Abstract
BACKGROUND
The St. Gallen International Expert Consensus of 2011 proposes a new classification system for breast cancer based on its division into five subgroups. The criteria to identify these subtypes were recently refined at the 2013 Conference. In this respect, the authors of this paper have conducted a retrospective analysis of breast cancer subtypes, related to Ki-67 and involvement of the axillary lymph nodes (ALNs). The analysis was performed only in the cases of invasive breast cancer in the pT2 stages. The research and results of the paper have shown that investigating the value of these parameters could be of great benefit in future treatment strategies of invasive breast cancer.
METHODS
A retrospective analysis of breast cancer subtypes, tumor nodal metastatic staging, and histopathological grading of 108 cases has been performed according to the methods recommended and provided by the St. Gallen International Expert Consensus Report, 2011. The estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and Ki-67 of 108 tumor samples were all investigated by immunohistochemistry according to the methods used to classify breast cancer subtypes as proposed in the St. Gallen Consensus Report, 2011. Invasive breast cancers (n = 108) were immunohistochemically classified as follows: 28 (25.92%) as Luminal A, 51 (47.22%) as Luminal B (HER2 negative), 21 (19.44%) as Luminal B-like (HER2 negative), 2 (1.85%) as HER2 positive, and 6 (5.55%) as being a triple-negative subtype.
RESULTS
The conclusion was made that when Ki-67 was found to be higher, patients also showed a higher involvement in their ALNs. The chi-square test shows the difference to be significant (chi-square = 4.757; P = 0.029). Luminal B subtypes had the highest percentage (54.9%) of involvement of lymph nodes when compared to the other four subtypes. The Luminal B subtype had a higher percentage (51.4%) of involvement of lymph nodes than did Luminal A (10.7%). The chi-square test also shows the difference to be significant (P < 0.05).
CONCLUSION
A combination of the Ki-67 index, HER negative tumors, PR negativity, and a low value that can be used to segregate ER positive pT2 tumors into prognostically significantly different clinical outcomes may be utilized clinically to guide patient management in accordance with these tumor characteristics.
Keywords: breast cancer, Luminal A, Luminal B, Ki-67, positive lymph node status, prognostic information
Introduction
Tumors from breast cancer are among the most common malignant tumors that occur in women worldwide and comprise 16% of all female incidences of cancer.1 Yet, despite the increase of the incidences of tumors from breast cancer, its mortality is slowly being reduced, owing to early diagnosis, as well as adequate and improved breast cancer treatment.2 Given the fatal nature and prevalence then of breast cancer tumors, the objective of this study has been to determine the prognostic significance of subgrouping estrogen receptor (ER) positive breast tumors into low- and high-risk luminal categories using a Ki-67 index, human epidermal growth factor receptor-2 (HER2) status, and progesterone receptor (PR) status.
The St. Gallen International Expert Consensus 2011 proposed a new classification system for breast cancer based on its division into five subgroups. The criteria to identify subtypes were further recently refined at the 2013 Conference, in that moderate of a strong expression of PR and Ki-67 level were both recognized as being important to the surrogate definition of a “Luminal A-like” disease. According to these criteria, the subtypes in question have been defined as: Luminal A – ER positive, HER2 negative, Ki-67 low, and PR high; Luminal B (HER2 negative) – ER positive, HER2 negative, and either Ki-67 high or PR low; Luminal B-like (HER2 positive) – ER positive, HER2 overexpressed or amplified, any Ki-67, and any PR; HER2 positive – HER2 over-expressed or amplified, ER and PR absent; and triple negative – ER and PR absent and HER2 negative.3 These exact criteria and their recognition of biology subtypes have been used here throughout the course of this study within the breast cancer spectrum.
A higher Ki-67 index has been found to correlate significantly with young age, large tumors, positive lymph nodes, negative ER/PR, p53 overexpression, and positive HER2. A higher Ki-67 index has also been found to correlate with a poorer prognosis and early recurrence (<2 years). On the other hand, a lower Ki-67 index has correlated with a favorable prognosis and late recurrence (>10 years). Thus, proliferative activity as determined by Ki-67 may reflect the aggressive behavior of breast cancer and predict the time of recurrence and the appropriate therapy required in treatment. It is therefore important to take the Ki-67 index into consideration in the treatment and follow-up of breast cancer patients,4 which is one aim of this study.
Ongoing research has aimed to identify patients who may be at a high risk of relapse and, by that nature, who may act as a guide for endocrine therapy, chemotherapy, and other treatments used for early breast cancer. In the same vein, the research undertaken here also aims to explore the connection between the expression of Ki-67 and the involvement of axillary lymph glands and the difference in frequency between the Luminal A and Luminal B subtypes in the aims of this service to better diagnose and treat breast cancer.
Subjects and Methods
Patients
A total of 108 patients suffering from invasive breast cancer in the stages T2, N0 or N1, M0, who underwent surgery at the Institute for Oncology and Radiology of Serbia, between 2003 and 2013, were retrospectively investigated for this study. Men with breast cancer, women with in situ carcinoma, bilateral breast cancer, or who underwent neoadjuvant chemotherapy or chemotherapy for another disease were excluded from the study. Patients had been originally treated with breast-conserving surgery or mastectomy. Informed consent was obtained from every patient involved.
Histopathological and immunohistochemical evaluations
All histopathological and immunohistopathological diagnoses were determined by several pathologists at the Institute’s Laboratory for Pathology. Individual tissue sections were used. Surgical specimens were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The Luminal A subtype was defined as ER and/or PR positive and HER2 negative. If the Ki-67 labeling index and/or nuclear grade was determined, the Luminal A subtype was also defined to a low Ki-67 labeling index (<14%) and/or nuclear grade 1 or 2. ER and PR positivity was confirmed by immunohistochemistry (IHC); greater than 1% of tumor cells staining positive was considered to be positive. A HER2-negative status was confirmed by the IHC (with 0, 1+, and 2+ scores indicating no cells stained, <10% of cells have membrane staining and >10% of cells with a low or medium membrane staining, respectively). Tumors that were 2+ by IHC were also examined by fluorescence in situ hybridization (with an amplification ratio <2.0, indicating a negative status). The Ki-67 labeling index was determined by IHC. Figure 1 shows the Ki-67 antigen staining in both its ER- and PR-positive sections.
Figure 1.
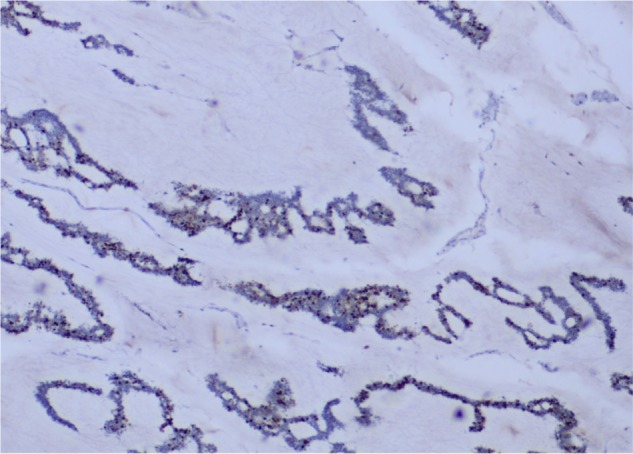
IHC nuclear staining of Ki-67. Here, the low Ki-67 labeling index (<14%) can be seen (H&E; ×4).
Statistics
A chi-square test was used to compare categorical variables. The major variables utilized were Ki-67, ER, PR, HER2, tumor grade, patient age, and lymph node status. Differences were considered significant when the P value was <0.05. SPSS 15.0 software was used for statistical analysis.
Results
Table 2 shows that when the Ki-67 level was high, it correlated with 32 patients (94.1%) whose lymph nodes had also been involved; however, when the Ki-67 was found to be low, it correlated with only two patients (5.9%) whose lymph nodes had also been involved. Given this correlation between high Ki-67 and lymph node involvement, the conclusion can be drawn that patients had a higher involvement of axillary lymph nodes (ALNs) when Ki-67 was also high. The chi-square test has shown the difference to be significant (chi-square = 4.757; P = 0.029).
Table 2.
The correlation between the Ki-67 index and lymph node status.
| KI67 | ||||
|---|---|---|---|---|
| <14 | ≥14 | |||
| Involved Lymph Nodes | No | N | 22 | 52 |
| % | 29.7% | 70.3% | ||
| Yes | N | 2 | 32 | |
| % | 5.9% | 94.1% | ||
The chi-square test has shown the difference to be significant (chi-square = 4.757; P = 0.029).
The chi-square test shows the difference to be significant (P < 0.05).
Table 3 shows that 89.3% of Luminal A subtypes had negative ALNs, Luminal B had 45.1%, Luminal B-like had 57.1%, HER2 had 100.0%, and triple negative had 50.0%. The Luminal B subtype had the highest percentages (54.9%) of involved lymph nodes.
Table 3.
The relationship between lymph node status and subtypes.
| INVOLVED LYMPH NODES | ||||
|---|---|---|---|---|
| NO | YES | |||
| Subtypes | Luminal A | N | 25 | 3 |
| % | 89.3% | 10.7% | ||
| Luminal B | N | 23 | 28 | |
| % | 45.1% | 54.9% | ||
| Luminal B Like | N | 12 | 9 | |
| % | 57.1% | 42.9% | ||
| HER2 | N | 2 | 0 | |
| % | 100.0% | 0.0% | ||
| Triple Negative | N | 3 | 3 | |
| % | 50.0% | 50.0% | ||
Luminal B (HER2 negative) and Luminal B-like (HER2 positive) were subsequently combined together into the same group of Luminal B (Table 4 and Fig. 2). Table 4 and Figure 2 show the relationship between the lymph node status and the Luminal A and Luminal B subtypes. The Luminal B subtypes had a higher percentage (51.4%) of involved lymph nodes than the Luminal A subtype (10.7%). The chi-square test shows the difference to be significant (P < 0.05).
Table 4.
The relationship between lymph node status with Luminal A and Luminal B subtypes.
| INVOLVED LYMPH NODES | ||||
|---|---|---|---|---|
| NO | YES | |||
| Subtypes | Luminal A | N | 25 | 3 |
| % | 89.3% | 10.7% | ||
| Luminal B | N | 35 | 37 | |
| % | 48.6% | 51.4% | ||
Figure 2.
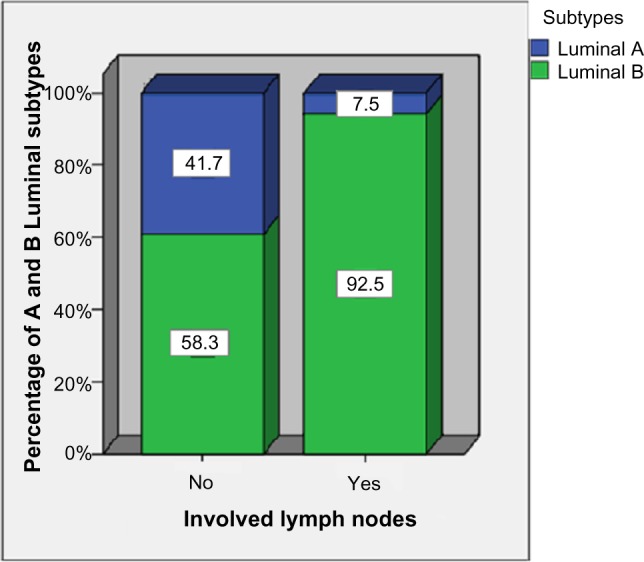
The relationship between lymph node status with Luminal A and B subtypes.
The chi-square test establishes that no statistically significant difference between the involved lymph nodes and patient age (P = 0.055) exists, but does show there to be a significant difference between the involved lymph nodes and the tumor grade (P < 0.05). Therein, negative ALNs scored 84% of tumor grade 1 patients, 71.4% of tumor grade 2, and 39.6% of tumor grade 3, the last group of whom were found to possess the highest percentage (60.4%) of involved lymph nodes.
The chi-square test shows the difference to be significant (P < 0.05).
Discussion
Recently, a molecular classification system has been proposed to categorize breast cancers into subtypes associated with optimal therapeutic modality, which has also become widely used.5 In concordance with this molecular classification system, this study has sought to compare IHC-based subtypes with Ki-67 and positive ALNs.
The consensus of the 12th St. Gallen Conference defined Luminal A breast cancer as ER+ and/or PR+, and HER2− tumors with a Ki-67 labeling index of <14%. At the 2013 Conference, the conclusion was made that Luminal A breast cancer treatment, in most cases, can be successfully treated with endocrine therapy alone, as well as in cases of multiple positive nodes where chemotherapy may be supplemented to treatment. Moreover, while Luminal A patients are less responsive to chemotherapy, this treatment may be supplemented by endocrine therapy in cases of high bulk disease (eg, multiple positive nodes). In this respect, when patients with hormone receptor (HR)-positive tumors were treated with adjuvant tamoxifen, their risk for the composite outcome of recurrence or death was reduced by more than 30%.6 However, many patients with lymph node positive, ER-positive breast tumors, gain minimal benefit from adjuvant chemotherapy.7
Generally speaking, the number of ALN metastases is the most reliable prognostic factor and an important indication for adjuvant therapy. ALN status is an important prognostic factor for locoregional control and survival in breast cancer patients; to wit, the 7th American Joint Committee on Cancer staging system for breast cancer is based on the absolute number of pathologically positive ALNs.4 HR status and c-erbB-2/HER2 status are markers of specific intrinsic subtypes of breast cancer. The Ki-67 index, a marker of cell proliferation, is likewise a marker of a specific intrinsic subtype5,8 and is also associated with breast cancer recurrence and death.9–12 Tumor size itself has been proven to be the most significant individual marker for predicting nodal metastases; when size is used in combination with Ki-67, the prediction results drop significantly.13
All 108 patients in this study had invasive breast cancer in the pT2 stage. Table 2 shows that when Ki-67 proved to be high and lymph node involvement was also high (32 patients, 94.1%). It was therefore concluded that when Ki-67 was higher, patients also had higher involvement in their ALNs and a higher risk of locoregional relapse. The chi-square test shows the difference between the two groupings to be significant (chi-square = 4.757; P = 0.029).
Table 3 shows that 89.3% of Luminal A subtypes were of negative ALNs. An important conclusion to be drawn from this datum was that Luminal B subtypes possess higher percentages of lymph node involvement (51.4%) than do Luminal A (10.7%). The chi-square test establishes the difference to be significant (P < 0.05). This study has also found that the tumor grade 3 group, the worst prognosis possible, was also found to possess the highest percentage (60.4%) of involved lymph nodes of all the groups.
The final conclusion of this study is that patients with Luminal B subtype will have a worse prognosis as well as a greater chance for local recurrence and survival than that in the case of patients with Luminal B subtype.
Although the optimal threshold of a Ki-67 index has been a matter of controversy due to interlaboratory variations, a Ki-67 index has been frequently used to divide breast cancers into tumors that have low and high proliferation activity.5,14–17 The expression level of the Ki-67 antigen is generally connected to the IHC-based subtypes.9 Not surprisingly, all Luminal A subtype tumors investigated in this study have been shown to have possessed a low Ki-67 index. In contrast, more than 85% of Luminal B, HER2, and basal-like subtypes had been of a high Ki-67 index. As it has been suggested that cell proliferation activity is much lower in Luminal A tumors than in other subtypes, it therefore may be concluded that such proliferation activity, as well as other cellular characteristics such as the HER2 expression, may prove to be a useful marker for categorizing breast cancer, which is primarily affected by the genomic status.
Conclusion
As the key to treating breast cancer successfully necessitates its early detection, as well as the severity or malignancy of the tumors present, many different tests and methodologies need to be explored that will allow the doctor or oncologist to better and more accurately predict the biology of the cancer present and to ensure a higher survival rate. As this paper has attempted to show, one such method to do so may be through the examination of a combination of Ki-67 index, HER negative tumors, PR negativity and a low value that can be used to segregate ER positive, pT2 tumors into prognostically significant different clinical outcomes. In short, these characteristics of tumors may be used clinically to guide patient management, better handle patient treatment, and ensure a better survival rate.
Table 1.
Shows that the median patient age was 62 (range: 33–84 years of age).
| AGE | |||||
|---|---|---|---|---|---|
| N | Mean | SD | Med | Min | Max |
| 108 | 60.66 | 12.44 | 62.00 | 33.00 | 84.00 |
Glossary
Abbreviations
- ER
estrogen receptor
- PR
progesterone receptor
Footnotes
Author Contributions
Conceived and designed the study: ZI. Performed medical operations: MI, MZ, IM, ID, ZK. Pathology: GP. Analyzed the data: II, MZ, SJ. Wrote the first draft of the manuscript: ZI. Contributed to the writing of the manuscript: ZI. Agreed with manuscript results and conclusions: ZI, MZ, MI, IM, ID, ZK, II, GP, SJ. Jointly developed the structure and arguments for the paper: ZI, MZ, MI, IM, ID, ZK, II, GP, SJ. Made critical revisions and approved final version: ZI. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: William CS Cho, Editor in Chief
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108(11):2205–40. doi: 10.1038/bjc.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yanagawa M, Ikemot K, Kawauchi S, et al. Luminal A and luminal B (HER2 negative) subtypes of breast cancer consist of a mixture of tumors with different genotype. BMC Res Notes. 2012;5:376. doi: 10.1186/1756-0500-5-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura R, Osako T, Okumura Y, Hayashi M, Toyozumi Y, Arima N. Ki-67 as a prognostic marker according to breast cancer subtype and a predictor of recurrence time in primary breast cancer. Exp Ther Med. 2010;133:747–54. doi: 10.3892/etm.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–47. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 7.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295(14):1658–67. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang MC, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28:1684–91. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 10.De Azambuja E, Cardoso F, de Castro G, Jr, et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–13. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones RL, Salter J, A’Hern R, et al. The prognostic significance of Ki67 before and after neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat. 2009;116:53–68. doi: 10.1007/s10549-008-0081-7. [DOI] [PubMed] [Google Scholar]
- 12.Selz J, Stevens D, Jouanneau L, Labib A, Le Scodan R. Prognostic value of molecular subtypes, Ki67 expression and impact of postmastectomy radiation therapy in breast cancer patients with negative lymph nodes after mastectomy. Int J Radiat Oncol Biol Phys. 2012;84:1123–32. doi: 10.1016/j.ijrobp.2012.02.047. [DOI] [PubMed] [Google Scholar]
- 13.Mojarad S, Venturini B, Fulgenzi P, et al. Prediction of nodal metastasis and prognosis of breast cancer by ANN-based assessment of tumour size and p53, Ki-67 and steroid receptor expression. Anticancer Res. 2013;33(9):3925–33. [PubMed] [Google Scholar]
- 14.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6:195–7. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki K, Matsumura K, Murakami T, Tsuji T. Measurement of bromodeoxyuridine labeling index, Ki-67 score and Ag-NOR count in breast carcinomas. Comparison with DNA ploidy. Oncology. 1992;49:147–53. doi: 10.1159/000227029. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki K, Matsumura K, Tsuji T, Shinozaki F, Takahashi M. Relationship between labeling indices of Ki-67 and BrdUrd in human malignant tumors. Cancer. 1988;62:989–93. doi: 10.1002/1097-0142(19880901)62:5<989::aid-cncr2820620525>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]


