Evidence suggests that advanced glycation end products (AGEs) contribute to cognitive decline (Yaffe et al., 2011). AGEs encourage the formation and deposition of neurofibrillary tangles and amyloid plaques, the hallmarks of Alzheimer’s Disease (AD). AGEs also appear to increase the levels and toxicity of beta-amyloid (Li et al., 2012; Li et al., 2013), higher levels of which are associated with accelerated memory decline in individuals with both normal cognition and mild cognitive impairment (Lim et al., 2014).
Numerous endogenous AGEs have been implicated in cognitive decline. AGE precursor methylglyoxal (MGO) has been found to be associated with poorer cognition and cerebral atrophy in older adults (Srikanth et al., 2012). As detailed below, Beeri et al. (2011) reported a decline in performance on the Mini Mental State Exam (MMSE) with higher levels of serum methylglyoxal (sMG). Albumin, a protein abundant in serum and cerebrospinal fluid, is found in higher glycated levels in AD patients compared to controls (Ramos-Fernández, et al., 2014). Higher levels of the AGE Nε-(carboxymethyl)-lysine (CML) are associated with severity of cognitive impairment in people with cerebrovascular disease, suggesting a relationship between AGEs and vascular dementia (Southern, et al., 2007). Finally, individuals with high levels of urinary pentosidine demonstrated greater decline on the digit symbol substitution test in older adults with and without diabetes (Yaffe et al., 2011).
Exogenous dietary AGEs (dAGEs) are found in higher levels in animal-derived foods and high-and dry-heat processed foods (Uribarri, 2010) and are associated with elevated circulating AGE levels (Uribarri, 2010). Clarifying the relationship between exogenous and endogenous AGEs and cognitive decline may provide promising directions for addressing and treating AD and vascular dementia.
We reported higher levels of sMG to be associated with cognitive decline, as determined by the Mini Mental State Exam (MMSE), a screen for cognitive impairment (Beeri et al., 2011), in a sample of very elderly that were cognitively normal at baseline and followed over time. Here we examined the relationships of dAGE and sMG with cognitive domains in substantially younger elderly, also cognitively normal at baseline, assessed with a broad neuropsychological battery.
dAGEs were estimated by using a published database as described in Vlassara et al. (2009). Methods to measure sMG, sociodemographic and cardiovascular risk factors and statistical analyses were the same as described in Beeri et al. (2011). Forty nine subjects participating in a study on the relationships of AGEs with inflammatory/cardiovascular risk factors (R37 AG023188), who had baseline assessments for dAGE, sMG, sociodemographic (age, sex, education), and cardiovascular (diabetes status, blood pressure, BMI, APOE genotype), were assessed for cognitive outcomes.
Subjects averaged 71.0 (±8.1 S.D.) years of age, 15.9 (2.5) years of education, at baseline and had 35.9 (±13.5) months of follow-up. At baseline, their mean dAGE was 11.9 AGE Eq/d (±6.9), their mean sMG was 0.91 nmol/ml (±0.21 nmol/ml), BMI 25.5 (±4.3), and 76% of the sample were female. The average MMSE at baseline was 28.9 (±1.6) reflecting a cognitively normal sample. Compared to Beeri et al. (2011), subjects averaged 12 years younger, but years of education, length of follow-up, baseline sMG, and BMI were very similar.
Factor analysis summarized the neuropsychological measures into four domains by summing z-scores (reversed if necessary): attention (diamond and letter cancellation), executive (Trails A and B), memory (immediate and delayed recall, recognition), and language (animal fluency, similarities, Boston). Mixed regression model were used to assess the effect of baseline dAGE and sMG on the association between cognitive domain measures and time, controlling for sociodemographic and cardiovascular risk factors.
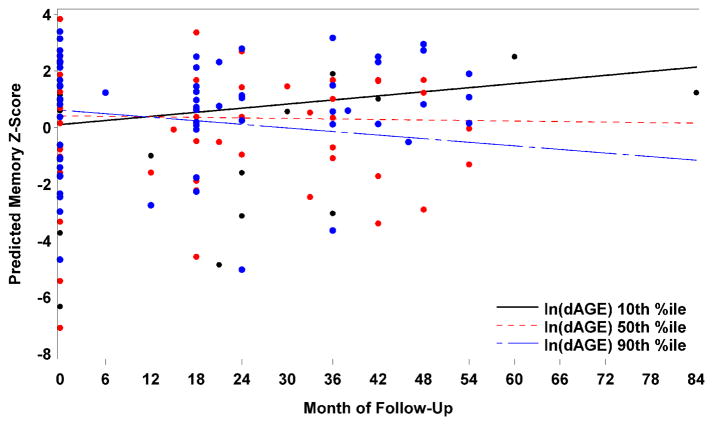
There was a significant interaction between dAGE and month of follow-up for memory scores—for each 1 unit increase in natural log of baseline dAGE, there was a decrease of .035 in the monthly rate of change of memory scores (p = 0.012). For descriptive purposes, Figure 1 presents the predicted memory scores over time for baseline dAGE levels of 1.6, 2.4, and 2.9 (the 10th, 50th, and 90th percentiles, respectively. Baseline dAGE was not a significant effect modifier of the associations between other neuropsychological measures and month of follow-up (Table 1). Interactions between sMG and month of follow-up were also significant for attention (p=.017). dAGE and sMG were highly correlated (r=.62; p<.001) but including each of them as covariates in the analysis of the other did not affect the results whatsoever.
Figure 1.
Predicted memory scores over time for 10th, 50th and 90th percentile of baseline dietary AGE (dAGE) – adjusted for sex, age, education, diabetes, systolic blood pressure, diastolic blood pressure, APOE4, and BMI. The points on the plot represent the observed memory scores.
Table 1.
Mixed Regression Model Estimates for interaction of baseline dAGE and sMG with time for cognitive domains, adjusted for months of follow-up, baseline of the cognitive outcome, age, years of education, sex, diabetes, systolic and diastolic blood pressure, APOE4 genotype, and BMI.
| Outcome | Estimate | SE | T-value | P-value | |
|---|---|---|---|---|---|
| Memory | sMG | −0.04847 | 0.03380 | −1.43 | 0.1560 |
| dAGE | −0.03465 | 0.01348 | −2.57 | 0.0123* | |
| Attention | sMG | 0.04644 | 0.02059 | 2.26 | 0.0273* |
| d AGE | 0.008015 | 0.008716 | 0.92 | 0.3610 | |
| Language | sMG | −0.03894 | 0.03297 | −1.18 | 0.2416 |
| dAGE | −0.00226 | 0.01403 | −0.16 | 0.8725 | |
| Executive | sMG | −0.00791 | 0.03969 | −0.20 | 0.8425 |
| dAGE | 0.01439 | 0.01619 | 0.89 | 0.3771 | |
| MMSE | sMG | −0.05648 | 0.04626 | −1.22 | 0.2281 |
| dAGE | −0.00625 | 0.01869 | −0.33 | 0.7398 |
indicates p<.05
To the best of our knowledge, this is the first report showing that high dAGE levels were associated with a faster rate of decline in memory over time in initially non-demented young elderly. Consistent with this result and with our previous report using a global measure of cognition, high sMG was associated with faster rate of decline in attention. dAGEs and sMG were not related to other cognitive factors in this small sample.
Higher levels of AGEs likely contribute to AD (Yaffe et al., 2011). Memory impairment, the primary symptom of AD, has been found to be linked to serum markers for AGEs (Srikanth et al., 2013). AGEs increase aggregation and cytotoxicity of amyloid-β (Li et al., 2012; Li et al., 2013) and are found in higher levels in individuals with AD (Krautwald & Munch, 2010). dAGEs are strongly connected to elevated circulating AGEs (Uribarri, 2010), and thus it is plausible that through increases in circulating AGEs, they affect cognition deleteriously. However, including dAGE as a covariate in the sMG analysis and vice-versa did not alter the results, suggesting that dAGEs and circulating AGEs have unique contributions to cognitive decline.
Increased baseline sMG levels were found to be related to attentional decline. Since the attention tests were all timed, our results suggest that high sMG may negatively affect processing speed (McGuinness et al., 2010). Impairments in attention and processing speed have been linked to cerebrovascular disease (Wiederkehr et al., 2009) and its risk factors, such as metabolic syndrome (Segura et al., 2009), which are associated with dementia (Wiederkehr et al., 2009; Segura et al., 2009). AGEs accumulate in, and thicken and stiffen, vascular walls, increasing risk of hypertension (Goh & Cooper, 2008) potentially leading to cognitive impairment via cerebrovascular disease.
Since subjects were cognitively normal at baseline, our findings suggest that elevated dAGEs may negatively impact memory before clinical symptoms of cognitive decline are expressed. A low-AGE diet may reduce circulating AGEs (Cai et al., 2013; Vlassara et al., 2009; Uribarri et al, 2010), potentially providing a simple intervention by which risk for cognitive compromise may be reduced. Since individuals with diabetes have higher circulating AGEs (Uribarri et al., 2010), our results suggest an explanation for the higher rates of cognitive decline and AD in diabetes (Krautwald & Munch, 2010; McCrimmon, Ryan, & Frier, 2012).
The study is limited by the small number of subjects and by use of a convenience sample but is strengthened by its longitudinal design, a broad neuropsychological battery that permits assessment of several cognitive domains, and by numerous, directly measured, potential confounders that were accounted for.
Table 2.
Participant characteristics
| Characteristic | Mean | SD | Minimum | Maximum |
|---|---|---|---|---|
| Follow-up Months | 35.94 | 13.50 | 15.00 | 84.00 |
| dAGE | 11.88 | 6.87 | 3.80 | 35.25 |
| sMG | 0.91 | 0.21 | 0.46 | 1.36 |
| Age at baseline | 70.99 | 8.06 | 60.00 | 89.69 |
| Years of education | 15.88 | 2.47 | 10.00 | 20.00 |
| Systolic blood pressure | 126.41 | 16.05 | 83.00 | 164.00 |
| Diastolic blood pressure | 69.16 | 8.94 | 49.25 | 90.00 |
| BMI | 25.47 | 4.33 | 16.00 | 34.50 |
| Diabetes | 3 (6%) | |||
| Female | 37 (76%) | |||
| APOE4 | 9 (18%) | |||
| Baseline MMSE | 28.90 | 1.56 | 24.00 | 30.00 |
| Baseline memory | 0.11 | 2.49 | −7.08 | 3.39 |
| Baseline attention | 0.06 | 1.81 | −2.88 | 4.21 |
| Baseline language | 0.01 | 3.65 | −13.95 | 4.18 |
| Baseline executive | −0.17 | 1.97 | −6.15 | 2.37 |
Highlights.
Advanced glycation end products are associated cognitive decline.
High levels of dietary AGEs (dAGEs) are associated with faster decline in memory.
High serum methylglyoxal levels are associated with faster decline in attention.
Modifying AGEs in the diet may be a strategy to diminish cognitive compromise.
Acknowledgments
This study was funded by the Alzheimer’s Association grant #NIRG-06-25559, the Alzheimer’s Disease Research Center of the Mount Sinai School of Medicine, and NIH R01 AG34087 and the Irma T. Hirschl Scholar Award to Dr. Beeri, and NIH R37 AG023188 to Dr. Vlassara.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beeri MS, Moshier E, Schmeidler J, Godbold J, Uribarri J, Reddy S, Sano M, Grossman HT, Cai W, Vlassara H, Silverman JM. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech Ageing Dev. 2011;132:583–587. doi: 10.1016/j.mad.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, Woodward M, Striker GE, Vlassara H. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. PNAS. 2012;111:4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goh SY, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 4.Krautwald M, Münch G. Advanced glycation end products as biomarkers and gerontotoxins – A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp Gerontol. 2010;45:744–751. doi: 10.1016/j.exger.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Liu D, Sun L, Lu Y, Zhang Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J Neurol Sci. 2012;317:1–5. doi: 10.1016/j.jns.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 6.Li XH, Du LL, Cheng XS, Jiang X, Zhang Y, Lv BL, Liu R, Wang JZ, Zhou XS. Glycation exacerbates the neuronal toxicity of β-amyloid. Cell Death Dis. 2013;4:e673. doi: 10.1038/cddis.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim YY, Maruff P, Piertrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, Savage G, Villemagne VL, Rowe CC. Aβ and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.11.005. In Press. [DOI] [PubMed] [Google Scholar]
- 8.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 9.McGuinness B, Barrett SL, Craig D, Lawson J, Passmore AP. Attention deficits in Alzheimer’s disease and vascular dementia. J Neurol Neurosurg Psychiatry. 2010;81:157–159. doi: 10.1136/jnnp.2008.164483. [DOI] [PubMed] [Google Scholar]
- 10.Ramos-Fernández E, Tajes M, Palomer E, Ill-Raga G, Bosch-Morató M, Guivernau B, Román-Dégano I, Eraso-Pichot A, Alcolea D, Fortea J, Nuñez L, Paez A, Alameda F, Fernández-Busquets X, Lleó A, Elosúa R, Boada M, Valverde MA, Muñoz FJ. Posttranslational Nitro-Glycative Modifications of Albumin in Alzheimer’s Disease: Implications in Cytotoxicity and Amyloid-β Peptide Aggregation. J Alzheimers Dis. 2014 doi: 10.3233/JAD-130914. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Segura B, Jurado MA, Freixenet N, Albuin C, Muniesa J, Junque C. Mental slowness and executive dysfunctions in patients with metabolic syndrome. Neuroscience Letters. 2009;462:49–53. doi: 10.1016/j.neulet.2009.06.071. [DOI] [PubMed] [Google Scholar]
- 12.Srikanth V, Westcott B, Forbes J, Phan TG, Beare R, Venn A, Pearson S, Greenaway T, Parameswaran V, Munch G. Methylglyoxal, Cognitive Function and Cerebral Atrophy in Older People. J Gerontol A Biol Sci Med Sci. 2013;68:68–73. doi: 10.1093/gerona/gls100. [DOI] [PubMed] [Google Scholar]
- 13.Southern L, Williams J, Esiri MM. Immunohistochemical study of N-epsilon-carboxymethyl lysine (CML) in human brain: relation to vascular dementia. BMC Neurol. 2007;7:35–42. doi: 10.1186/1471-2377-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in food and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911–916. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vlassara H, Cai W, Goodman S, Pyzik R, Yong A, Chen X, Zhu L, Neade T, Beeri M, Silverman JM, Ferruci L, Tansman L, Striker GE, Uribarri J. Protection against loss of innate defenses in adulthood by low advanced glycation end products (AGE) intake: role of the antiinflammatory AGE receptor-1. J Clin Endocrinol Metab. 2009;94:4483–91. doi: 10.1210/jc.2009-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiederkehr S, Laurin D, Simard M, Verrault R, Lindsay J. Vascular risk factors and cognitive functions in nondemented elderly individuals. J Geriatr Psychiatry. 2009;22:196–206. doi: 10.1177/0891988709335797. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe K, Lindquist K, Schwartz AV, Vitartis C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011;77:1351–1356. doi: 10.1212/WNL.0b013e3182315a56. [DOI] [PMC free article] [PubMed] [Google Scholar]