Abstract
Ku70, a DNA repair factor in the nucleus, also regulates cell death by binding to the apoptotic protein Bax in the cytoplasm. Acetylation of Ku70 triggers Bax release resulting in Bax dependent cell death. Thus dissociating Bax from Ku70, either by inhibiting histone deacetylase 6 (HDAC6) that deacetylates Ku70 or by increasing Ku70 acetylation induces cell death. Our results showed that in neuroblastoma cells, the depletion of Ku70 results in Bax-dependent cell death. This model provides a rationale for screening Ku70 acetylation modulators that can be tested in clinical trials, either alone or in combination with radiotherapy or DNA-damaging agents for the treatment of cancer.
Keywords: Ku70, Bax, HDAC6, CBP, apoptosis
Introduction
Ku70 was originally discovered as an auto-antigen and later characterized as a DNA binding component of the non-homologous end joining (NHEJ) double strand break (DSB) repair.1 Ku70 dimerizes with Ku80, and the Ku70–Ku80 complex binds to DSB DNA. The Ku70/80 heterodimer then recruits DNA dependent protein kinase catalytic subunit (DNA-PKcs) to the DSB, followed by auto-phosphorylation of the DNA-PKcs and other DNA repair proteins, including Ku70 and XRCC4.2 Over the last several years, our laboratory has investigated Ku70’s cytoplasmic function, the regulation of cell death through interaction with an apoptotic protein, Bax. Here, we will describe our model and discuss the implications of this model.
Model of Ku70 and Implications
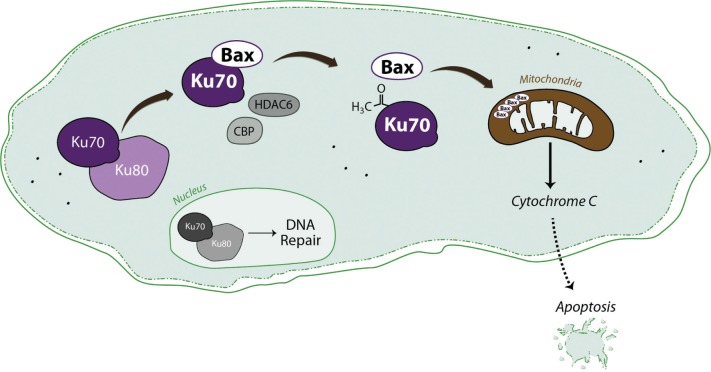
Although Ku70 was originally found in the nucleus, it was found to bind to Bax in the cytoplasm.3–5 The Bax-binding domain of Ku70 was mapped to the residues between residues 578–609 of Ku70.6 A five-residue peptide corresponding to the Ku70 596–600 has been demonstrated to bind to Bax and block Bax-mediated cell death.7 A study by Cohen et al has mapped the acetylation sites of Ku70.8 When two of these sites, K539 and K542, are acetylated, Ku70 dissociates from Bax. However, whether dissociated Bax will induce cell death is not clear as when the HEK293 and HeLa cells were treated with class 1 and class 2 histone deacetylase inhibitors (HDACI) to increase Ku70 acetylation, the cells did not die.8 Thus, how Ku70-Bax regulated cell death is uncertain.
Using neuroblastoma (NB) cells as model, we have demonstrated a key role for Ku70 in regulating cell death. In NB cells, especially the neuroblastic type (N-type) cells, Ku70 binds to Bax in an acetylation-sensitive manner.3 Upon the inhibition of HDAC activity, Ku70 is acetylated. Acetylated Ku70 releases Bax allowing it to translocate to mitochondria and trigger cytochrome c release, resulting in caspase-dependent death. Importantly, depleting Ku70 in NB cells triggers cell death, but the cell killing can be stopped by simultaneous depletion of Bax, suggesting that leaving Bax unbound when Ku70 is absent will lead to cell death.4 Our findings are contrary to the findings in HEK293 and HeLa cells.8 Our results suggest that in addition to its role in NHEJ repair, Ku70 may act as a survival factor, at least in NB cells, to block Bax-triggered cell death.9 These results indicate that Ku70 is a regulatory factor for Bax activity, and that this interaction may be therapeutically targeted in NB cells.
To investigate how the Ku70–Bax complex is regulated by acetylation, we and others have shown that the cAMP-response-element binding protein (CREB)-binding protein (CBP), a transcriptional co-activator and an acetyltransferase, acetylates Ku70 in NB cells.4,8 CBP depletion causes the down regulation of Ku70 acetylation, resulting in increased resistance to HDAC inhibitor induced cell death. Mutation of K539/K542 of Ku70 to arginine also blocks HDAC inhibitor-induced cell death and blocks Bax release following HDACI treatment, suggesting that K539 and K542 play an important role in regulating Bax binding. In addition to K539 and K542, Ku70 is also acetylated at K282, K317, K331, K338, K544, K553, and K556.8 Two of these acetylation sites (K282, K317) lie within the Ku70 DNA binding domain. Previous studies have suggested that the acetylation of these lysines in Ku70 down-regulates its DNA binding activity.10
Five of the acetylable lysines (K539, K542, K544, K553, and K556) of Ku70 are found within the nuclear localization signal (NLS). As the acetylation of lysine residues within the NLS may regulate nuclear translocation,11 the acetylation of lysine residues within the NLS of Ku70 may thus also regulate Ku70 nuclear translocation. Thus, it is possible that the acetylation of cytoplasmic Ku70 results in Bax release. Acetylated Ku70 may then be free from Bax and translocate into the nucleus. Our recent results, in fact, have demonstrated that following ionization radiation, both cytoplasmic and nuclear Ku70 are acetylated.1 Interestingly, cytoplasmic Ku70 was redistributed to the nucleus following irradiation. However, the role of Ku70 that translocates into the nucleus is still not clear.
We have shown that depleting CBP in NB cells reduces Ku70 acetylation and enhances DNA repair activity, suggesting that Ku70 acetylation may have an inhibitory role in DNA repair.12 Several studies, however, have demonstrated that the level of Ku70 correlates with radiosensitivity.13 Ku70 knock down increases the sensitivity of radiation treatment in human cancer cell lines14 while increasing Ku70 levels in cells reduces radiosensitivity. It has been proposed that the altered sensitivity to irradiation is because of changes in Ku70 levels that may be the result of Ku70 mediated DNA repair following irradiation. However, our results provide an additional rationale for testing modulators of Ku70 acetylation in treatment with radiotherapy or DNA-damaging agents for the treatment of NB.
As lysine acetylation is regulated by both acetyltransferases and deacetylases, we sought to identify the deacetylase that deacetylates Ku70 in the cytoplasm. A previous study has shown that a class III HDAC, SIRT3, was able to deacetylate Ku70,15 but it is uncertain how SIRT3, which is mainly localized in the mitochondria, is involved in regulating Ku70 acetylation resulting in Bax dissociation. In our studies, we used class specific HDAC inhibitors to inhibit HDAC activity in cells. We have shown that a HDAC6 specific inhibitor, tubacin, induces cell death in NB cells.4 HDAC6 is a class IIb HDAC containing two catalytic domains.16 HDAC6 is mainly localized in the cytoplasm and has been associated with many cell functions including tubulin stabilization, cell motility, and regulation of the binding between Hsp90 and its cochaperone.17 In NB cells, HDAC6 forms a complex with Ku70 and Bax, and that depleting HDAC6 has a similar effect as that of tubacin treatment. Furthermore, depleting HDAC6 also increases Ku70 acetylation, releasing Bax from Ku70, causing cell death.18 Thus, our results show the feasibility of targeting a single HDAC to therapeutically target the Ku70-Bax complex.
The two established functions of Ku70, one in the nucleus (to repair DNA) and one in the cytoplasm (to block Bax activity) are to protect the cell from dying (Fig. 1). However, following an apoptotic stimulation (radiation or DNA-damaging agent treatment), while nuclear Ku70 may still protect cells from dying (discussed below) by repairing DSB DNA, cytoplasmic Ku70 will become an apoptotic protein by releasing Bax following Ku70 acetylation. Whether these two functions of Ku70 following apoptotic stimulations are related or will affect each other is currently not clear. Furthermore, we have shown that following radiation, both cytoplasmic and nuclear Ku70 are acetylated.1 The cytoplasmic Ku70 was observed to translocate into the nucleus following radiation. Thus, if the Ku70 that enters the nucleus is still acetylated after entering the nucleus, these acetylated Ku70 will have a reduced binding activity for DSB DNA, resulting in lower DNA repair and more cell death. This model predicts that following apoptotic stimuli, Ku70 changes from an anti-apoptotic factor into a pro-apoptotic factor. Currently it is not known to what extent that cytoplasmic Ku70 affects the DNA-repair function of Ku70 in the nucleus. Is it possible that nuclear translocation of Ku70 following radiation depends on the degree of DNA damage caused by radiation, such that larger DNA damage will allow cytoplasmic, acetylated Ku70 to translocate into the nucleus, reducing the DNA-repair activity and allowing the cell to die? If this model is correct, how is it regulated? Currently, we have no answers for these questions.
Figure 1.
A model for the regulation of Ku70-Bax complex in cells.
Conclusions
Our results established a role of Ku70 in regulating cell death by suppressing Bax activity. Dissociating Bax from Ku70, either by pharmacological means, such as HDAC6 specific inhibitors, or by agents that block the interaction between Ku70 and Bax, will result in cell death, at least in NB cells. Our recent results have shown that this model may be specific for NB cells as Ku70 depletion in other cell types does not induce cell death, but sensitizes the cells to radiation or DNA damaging agents (manuscript submitted). Thus, this model provides a rationale for screening small molecules that enhance or block Ku70–Bax binding, or modulate Ku70 acetylation. Agents that block Ku70–Bax binding or increase Ku70 acetylation may be tested in clinical trials either alone or in combination with radiotherapy or DNA-damaging agents for the treatment of cancer.
Footnotes
Author Contributions
Conceived and designed the experiments: MH, RK. Analyzed the data: MH, RK. Wrote the first draft of the manuscript: RK. Contributed to the writing of the manuscript: MH, RK. Agree with manuscript results and conclusions: MH, RK. Jointly developed the structure and arguments for the paper: MH, RK. Made critical revisions and approved final version: MH, RK. All authors reviewed and approved of the final manuscript.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
ACADEMIC EDITOR: Garry Walsh, Editor in Chief
FUNDING: Cited authors’ work was partly supported by a National Institute of Health R01 grant (DK067102 to RK) and a research fund provided by the Department of Obstetrics and Gynecology, University of Michigan Medical School.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
REFERENCES
- 1.Hurwitz JL, et al. Vorinostat/SAHA-induced apoptosis in malignant mesothelioma is FLIP/caspase 8-dependent and HR23B-independent. Eur J Cancer. 2012;48(7):1096–1107. doi: 10.1016/j.ejca.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Morio T, Kim H. Ku, Artemis, and ataxia-telangiectasia-mutated: signalling networks in DNA damage. Int J Biochem Cell Biol. 2008;40(4):598–603. doi: 10.1016/j.biocel.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Subramanian C, et al. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102(13):4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kerr E, et al. Identification of an acetylation-dependant Ku70/FLIP complex that regulates FLIP expression and HDAC inhibitor-induced apoptosis. Cell Death Differ. 2012;19(8):1317–1327. doi: 10.1038/cdd.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou H, Volonte D, Galbiati F. Interaction of caveolin-1 with Ku70 inhibits Bax-mediated apoptosis. PLoS One. 2012;7(6):e39379. doi: 10.1371/journal.pone.0039379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida T, et al. Bax-inhibiting peptide derived from mouse and rat Ku70. Biochem Biophys Res Commun. 2004;321(4):961–966. doi: 10.1016/j.bbrc.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 7.Gomez JA, et al. Bax-inhibiting peptides derived from Ku70 and cell- penetrating pentapeptides. Biochem Soc Trans. 2007;35(pt 4):797–801. doi: 10.1042/BST0350797. [DOI] [PubMed] [Google Scholar]
- 8.Cohen HY, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13(5):627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 9.Subramanian C, et al. CREB-binding protein is a mediator of neuroblastoma cell death induced by the histone deacetylase inhibitor trichostatin A. Neoplasia. 2007;9(6):495–503. doi: 10.1593/neo.07262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CS, et al. Histone deacetylase inhibitors sensitize prostate cancer cells to agents that produce DNA double-strand breaks by targeting Ku70 acetylation. Cancer Res. 2007;67(11):5318–5327. doi: 10.1158/0008-5472.CAN-06-3996. [DOI] [PubMed] [Google Scholar]
- 11.Spilianakis C, Papamatheakis J, Kretsovali A. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol Cell Biol. 2000;20(22):8489–8498. doi: 10.1128/mcb.20.22.8489-8498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subramanian C, et al. CREB-binding protein regulates Ku70 acetylation in response to ionization radiation in neuroblastoma. Mol Cancer Res. 2013;11(2):173–181. doi: 10.1158/1541-7786.MCR-12-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu Y, et al. Ku70-deficient embryonic stem cells have increased ionizing radio-sensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci U S A. 1997;94(15):8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandersickel V, et al. The radiosensitizing effect of Ku70/80 knockdown in MCF10A cells irradiated with X-rays and p(66) + Be(40) neutrons. Radiat Oncol. 2010;5:30. doi: 10.1186/1748-717X-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sundaresan NR, et al. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol. 2008;28(20):6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci U S A. 1999;96(9):4868–4873. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, et al. The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 2008;68(18):7561–7569. doi: 10.1158/0008-5472.CAN-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian C, et al. HDAC6 deacetylates Ku70 and regulates Ku70-Bax binding in neuroblastoma. Neoplasia. 2011;13(8):726–734. doi: 10.1593/neo.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]