Abstract
BACKGROUND
One in 88 children in the US is thought to have one of the autism spectrum disorders (ASDs). ASDs are characterized by social impairments and communication problems. Growth factors and their receptors may play a role in the etiology of ASDs. Research has shown that epidermal growth factor receptor (EGFR) activation is associated with nerve cell development and repair. This study was designed to measure plasma levels of EGFR in autistic children and correlate these levels with its ligand, epidermal growth factor, other related putative biomarkers such as hepatocyte growth factor (HGF), the ligand for MET (MNNG HOS transforming gene) receptor, as well as the symptom severity of 19 different behavioral symptoms.
SUBJECTS AND METHODS
Plasma EGFR concentration was measured in 33 autistic children and 34 age- and gender-similar neurotypical controls, using an enzyme-linked immunosorbent assay. Plasma EGFR levels were compared to putative biomarkers known to be associated with EGFR and MET and severity levels of 19 autism-related symptoms.
RESULTS
We found plasma EGFR levels significantly higher in autistic children, when compared to neurotypical controls. EGFR levels correlated with HGF and high-mobility group protein B1 (HMGB1) levels, but not other tested putative biomarkers, and EGFR levels correlated significantly with severity of expressive language, conversational language, focus/attention, hyperactivity, eye contact, and sound sensitivity deficiencies.
CONCLUSIONS
These results suggest a relationship between increased plasma EGFR levels and designated symptom severity in autistic children. A strong correlation between plasma EGFR and HGF and HMGB1 suggests that increased EGFR levels may be associated with the HGF/Met signaling pathway, as well as inflammation.
Keywords: EGFR, EGF, HGF, HMGB1, autism, symptom severity
Introduction
Autism spectrum disorders (ASDs) comprise a diverse group of conditions characterized by problems in social actions, communication deficits, and stereotypical repetitive behaviors. One in 88 children in the US is thought to have ASD.1
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a member of the ErbB family of receptors, and a member of a subfamily of four closely related receptor tyrosine kinases (RTKs): EGFR (ErbB-1), HER2/c-neu (ErbB-2), Her 3 (ErbB-3), and Her 4 (ErbB-4). These cell-surface receptors are members of the epidermal growth factor (EGF) family of extracellular protein ligands.2 EGFR is activated by binding to its specific ligands, including EGF and transforming growth factor α. Upon activation, EGFR changes to a homodimer,3 which stimulates its intrinsic intracellular protein-tyrosine kinase activity.4 This autophosphorylation elicits downstream activation of several signal transduction cascades, principally the MAPK/ERK (mitogen activated kinases/extracellular signal regulated kinases), Akt and JNK pathways, leading to DNA synthesis and cell proliferation.5
EGFR is important in nerve cell development and repair. Activation of EGFR is a common, regulatory pathway that triggers quiescent astrocytes into reactive astrocytes in response to neural injuries in the optic nerve, and perhaps other parts of the CNS.6 An EGFR inhibitor, erlotinib, has shown delayed disease progression in a mouse model of ALS.7 Alzheimer’s-like memory loss has been reversed in animal models by blocking EGFR signaling.8 Inhibition of EGFR/MAPK suppresses microglia activation and associated cytokine production. This reduces neuroinflammation-associated secondary damage, thus providing neuroprotection to spinal cord injury rats.9 Recently, research has demonstrated that EGFR is a preferred target for treating Amyloid-β–induced memory loss.9
Genetic variants of RTKs, such as MET, have been implicated in the etiology of autism.10,11 Growth factors, hepatocyte growth factor (HGF) and EGF, which are the signaling ligands for MET and EGFR, respectively, are decreased in autism.12,13
Attachment of growth factors to their receptors regulates many aspects of neuronal growth and differentiation. Its signaling also is in part responsible for neuronal survival, migration, and synaptic signaling.14 These growth factors also act as immune modulators.15–18 Since there is immune dysfunction in the nervous system19–22 in children with autism, it is plausible to think that growth factors are involved.
EGF helps in controlling cell division and differentiation of nervous tissue23,24 and has been found to promote wound healing.25
EGF concentration and gene mutation has been linked to autism. Frequency of EGF single-nucleotide polymorphisms is in children with autism.26 Plasma EGF levels in adults27 and children with autism11,28 were found to be significantly decreased. However, in one report of younger autistic children, EGF was increased.29
High-mobility group protein B1 (HMGB1) is a marker of inflammatory diseases. It acts by binding to lipopolysaccharides and IL-1 (Interleukin-1), to initiate Toll-like receptor 4–mediated inflammation30 and then is released from activated macrophages. HMGB1 is dependent on various processes such as phosphorylation by calcium-dependent protein kinase C,31 acetylation, and methylation32 and was found to be associated with the generation and recurrence of seizures in experimental animals.33,34 This inflammatory marker is increased in autistic children,35 and in neural tissue and malignant cells, and receptor activation by HMGB1 leads to MAPK activation with increased cell growth.36
Because several studies have associated abnormal levels of EGF and HGF with autism, and the EGF/EGFR signaling pathway plays a role in regulating neural growth, proliferation, differentiation, and migration, it is important to continue studying the elements of this pathway to determine if abnormal levels are associated with abnormal neurodevelopment and symptoms in individuals with autism.
This study was designed to determine plasma levels of EGFR in children with autism and evaluate the correlation of these EGFR levels with inflammatory and regulatory biomarkers, as well as severity of 19 different symptoms related to autism.
Materials and Methods
Enzyme-linked immunosorbent assays (ELISAs) were used to measure plasma EGFR and other biomarkers (ELISA kits, R and D Systems, Minneapolis, MN, and USCN Life Sciences, Wuhan, China). The ELISA and serum protocols have been previously reported and are discussed below.37
All reagents and specimens were equilibrated to room temperature before each assay was performed. A 1:51 dilution of the patient samples was prepared by mixing 10 μL of the patient’s plasma with 0.5 mL of plasma diluent. One hundred microliters of calibrators (20–200 Eu/mL antibodies), positive and negative control plasma, plasma diluent alone, and diluted patient samples were added to the appropriate microwells of a microculture plate (each well contained affinity-purified polyclonal IgG to the appropriate marker). Wells were incubated for 60 minutes (±5 minutes) at room temperature, and then washed 4× with wash buffer. One hundred microliters of prediluter anti-human IgG conjugated with HRP (horseradish peroxidase) was added to all microwells, incubated for 30 minutes (±5 minutes) at room temperature, and then washed 4× with wash buffer. One hundred microliters of enzyme substrate was added to each microwell. After approximately 30 minutes at room temperature, the reaction was stopped by adding 50 μL of 1 M sulfuric acid, and then the wells were read at 405 nm with an ELISA reader (BioRad Laboratories, Inc., Hercules, CA, USA).
Serums
All serums, experimental and control serums, were treated in an identical manner, frozen at −70 °C immediately after collection and cell/serum separation, and then stored at −70 °C until thawing for use in ELISAs.
Subjects
Plasma and diagnostic criteria (below) have been previously reported.37 Plasma EGFR concentration was measured in 33 autistic children and 34 age- and gender-similar neurotypical controls.
The diagnostic criteria used in this study were defined by DSM-IV (Diagnostic and Statistical Manual of Mental Disorders) criteria. In 2012, the separate diagnostic labels of autistic disorder, Asperger’s disorder, and pervasive developmental disorder-not otherwise specified were replaced by one umbrella termed “Autism Spectrum Disorder.”
Plasma from consecutive individuals with diagnosed autism (n = 33; 28 male; mean age 10.3 years) and controls (n = 34; 25 male; mean age 9.6 years) was obtained from patients presenting at the Health Research Institute (HRI, which is a comprehensive treatment and research center, specializing in the care of persons with neurological disorders, including autism) over a 2-year period. All autistic individuals who presented to HRI were asked to participate. Patients who participated in this study were randomly chosen from all patients who volunteered. Neurotypical control plasma was obtained from HRI and the Autism Genetic Resource Exchange (AGRE, which is a repository of biomaterials and phenotypic and genotypic data to aid research on ASDs) and randomly chosen from a selection of about 200 samples. The autistic individuals in this study met the DSM-IV criteria, and many were diagnosed using the Autism Diagnostic Interview–Revised before presenting to the HRI.
Patient consent was obtained from all patients involved in this study, and this study was approved by the IRB of the HRI.
Severity of disease
The Pfeifer questionnaire, severity criteria, and statistical methodology have been previously reported.37
An autism symptom severity questionnaire was used to evaluate symptoms. The questionnaire (Pfeiffer questionnaire) asked parents or caregivers to assess the severity of the following symptoms: awareness, expressive language, receptive language, (conversational) pragmatic language, focus, attention, hyperactivity, impulsivity, perseveration, fine motor skills, gross motor skills, hypotonia (low muscle tone), tip toeing, rocking/pacing, stimming, obsessions/fixations, eye contact, sound sensitivity, light sensitivity, and tactile sensitivity. The symptoms were rated by parents/guardians on a scale of 0–5 (5 being the highest severity) for each of these behaviors.
Statistics
Inferential statistics were derived from unpaired t-test and odds ratios with 95% confidence intervals. Pearson moment correlation test was used to establish the degree of correlation between the groups (r > 0.3 or < −0.3; P < 0.1).
Results
We found that plasma levels of EGFR in children with autism (m = 13,200 ± 804 pg/mL) was significantly higher when compared to neurotypical controls (m = 11,312 ± 369 pg/mL) (P = 0.03) (Fig. 1).
Figure 1.
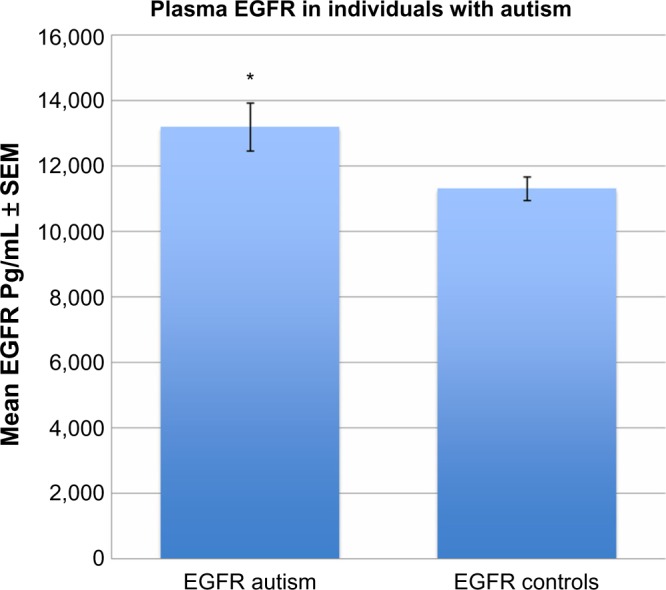
EGFR plasma levels in autistic children (*) are significantly higher than controls (P = 0.03).
EGFR levels correlated with HGF levels (r = 0.55; P = 0.007) (Fig. 2) and HMGB1 (r = 0.42; P = 0.003) (Fig. 3), and increased EGFR plasma levels correlated with severity of symptoms related to expressive language, conversational language, focus/attention, hyperactivity, eye contact, and sound sensitivity (Table 1).
Figure 2.
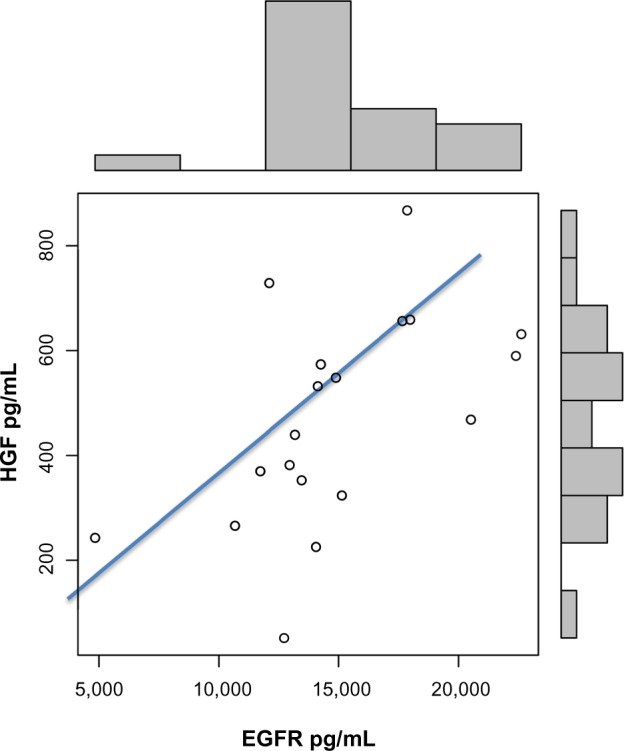
Correlation between EGFR and HGF in autistic children (r = 0.55; P = 0.007).
Figure 3.
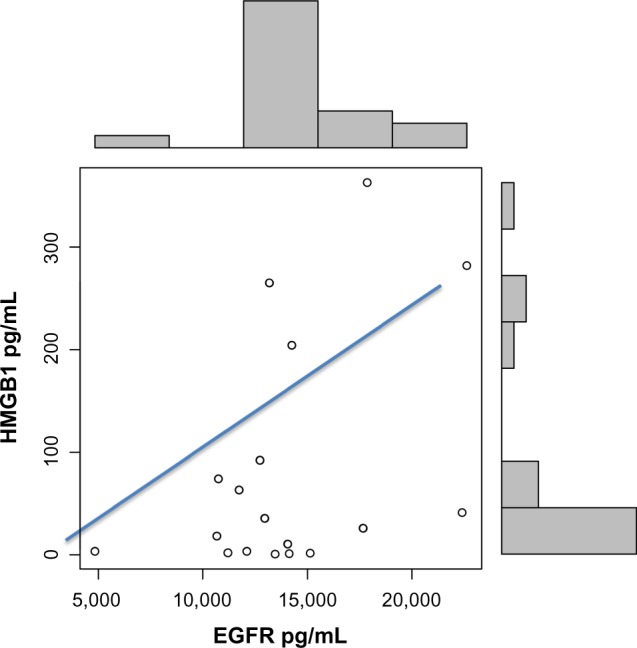
Correlation between EGFR and HMGB1 in autistic children (r = 0.42; P = 0.03).
Table 1.
Correlation between EGFR levels and sympton severity in autistic children.
| SYMPTON | SYMPTON SEVERITY CORRELATION WITH EGFR |
|---|---|
| Expressive language | r = 0.41; P = 0.01 |
| Conversational language | r = 0.31; P = 0.05 |
| Focus/Attention | r = 0.3; P = 0.06 |
| Hyperactivity | r = 0.32; P = 0.04 |
| Eye contact | r = 0.4; P = 0.02 |
| Sound sensitivity | r = 0.41; P = 0.01 |
Discussion
The MET and EGFR RTKs when signaled by their ligands, HGF and EGF, respectively, through a cascade of signaling reactions, modulate the ERK and PI3K intracellular regulatory pathways. ERK and PI3K activate mammalian targets, which, through other kinases, increase messenger RNA translation to influence developmental functions as diverse as the cell cycle, cell survival, differentiation, and motility. The key genes involved in met proto-oncogene–RTK have been implicated in ASD risk.10
Different genetic routes to altered RTKs, such as MET and EGFR, function by way of modulation of ERK/PI3K signaling pathways. It has been proposed that, combined with environmental factors, such as biochemical stressors, they may modulate the degree of dysfunction of the clinical features of autism.10
Our laboratory has demonstrated that plasma HGF and EGF (the ligands for MET and EGFR, respectively) are significantly decreased in autistic children,10,11 and in the data reported here, we have found that EGFR is increased. We also found in this study and previous studies that EGF and EGFR levels, but not HGF levels, correlate with symptom severity in autistic children.10,11
It is not surprising that RTKs are associated with autism, because other neurobehavioral and mood disorders such as bipolar disorder and schizophrenia are linked to altered markers associated with these pathways.38–40
It is plausible that higher EGFR levels are the result of altered RTK suppressor proteins such as mitogen-inducible gene protein (MIG-6). MIG-6 can be induced by HGF and functions as a negative feedback regulator of the MET pathway by inhibiting HGF-induced cell migration and proliferation.41 Overall, this RTK cascade, initiated by EGFR signaling, functions in cellular proliferation, differentiation, and survival, and its inappropriate activation is a common occurrence in human cancers. Specifically, as an example of the effect of a decreased suppressor protein, low Mig-6 expression is associated with high levels of EGFR and altered ERK phosphorylation in certain cancers.42
EGF has been found to play an integral role in nerve cell development,24,43 as well as intestinal mucosa development.44–46 The inflammatory marker HMGB1 is increased in autistic children.35 Our laboratory has previously reported a relationship between decreased EGF and increased HMGB1, as well as hyperactivity-related symptom severity in a group of autistic children.13
We also reported significantly decreased HGF in autistic children (mean age of approx. 10 years) with severe GI disease,12 which did not correlate with symptom severity. Since we previously found that EGF correlated with hyperactivity- related symptoms, it is reasonable to suggest that EGF is more important than HGF as a putative biomarker.
In this study, we found that plasma EGFR was significantly higher in autistic children. EGFR levels correlated with HGF and HMGB1 (as we had found with EGF), although the clustering of these data suggests that there may be more than one population distribution represented, and therefore may require a larger sample size to confirm. We also found that EGFR correlated with high symptom severity in six autism-related behaviors, including hyperactivity.
Our observations of decreased EGF and increased EGFR in autistic children suggest that decreased EGF may be the result of increased ligand binding to its receptor, resulting in decreased plasma/serum EGF. Decreased EGF has been associated with inflammatory conditions such as colitis.44,45 We did not find a correlation between decreased EGF and EGFR in this patient group. However, both EGF and EGFR correlate with HMGB1, suggesting that their abnormal levels may be associated with inflammation and generally increased neuroimmune activity. Our data suggest that decreased EGF and increased EGFR are associated with higher symptom severity, suggesting that their levels are associated with abnormal behavior and, as a result, their association with the etiology of autism.
In summary, these data support the notion that the EGFR/ERK pathway, associated with cell growth, differentiation, and division, may be associated with the etiology of autism. High EGFR levels are associated with many cancers. These increased levels are, in turn, associated with unregulated cell division. The data reported in this study demonstrate a correlation between increased EGFR and increased severity of autistic symptoms. We also found a correlation between EGFR and HGF and the inflammatory marker, HMGB1. This supports evidence that this (EGFR) RTK pathway, possibly associated with decreased HGF levels and inflammation, is associated with the etiology of autism.
Acknowledgments
The author would like to acknowledge the resources provided by AGRE Consortium and the participating AGRE families.
Footnotes
Author Contributions
Conceived and designed the experiments: AJR. Analyzed the data: AJR. Wrote the first draft of the manuscript: AJR. Made critical revisions: AJR. The author reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Alexander Rotenberg, Editor in Chief
FUNDING: This work received financial support from the Autism Research Institute, and resources from The Autism Genetic Resource Exchange. The AGRE is a program of Autism Speaks and is supported, in part, by grant 1U24MH081810 from the National Institute of Mental Health to Clara M. Lajonc.
COMPETING INTERESTS: Author discloses no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DMS-IV-TR) Washington, DC, USA: American Psychiatric Association; 2000. [Google Scholar]
- 2.Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59(2 suppl):21–6. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Schlessinger J. Epidermal growth-factor induces rapid, reversible aggregation of the purified epidermal growth-factor receptor. Biochemistry. 1987;26(5):1443–51. doi: 10.1021/bi00379a035. [DOI] [PubMed] [Google Scholar]
- 4.Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311(5985):483–5. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- 5.Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;12005(1):0010. doi: 10.1038/msb4100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26(28):7532–40. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Pichon CE, Dominguez SL, Solanoy H, et al. EGFR inhibitor erlotinib delays disease progression but does not extend survival in the SOD1 mouse model of ALS. PLoS One. 2013;8(4):e62342. doi: 10.1371/journal.pone.0062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu WS, Tian DS, Guo ZB, et al. Inhibition of EGFR/MAPK signaling reduces microglial inflammatory response and the associated secondary damage in rats after spinal cord injury. J Neuroinflammation. 2012;9:178. doi: 10.1186/1742-2094-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Chiang HC, Wu W, et al. Epidermal growth factor receptor is a preferred target for treating amyloid-β–induced memory loss. Proc Natl Acad Sci USA. 2012;109:16743–8. doi: 10.1073/pnas.1208011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. J Clin Invest. 2009;119(4):747–54. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eagleson KL, Campbell DB, Thompson BL, Bergman MY, Levitt P. The autism risk genes MET and PLAUR differentially impact cortical development. Autism Res. 2011;4:68–83. doi: 10.1002/aur.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo AJ, Krigsman A, Jepson B, Wakefield A. Decreased serum hepatocyte growth factor (HGF) in autistic children with severe gastrointestinal disease. Biomark Insights. 2009;4:181–90. doi: 10.4137/bmi.s3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo AJ. Decreased epidermal growth factor (EGF) associated with HMGB1 and increased hyperactivity in children with autism. Biomark Insights. 2013;8:35–41. doi: 10.4137/BMI.S11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickl-Jockschat T, Michel TM. The role of neurotrophic factors in autism. Mol Psychiatry. 2011;16:478–90. doi: 10.1038/mp.2010.103. [DOI] [PubMed] [Google Scholar]
- 15.Heck DE, Laskin DL, Gardner CR, Laskin JD. Epidermal growth factor suppresses nitric oxide and hydrogen peroxide production by keratinocytes. Potential role for nitric oxide in the regulation of wound healing. J Biol Chem. 1992;267:21277–80. [PubMed] [Google Scholar]
- 16.Okunishi K, Dohi M, Nakagome K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J Immunol. 2005;175:4745–53. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- 17.Vega JA, Garcćıa-Suaárez O, Hannestad J, Peérez-Peérez M, Germanà A. Neurotrophins and the immune system. J Anat. 2003;203:1–19. doi: 10.1046/j.1469-7580.2003.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P. Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res. 2004;146:387–401. doi: 10.1016/s0079-6123(03)46024-x. [DOI] [PubMed] [Google Scholar]
- 19.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Chauhan A, Sheikh AM, et al. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207:111–6. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral out-come. Brain Behav Immun. 2011;25:40–5. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xian CJ, Zhou XF. Roles of transforming growth factor-α and related molecules in the nervous system. Mol Neurobiol. 1999;20:157–83. doi: 10.1007/BF02742440. [DOI] [PubMed] [Google Scholar]
- 24.Xian CJ, Zhou XF. EGF family of growth factors: essential roles and functional redundancy in the nerve system. Front Biosci. 2004;9:85–92. doi: 10.2741/1210. [DOI] [PubMed] [Google Scholar]
- 25.Pastore S, Mascia F. Novel acquisitions on the immunoprotective roles of the EGF receptor in the skin. Expert Rev Dermatol. 2008;3:525–7. doi: 10.1586/17469872.3.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K, Hashimoto K, Iwata Y, et al. Decreased serum levels of epidermal growth factor in adult subjects with high-functioning autism. Biol Psychiatry. 2007;62:267–9. doi: 10.1016/j.biopsych.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Toyoda T, Nakamura K, Yamada K, et al. SNP analyses of growth factor genes EGF, TGFβ-1, and HGF reveal haplotypic association of EGF with autism. Biochem Biophys Res Commun. 2007;360:715–20. doi: 10.1016/j.bbrc.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Onore C, Van de Water J, Ashwood P. Decreased levels of EGF in plasma of children with autism spectrum disorder. Autism Res Treat. 2012;2012:205362. doi: 10.1155/2012/205362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Işeri E, Güney E, Ceylan MF, et al. Increased serum levels of epidermal growth factor in children with autism. J Autism Dev Disord. 2011;41(2):237–41. doi: 10.1007/s10803-010-1046-3. [DOI] [PubMed] [Google Scholar]
- 30.Youn JH, Oh YJ, Kim ES, Choi JE, Shin JS. High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-{alpha} production in human monocytes. J Immunol. 2008;180:5067–74. doi: 10.4049/jimmunol.180.7.5067. [DOI] [PubMed] [Google Scholar]
- 31.Oh YJ, Youn JH, Ji Y, et al. HMGB1 is phosphorylated by classical protein kinase C and is secreted by a calcium-dependent mechanism. J Immunol. 2009;182:5800–9. doi: 10.4049/jimmunol.0801873. [DOI] [PubMed] [Google Scholar]
- 32.Rauvala H, Rouhiainen A. Physiological and pathophysiological outcomes of the interactions of HMGB1 with cell surface receptors. Biochim Biophys Acta. 2010;1799:164–70. doi: 10.1016/j.bbagrm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Maroso M, Balosso S, Ravizza T, et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–9. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 34.Vezzani A, French J, Bartfai T, Baram TZ. The role of inflammation in epilepsy. Nat Rev Neurol. 2011;7:31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Emanuele E, Boso M, Brondino N, et al. Increased serum levels of high mobility group box 1 protein in patients with autistic disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):681–3. doi: 10.1016/j.pnpbp.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Palumbo R, Sampaolesi M, De Marchis F, et al. Extracellular HMGB1, a signal of tissue damage, induces mesoangioblast migration and proliferation. J Cell Biol. 2004;164(3):441–9. doi: 10.1083/jcb.200304135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russo AJ. Correlation between hepatocyte growth factor (HGF) and gamma-aminobutyric acid (GABA) plasma levels in autistic children (PubMed) Biomark Insights. 2013;8:69–75. doi: 10.4137/BMI.S11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Einat H, Manji HK, Gould TD, Du J, Chen G. Possible involvement of the ERK signaling cascade in bipolar disorder: behavioral leads from the study of mutant mice. Drug News Perspect. 2003;16(7):453–63. doi: 10.1358/dnp.2003.16.7.829357. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, Shin SY, Lee KY, et al. The genetic association of DUSP6 with bipolar disorder and its effect on ERK activity. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37(1):41–9. doi: 10.1016/j.pnpbp.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno M. ErbB inhibitors ameliorate behavioral impairments of an animal model for schizophrenia: implication of their dopamine-modulatory actions. Transl Psychiatry. 2013;3:e252. doi: 10.1038/tp.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pante G, Thompson J, Lamballe F, Iwata T, Ferby I. Mitogen-inducible gene 6 is an endogenous inhibitor of HGF/Met-induced cell migration and neurite growth. J Cell Biol. 2005;171:337–48. doi: 10.1083/jcb.200502013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin CI, Du J, Shen WT, et al. Mitogen-inducible gene-6 is a multifunctional adaptor protein with tumor suppressor-like activity in papillary thyroid cancer. J Clin Endocrinol Metab. 2011;96(3):E554–65. doi: 10.1210/jc.2010-1800. [DOI] [PubMed] [Google Scholar]
- 43.Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van Der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–58. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1993;376:337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 45.Farrell RJ. Epidermal growth factor for ulcerative colitis. N Engl J Med. 2003;349:395–7. doi: 10.1056/NEJMe030075. [DOI] [PubMed] [Google Scholar]
- 46.Menard D, Tremblay E, Ferretti E, et al. Anti-inflammatory effects of epidermal growth factor on the immature human intestine. Physiol Genomics. 2012;44(4):268–80. doi: 10.1152/physiolgenomics.00101.2011. [DOI] [PubMed] [Google Scholar]


