significant survival-prolonging factors seem to exist because delayed apoptosis of blood and nasal polyp tissue eosinophils was only partly prevented by anti-GM-CSF, anti-IL-5, and/or anti-IL-3 antibodies.56,57 Many pathogenic components, cytokines (eg TNF-α, leptin, interferons (IFNs)) and allergens also prolong eosinophil survival.10,58–65 Generally, NF-κB may be the most important transcription factor mediating eosinophil survival, as its inhibition turns eosinophils into the apoptotic cascade.64,66
TNF-α, which can be produced locally by mast cells, was demonstrated to be an anti-apoptotic factor for eosinophils if NF-κB was not inhibited. This effect was proposed to be mediated via TNF receptors, NF-κB, and induction of GM-CSF production.60,67 IFN-γ is produced by T helper 1 cells, and its effects on eosinophils seem to be complex. IFN-γ inhibited IL-3- or IL-5-induced differentiation of eosinophils from cord blood mononuclear cells68 but prolonged eosinophil survival in vitro.69 Leptin is a cytokine that is mainly produced by adipocytes of white adipose tissue with the main function related to inhibition of appetite. Leptin has been shown to increase eosinophil survival even though it remains unclear whether the survival-prolonging concentrations may be reached in vivo.70
In addition, CD40–CD40L interaction has been shown to prolong eosinophil survival. Freshly isolated blood eosinophils did not express CD40, but the expression was strong after 48 h of culture. The mechanism of CD40L-induced survival prolongation involved induced expression of cellular inhibitor of apoptosis proteins (cIAPs).71 CD40L- deficient mice showed decreased eosinophilic lung inflammation 72 h but not 24 h after allergen challenge72 suggesting that CD40–CD40L interaction affects maintenance of eosinophilic airway inflammation. In the absence of any of these or other survival-prolonging factors, eosinophils proceed into spontaneous apoptosis.
Progression of Spontaneous Apoptosis
When eosinophils isolated from human blood are cultured in the absence of any inducers or inhibitors of apoptosis, approximately half of them undergo spontaneous apoptosis in 2 days.64,73 We have applied several methods for the determination of eosinophil apoptosis providing basic information on the progression of apoptosis and the cascade of apoptotic events in spontaneously dying eosinophils. According to our combined data over time, apoptotic values obtained with Annexin-V/propidium iodide double-staining are continuously higher when compared to those obtained with other standard methods of apoptosis determination (DNA fragmentation assay, morphological examination, ΔΨm dissipation) (Fig. 2, unpublished observation). This suggests that cell surface expression of phosphatidylserine (PS) precedes many well-known manifestations of apoptosis in eosinophils. Early occurrence of PS exposure has been previously demonstrated in eosinophils,74 and evidence exists also of PS exposure as a caspase-dependent event.52,74,75 Mitochondrial events such as cytochrome c release and ΔΨm dissipation were shown to occur after PS exposure in eosinophils, which was in contrast to lymphocytes.74 It seems that the order of events is stimulus dependent as well as cell-type dependent.74,76,77 To further support early time-course of PS exposure, it was shown that PS exposure preceded cell shrinkage and DNA fragmentation in a lymphoma cell line by using three different stimulants to induce apoptosis.78 During the process of apoptosis, early appearance of PS is logical since PS functions as a cell surface signal for phagocytes to ingest the apoptotic cells and attraction of phagocytes and phagocytosis may be considered as one of the most important events for occurrence of apoptosis in a non-inflammatory fashion.37 Indeed, inhibition of PS exposure during apoptosis led to more than 50% reduction in engulfment of apoptotic cells.79 However, from the methodological point of view, it is recommended to measure apoptosis using a combination of different methods, not solely Annexin-V-assay despite the early appearance of PS in apoptotic cells. All methodologies to analyze apoptosis have their drawbacks.80,81
Figure 2.
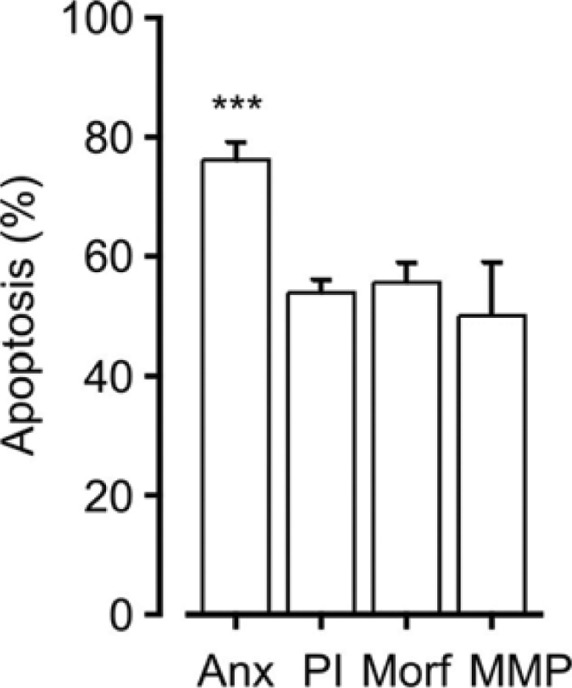
Comparison of percentages of spontaneous eosinophil apoptosis obtained by different apoptosis determination methods. Apoptosis was determined by Annexin-V FITC/propidium iodide double-staining (Anx, n = 12), DNA fragmentation assay carried out by propidium iodide staining of permeabilized eosinophils (PI, n = 56), morphological analysis of May-Grunwald–Giemsa-stained eosinophils (Morf, n = 23), or determination of mitochondrial membrane potential by JC-1 staining (MMP, n = 5) after 40 h of incubation. Descriptions of the methods used can be found at Ref. 73.
Note: ***Indicates P < 0.001 as compared to all other columns by using ANOVA analysis.
Mediators of Spontaneous Eosinophil Apoptosis
Bcl-2 members and mitochondrial events during spontaneous eosinophil apoptosis
Members of Bcl-2 family are critical in monitoring intracellular damage and important for mitochondrial membrane permeabilization (MMP) to occur, especially in the intrinsic pathway of apoptosis. The Bcl-2 family consists of a group of anti-apoptotic proteins and two groups of pro-apoptotic proteins.82,83 Because eosinophils undergo apoptosis quite rapidly, the expression of proteins regulating longevity is balanced toward pro-apoptotic members. Generally, pro-apoptotic Bcl-2 family members Bax and Bid are strongly expressed in untreated human eosinophils.49,84,85 Cleavage of Bax and Bid into pore-forming fragments enables permeabilization of the outer mitochondrial membrane and release of cytochrome c. It was shown that during spontaneous eosinophil apoptosis, Bax is clustered and re-localized into mitochondria, independent from caspases, and this leads to the release of cytochrome c to the cytosol and activation of caspases.52,86 An accelerated Bax translocation is observed in dexamethasone-treated eosinophils.86 Also Bid is processed during spontaneous apoptosis and at a faster rate during Fas- and glucocorticoid-induced apoptosis.84 Spontaneous, FasL-, and dexamethasone-mediated eosinophil apoptosis were reduced by 30, 50, and 25%, respectively, in cultured bronchoalveolar lavage (BAL) eosinophils from Bid-deficient mice, suggesting that Bid has a lesser role in spontaneous and glucocorticoid-induced apoptosis and is a more critical mediator in FasL-induced apoptosis.85 It seems clear that extrinsic (FasL-induced) apoptosis requires an additional mitochondrial loop in eosinophils.
As expected in cells prone to undergo apoptosis, the expression of anti-apoptotic Bcl-2 members Bcl-2, Bcl-xL, and Mcl-1L is generally low in eosinophils.87–90 However, the level of Bcl-2 expression seems to depend on the status of the patient and origin of eosinophils, because higher expression of Bcl-2 was found in the lung eosinophils of patients with asthma and children with severe exacerbations when compared to eosinophils of healthy individuals or children with mild-to-moderate exacerbations, respectively.91–93 Anti-apoptotic Mcl-1L is degraded during spontaneous apoptosis and in an accelerated manner during glucocorticoid-induced apoptosis.90
In addition to the pore-forming activity of cleaved Bax or Bid, MMP can be mediated via mitochondrial permeability transition (mPT) pore.11,94 It is a channel formed to the merging point of inner and outer mitochondrial membranes in response to Ca2+, oxidants, or pro-apoptotic Bcl-2 family members leading to free passage of solutes and molecules up to 1.5 kDa.94,95 mPT does not seem to be important for spontaneous apoptosis or Fas-induced apoptosis but is a critical mediator of eosinophil apoptosis induced by glucocorticoids.73,96,97 As discussed above, pores formed in the outer mitochondrial membrane by the cleaved Bax and/or Bid are probably responsible for the MMP in the pathways of spontaneous and Fas-induced eosinophil apoptosis.84,90
Caspases and calpains
Caspases are cysteine-dependent aspartate-specific proteases involved in the execution phase of apoptosis and are further divided into initiators (caspases-8, -9, and -10) and effectors (caspases-3, -6, and -7). Initiator caspases are synthesized as inactive proenzymes and require dimerization for activation that is enabled by platforms such as death-inducing signaling complex (DISC) or apoptosome. Effector caspases occur as inactive dimers and require cleavage by initiator or other effector caspases to become activated. When activated, caspases cleave cellular components into tetrapeptide sequences optimal enough to fit their catalytic site.98,99
Eosinophils have been shown to express caspases-3, -6, -7, -8, and -9.52,75,87 Many apoptotic events during spontaneous or induced eosinophil apoptosis are reduced or prevented by pan-caspase inhibitors suggesting that eosinophil apoptosis is mediated by the activation of the caspase cascade.52,75,96,100 Caspase-9, accounted as the initiator caspase activated in response to mitochondrial apoptotic pathway, has been shown to be processed during spontaneous and induced apoptosis.52,84,101–103 However, its inhibition by Z-LEHD-FMK did not prevent spontaneous apoptosis or FasL-mediated apoptosis, suggesting that it may not function as a critical initiator caspase in these pathways.84,101 However, a possibility exists that the inhibitor used was inefficient, because according to our data it inhibited only 65% of caspase-9 activity in eosinophils. It can be suspected that the residual 35% of caspase-9 activity was enough to activate effector caspases.101 Also caspase-8 activity has been detected in spontaneously dying eosinophils in some but not all studies. But similar to caspase-9, its inhibition did not prevent apoptotic events during spontaneous apoptosis.52,84,101–103 Altogether, the initiator caspase responsible for the proceeding of spontaneous apoptosis is not clear. In neutrophils, activation of caspase-8 was shown to be dependent on initiator caspase-9104 and may be actually activated by effector caspase-3, as previously described.105 In eosinophils, activation of both initiator caspases (8 and 9) has been detected during Fas- and glucocorticoid-induced apoptosis.84,106,107 The evidence indicates that caspase-8 functions as the critical initiator caspase in FasL-mediated eosinophil apoptosis as its inhibition was reported to prevent Bid-cleavage and reduce apoptosis.84
Activation of effector caspases-3 and -6 seems to be a general feature of eosinophil apoptosis. Involvement of these caspases has been found in spontaneous eosinophil apoptosis and apoptosis induced by various stimuli.52,75,87,96,103 Lamin degradation and DNA fragmentation are caspase-6- dependent events in eosinophils, and inhibition of caspase-6 delayed or halted apoptosis at the level of chromatin condensation but did not prevent apoptosis.75 This is consistent with the results in other cell types.108–111 Also PS externalization was shown to be partly dependent on caspase-6.75 Inhibition of caspase-3 partially prevented DNA fragmentation in eosinophils.75,96
Calpains are calcium-activated (papain-like) neutral proteases that are involved in the execution of both apoptosis and necrosis. At least 14 isoforms of calpains exist. Similar to caspases, calpains are cysteine proteases; but in contrast to caspases, they require no particular amino acid in the substrate peptide sequence. Calpains are activated by increased intracellular calcium and their substrates include X-linked IAP (XIAP), Bcl-xL, Bid, and pro-caspases-3, -7, -8, and -9.112,113 DNA fragmentation during spontaneous apoptosis was prevented by inhibition of calpains 1 and 2. Unfortunately, no information exists on the role of calpains in FasL- or glucocorticoid-induced eosinophil apoptosis. However, lack of role of calpains in nitric oxide-induced apoptosis suggests that calpains are not activated in analogous situations to caspases. Calpains have been shown to be involved in the cleavage of Bax in spontaneous eosinophil apoptosis. Bax cleavage is a pro-apoptotic event leading to its mitochondrial targeting.49
Reactive oxygen species (ROS)
ROS induce apoptosis of human eosinophils and are often involved in the mitochondrial pathway of apoptosis.114,115 Thiol-antioxidant glutathione is considered to form the most important antioxidant defence in mitochondria.116 Spontaneous eosinophil apoptosis was reduced by antioxidants that elevate intracellular levels of glutathione and by hypoxia86,115,117,118 suggesting a role of ROS in mediating spontaneous apoptosis and importance of glutathione in the regulation of intracellular oxidant levels in eosinophils. In a similar manner, the antioxidants increasing glutathione reduced Fas-induced apoptosis.115 Also, glucocorticoid-induced apoptosis was prevented by mimetic of superoxide dismutase (SOD) and hypoxia, indicating the involvement of ROS.86 ROS seem to mediate also eosinophil apoptosis induced by many other stimulants.73,115,119 However, the exact mechanism of increased oxidant levels remains unclear. Decrease in the levels of an important mitochondrial antioxidant MnSOD was demonstrated during spontaneous apoptosis as well as in glucocorticoid-treated cells.86 Levels of a cytosolic antioxidant were not similarly decreased. It is possible that loss of mitochondrial antioxidant defence at least partly explains the increased ROS during spontaneous and glucocorticoid-induced eosinophil apoptosis.
Mitogen-activated protein kinases (MAPKs) and mammalian sterile 20-like kinase (Mst)
MAPKs are serine/threonine kinases mainly activated by proinflammatory cytokines, growth factors, and environmental stress. MAPK family consists of c-Jun N-terminal kinases (JNK) 1-3, ERK 1/2, 3, 5 and 7, and p38 family members and a serial of phosphorylation cascades leads to activation of MAPK. MAPKs phosphorylate transcription factors resulting in transcription of genes involved in apoptosis, survival, proliferation, and differentiation. Additionally, MAPKs affect function of numerous other proteins via phosphorylation.120,121 JNK has been previously shown to mediate apoptosis through several pathways: AP-1-mediated transcription of FasL and TRAIL-receptor 1,122,123 phosphorylation of Bcl-2 family protein members,120,124,125 mPT induction,126,127 and phosphorylation of histone H2AX required for DNA fragmentation.128
Some evidence has been gathered regarding the role of JNK as a mediator of spontaneous eosinophil apoptosis, even though results are contradictory. Spontaneous eosinophil apoptosis was decreased by a peptide inhibitor of JNK but not by the other JNK inhibitors tested. Furthermore, modest or no activation of JNK and lack of activation of c-Jun has been demonstrated in spontaneously dying eosinophils.73,129 Instead, JNK was involved in glucocorticoid-induced eosinophil apoptosis and its activation was dependent on oxidants.86 Indeed, increased level of ROS is one possible general activation mechanism for JNK in eosinophils proceeding toward apoptosis. Additionally, activation of JNK pathway has been previously demonstrated to occur in response to FasL in lymphocytes,130,131 and some evidence points to the role of JNK in FasL-induced eosinophil apoptosis.132 The other MAP kinases ERK 1/2 and p38 seem to mediate eosinophil survival, not apoptosis.49,50 Interestingly, p38 MAP kinase seems to be active in isolated eosinophils, and its inhibition by a pharmacological inhibitor induces apoptosis.50
Mst1 belongs to a group of germinal center kinases (GSKs) that is involved in many functions of immune cells such as trafficking, proliferation, and apoptosis.133 Mst1 has been shown to be involved in the activation of MAPKs such as JNK.134 Caspase-mediated cleavage and release of 36 kDa fragment of Mst1 was demonstrated to correlate with eosinophil apoptosis but not with neutrophil apoptosis. Cleavage of Mst1 was increased by FasL and decreased by IL-5, suggesting an important role of this kinase in mediating eosinophil apoptosis.135
Summary and Conclusions
Eosinophil apoptosis induced by FasL or glucocorticoids is a physiologically or clinically relevant mechanism of eosinophil clearance. Plenty of evidence exist about the clinical relevance of steroid-induced eosinophil apoptosis in the airways of steroid-treated asthmatics.28–31 Most likely, spontaneous eosinophil apoptosis occurs in a physiological situation, even though direct evidence is difficult to obtain. The signaling pathway of spontaneous apoptosis seems to overlap with the pathways of FasL or glucocorticoid-stimulated apoptosis. A summary of different pathways is shown in Table 1. Premitochondrial phases of FasL-stimulated apoptosis have unique features such as Fas-associated protein with death domain (FADD) phosphorylation and activation of initiator caspase-8. However, mitochondrion has an important role in all of these pathways of apoptosis as suggested by common dependence on mitochondrial ROS and processing of pro-apoptotic Bcl-2 family members. The mechanism of MMP may differ between these pathways of apoptosis. Bax and Bid seem to be important mediators of MMP in eosinophils undergoing spontaneous apoptosis, while FasL-stimulated apoptosis was dependent on Bid. mPT is emphasized during glucocorticoid-induced apoptosis. Effector caspases seem to be similarly activated in all three apoptotic routes. Activation of JNK may also be common for these pathways of apoptosis, even though additional evidence is required to address it. In a physiological situation, some level of mitochondrial disruption may already be ongoing at the time the cell encounters a pro-apoptotic stimulant, and the stimulant probably adds pro-apoptotic signals that merge at the level of mitochondria to augment, amplify, and finalize the ongoing process of (spontaneous) apoptosis. Thereby, enhancement of the ongoing spontaneous apoptosis is a relevant therapeutic strategy to treat diseases with eosinophilic inflammation.
Table 1.
A summary of the mechanisms involved in the regulation of spontaneous eosinophil apoptosis when compared to extrinsic apoptosis induced by Fas or intrinsic apoptosis induced by glucocorticoids.
| FAS-MEDIATED APOPTOSIS | SPONTANEOUS APOPTOSIS | APOPTOSIS INDUCED BY GLUCOCORTICOIDS | REF. | |
|---|---|---|---|---|
| Caspase-3 | ++ | ++ | ++ | 52, 75, 87, 96, 101–103 |
| Caspase-6 | ND | ++ | ND | 75 |
| Caspase-8 | +++ | + | + | 52, 84, 87, 96, 101–103, 106 |
| Caspase-9 | + | + | + | 52, 84, 101, 102, 106, 107 |
| Calpains | ND | ++ | ND | 49, 75 |
| mPT | – | + | +++ | 73, 96, 97 |
| Bid | +++ | ++ | ++ | 84, 106, 85 |
| Bax | ND | ++ | ++ | 49, 52, 86, 90 |
| JNK | ++ (id) | ++? | ++ | 73, 86, 129, 132 |
| ROS | ++ | ++ | ++ | 73, 86, 115, 117, 135 |
| Mst 1/2 | ++ | ++ | ND | 135 |
Abbreviations: +++, apoptosis is completely dependent; ++, apoptosis is partially (approximately 50%) dependent or clearly involved in apoptosis; +, minor role in apoptosis; −, no role in apoptosis; ND, not determined, id, indirect evidence; Bax, Bcl-2-associated X protein; Bid, BH3-interacting domain death agonist; JNK, c-Jun N-terminal kinase; Mst, mammalian sterile 20-like kinase, mPT, mitochondrial permeability transition; ROS, reactive oxygen species.
Footnotes
Author Contributions
PI wrote the first draft of the manuscript. HK contributed to the writing of the manuscript. HK and EM made critical revisions. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
ACADEMIC EDITOR: Garry Walsh, Editor in Chief
FUNDING: This study was financially supported by the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (Tampere, Finland), grant numbers VTR 15 and VTR 224; Competitive Research Funding of Seinäjoki Central Hospital (Seinäjoki, Finland); Tampere Tuberculosis Foundation (Tampere, Finland); and Finnish Anti-Tuberculosis Association Foundation (Helsinki, Finland), which is gratefully acknowledged.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
REFERENCES
- 1.Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004;113:30–37. doi: 10.1016/j.jaci.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci. 2007;64:1269–1289. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuo L, Rothenberg ME. Gastrointestinal eosinophilia. Immunol Allergy Clin North Am. 2007;27:443–455. doi: 10.1016/j.iac.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gleich GJ, Leiferman KM. The hypereosinophilic syndromes: current concepts and treatments. Br J Haematol. 2009;145:271–285. doi: 10.1111/j.1365-2141.2009.07599.x. [DOI] [PubMed] [Google Scholar]
- 5.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 6.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–993. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 7.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, et al. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 10.Park YM, Bochner BS. Eosinophil survival and apoptosis in health and disease. Allergy Asthma Immunol Res. 2010;2:87–101. doi: 10.4168/aair.2010.2.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kankaanranta H, Moilanen E, Zhang X. Pharmacological regulation of human eosinophil apoptosis. Curr Drug Targets Inflamm Allergy. 2005;4:433–445. doi: 10.2174/1568010054526395. [DOI] [PubMed] [Google Scholar]
- 13.Walsh G. Eosinophil apoptosis and clearance in asthma. J Cell Death. 2013;6:17–25. doi: 10.4137/JCD.S10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet. 2006;368:804–813. doi: 10.1016/S0140-6736(06)69290-8. [DOI] [PubMed] [Google Scholar]
- 15.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farahi N, Singh NR, Heard S, Loutsios C, Summers C, Solanki CK, et al. Use of 111-indium-labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood. 2012;120:4068–4071. doi: 10.1182/blood-2012-07-443424. [DOI] [PubMed] [Google Scholar]
- 17.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- 18.Lukawska JJ, Livieratos L, Sawyer BM, Lee T, O’Doherty M, Blower PJ, et al. Real-time differential tracking of human neutrophil and eosinophil migration in vivo. J Allergy Clin Immunol. 2014;133:233–239. doi: 10.1016/j.jaci.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Canonico B, Betti M, Luchetti F, Battistelli M, Falcieri E, Ferri P, et al. Flow cytometric profiles, biomolecular and morphological aspects of transfixed leukocytes and red cells. Cytometry B Clin Cytom. 2010;78:267–278. doi: 10.1002/cyto.b.20510. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann N, McBride ML, Yamada Y, Hudson SA, Jones C, Cromie KD, et al. Siglec-F antibody administration to mice selectively reduces blood and tissue eosinophils. Allergy. 2008;63:1156–1163. doi: 10.1111/j.1398-9995.2008.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Moilanen E, Kankaanranta H. Enhancement of human eosinophil apoptosis by fluticasone propionate, budesonide, and beclomethasone. Eur J Pharmacol. 2000;406:325–332. doi: 10.1016/s0014-2999(00)00690-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X, Moilanen E, Adcock IM, Lindsay MA, Kankaanranta H. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002;71:1523–1534. doi: 10.1016/s0024-3205(02)01921-5. [DOI] [PubMed] [Google Scholar]
- 23.Lamas AM, Leon OG, Schleimer RP. Glucocorticoids inhibit eosinophil responses to granulocyte-macrophage colony-stimulating factor. J Immunol. 1991;147:254–259. [PubMed] [Google Scholar]
- 24.Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine- mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- 25.Lee E, Robertson T, Smith J, Kilfeather S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am J Respir Crit Care Med. 2000;161:1881–1886. doi: 10.1164/ajrccm.161.6.9907054. [DOI] [PubMed] [Google Scholar]
- 26.Yasui K, Hu B, Nakazawa T, Agematsu K, Komiyama A. Theophylline accelerates human granulocyte apoptosis not via phosphodiesterase inhibition. J Clin Invest. 1997;100:1677–1684. doi: 10.1172/JCI119692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto K, Terakawa M, Miura K, Fukuda S, Nakajima T, Saito H. Extremely rapid and intense induction of apoptosis in human eosinophils by anti-CD30 antibody treatment in vitro. J Immunol. 2004;172:2186–2193. doi: 10.4049/jimmunol.172.4.2186. [DOI] [PubMed] [Google Scholar]
- 28.Woolley KL, Gibson PG, Carty K, Wilson AJ, Twaddell SH, Woolley MJ. Eosinophil apoptosis and the resolution of airway inflammation in asthma. Am J Respir Crit Care Med. 1996;154:237–243. doi: 10.1164/ajrccm.154.1.8680686. [DOI] [PubMed] [Google Scholar]
- 29.Druilhe A, Wallaert B, Tsicopoulos A, Lapa e Silva JR, Tillie-Leblond I, Tonnel AB, et al. Apoptosis, proliferation, and expression of Bcl-2, Fas, and Fas ligand in bronchial biopsies from asthmatics. Am J Respir Cell Mol Biol. 1998;19:747–757. doi: 10.1165/ajrcmb.19.5.3166. [DOI] [PubMed] [Google Scholar]
- 30.Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- 31.Duncan CJ, Lawrie A, Blaylock MG, Douglas JG, Walsh GM. Reduced eosinophil apoptosis in induced sputum correlates with asthma severity. Eur Respir J. 2003;22:484–490. doi: 10.1183/09031936.03.00109803a. [DOI] [PubMed] [Google Scholar]
- 32.Gochuico BR, Miranda KM, Hessel EM, De Bie JJ, Van Oosterhout AJ, Cruikshank WW, et al. Airway epithelial Fas ligand expression: potential role in modulating bronchial inflammation. Am J Physiol. 1998;274:L444–L449. doi: 10.1152/ajplung.1998.274.3.L444. [DOI] [PubMed] [Google Scholar]
- 33.Solarewicz-Madejek K, Basinski TM, Crameri R, Akdis M, Akkaya A, Blaser K, et al. T cells and eosinophils in bronchial smooth muscle cell death in asthma. Clin Exp Allergy. 2009;39:845–855. doi: 10.1111/j.1365-2222.2009.03244.x. [DOI] [PubMed] [Google Scholar]
- 34.Darveau ME, Jacques E, Rouabhia M, Hamid Q, Chakir J. Increased T-cell survival by structural bronchial cells derived from asthmatic subjects cultured in an engineered human mucosa. J Allergy Clin Immunol. 2008;121:692–699. doi: 10.1016/j.jaci.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Spinozzi F, Agea E, Fizzotti M, Bassotti G, Russano A, Droetto S, et al. Role of T-helper type 2 cytokines in down-modulation of Fas mRNA and receptor on the surface of activated CD4(+) T cells: molecular basis for the persistence of the allergic immune response. FASEB J. 1998;12:1747–1753. doi: 10.1096/fasebj.12.15.1747. [DOI] [PubMed] [Google Scholar]
- 36.Sharma SK, Almeida FA, Kierstein S, Hortobagyi L, Lin T, Larkin A, et al. Systemic FasL neutralization increases eosinophilic inflammation in a mouse model of asthma. Allergy. 2012;67:328–335. doi: 10.1111/j.1398-9995.2011.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uller L, Rydell-Tormanen K, Persson CG, Erjefalt JS. Anti-Fas mAb-induced apoptosis and cytolysis of airway tissue eosinophils aggravates rather than resolves established inflammation. Respir Res. 2005;6:90. doi: 10.1186/1465-9921-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 39.Park CS, Choi YS, Ki SY, Moon SH, Jeong SW, Uh ST, et al. Granulocyte macrophage colony-stimulating factor is the main cytokine enhancing survival of eosinophils in asthmatic airways. Eur Respir J. 1998;12:872–878. doi: 10.1183/09031936.98.12040872. [DOI] [PubMed] [Google Scholar]
- 40.Adachi T, Motojima S, Hirata A, Fukuda T, Makino S. Eosinophil viability-enhancing activity in sputum from patients with bronchial asthma, contributions of interleukin-5 and granulocyte/macrophage colony-stimulating factor. Am J Respir Crit Care Med. 1995;151:618–623. doi: 10.1164/ajrccm.151.3.7881646. [DOI] [PubMed] [Google Scholar]
- 41.Tai PC, Sun L, Spry CJ. Effects of IL-5, granulocyte/macrophage colony-stimulating factor (GM-CSF) and IL-3 on the survival of human blood eosinophils in vitro. Clin Exp Immunol. 1991;85:312–316. doi: 10.1111/j.1365-2249.1991.tb05725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Bruggen T, Caldenhoven E, Kanters D, Coffer P, Raaijmakers JA, Lammers JW, et al. Interleukin-5 signaling in human eosinophils involves JAK2 tyrosine kinase and Stat1 alpha. Blood. 1995;85:1442–1448. [PubMed] [Google Scholar]
- 43.Yousefi S, Hoessli DC, Blaser K, Mills GB, Simon HU. Requirement of lyn and syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R. Lyn, Jak2, and raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only raf-1 kinase is essential for eosinophil activation and degranulation. J Exp Med. 1998;188:421–429. doi: 10.1084/jem.188.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosas M, Dijkers PF, Lindemans CL, Lammers JW, Koenderman L, Coffer PJ. IL-5-mediated eosinophil survival requires inhibition of GSK-3 and correlates with beta-catenin relocalization. J Leukoc Biol. 2006;80:186–195. doi: 10.1189/jlb.1105636. [DOI] [PubMed] [Google Scholar]
- 46.Pazdrak K, Schreiber D, Forsythe P, Justement L, Alam R. The intracellular signal transduction mechanism of interleukin 5 in eosinophils: the involvement of lyn tyrosine kinase and the ras-raf-1-MEK-microtubule-associated protein kinase pathway. J Exp Med. 1995;181:1827–1834. doi: 10.1084/jem.181.5.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pazdrak K, Stafford S, Alam R. The activation of the jak-STAT 1 signaling pathway by IL-5 in eosinophils. J Immunol. 1995;155:397–402. [PubMed] [Google Scholar]
- 48.Coffer PJ, Schweizer RC, Dubois GR, Maikoe T, Lammers JW, Koenderman L. Analysis of signal transduction pathways in human eosinophils activated by chemoattractants and the T-helper 2-derived cytokines interleukin-4 and interleukin-5. Blood. 1998;91:2547–2557. [PubMed] [Google Scholar]
- 49.Shen ZJ, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat Immunol. 2009;10:257–265. doi: 10.1038/ni.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kankaanranta H, De Souza PM, Barnes PJ, Salmon M, Giembycz MA, Lindsay MA. SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils. J Pharmacol Exp Ther. 1999;290:621–628. [PubMed] [Google Scholar]
- 51.Miike S, Nakao A, Hiraguri M, Kurasawa K, Saito Y, Iwamoto I. Involvement of JAK2, but not PI 3-kinase/Akt and MAP kinase pathways, in anti-apoptotic signals of GM-CSF in human eosinophils. J Leukoc Biol. 1999;65:700–706. doi: 10.1002/jlb.65.5.700. [DOI] [PubMed] [Google Scholar]
- 52.Dewson G, Cohen GM, Wardlaw AJ. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood. 2001;98:2239–2247. doi: 10.1182/blood.v98.7.2239. [DOI] [PubMed] [Google Scholar]
- 53.Alam R, Forsythe P, Stafford S, Fukuda Y. Transforming growth factor beta abrogates the effects of hematopoietins on eosinophils and induces their apoptosis. J Exp Med. 1994;179:1041–1045. doi: 10.1084/jem.179.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pazdrak K, Justement L, Alam R. Mechanism of inhibition of eosinophil activation by transforming growth factor-beta. Inhibition of Lyn, MAP, Jak2 kinases and STAT1 nuclear factor. J Immunol. 1995;155:4454–4458. [PubMed] [Google Scholar]
- 55.Xie Q, Shen ZJ, Oh J, Chu H, Malter JS. Transforming growth factor-1 antagonizes interleukin-5 pro-survival signaling by activating calpain-1 in primary human eosinophils. J Clin Cell Immunol. 2011;(Suppl 1):003. doi: 10.4172/2155-9899.S1-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kankaanranta H, Lindsay MA, Giembycz MA, Zhang X, Moilanen E, Barnes PJ. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106:77–83. doi: 10.1067/mai.2000.107038. [DOI] [PubMed] [Google Scholar]
- 57.Simon HU, Yousefi S, Schranz C, Schapowal A, Bachert C, Blaser K. Direct demonstration of delayed eosinophil apoptosis as a mechanism causing tissue eosinophilia. J Immunol. 1997;158:3902–3908. [PubMed] [Google Scholar]
- 58.Hoontrakoon R, Chu HW, Gardai SJ, Wenzel SE, McDonald P, Fadok VA, et al. Interleukin-15 inhibits spontaneous apoptosis in human eosinophils via autocrine production of granulocyte macrophage-colony stimulating factor and nuclear factor-kappa B activation. Am J Respir Cell Mol Biol. 2002;26:404–412. doi: 10.1165/ajrcmb.26.4.4517. [DOI] [PubMed] [Google Scholar]
- 59.Wong CK, Cheung PF, Lam CW. Leptin-mediated cytokine release and migration of eosinophils: implications for immunopathophysiology of allergic inflammation. Eur J Immunol. 2007;37:2337–2348. doi: 10.1002/eji.200636866. [DOI] [PubMed] [Google Scholar]
- 60.Temkin V, Levi-Schaffer F. Mechanism of tumour necrosis factor alpha mediated eosinophil survival. Cytokine. 2001;15:20–26. doi: 10.1006/cyto.2001.0890. [DOI] [PubMed] [Google Scholar]
- 61.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2010;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 62.Coward WR, Sagara H, Wilson SJ, Holgate ST, Church MK. Allergen activates peripheral blood eosinophil nuclear factor-kappa B to generate granulocyte macrophage-colony stimulating factor, tumour necrosis factor-alpha and interleukin-8. Clin Exp Allergy. 2004;34:1071–1078. doi: 10.1111/j.1365-2222.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- 63.Wong CK, Cheung PF, Ip WK, Lam CW. Intracellular signaling mechanisms regulating toll-like receptor-mediated activation of eosinophils. Am J Respir Cell Mol Biol. 2007;37:85–96. doi: 10.1165/rcmb.2006-0457OC. [DOI] [PubMed] [Google Scholar]
- 64.Ilmarinen P, Hasala H, Sareila O, Moilanen E, Kankaanranta H. Bacterial DNA delays human eosinophil apoptosis. Pulm Pharmacol Ther. 2009;22:167–176. doi: 10.1016/j.pupt.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Ilmarinen P, Kankaanranta H. Eosinophil apoptosis as a therapeutic target in allergic asthma. Basic Clin Pharmacol Toxicol. 2014;114(1):109–117. doi: 10.1111/bcpt.12163. [DOI] [PubMed] [Google Scholar]
- 66.Fujihara S, Jaffray E, Farrow SN, Rossi AG, Haslett C, Hay RT. Inhibition of NF-kappa B by a cell permeable form of I kappa B alpha induces apoptosis in eosinophils. Biochem Biophys Res Commun. 2005;326:632–637. doi: 10.1016/j.bbrc.2004.11.090. [DOI] [PubMed] [Google Scholar]
- 67.Levi-Schaffer F, Temkin V, Malamud V, Feld S, Zilberman Y. Mast cells enhance eosinophil survival in vitro: role of TNF-alpha and granulocyte- macrophage colony-stimulating factor. J Immunol. 1998;160:5554–5562. [PubMed] [Google Scholar]
- 68.Ochiai K, Iwamoto I, Takahashi H, Yoshida S, Tomioka H, Yoshida S. Effect of IL-4 and interferon-gamma (IFN-gamma) on IL-3- and IL-5-induced eosinophil differentiation from human cord blood mononuclear cells. Clin Exp Immunol. 1995;99:124–128. doi: 10.1111/j.1365-2249.1995.tb03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valerius T, Repp R, Kalden JR, Platzer E. Effects of IFN on human eosinophils in comparison with other cytokines. A novel class of eosinophil activators with delayed onset of action. J Immunol. 1990;145:2950–2958. [PubMed] [Google Scholar]
- 70.Conus S, Bruno A, Simon HU. Leptin is an eosinophil survival factor. J Allergy Clin Immunol. 2005;116:1228–1234. doi: 10.1016/j.jaci.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 71.Bureau F, Seumois G, Jaspar F, Vanderplasschen A, Detry B, Pastoret PP, et al. CD40 engagement enhances eosinophil survival through induction of cellular inhibitor of apoptosis protein 2 expression: possible involvement in allergic inflammation. J Allergy Clin Immunol. 2002;110:443–449. doi: 10.1067/mai.2002.126781. [DOI] [PubMed] [Google Scholar]
- 72.Lei XF, Ohkawara Y, Stampfli MR, Mastruzzo C, Marr RA, Snider D, et al. Disruption of antigen-induced inflammatory responses in CD40 ligand knockout mice. J Clin Invest. 1998;101:1342–1353. doi: 10.1172/JCI1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilmarinen-Salo P, Moilanen E, Kinnula VL, Kankaanranta H. Nitric oxide-induced eosinophil apoptosis is dependent on mitochondrial permeability transition (mPT), JNK and oxidative stress: apoptosis is preceded but not mediated by early mPT-dependent JNK activation. Respir Res. 2012;13:73. doi: 10.1186/1465-9921-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nopp A, Lundahl J, Stridh H. Caspase activation in the absence of mitochondrial changes in granulocyte apoptosis. Clin Exp Immunol. 2002;128:267–274. doi: 10.1046/j.1365-2249.2002.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ilmarinen-Salo P, Moilanen E, Kankaanranta H. Nitric oxide induces apoptosis in GM-CSF-treated eosinophils via caspase-6-dependent lamin and DNA fragmentation. Pulm Pharmacol Ther. 2010;23:365–371. doi: 10.1016/j.pupt.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Bailey RW, Nguyen T, Robertson L, Gibbons E, Nelson J, Christensen RE, et al. Sequence of physical changes to the cell membrane during glucocorticoid-induced apoptosis in S49 lymphoma cells. Biophys J. 2009;96:2709–2718. doi: 10.1016/j.bpj.2008.12.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiegand UK, Corbach S, Prescott AR, Savill J, Spruce BA. The trigger to cell death determines the efficiency with which dying cells are cleared by neighbours. Cell Death Differ. 2001;8:734–746. doi: 10.1038/sj.cdd.4400867. [DOI] [PubMed] [Google Scholar]
- 78.Jessel R, Haertel S, Socaciu C, Tykhonova S, Diehl HA. Kinetics of apoptotic markers in exogeneously induced apoptosis of EL4 cells. J Cell Mol Med. 2002;6:82–92. doi: 10.1111/j.1582-4934.2002.tb00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kenis H, van Genderen H, Deckers NM, Lux PA, Hofstra L, Narula J, et al. Annexin A5 inhibits engulfment through internalization of PS-expressing cell membrane patches. Exp Cell Res. 2006;312:719–726. doi: 10.1016/j.yexcr.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 80.Archana M, Bastian, Yogesh TL, Kumaraswamy KL. Various methods available for detection of apoptotic cells—a review. Indian J Cancer. 2013;50:274–283. doi: 10.4103/0019-509X.118720. [DOI] [PubMed] [Google Scholar]
- 81.Wlodkowic D, Skommer J, Darzynkiewicz Z. Cytometry of apoptosis. Historical perspective and new advances. Exp Oncol. 2012;34:255–262. [PMC free article] [PubMed] [Google Scholar]
- 82.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: orchestrators of apoptosis. Biochim Biophys Acta. 2011;1813:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 83.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 84.Segal M, Niazi S, Simons MP, Galati SA, Zangrilli JG. Bid activation during induction of extrinsic and intrinsic apoptosis in eosinophils. Immunol Cell Biol. 2007;85:518–524. doi: 10.1038/sj.icb.7100075. [DOI] [PubMed] [Google Scholar]
- 85.Maret M, Ruffie C, Letuve S, Phelep A, Thibaudeau O, Marchal J, et al. A role for bid in eosinophil apoptosis and in allergic airway reaction. J Immunol. 2009;182:5740–5747. doi: 10.4049/jimmunol.0800864. [DOI] [PubMed] [Google Scholar]
- 86.Gardai SJ, Hoontrakoon R, Goddard CD, Day BJ, Chang LY, Henson PM, et al. Oxidant-mediated mitochondrial injury in eosinophil apoptosis: enhancement by glucocorticoids and inhibition by granulocyte-macrophage colony- stimulating factor. J Immunol. 2003;170:556–566. doi: 10.4049/jimmunol.170.1.556. [DOI] [PubMed] [Google Scholar]
- 87.Zangrilli J, Robertson N, Shetty A, Wu J, Hastie A, Fish JE, et al. Effect of IL-5, glucocorticoid, and Fas ligation on Bcl-2 homologue expression and caspase activation in circulating human eosinophils. Clin Exp Immunol. 2000;120:12–21. doi: 10.1046/j.1365-2249.2000.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Druilhe A, Arock M, Le Goff L, Pretolani M. Human eosinophils express Bcl-2 family proteins: modulation of Mcl-1 expression by IFN-gamma. Am J Respir Cell Mol Biol. 1998;18:315–322. doi: 10.1165/ajrcmb.18.3.3019. [DOI] [PubMed] [Google Scholar]
- 89.Dewson G, Walsh GM, Wardlaw AJ. Expression of Bcl-2 and its homologues in human eosinophils. Modulation by interleukin-5. Am J Respir Cell Mol Biol. 1999;20:720–728. doi: 10.1165/ajrcmb.20.4.3453. [DOI] [PubMed] [Google Scholar]
- 90.Sivertson KL, Seeds MC, Long DL, Peachman KK, Bass DA. The differential effect of dexamethasone on granulocyte apoptosis involves stabilization of Mcl-1L in neutrophils but not in eosinophils. Cell Immunol. 2007;246:34–45. doi: 10.1016/j.cellimm.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vignola AM, Chanez P, Chiappara G, Siena L, Merendino A, Reina C, et al. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- 92.Maa SH, Wang CH, Liu CY, Lin HC, Huang KH, Kuo HP. Endogenous nitric oxide downregulates the Bcl-2 expression of eosinophils through mitogen-activated protein kinase in bronchial asthma. J Allergy Clin Immunol. 2003;112:761–767. doi: 10.1016/s0091-6749(03)02009-8. [DOI] [PubMed] [Google Scholar]
- 93.El-Gamal Y, Heshmat N, Mahran M, El-Gabbas Z. Expression of the apoptosis inhibitor bcl-2 in sputum eosinophils from children with acute asthma. Clin Exp Allergy. 2004;34:1701–1706. doi: 10.1111/j.1365-2222.2004.02089.x. [DOI] [PubMed] [Google Scholar]
- 94.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 95.Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)- dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–233. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 96.Letuve S, Druilhe A, Grandsaigne M, Aubier M, Pretolani M. Involvement of caspases and of mitochondria in Fas ligation-induced eosinophil apoptosis: modulation by interleukin-5 and interferon-gamma. J Leukoc Biol. 2001;70:767–775. [PubMed] [Google Scholar]
- 97.Letuve S, Druilhe A, Grandsaigne M, Aubier M, Pretolani M. Critical role of mitochondria, but not caspases, during glucocorticosteroid-induced human eosinophil apoptosis. Am J Respir Cell Mol Biol. 2002;26:565–571. doi: 10.1165/ajrcmb.26.5.4671. [DOI] [PubMed] [Google Scholar]
- 98.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fuentes-Prior P, Salvesen GS. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem J. 2004;384:201–232. doi: 10.1042/BJ20041142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang JP, Wong CK, Lam CW. Role of caspases in dexamethasone-induced apoptosis and activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase in human eosinophils. Clin Exp Immunol. 2000;122:20–27. doi: 10.1046/j.1365-2249.2000.01344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kankaanranta H, Ilmarinen P, Zhang X, Nissinen E, Moilanen E. Antieosinophilic activity of orazipone. Mol Pharmacol. 2006;69:1861–1870. doi: 10.1124/mol.105.021170. [DOI] [PubMed] [Google Scholar]
- 102.Kankaanranta H, Janka-Junttila M, Ilmarinen-Salo P, Ito K, Jalonen U, Ito M, et al. Histone deacetylase inhibitors induce apoptosis in human eosinophils and neutrophils. J Inflamm (Lond) 2010;7:9. doi: 10.1186/1476-9255-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hasala H, Giembycz MA, Janka-Junttila M, Moilanen E, Kankaanranta H. Histamine reverses IL-5-afforded human eosinophil survival by inducing apoptosis: pharmacological evidence for a novel mechanism of action of histamine. Pulm Pharmacol Ther. 2008;21:222–233. doi: 10.1016/j.pupt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Daigle I, Simon HU. Critical role for caspases 3 and 8 in neutrophil but not eosinophil apoptosis. Int Arch Allergy Immunol. 2001;126:147–156. doi: 10.1159/000049506. [DOI] [PubMed] [Google Scholar]
- 105.Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niazi S, Robertson NM, Agrawal A, Hastie AT, Peters SP, Zangrilli J. Overlap between death receptor and non-receptor-mediated mechanisms during apoptosis in human eosinophils. Chest. 2003;123:345S. doi: 10.1378/chest.123.3_suppl.345s. [DOI] [PubMed] [Google Scholar]
- 107.Oh J, Malter JS. Pin1-FADD interactions regulate Fas-mediated apoptosis in activated eosinophils. J Immunol. 2013;190:4937–4945. doi: 10.4049/jimmunol.1202646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allsopp TE, McLuckie J, Kerr LE, Macleod M, Sharkey J, Kelly JS. Caspase 6 activity initiates caspase 3 activation in cerebellar granule cell apoptosis. Cell Death Differ. 2000;7:984–993. doi: 10.1038/sj.cdd.4400733. [DOI] [PubMed] [Google Scholar]
- 110.Ruchaud S, Korfali N, Villa P, Kottke TJ, Dingwall C, Kaufmann SH, et al. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. EMBO J. 2002;21:1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 112.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer. 2011;11:364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- 113.Harwood SM, Yaqoob MM, Allen DA. Caspase and calpain function in cell death: bridging the gap between apoptosis and necrosis. Ann Clin Biochem. 2005;42:415–431. doi: 10.1258/000456305774538238. [DOI] [PubMed] [Google Scholar]
- 114.Kankaanranta H, Giembycz MA, Barnes PJ, Haddad el-B, Saarelainen S, Zhang X, et al. Hydrogen peroxide reverses IL-5 afforded eosinophil survival and promotes constitutive human eosinophil apoptosis. Int Arch Allergy Immunol. 2002;127:73–78. doi: 10.1159/000048171. [DOI] [PubMed] [Google Scholar]
- 115.Wedi B, Straede J, Wieland B, Kapp A. Eosinophil apoptosis is mediated by stimulators of cellular oxidative metabolisms and inhibited by antioxidants: involvement of a thiol-sensitive redox regulation in eosinophil cell death. Blood. 1999;94:2365–2373. [PubMed] [Google Scholar]
- 116.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 117.Martinez-Losa M, Cortijo J, Juan G, Ramon M, Sanz MJ, Morcillo EJ. Modulatory effects of N-acetyl-L-cysteine on human eosinophil apoptosis. Eur Respir J. 2007;30:436–442. doi: 10.1183/09031936.00073706. [DOI] [PubMed] [Google Scholar]
- 118.Nissim Ben Efraim AH, Eliashar R, Levi-Schaffer F. Hypoxia modulates human eosinophil function. Clin Mol Allergy. 2010;8:10. doi: 10.1186/1476-7961-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kano G, Almanan M, Bochner BS, Zimmermann N. Mechanism of siglec-8- mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437–445. doi: 10.1016/j.jaci.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 121.Wancket LM, Frazier WJ, Liu Y. Mitogen-activated protein kinase phosphatase (MKP)-1 in immunology, physiology, and disease. Life Sci. 2012;90:237–248. doi: 10.1016/j.lfs.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eichhorst ST, Muller M, Li-Weber M, Schulze-Bergkamen H, Angel P, Krammer PH. A novel AP-1 element in the CD95 ligand promoter is required for induction of apoptosis in hepatocellular carcinoma cells upon treatment with anticancer drugs. Mol Cell Biol. 2000;20:7826–7837. doi: 10.1128/mcb.20.20.7826-7837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Guan B, Yue P, Lotan R, Sun SY. Evidence that the human death receptor 4 is regulated by activator protein 1. Oncogene. 2002;21:3121–3129. doi: 10.1038/sj.onc.1205430. [DOI] [PubMed] [Google Scholar]
- 124.Schroeter H, Boyd CS, Ahmed R, Spencer JP, Duncan RF, Rice-Evans C, et al. c-Jun N-terminal kinase (JNK)-mediated modulation of brain mitochondria function: new target proteins for JNK signalling in mitochondrion-dependent apoptosis. Biochem J. 2003;372:359–369. doi: 10.1042/BJ20030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bogoyevitch MA, Kobe B. Uses for JNK: the many and varied substrates of the c-Jun N-terminal kinases. Microbiol Mol Biol Rev. 2006;70:1061–1095. doi: 10.1128/MMBR.00025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lin X, Wang YJ, Li Q, Hou YY, Hong MH, Cao YL, et al. Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. FEBS J. 2009;276:2022–2036. doi: 10.1111/j.1742-4658.2009.06938.x. [DOI] [PubMed] [Google Scholar]
- 128.Lu C, Zhu F, Cho YY, Tang F, Zykova T, Ma WY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hasala H, Zhang X, Saarelainen S, Moilanen E, Kankaanranta H. c-Jun N-terminal kinase mediates constitutive human eosinophil apoptosis. Pulm Pharmacol Ther. 2007;20:580–587. doi: 10.1016/j.pupt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 130.Wilson DJ, Fortner KA, Lynch DH, Mattingly RR, Macara IG, Posada JA, et al. JNK, but not MAPK, activation is associated with Fas-mediated apoptosis in human T cells. Eur J Immunol. 1996;26:989–994. doi: 10.1002/eji.1830260505. [DOI] [PubMed] [Google Scholar]
- 131.Cahill MA, Peter ME, Kischkel FC, Chinnaiyan AM, Dixit VM, Krammer PH, et al. CD95 (APO-1/Fas) induces activation of SAP kinases downstream of ICE-like proteases. Oncogene. 1996;13:2087–2096. [PubMed] [Google Scholar]
- 132.Hebestreit H, Dibbert B, Balatti I, Braun D, Schapowal A, Blaser K, et al. Disruption of Fas receptor signaling by nitric oxide in eosinophils. J Exp Med. 1998;187:415–425. doi: 10.1084/jem.187.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yin H, Shi Z, Jiao S, Chen C, Wang W, Greene MI, et al. Germinal center kinases in immune regulation. Cell Mol Immunol. 2012;9:439–445. doi: 10.1038/cmi.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ura S, Nishina H, Gotoh Y, Katada T. Activation of the c-Jun N-terminal kinase pathway by MST1 is essential and sufficient for the induction of chromatin condensation during apoptosis. Mol Cell Biol. 2007;27:5514–5522. doi: 10.1128/MCB.00199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Souza PM, Kankaanranta H, Michael A, Barnes PJ, Giembycz MA, Lindsay MA. Caspase-catalyzed cleavage and activation of Mst1 correlates with eosinophil but not neutrophil apoptosis. Blood. 2002;99:3432–3438. doi: 10.1182/blood.v99.9.3432. [DOI] [PubMed] [Google Scholar]