Abstract
Introduction
Information about sepsis in mainland China remains scarce and incomplete. The purpose of this study was to describe the epidemiology and outcome of severe sepsis and septic shock in mixed ICU in mainland China, as well as the independent predictors of mortality.
Methods
We performed a 2-month prospective, observational cohort study in 22 closed multi-disciplinary intensive care units (ICUs). All admissions into those ICUs during the study period were screened and patients with severe sepsis or septic shock were included.
Results
A total of 484 patients, 37.3 per 100 ICU admissions were diagnosed with severe sepsis (n = 365) or septic shock (n = 119) according to clinical criteria and included into this study. The most frequent sites of infection were the lung and abdomen. The overall ICU and hospital mortality rates were 28.7% (n = 139) and 33.5% (n = 162), respectively. In multivariate analyses, APACHE II score (odds ratio[OR], 1.068; 95% confidential interval[CI], 1.027–1.109), presence of ARDS (OR, 2.676; 95%CI, 1.691–4.235), bloodstream infection (OR, 2.520; 95%CI, 1.142–5.564) and comorbidity of cancer (OR, 2.246; 95%CI, 1.141–4.420) were significantly associated with mortality.
Conclusions
Our results indicated that severe sepsis and septic shock were common complications in ICU patients and with high mortality in China, and can be of help to know more about severe sepsis and septic shock in China and to improve characterization and risk stratification in these patients.
Introduction
Severe sepsis and septic shock are among the main factors contributing to mortality in intensive care units (ICUs), and exhibit a significant disease burden and negative economic impact [1]–[3].
The incidence of sepsis varies among different racial and ethnic groups [4]–[7]. Between 6 and 54% of patients admitted to ICUs have severe sepsis [2], [3], [6], [8]–[10], and the mortality rate for these patients varies from 20 to 60% [6], [10]–[12], which will increase stepwise with increasing disease severity [13]. Although the mortality rate may have decreased in recent years [5], [7], the incidence of severe sepsis and septic shock is increasing, so that overall deaths are increasing [2], [4], [7]. Even death has been avoided, the patient who survives sepsis would have a significantly compromised long-term health-related quality of life than general population [1], [14].
There have been a number of studies describing the epidemiology, risk factor and outcome of severe sepsis and septic shock in different countries [2], [4], [7], [10], [11]. Yet, information about sepsis in mainland China remains scarce and incomplete. Cheng et al [3] have described the epidemiology of severe sepsis in surgical ICUs, but data concerning the epidemiology of severe sepsis/septic shock in mixed ICUs are limited. So the China Critical Care Clinical Trials Group (CCCCTG) conducted an inception cohort study to investigate the epidemiology and outcome of severe sepsis and septic shock in mixed ICUs in China.
Patients and Methods
Study development
This was a secondary analysis of a prospective cohort study aiming to describe the demographics, case mix, interventions, and clinical outcome of critically ill patients admitted to ICUs in Mainland China and performed from 1 July 2009 to 31 August 2009 in 22 ICUs [15], so the data in the current study were collected prospectively but the analysis was done retrospectively. The participating ICUs were members of the CCCCTG and located in different provinces of China. The detailed characteristics of those ICUs, such as number of ICU beds, types of ICU, number of intensivists and nurses, and number of admissions in 2009 are showed in table 1. This study was approved by the institutional review board of Fuxing hospital (Number: 2009FXHEC-KY032), and the need for informed consent was waived. The ethical approval of Fuxing hospital was endorsed by the institutional review boards of all other participating centers (see the Appendix S1 for the full names and affiliation of participating hospitals) before data collection.
Table 1. Characteristics of participating ICUs.
| * | |
| Participating ICUs (n = 22) | |
| Type of hospital | |
| University affiliated | 19 |
| Public | 3 |
| Number of hospital beds (median, IQR) | 1730 (1402–2100) |
| Type of ICU | |
| Medical/Surgical | 18 |
| Surgical | 3 |
| Medical | 1 |
| Number of ICU beds (median, IQR) | 20.5 (12.0–28.0) |
| Total number of intensivists | 12.0 (8.5–13.8) |
| Total number of nurses | 33.5 (26.3–45.0) |
| Total ICU admissions in 2009 (median, IQR) | 791 (446–1353) |
| Hospital mortality (%) | 12.1 (8.2–18.7) |
ICU, intensive care unit; IQR, interquartile range.
We used a case report form (CRF) to collect data. Every participating ICU nominated a study coordinator who was responsible for screening and enrollment of patients and data collection. The CCCCTG data monitoring team was responsible for auditing the integrity of data.
Selection of participants, data collection, and definitions
During the study period, all admissions of participating ICUs were screened for eligibility. Patients less than 15 years old or with an ICU length of stay (LOS) less than 24 hours were excluded. Patients with severe sepsis/septic shock at ICU admission or during ICU hospitalization were included in the study cohort, and only the first episode of severe sepsis or septic shock was counted. Patients readmitted into ICU during the same hospitalization were not screened again. The following information was recorded: demographic characteristics, admission category, comorbidities and preexisting organ insufficiency. The Acute Physiology and Chronic Health Evaluation (APACHE) II score [16] and Sequential Organ Failure Assessment (SOFA) score [17] on the first day of ICU were recorded to evaluate the severity of illness. Severe sepsis and septic shock were defined according to the American College of Chest Physicians/Society of Critical Care Medicine consensus conference definitions [18]. Acute respiratory distress syndrome (ARDS) were defined according to the American-European Consensus Conference criteria [19]. Acute kidney injury (AKI) was defined based on the Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function and End-stage kidney disease(RIFLE) criteria [20]. Chronic organ failure and immunocompromise were diagnosed according to the criteria in APACHE II score. ICU-acquired infection was defined as the infection identified at least 48 hours after ICU admission, and ICU-acquired severe sepsis was defined as one occurring at least 48 hours following ICU admission. The reasons for ICU admission were based on disease categories of APACHE II scores.
Outcome measures
All enrolled patients were followed up till death in the hospital or hospital discharge or until November 30, 2009, whichever occurred earlier. The primary outcome measure was incidence and crude hospital mortality of severe sepsis and septic shock, as well as the risk factors for death. ICU mortality, ICU LOS and hospital LOS were also assessed. Patients who were still in hospital on November 30, 2009 were deemed survivors.
Statistical analysis
Data were analyzed using the SPSS 16.0 software program. Data are presented as mean and standard deviation (SD) for variables that exhibited normal distributions. On rejection of the normality hypothesis, we used median and interquartile range (IQR). Student's t-test for independent groups was applied to data with a normal distribution. When normality was rejected, the Mann–Whitney U-test was used for independent groups. For categorical variables the chi-square or Fisher's exact test was applied as appropriate. For determination of independent predictors for hospital mortality in severe sepsis patients, odds ratios (OR) and respective 95% confidence intervals (CIs) were estimated by means of multivariate logistic regression analysis. Variables including demographics, underlying diseases, severity of illness, admission status, and complications were entered into the model if p<0.2 in univariate analysis. The Hosmer-Lemeshow test was used to assess the calibration of the regression model. All comparisons were unpaired and all tests of significance were two-tailed. A p value <0.05 was considered statistically significant.
Results
Population characteristics and incidence of severe sepsis and septic shock
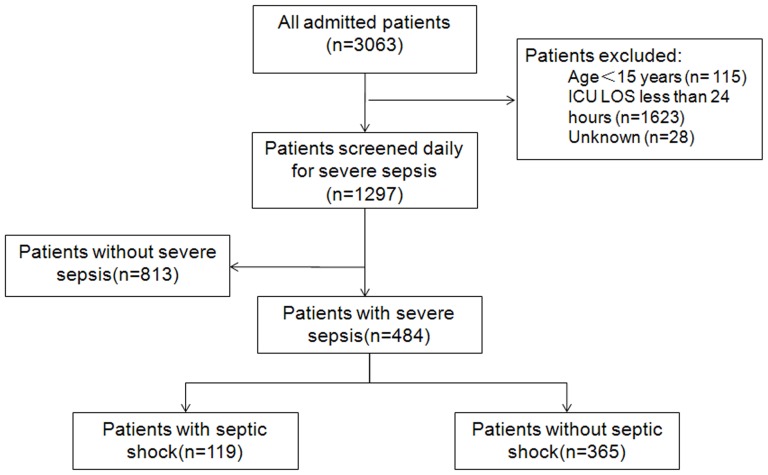
We screened 3063 admissions during the study period and 1297 patients (42.3%) were enrolled (Figure 1). A total of 484 patients developed severe sepsis or septic shock, including 336 males (69.4%), and their median age was 66 (interquartile range [IQR], 51–77) years. More than half of the patients were admitted into ICU because of respiratory diseases (53.5%), and two-thirds (67.4%) had at least one underlying disease or chronic organ system dysfunction. The median APACHE II score was 21 (IQR, 16–27), and the median SOFA score on ICU day 1 was 7.5 (IQR, 5–10). Further baseline characteristics are shown in Table 2.The incidence of severe sepsis and septic shock was 37.3 per 100 ICU admissions.
Figure 1. Flow diagram of enrolled patients and their outcome.
ICU, intensive care unit; LOS, length of stay.
Table 2. Characteristics and outcome of patients with severe sepsis.
| Variables | All patients (n = 484) | Survivors (n = 322) | Non-survivors (n = 162) | P Value |
| Age, median (IQR) | 66 (51–77) | 62 (47–74) | 71 (56–80) | <0.001 |
| Male sex | 336 (69.4%) | 216 (67.1%) | 120 (74.1%) | 0.115 |
| ICU admission categories | ||||
| Medical | 371 (76.7%) | 239 (74.2%) | 132 (81.5%) | 0.075 |
| Scheduled surgery | 39 (8.1%) | 27 (8.4%) | 12 (7.4%) | 0.709 |
| Emergency surgery | 74 (15.3%) | 56 (17.4%) | 18 (11.1%) | 0.070 |
| Reasons for ICU admission | ||||
| Respiratory disease | 259 (53.5%) | 163 (50.6%) | 96 (59.3%) | 0.072 |
| Gastrointestinal disease | 59 (12.2%) | 46 (14.3%) | 13 (8.0%) | 0.047 |
| Neurological disease | 49 (10.1%) | 35 (10.9%) | 14 (8.6%) | 0.443 |
| Cardiovascular disease | 46 (9.5%) | 27 (8.4%) | 19 (11.7%) | 0.237 |
| Trauma | 34 (7.0%) | 28 (8.7%) | 6 (3.7%) | 0.043 |
| Renal disease | 26 (5.4%) | 14 (4.3%) | 12 (7.4%) | 0.159 |
| Miscellaneous | 11 (2.3%) | 9 (2.8%) | 2 (3.7%) | 0.277 |
| Comorbidities | ||||
| No comorbidity | 158(32.6%) | 119(37.0%) | 39(24.1%) | 0.004 |
| Hypertension | 166(34.3%) | 102(31.7%) | 64(39.5%) | 0.087 |
| Diabetes mellitus | 85(17.6%) | 53(16.5%) | 32(19.8%) | 0.369 |
| Coronary artery disease | 83(17.1%) | 53(16.5%) | 30(18.5%) | 0.571 |
| COPD | 80(16.5%) | 48(14.9%) | 32(19.8%) | 0.176 |
| Cancer | 55(11.4%) | 27(8.4%) | 28(17.3%) | 0.004 |
| Hematologic malignancy | 10(2.1%) | 7(2.2%) | 3(1.9%) | 1.000 |
| Organ transplantation | 9(1.9%) | 4(1.2%) | 5(3.1%) | 0.169 |
| Chronic respiratory failure | 73(15.1%) | 44(13.7%) | 29(17.9%) | 0.219 |
| Chronic heart failure | 56(11.6%) | 23(7.1%) | 33(20.4%) | <0.001 |
| Immunocompromise | 48(9.9%) | 25(7.8%) | 23(14.2%) | 0.025 |
| Chronic renal failure | 13(2.7%) | 6(1.9%) | 7(4.3%) | 0.138 |
| Chronic liver dysfunction | 11(2.3%) | 7(2.2%) | 4(2.5%) | 1.000 |
| APACHE II, median (IQR) | 21(16–27) | 18(14–24) | 25(19–32) | <0.001 |
| SOFA on ICU day1, median (IQR) | 7.5(5–10) | 7(5–9) | 9(6.75–12) | <0.001 |
| Complications | ||||
| Septic shock | 119(24.6%) | 68(21.1%) | 51(31.5%) | 0.012 |
| Acute kidney injury | 201(41.5%) | 119(37.0%) | 82(50.6%) | 0.004 |
| ARDS | 265(54.8%) | 150(46.6%) | 115(71.0%) | <0.001 |
| ICU stay, days, median (IQR) | 7(4–15) | 7(4–14) | 9(4–17) | 0.067 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; COPD, Chronic obstructive pulmonary disease; ICU, Intensive care unit; IQR, interquartile range; SOFA, Sequential Organ Failure Assessment.
Sources of infection and microbiology
The lung (85.7%) and the abdomen (18.0%) were the most common sites of infection (Table 3). One hundred and sixty-seven patients (34.5%) had two or more infection sites. Only 37 patients (7.6%) had bloodstream infection.
Table 3. The source of infection of patients with severe sepsis (total >100% because 167 patients had more than one infection sites).
| Variables | All patients (n = 484) | Survivors (n = 322) | Non-survivors (n = 162) | P Value |
| Pneumonia | 419 (86.6%) | 276 (85.7%) | 143 (88.3%) | 0.436 |
| Intra-abdominal infection | 80 (16.5%) | 58 (18.0%) | 22 (13.6%) | 0.215 |
| Gastroenteritis | 41 (8.5%) | 27 (8.4%) | 14 (8.6%) | 0.924 |
| Urinary tract infection | 37 (7.6%) | 25 (7.8%) | 12 (7.4%) | 0.889 |
| Bloodstream infection | 37 (7.6%) | 16 (5%) | 21 (13%) | 0.002 |
| Soft tissue infection | 34 (7.0%) | 19 (5.9%) | 15 (9.3%) | 0.172 |
| Central nervous system infection | 23 (4.8%) | 19 (5.9%) | 4 (2.5%) | 0.094 |
| Multiple-site infection (≥2) | 167 (34.5%) | 111 (34.5%) | 56 (34.6%) | 0.983 |
Only half of the ICU (11/22) reported the microbiology, and 148 patients (30.6%) had microbiological documentations associated with severe sepsis and septic shock. Out of these 148 patients with microbiological results, Gram-negative bacilli were isolated in 111 patients (75.0%), and Gram-positive organisms were isolated in 32 patients (21.6%). Only six patients were diagnosed as invasive fungal infection or fungemia (4.1%). Forty-nine patients (33.1%) had polymicrobial (≥2 infection agents) infections. The most prevalent species were Acinetobacter baumannii, Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, methicillin-resistant Staphylococcus aureus, and Stenotrophomonas maltophilia (Table 4).
Table 4. Distribution of microorganisms isolated from 148 patients.
| Microorganism | Total (n = 269) | ICU-acquired (n = 221) | Non-ICU-acquired (n = 48) |
| Gram positives | 39 (14.5%) | 34 (15.4%) | 5 (10.4%) |
| Methicillin-resistant Staphylococcus aureus | 17 (6.3%) | 14 (6.3%) | 3 (6.3%) |
| Coagulase-negative Staphylococcus | 10 (3.7%) | 8 (3.6%) | 2 (4.2%) |
| Enterococcus faecium | 8 (3.0%) | 8 (3.6%) | 0 |
| Enterococcus faecalis | 3 (1.1%) | 3 (1.4%) | 0 |
| Streptococcus viridans | 1 (0.4%) | 1 (0.5%) | 0 |
| Gram negatives | 168 (62.5%) | 137 (62.0%) | 31 (64.6%) |
| Acinetobacterbaumannii | 38 (14.1%) | 30 (13.6%) | 8 (16.7%) |
| Pseudomonas aeruginosa | 33 (12.3%) | 27 (12.2%) | 6 (12.5%) |
| Escherichia coli | 26 (9.7%) | 24 (10.9%) | 2 (4.2%) |
| Klebsiellapneumoniae | 25 (9.3%) | 21 (9.5%) | 4 (8.3%) |
| Stenotrophomonasmaltophilia | 16 (5.9%) | 12 (5.4%) | 4 (8.3%) |
| Proteus mirabilis | 5 (1.9%) | 3 (1.4%) | 2 (4.2%) |
| Serratiamarcescens | 4 (1.5%) | 3 (1.4%) | 1 (2.1%) |
| Other Gram negatives* | 21 (7.8%) | 17 (7.7%) | 4 (8.3%) |
| Fungi** | 6 (2.2%) | 6 (2.7%) | 0 |
| Candida albicans | 1 (0.4%) | 1 (0.5%) | 0 |
| Aspergillus spp. | 4 (1.5%) | 4 (1.8%) | 0 |
| Pneumocystis | 1 (0.4%) | 1 (0.5%) | 0 |
*including Burkholderia cepacia, Chryseobacter iumindologenes, Enterobacter cloacae, Enterobacteraerogenes, and Serratialiquefaciens.
**fungal infection here refers to the invasive fungal infection and fungemia.
Outcome of patients
Among the 484 patients included in the cohort, 139 died in ICU, and 23 died during hospitalization after transfer to general wards. Twenty patients (4.1%) were still in hospital at the end of follow-up (i.e. November 30, 2009) and were deemed survivors. The crude ICU and hospital mortality rates were 28.7% and 33.5%, respectively. The median ICU LOS was 7 days (IQR, 4–15) and hospital LOS was 18 days (IQR, 10–38). About three fourths (72.3%) of patients had stayed in ICU for less than 2 weeks, while 9.5% of patients had an ICU LOS of more than 4 weeks. Compared with patients without shock, patients with septic shock were more likely to receive mechanical ventilation (78.2% vs. 63.3%, p = 0.003), and had a longer ICU LOS (9 [IQR, 4–17.5] vs. 7 [IQR, 4–15], p = 0.011).
Prognostic factors
Variables added into the multivariate model included age, sex, comorbidity of cancer, hypertension, COPD, and organ transplantation, chronic heart failure, immune-compromised status, type of ICU admission (respiratory disease, gastrointestinal disease, renal disease and trauma), ICU admission categories (medical, scheduled surgery and emergency surgery), APACHE II score, SOFA score of day1 in ICU, presence of septic shock, ARDS and AKI, bloodstream infection, soft tissue infection, central nervous system infection. As shown in Table 5, APACHE II score, presence of ARDS, bloodstream infection and comorbidity of cancer were independent risk factors for hospital mortality. The Chi-square value of Hosmer-Lemeshow test was 4.868, and the p value was 0.772.
Table 5. Multivariate logistic regression analysis of independent predictors of hospital mortality in patients with severe sepsis and septic shock.
| Risk factor | OR (95% CI) | P value | ||
| Univariate | Multivariate | Univariate | Multivariate | |
| APACHE II | 1.113(1.083–1.144) | 1.068(1.027–1.109) | <0.001 | 0.001 |
| ARDS | 3.173(2.129–4.730) | 2.676(1.691–4.235) | <0.001 | <0.001 |
| Bloodstream infection | 2.848(1.443–5.624) | 2.520(1.142–5.564) | 0.002 | 0.022 |
| Cancer | 2.283(1.295–4.024) | 2.246(1.141–4.420) | 0.004 | 0.019 |
APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, Acute respiratory distress syndrome; CI, confidence interval; OR, odds ratio.
Discussion
The incidence of severe sepsis in the present study was 37.3 cases per 100 ICU admissions, and 9.2% (n = 119) of the patients admitted into ICU developed septic shock. The lung and abdomen were the most frequent sites of infection. The ICU and hospital mortality rates of severe sepsis were 28.7% and 33.5%, respectively. Patients with shock had a much higher mortality rate (42.9%) than those without shock (30.4%). Patients with higher APACHE II score, presence of ARDS, bloodstream infection and comorbidity of cancer may have higher risk of death.
In comparison with previous studies [3], [6], [7], [21], the treated incidence of severe sepsis in the study ICUs is very high. This difference may be due to the increasing trend of severe sepsis [4], [5], as our study was conducted much later. However, secular trend may not be the only cause of incidence variation, because even higher incidence of severe sepsis has been reported in previous studies [9]. Different inclusion criteria may also explain the reported discrepancy. For example, Adrie et al included patients with ICU LOS of at least 48 hours [9], while Padkin et al only screened patients for severe sepsis within the first 24 hours of ICU admission [21]. In order to include cases with early recovery from or late onset of severe sepsis, we screened all ICU admissions with ICU LOS no less than 24 hours. Second, patient population in different studies may be quite different. Cheng et al only studied patients admitted to surgical ICUs [3], while, in the current study, 18 out of the 22 participating ICUs were general ICUs, and 76.7% of our cohort were medical patients. Another possible reason is the relative lack of ICU beds in China compared to other countries, which might have led to admission of sicker patients into the study ICUs [22], [23]. Finally, definitions of severe sepsis employed in various studies may be also different [21].
The outcome of severe sepsis patients varies considerably in different studies. In SOAP study, ICU mortality rate was 32.2% for severe sepsis and 54.1% for septic shock [24]. In France, patients with severe sepsis had a hospital mortality rate of 59% [8], whereas patients with septic shock had a hospital mortality of 61.2% [4]. Finfer et al reported that overall ICU and hospital mortality rates were 26.5% and 37.5% for patients with severe sepsis in Australia and New Zealand [6]. In comparison, the mortality rates of patients with severe sepsis (30.4%) and septic shock (42.9%) in the current study were lower than that in most studies [6], [12], [25]. Many factors can explain the difference. First, the severity of acute illness might be different. For example, patients included in the study of Khwannimit et al [12] were more severely ill, as suggested by a higher APACHE II score (26.8 vs. 21), and were more likely to die than our cohort (49.7% vs. 33.5%). Second, previous studies found that, compared with patients who developed sepsis outside ICU, patients with ICU-acquired sepsis had a higher mortality [24], [26]. Only 12.6% of patients in the current study developed ICU-acquired severe sepsis or septic shock, while 25% episodes of severe sepsis in France were ICU-acquired [8]. Despite the fact that hospital mortality rate in the current study was lower than that in many studies, it was still higher with a median APACHE II score of only 21. This was possibly attributable to the very low compliance with sepsis resuscitation and management bundles, although not reported in the current study but consistently observed in other studies involving Chinese patients [27]. There should be no doubt that clinical outcome of severe sepsis/septic shock could be improved significantly by better understanding of pathogenesis, as well as increasing the uniform compliance with standard therapy and other treatments proven effective for severe sepsis in the future. Due to the high mortality rate in patients with septic shock, patients with risk factors [28], [29], such as higher SOFA score should be observed closely.
Similar to other studies [4], [6], [8], we found that lung and abdomen were the most common source of infection. The potential implication of this finding is that, when the source of infection remains unknown in a patient with severe sepsis/septic shock, clinicians should consider pulmonary and intra-abdominal sources. Furthermore, majority of our patients had pneumonia, indicating the importance of implementing effective strategies to prevent both community-acquired pneumonia (such as public education, and vaccination against influenza and pneumococcus in high risk population) and hospital-acquired pneumonia (such as hand hygiene, and selective digestive decontamination). In accordance with other studies [3], [12], most isolated pathogens in our study were Gram-negative bacilli, although some studies in developed countries reported predominance of Gram-positive bacteria [2], [6], [24]. Many factors, including geographic variation, case mix, and antibiotic prescription habits, may explain the observed difference. Moreover, clinical significance of the same pathogens may vary in different studies. Cheng et al reported that Acinetobacter baumannii (25.8%) and Escherichia coli (13.8%) were the most common pathogens, while only 13.8% of the infections were caused by Pseudomonas aeruginosa. Despite a similar frequency of Acinetobacter baumannii (25.7%), Pseudomonas aeruginosa and Klebsiella pneumonia were much more common in our study. The dramatic increase in the incidence of Pseudomonas aeruginosa is worth mentioning as Pseudomonas species may be associated with increased mortality rates [24]. There might be bias in the results of microorganisms distribution as not all units reported microbiology results. Furthermore, we cannot rule out the possibility that many patients included in our study were nosocomial cases of sepsis, which might have resulted in the high frequency of Acinetobacter baumannii.
Independent risk factors associated with increased mortality in severe sepsis include higher APACHE II score [12], [27], presence of ARDS [12], [30], bloodstream infection [8] and comorbid cancer [2], [3], [24], [31], which was a consistent finding across literatures. Although patients with shock had a higher mortality, the presence of shock was not an independent risk factor for death in our study.
As we know, there are few data about the epidemiology of severe sepsis and septic shock in mixed ICU in China. Our report may add some valuable information. Nonetheless, some limitations merit discussion. First, there may have been bias only concerning ICU admissions. In general, patients with severe sepsis would be treated in ICU in those participating hospitals, unless they responded to simple measures such as fluid resuscitation and antibiotics. Some patients may not be admitted into ICU because of personal willingness. However, most Chinese people tend to reject advance directives, and prefer family-centered decision making than other ethnic and cultural groups. Even if the illness is irreversible, families may strongly advocate aggressive treatment, as they endorse the cultural belief that withdrawing or withholding support of their family member with critical illness is disgraceful or not filial piety [32]. Chinese doctors seldom advise families to withdraw treatment because of the possibility of involvement in medical disputes and undertaking legal liability [33]. Second, the results of the present study might not be able to generalize to ICUs in small local hospitals. Although about 10% (47/484) of our cohort are transferred from other hospitals, we believe that even more patients with severe sepsis/septic shock are treated in local hospitals, possibly with higher mortality rate. Third, only a minority of patients had microbiological documentation, and the rest were with negative or unreported positive culture results. But the distribution of pathogens obtained in this research was similar to the result of Cheng and colleagues' research [3], and there was no significant difference in patient demographics and mortality rate (38% vs. 31.3%, p = 0.144) between those ICUs with and without microbiological results. Zahar et al have also found that microbiological characteristics of infection did not influence the outcome of patients with severe sepsis [34]. Fourth, the exclusion criterion of ICU LOS<24 hours would have the effect of excluding the sickest patients who died early and might have bias the study results. Finally, the data were not collected for the primary purpose of identifying severe sepsis. Some important information related to severe sepsis was not reported, such as the compliance with sepsis bundle which might affect the clinical outcome of severe sepsis and the results of multivariate regression analysis. Further studies focusing on the rate of compliance with the resuscitation bundle and its influence factors should be conducted in China.
Conclusions
Severe sepsis is an important public health problem and a frequent cause of ICU admission with a high mortality rate. Higher APACHE II score, presence of ARDS, bloodstream infection and comorbidity of cancer are risk factors contributing to fatal outcome. This argues that severe sepsis/septic shock represents a major disease burden in mainland China. Future clinical research is warranted to ensure early identification of high risk patient population, prompt implementation of validated treatment, and significant improvement of clinical outcome.
Supporting Information
The full names and affiliation of participating hospitals.
(DOC)
Acknowledgments
The China Critical Care Clinical Trials Group (CCCCTG) is composed of 24 ICUs in different hospitals in China, and the individual authors (their affiliations) within this group are listed as follows: Bin Du, Li Weng (Medical ICU, Peking Union Medical College Hospital, Peking Union Medical College & Chinese Academy of Medical Sciences, Beijing, China); Chuanyun Qian, Wei Zhang (Department of Emergency Medicine and Medical ICU, The First Affiliated Hospital of Kunming Medical University, Kunming, China); Mingyan Zhao, Dongsheng Fei (Department of Critical Care Medicine, The First Affiliated Hospital of Harbin Medical University, Harbin, China); Xiangyou Yu, Yi Wang (Department of Critical Care Medicine, First Affiliated Hospital, Xinjiang Medical University, Urumqi, China); Yan Kang, Xuelian Liao (Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China); Xiaochun Ma, Yini Sun (Department of Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China); Yuhang Ai, Li Huang (Department of Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, China); Yuan Xu, Wei He (Department of Critical Care Medicine, Beijing Tongren Hospital, Capital Medical University, Beijing, China); Yongjie Yin, Dexin Liu (Department of Critical Care Medicine, The Second Hospital of Jilin University, Changchun, China); Youzhong An, Huiying Zhao (Department of Critical Care Medicine, Peking University People's Hospital, Beijing, China); Dawei Wu, Chen Li (Department of Critical Care Medicine, Qilu Hospital of Shandong University, Jinan, China); Renhua Sun, Qian Li (Department of Critical Care Medicine, Zhejiang Provincial People's Hospital, Hangzhou, China); Shusheng Li, Xiao Ran (Department of Critical Care Medicine, Tongji Hospital of Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China); Zhenjie Hu, Yan Huo (Department of Critical Care Medicine, Hebei Medical University Fourth Hospital, Shijiazhuang, China); Xiangyuan Cao, Xigang Ma (Department of Critical Care Medicine, General Hospital of Ningxia Medical University, Yinchuan, China); Fachun Zhou, Fang Xu (Department of Emergency and Intensive Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China); Li Jiang, Qi Zhang (Department of Critical Care Medicine, Fuxing Hospital, Capital Medical University, Beijing, China); Jiandong Lin, Xiongjian Xiao (Department of Critical Care Medicine, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China); Enqiang Mao, Cheng Zhu (Emergency ICU, Ruijin Hospital, Shanghai Jiao Tong University, Shanghai, China); Tiehe Qin, Shouhong Wang (Department of Critical Care Medicine, Guangdong General Hospital, Guangzhou, China); Zhenyang He, Rui Li (Department of Critical Care Medicine, Hainan Provincial People's Hospital, Haikou, China); Lihua Zhou, Lipeng Zhang (Department of Critical Care Medicine, The Affiliated Hospital of Inner Mongolia Medical University, Huhhot, China); Rongqing Sun, Yuexia Li (Intensive Care Unit, The First Affiliated Hospital, Zhengzhou University); Xijing Zhang, Binxiao Su (Surgical Intensive Care Unit, Xijing Hospital). The lead author for this group is Bin Du, and his contact email address isdubin98@gmail.com.
We would like to thank persons listed above but not designated as the author of this article in those 22 participating ICUs for their help with data collection.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported in part, by the Capital Clinical Application Research grant (Number: Z131107002213112) from the Science and Technology Commission of Beijing, and the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Heyland DK, Hopman W, Coo H, Tranmer J, McColl MA (2000) Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med 28: 3599–3605. [DOI] [PubMed] [Google Scholar]
- 2. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 3. Cheng B, Xie G, Yao S, Wu X, Guo Q, et al. (2007) Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. Crit Care Med 35: 2538–2546. [DOI] [PubMed] [Google Scholar]
- 4. Annane D, Aegerter P, Jars-Guincestre MC, Guidet B (2003) Current epidemiology of septic shock: the CUB-Rea Network. Am J Respir Crit Care Med 168: 165–172. [DOI] [PubMed] [Google Scholar]
- 5. Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 6. Finfer S, Bellomo R, Lipman J, French C, Dobb G, et al. (2004) Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 30: 589–596. [DOI] [PubMed] [Google Scholar]
- 7. Harrison DA, Welch CA, Eddleston JM (2006) The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 10: R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, et al. (1995) Incidence, risk factors, and outcome of severe sepsis and septic shock in adults. A multicenter prospective study in intensive care units. French ICU Group for Severe Sepsis. Jama 274: 968–974. [PubMed] [Google Scholar]
- 9. Adrie C, Alberti C, Chaix-Couturier C, Azoulay E, De Lassence A, et al. (2005) Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquisition (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care 20: 46–58. [DOI] [PubMed] [Google Scholar]
- 10. Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, et al. (2008) Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 12: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez F, Barrera L, De La Rosa G, Dennis R, Duenas C, et al. (2011) The epidemiology of sepsis in Colombia: a prospective multicenter cohort study in ten university hospitals. Crit Care Med 39: 1675–1682. [DOI] [PubMed] [Google Scholar]
- 12. Khwannimit B, Bhurayanontachai R (2009) The epidemiology of, and risk factors for, mortality from severe sepsis and septic shock in a tertiary-care university hospital setting. Epidemiol Infect 137: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 13. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, et al. (1995) The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. Jama 273: 117–123. [PubMed] [Google Scholar]
- 14. Perl TM, Dvorak L, Hwang T, Wenzel RP (1995) Long-term survival and function after suspected gram-negative sepsis. Jama 274: 338–345. [PubMed] [Google Scholar]
- 15. Du B, An Y, Kang Y, Yu X, Zhao M, et al. (2013) Characteristics of critically ill patients in ICUs in Mainland China. Crit Care Med 41: 84–92. [DOI] [PubMed] [Google Scholar]
- 16. Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818–829. [PubMed] [Google Scholar]
- 17. Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, et al. (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 18. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, et al. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 19. Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, et al. (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 20. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P (2004) Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padkin A, Goldfrad C, Brady AR, Young D, Black N, et al. (2003) Epidemiology of severe sepsis occurring in the first 24 hrs in intensive care units in England, Wales, and Northern Ireland. Crit Care Med 31: 2332–2338. [DOI] [PubMed] [Google Scholar]
- 22. Austin S, Murthy S, Wunsch H, Adhikari NK, Karir V, et al. (2014) Access to urban acute care services in high- vs. middle-income countries: an analysis of seven cities. Intensive Care Med 40: 342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du B, Xi X, Chen D, Peng J (2010) Clinical review: critical care medicine in mainland China. Crit Care 14: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, et al. (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 25. Silva E, Pedro Mde A, Sogayar AC, Mohovic T, Silva CL, et al. (2004) Brazilian Sepsis Epidemiological Study (BASES study). Crit Care 8: R251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakr Y, Elia C, Mascia L, Barberis B, Cardellino S, et al. (2013) Epidemiology and outcome of sepsis syndromes in Italian ICUs: a muticentre, observational cohort study in the region of Piedmont. Minerva Anestesiol 79: 993–1002. [PubMed] [Google Scholar]
- 27. Phua J, Koh Y, Du B, Tang YQ, Divatia JV, et al. (2011) Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ 342: d3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glickman SW, Cairns CB, Otero RM, Woods CW, Tsalik EL, et al. (2010) Disease progression in hemodynamically stable patients presenting to the emergency department with sepsis. Acad Emerg Med 17: 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song YH, Shin TG, Kang MJ, Sim MS, Jo IJ, et al. (2012) Predicting factors associated with clinical deterioration of sepsis patients with intermediate levels of serum lactate. Shock 38: 249–254. [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen ME, Shah CV, Meyer NJ, Gaieski DF, Lyon S, et al. (2013) The Epidemiology of Acute Respiratory Distress Syndrome in Patients Presenting to the Emergency Department With Severe Sepsis. Shock. [DOI] [PMC free article] [PubMed]
- 31. de Montmollin E, Tandjaoui-Lambiotte Y, Legrand M, Lambert J, Mokart D, et al. (2013) Outcomes in critically ill cancer patients with septic shock of pulmonary origin. Shock 39: 250–254. [DOI] [PubMed] [Google Scholar]
- 32. Blumenthal D, Hsiao W (2005) Privatization and its discontents–the evolving Chinese health care system. N Engl J Med 353: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 33. Li LB (2013) Clinical review: Ethics and end-of-life care for critically ill patients in China. Crit Care 17: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesim A, et al. (2011) Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med 39: 1886–1895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The full names and affiliation of participating hospitals.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.