Abstract
During the development of the neuromuscular junction, motor axons induce the clustering of acetylcholine receptors (AChRs) and increase their metabolic stability in the muscle membrane. Here, we asked whether the synaptic organizer agrin might regulate the metabolic stability and density of AChRs by promoting the recycling of internalized AChRs, which would otherwise be destined for degradation, into synaptic sites. We show that at nerve-free AChR clusters induced by agrin in extrasynaptic membrane, internalized AChRs are driven back into the ectopic synaptic clusters where they intermingle with pre-existing and new receptors. The extent of AChR recycling depended on the strength of the agrin stimulus, but not on the development of junctional folds, another hallmark of mature postsynaptic membranes. In chronically denervated muscles, in which both AChR stability and recycling are significantly decreased by muscle inactivity, agrin maintained the amount of recycled AChRs at agrin-induced clusters at a level similar to that at denervated original endplates. In contrast, AChRs did not recycle at agrin-induced clusters in C2C12 or primary myotubes. Thus, in muscles in vivo, but not in cultured myotubes, neural agrin promotes the recycling of AChRs and thereby increases their metabolic stability.
Keywords: nicotinic acetylcholine receptors, agrin, neuromuscular junctions, recycled receptors, imaging
INTRODUCTION
The presence of a high density of neurotransmitter receptors (AChRs) in the postsynaptic muscle membrane is the hallmark of the neuromuscular junction. The molecular machinery that initiates and maintains receptor clusters is organized by agrin, a protein secreted from motor nerve terminals, which binds to the transmembrane protein LRP4 (low-density lipoprotein receptor-related protein 4, a member of the LDLR family) and stimulates MuSK (muscle skeletal receptor tyrosine-protein kinase) activity (DeChiara et al., 1996; Kim et al., 2008; Lin et al., 2001; Sanes and Lichtman, 2001; Zhang et al., 2008). The latter, through several pathways, acts both as a scaffold and a signaling molecule to form and stabilize the AChR clusters at the NMJ (Wu et al., 2010).
Recently, evidence has accumulated that the density of synaptic AChRs at the mature NMJ is maintained both through the insertion of newly synthetized AChRs and by the reinsertion of synaptic AChRs that had been internalized and then redirected back to the synaptic muscle membrane, a process called AChR recycling. Upon denervation, however, only a small fraction of the internalized AChRs are able to recycle back to the synaptic muscle membrane, and most internalized AChRs are degraded, a process that is reversed by muscle stimulation (Bruneau and Akaaboune, 2006). This shift to the degradation pathway by denervation and its reversal by muscle activity might explain the decrease in half-life of synaptic AChRs (from about 10–14 to 3–5 days as estimated through AChR pulse labeling) observed upon chronic denervation (Shyng and Salpeter, 1989). This change in receptor stability can also be reversed by muscle stimulation, apparently through Ca++ influx associated with action potential activity (Caroni et al., 1993). These data combined suggest that muscle activity, while suppressing extrasynaptic AChR expression (Lomo and Rosentha.J, 1972), directs the recyling of internalized AChRs into the postsynaptic membrane (Bruneau and Akaaboune, 2006; Rotzler et al., 1991), thus increasing their metabolic half-life.
Neural agrin alone is sufficient to induce nerve-free, ectopic specializations of sub-synaptic nuclei and formation of the postsynaptic apparatus in non-synaptic regions of muscle fibers (Bezakova et al., 2001b; Cohen et al., 1997; Meier et al., 1997). These agrin-induced ectopic postsynaptic-like membranes are functional and contain most of postsynaptic proteins that are present at innervated NMJs (Jones et al., 1997). Neural agrin appears also to be involved in setting-up the machinery that controls the metabolic stability of synaptic AChRs, because high neural concentrations of recombinant agrin can enhance the life-time of AChRs at ectopic agrin-induced clusters in both innervated and denervated muscles to levels comparable to those at innervated NMJs (Bezakova et al., 2001a; Bezakova and Lomo, 2001a; Bezakova et al., 2001b). Thus, we hypothesized that the effect of high agrin to stabilize AChRs could be mediated by increasing the amount of recycled AChRs.
In the current work we sought to test whether recombinant agrin is sufficient to induce the recycling of AChR at nerve-free, ectopic postsynaptic membranes. Analysis of receptor pools at such ectopic clusters showed that agrin is sufficient to induce the recycling in a dose-dependent manner, but that it is insufficient to trigger recycling at receptor clusters in cultured myotubes.
EXPERIMENTAL PROCEDURES
Intramuscular Injection of plasmid containing agrin
Adult female Wistar rats (~250 g body weight) were anaesthetized by i.p injection of ketamine and xylazine (0.4 ml/100 g body weight). Soleus muscles were exposed and different concentration of plasmids containing full length chicken agrin (NtAcagrin748) and nls-GFP (nuclear marker) were injected into individual muscle fibers as described previously (Jones et al., 1997). Mouse sternomastoid muscles were studied using identical methods.
NMJ labeling and confocal microscopy
Three to four weeks after injection, the soleus muscle was exposed; AChR were labeled with a saturating dose of α-bungarotoxin coupled to biotin (BTX-biotin; 5 µg/ml, 60 min) and then washed out with Ringer’s. Muscles were then bathed with a saturating dose of streptavidin-Alexa 594 and the incisions were sutured and the animals returned back to their cage. On subsequent days (typically 4–5 days later), the animals were re-anesthetized and muscles were exposed and bathed with a second saturating dose of streptavidin-Alexa 488 and BTX-Alexa 430 to label the recycled and newly synthesized AChRs respectively. Control experiments for ruling out dissociation of streptavidin from biotin on the surface of the muscle cells were described in our previous work (Bruneau et al., 2005). Muscles were removed and fixed with 4% paraformaldehyde, mounted on coverslips and scanned with a confocal microscope (Leica; model SPE) using a 100×, 1.46 numerical aperture (NA) oil-immersion objective (Leica; HCX Apochromat). The zstacks were then collapsed and the contrast of images was adjusted with Adobe Photoshop CS2.
In another set of experiments, Soleus muscles were exposed and bathed with a low dose of BTX-biotin (1 µg/ml, 30 min); so synapses remain fully functional, followed by a saturating dose of streptavidin Alexa 594 or unlabeled streptavidin (10 µg/ml, 3 hours). Four to five days later, the animals were anaesthetized and muscles were relabeled with streptavidin Alexa 488 to label specifically the recycled AChR pool. The analysis of fluorescence intensity of both recycled and pre-existing AChR pools was performed by using Image J (Version 1.45). Only receptor clusters that did not exhibit fluorescence saturation at ectopic clusters and original NMJs were analyzed. The magic wand function was used to select clusters of fluorescently labeled receptors and a region of the image with no signal, which was used to calculate a background level that was subtracted from fluorescence intensity of measured receptor clusters.
Denervation
Soleus muscles of rat were denervated by removing a 5 mm piece of sciatic nerve at the level of the thigh to prevent muscles from reinnervation and then injected with agrincDNA. Three to four weeks later the muscles were exposed and labeled as described above.
Immunocytochemistry
Soleus muscles injected with agrin cDNA (three to four weeks after injection) were labeled with BTX-biotin followed by streptavidin Alexa 594 (to label pre-existing AChRs) and four to five days later recycled AChRs were labeled with streptavidin Alexa 660. The muscles were then removed, permeabilized with 1% Triton X-100 for 15 min, incubated for 10 min with 100 mM glycine, blocked for 30 min with 1% BSA in PBS, incubated overnight at 4°C with anti-agrin antibody, washed 3 times in 1 h with in PBS containing 1% BSA, and incubated for 1 h with FITC-conjugated anti–rabbit. The bundles were then examined with a confocal laser–scanning microscope (Leica; model SPE).
Primary and C2C12 culture muscle cells
Primary and C2C12 myotubes (American Type Cell Culture) were cultured on laminincoated dishes focally impregnated with agrin (DMEM supplemented with 20% fetal bovine serum at 37°C), as described previously (Jones et al., 1997). Cells were differentiated 2 days later by replacing the media with DMEM supplemented with 5% horse serum. 4–6 days after differentiation, cells were bathed with a saturating dose of BTX-biotin followed by a saturating dose of fluorescently tagged streptavidin as describe above. 6 hours after initial labeling myotubes were bathed with (red) streptavidin-Alexa594 to label recycled AChR and with BTX-Alexa430 to label newly synthesized AChRs and receptor clusters were imaged.
Electron microscopy
Plasmid injected muscles were removed and prefixed for 1 hr with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, pH 7.4, at room temperature. Muscles were stained for acetylcholine esterase (AChE) activity according to Koelle and Friednewald (Koelle and Friedenwald, 1949), except that acetyl thiocholine concentration was reduced to keep AChE-dependent histochemical reaction product minimal. Muscles were then washed in PBS as soon as reaction product was observed, and they were postfixed in glutaraldehyde /cacodylate buffer overnight. Pieces of tissue were cut as described in Meier and colleagues (Meier et al., 1998) and then were dehydrated and embedded in Epon. Ultrathin sections were prepared and stained with uranylacetate and lead citrate, and viewed in a Hitachi 7100 electron microscope.
RESULTS
Recycled receptors at agrin-induced ectopic clusters in vivo
To examine whether internalized AChRs were able to recycle to agrin-induced ectopic AChR clusters, we co-injected fibers of rat soleus muscle with expression plasmids for nls-GFP (allowing later identification of injected fibers) and for full length chicken agrin (NtAcagrin748) (Denzer et al., 1995) at a concentration of 1 mg/ml, i.e. a high concentration inducing clusters of relatively high AChR density. Three to four weeks later, the muscles were bathed in situ with a saturating dose of bungarotoxin-biotin (BTX-biotin) to label surface receptors, followed by a saturating dose of streptavidin-Alexa594 (red: see Figure 1) to label all biotin sites as described by Bruneau et al. (Bruneau et al., 2005). Four days later, the soleus muscle was exposed and sequentially labeled with streptavidin-Alexa488 (green) to specifically label the recycled receptor pool (receptors that had lost their initial streptavidin-Alexa594 tag while retaining BTX-biotin during the process of internalization and reinsertion in the postsynaptic membrane receptors) and BTX-Alexa430 (blue) to label newly synthesized receptors that had been inserted after the initial labeling.
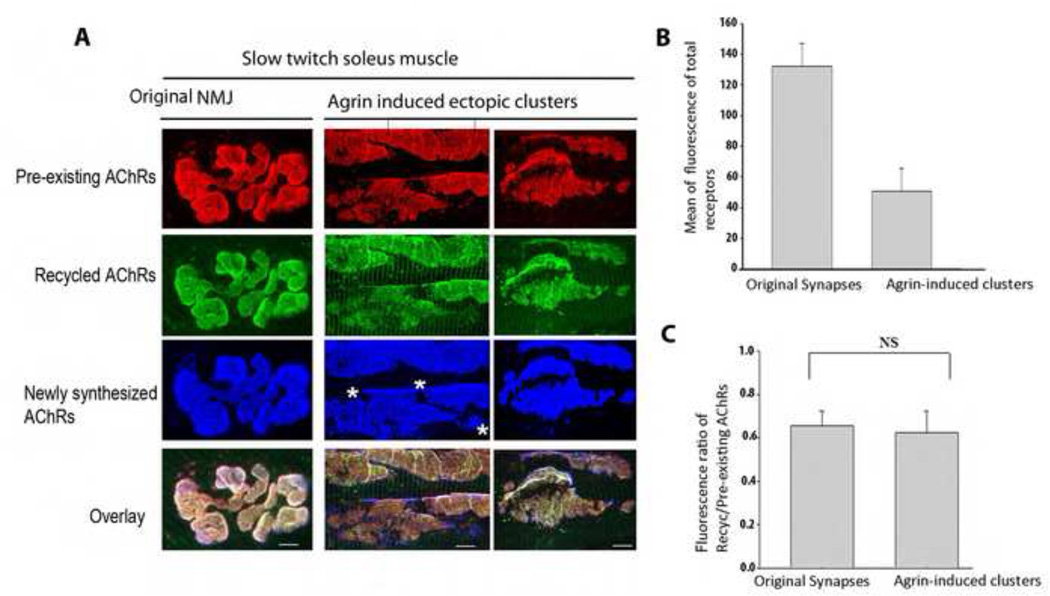
Figure 1.
Identification of a recycled receptor pool at ectopic agrin-induced acetylcholine receptor clusters in the Soleus muscles of adult rats. A) Examples of agrin-induced ectopic clusters and receptors at the postsynaptic membrane of a NMJ. Note the existence of all three receptor pools (recycled, pre-existing and newly synthesized) at both ectopic and the original NMJ. B) Graph summarizing the quantification of postsynaptic receptor density at agrin-induced clusters (N=18) and original NMJs (N=14). C) Graph summarizing the quantification of fluorescence ratio between the recycled AChR and pre-existing AChRs at agrin-induced and original synaptic clusters (as illustrated in panel A). Note that there is no significant difference between the size of the recycled receptor pool in agrin-induced clusters (N=24) and original NMJs (N=23) (Student test p=0.24), indicating unchanged internalization rates. Bar graph (±s.d). Images in A have been adjusted in Photoshop (Adobe) to maximize contrast. Bars, 10 µm. * indicates where newly synthesized AChRs are inserted. At least three rats were used in each experiment.
Analysis of the AChR pools demonstrated that agrin induced the formation of large contiguous AChR clusters and aggregates of small clusters (Fig. 1). Independent of their shapes and sizes, AChR aggregates were often observed around clustered nuclei (see Fig. 2C). In each cluster, we found that AChRs were able to recycle and intermingle with pre-existing AChRs and with new AChRs inserted after the first AChR labeling. Similar observations were made in mouse sternomastoid muscles (data not shown), although in the latter the induction of ectopic AChR clusters by agrin was less robust. These data show that agrin can induce clusters at which recycling of internalized receptors occurs in muscle fibers in vivo. Examination of the three AChR pools showed, however, that density was lower than the respective receptor densities at the original NMJs of the same muscle (Fig. 1A). To determine whether the lower fluorescence of recycled AChRs at agrin-induced clusters was due to a lower level of receptor recycling, we determined the ratio of fluorescence intensities of recycled to preexisting AChRs at each labeled cluster. These ratios were then compared with the ratio of recycled to preexisting AChR fluorescence at the original NMJs of the same muscle. We found that the fluorescence ratios were similar for agrin-induced ectopic AChR clusters and for original NMJs, demonstrating that recycling was similar for both (Fig. 1B). It should be noted that these ratios do not reflect true density ratios of the two AChR pools, as different fluorophores were used for their labeling. Interestingly, new AChR clusters containing only newly synthesized AChRs were formed after initial labeling at areas where no original aggregates (pre-existing/recycled AChRs) were present (see Fig. 1A, asterisks).
Figure 2.
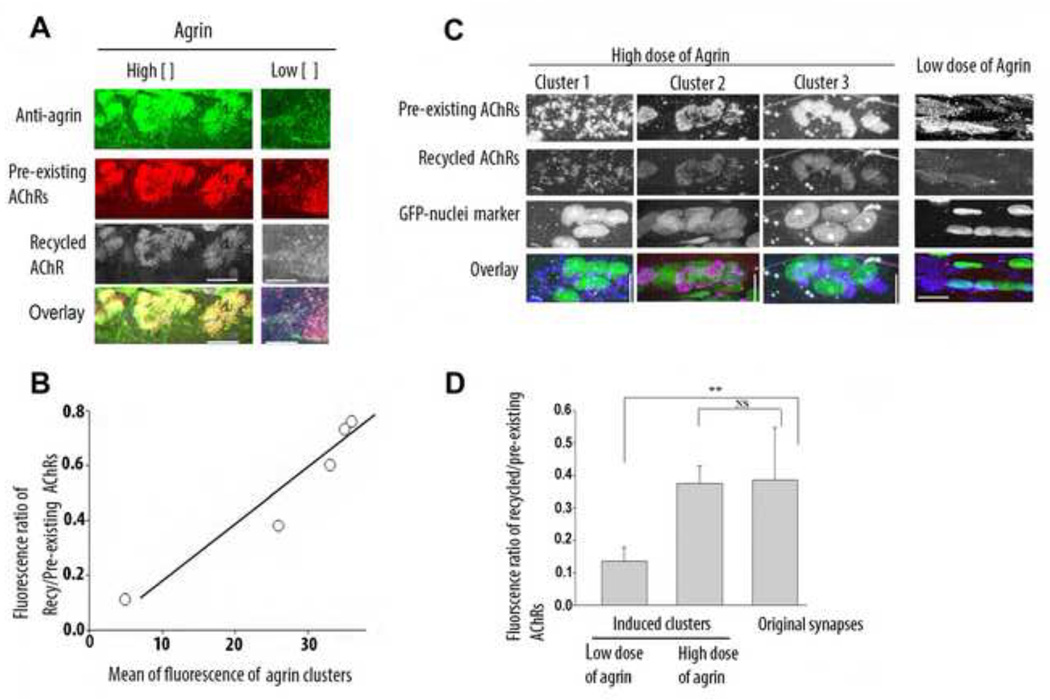
The size of the recycled receptor pool at agrin-induced acetylcholine receptor clusters depends on the strength of the agrin stimulus. A) Soleus muscle fibers were either injected with a high dose (1mg/ml) or a low dose (0.1 mg/ml) of plasmids coding for full length chicken agrin, receptor pools (recycled and pre-existing) B) Graph showing that the ratio of recycled/pre-existing AChRs increased with the strength of agrin stimulus. C) Soleus muscles of adult rats were denervated and then injected with either a low or a high concentration of agrin plasmid and a plasmid encoding nls-GFP. Shown are three examples of induced ectopic clusters with a high dose of agrin and one example with a low dose of agrin. D) Graph summarizing the quantification of fluorescence ratios of recycled/pre-existing AChRs at agrin-induced clusters (with a low (N=5) and high dose (N=9) of agrin) and original denervated NMJs (n=6). **P<0.01(One-way ANOVA followed by Tukey’s post test). Note that denervated muscles injected with a high dose of agrin maintained a high level of recycled AChRs at induced ectopic clusters comparable to that at original endplates. Bars, 10 µm for A and 15 µm for B. Bar graph (±s.d).
The level of the AChR recycling increases with the strength of the agrin stimulus
Previous studies have shown that the metabolic stability of the receptors at ectopic AChR clusters increases with agrin concentration (Bezakova et al., 2001b). To examine the possibility that this was due to agrin increasing the level of AChR recycling, soleus muscle fibers were injected with high (1 mg/ml) or low (0.1 mg/ml) concentrations of the agrin expression plasmid, the latter inducing AChR clusters of lower receptor density. The recycled and preexisting AChR pools at the ectopic AChR clusters were then stained and quantified as described above. The relative concentration of agrin on the fiber surface was estimated from the fluorescence intensity upon staining fibers with an anti-chicken agrin antibody (Denzer et al., 1995). In fibers that were injected with low plasmid concentration, agrin deposits tended to be weaker, and fewer recycled receptors were observed relative to preexisting receptors (see, e.g., Fig. 2). As the concentration of agrin at the fiber surface increased, the density of recycled AChRs increased (Fig. 2b). Thus, AChR recycling increased with the strength of the agrin stimulus (Fig. 2b).
Agrin is sufficient to maintain a high level of receptor recycling in denervated muscle
Previous studies have shown that, following denervation, the synaptic AChRs at the NMJ are less stable, and that they can be re-stabilized by exogenous muscle stimulation via implanted electrodes (Rotzler and Brenner, 1990). Comparison of recycled AChR pools in normal vs. chronically denervated muscles showed that in the absence of muscle stimulation most internalized receptors are targeted for degradation, and fewer receptors are able to recycle back into denervated NMJs (Bruneau and Akaaboune, 2006). Conversely, stimulation of denervated muscles increased AChR recycling (Martinez-Pena y Valenzuela et al., 2010), suggesting that stimulation-induced AChR re-stabilization in denervated muscle could be explained by increased AChR recycling. Therefore, we asked whether agrin could also increase AChR recycling at agrin-induced AChR clusters in denervated muscle. To this end, denervated muscles were injected with high and low doses of the agrin expression plasmid and 14 days after chronic denervation the recycled receptor pool at ectopic clusters was analyzed. We found that agrin induced recycling at ectopic AChR clusters in a dose-dependent manner. At low agrin concentrations, the amount of recycled AChRs in clusters was small compared to the number of recycled AChRs at clusters induced by high levels of agrin, which was similar to that observed at the original NMJs of the denervated muscle (Fig. 2D). Nevertheless, the levels of recycled AChR at high agrin-induced AChR clusters and at original NMJs were lower in the chronically denervated, inactive fibers than in the innervated, electrically active fibers (compare values of respective columns in Fig.s 2C and 1B). These results are in agreement with the previous observation that full metabolic stabilization of AChRs at agrin-induced AChR clusters to a half-life of about 10 days in denervated muscles (comparable to half-life of AChR at innervated NMJs) requires both agrin and electrical muscle activity (Bezakova et al., 2001b). This current result argues that agrin is able to induce AChR recycling autonomously and independently of muscle activity.
Agrin does not induce AChR recycling in cultured myotubes
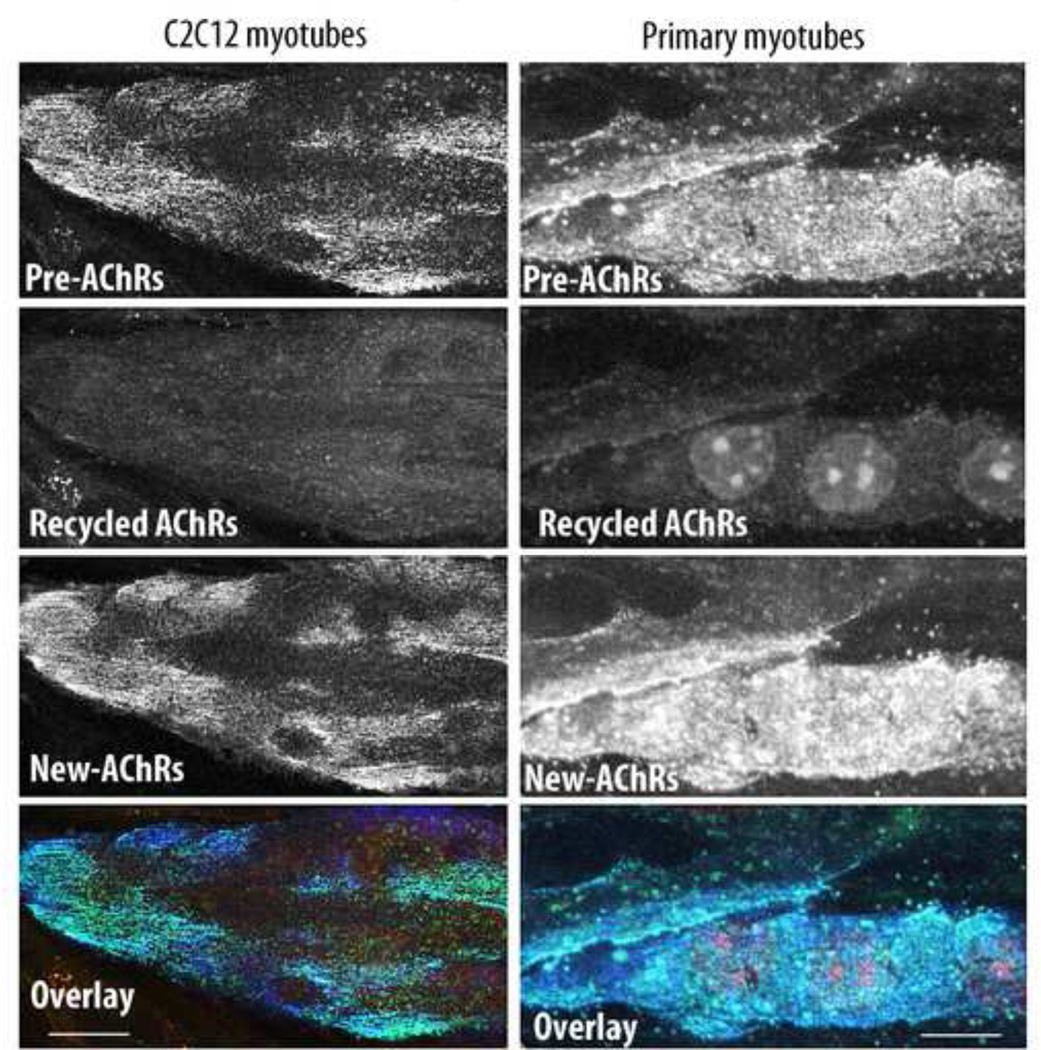
Having found that receptors are able to recycle back into agrin-induced clusters that lack innervation in living animals, we asked whether neuronal agrin alone could generate receptor recycling in cultured muscle cells. To test this, C2C12 myotubes were cultured on laminin-coated dishes focally impregnated with agrin expressed by COS-1 cells transfected with a plasmid coding for full-length chicken agrin. Differentiated myotubes were labeled with a saturating dose of BTX-biotin followed by a saturating dose of streptavidin-Alexa488 (green), and six hours later myotubes were bathed with (red) streptavidin-Alexa594 to label recycled AChR and BTX-Alexa430 to label newly synthesized AChRs. Extensive staining of newly synthesized AChRs was observed in original clusters, but the staining of recycled receptors was barely above the background. Similar data were obtained in cultures of primary myotubes (Fig. 3). To rule out the possibility that AChR recycling in cultured myotubes was missing due to poor viability, we used only viable cultures in which spontaneous twitching was observed. Furthermore, the insertion of new AChRs into pre-existing clusters during the experiment (see Fig. 3, lower panels), argues strongly that our myotubes are healthy. These results demonstrate that in cultured myotubes, agrin is not sufficient to induce their recycling, even though it was presented in a form attached to the substrate, i.e. similar to its presentation attached to the basal lamina at the NMJ in vivo.
Figure 3.
Agrin does not induce receptor recycling at AChR clusters on cultured myotubes. C2C12 and primary muscle cells were cultured on dishes focally impregnated with agrin expressed by COS-1 cells and were labeled with a saturating dose of BTX-biotin followed by a saturating dose of green streptavidin. 6 hours later, myotubes were bathed with (red) streptavidin-Alexa594 to label recycled AChRs and with BTX-Alexa430 to label newly synthesized AChRs. Note that few or no recycled receptors were present at agrin induced clusters. Bars, 10 µm
AChR recycling is independent of synaptic fold formation
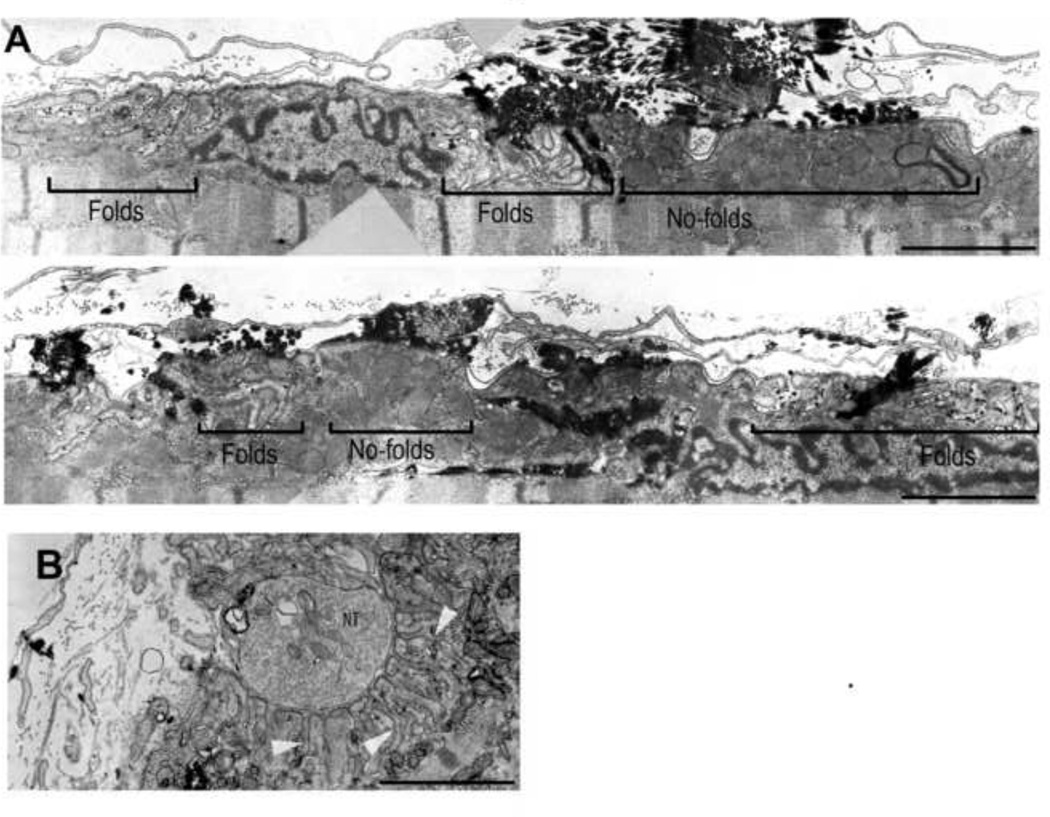
The ability of agrin to induce AChR recycling in muscle fibers in vivo as opposed to cultured myotubes could be due to a difference in the degree of postsynaptic differentiation. Specifically, a subsynaptic apparatus which is thought to be essential for AChR anchoring and stabilization, has not been reported at agrin-induced clusters in myotubes. Also, while agrin does induce receptor recycling in vivo (see above), the AChR density at agrin-induced ectopic clusters is lower than at the original NMJ (see above, and also Brenner et al., (Brenner et al., 1994)). To see whether these differences correlated with differences in postsynaptic differentiation, we examined agrin-induced clusters and original NMJs in longitudinal sections in the electron microscope. Ectopic clusters were identified in ultra-thin sections by staining the muscles lightly for AChE activity (Karnovsky and Roots, 1964). As shown in Fig. 4 agrin induced accumulations of myonuclei, mitochondria and subsynaptic folds (Cohen et al., 1997; Meier et al., 1997). However, within the region of AChE stain, the folds were restricted to relatively small regions intermingled with wide regions free of folds; furthermore, they appeared less regularly arranged and their troughs were shallower than at original NMJs of the same muscle (Fig. 4). Conspicuously, comparison of this pattern with, e.g., Fig. 1 showed that in contrast to fold formation, AChR recycling is present over the entire range of agrin-induced AChR clusters although the level of recycled AChRs is not uniform in the entire cluster. This difference suggests, therefore, that synaptic folds per se are not required for AChR recycling.
Figure 4.
Comparison of ultrastructure of agrin-induced, ectopic postsynaptic membrane and original neuromuscular junction in muscle fiber of soleus muscle. A) Montage of images taken along a longitudinal section of agrin-induced postsynaptic cluster. B) Electron microscopy of an innervated NMJ. Note the scattered distribution of synaptic folds along the ectopic postsynaptic membrane compared to the original synapse where folds are denser and deeper (see arrowheads). Black electrondense material in (A) marks reaction product from acetylcholine esterase activity used to locate the site of the ectopic AChR cluster. NT: nerve terminal. Bars, 3 µm
DISCUSSION
At innervated NMJs the recycling of receptors significantly contributes to the steady-state of the postsynaptic receptor density. However, in aneural cultured muscle cells (nerve-free) or in inactive muscles (denervated muscles or muscles blocked with pharmacological agents), the recycling of AChR is severely depressed or nonexistent (Bruneau et al., 2005; Bruneau and Akaaboune, 2006). We asked whether the nerve-derived factor agrin is sufficient to induce the recycling of AChRs, and, if so, whether this could explain the previously reported metabolic stabilization of receptors at ectopic AChRs by agrin. Our principal results are that: 1) in agrin-induced, nerve-free synaptic membranes, the machinery involved in the recycling of AChRs is activated. Receptors were able to recycle back and contribute to the postsynaptic density of clusters in both slow twitch muscles of rats and fast twitch muscles of mice; this process is independent of the formation of synaptic folds; 2) the degree of AChR recycling is dependent on agrin concentration; 3) in chronically denervated muscle, agrin-induced AChR recycling is quantitatively comparable to that at the original endplates; 4) in cultured myotubes, agrin is not sufficient to induce the recycling of AChRs at clusters. These results show that agrin is sufficient to activate AChR recycling in living mice, but not in cultured myotubes.
Previous reports indicate that agrin maintains the metabolic stability of AChRs in ectopic postsynaptic membranes in denervated soleus muscle fibers in a dose-dependent manner (16, 17). Consistent with this observation, our present data show that in agrin-induced clusters on denervated soleus muscle, a significant amount of recycling was observed at the clusters where they mixed with pre-existing AChRs. It is well documented that denervation increases the turnover rate of AChRs, and that high concentrations of agrin can increase the metabolic stability of receptors at ectopic clusters (13). The present experiments add an essential step toward understanding the mechanism by which the motor axon regulates the metabolic stability of the AChRs at the NMJ. Specifically, they show that the primary signal from the nerve regulating this process is agrin. It does so by inducing the recycling of AChRs in AChR clusters. However, as shown previously for synaptic AChRs, full stabilization of AChRs in ectopic, agrin-induced AChR clusters to a half-life of 10–14 days also requires muscle activity. Of note, muscle stimulation can promote the translocation of internalized receptors from the internal pool into the postsynaptic membrane in denervated synapses (Martinez-Pena y Valenzuela et al., 2010). Thus, the increase in AChR stability by muscle activity could be explained at least in part by an increase in the delivery of recycled AChR into induced clusters.
A hallmark of mature vertebrate NMJs is that the synaptic muscle membrane is deeply folded(Marques et al., 2000), and that molecules relevant to neuromuscular transmission such as AChRs and voltage gated Na+-channels are differentially distributed to specific regions of the folds (Flucher and Daniels, 1989). This suggests that the folds are involved in their anchoring to specific regions of the synaptic cytoskeleton, which in turn may affect their stability. An interesting finding of this work is, therefore, that junctional folds per se appear not to be involved in the process of the recycling of receptors. Specifically, recycled receptors were observed evenly throughout the entire ectopic clusters which comprise both folded and non-folded regions (Figure 4), and the ratio of recycled/pre-existing AChRs was roughly similar between ectopic, nerve-free clusters and innervated NMJs (Figure1). However, given the concentration of AChRs at the crests of the folds of mature NMJs, the lower fold density at ectopic AChR clusters offers a plausible explanation for their lower AChR density. It should be noted that the mechanisms involved in the formation of junctional folds remain unknown.
In conclusion, we have shown that neural agrin alone can induce recycling of clustered AChRs in a dose-dependent manner in muscle fibers of living animals, and that this can explain, at least in part, the regulation of metabolic AChR stability at the NMJ and at ectopic, agrin-induced AChR clusters observed previously by others (Bezakova et al., 2001b). In contrast, agrin is not sufficient to activate the recycling pathway in cultured muscle cells, even when it is presented in a substrate-attached, i.e. stable fashion similar to its presentation in differentiated muscle fibers in vivo, i.e. attached to the synaptic basal lamina. This indicates that in addition to neural agrin, other factors secreted either by nerve terminals or fully differentiated muscle fibers are required for AChR recycling and for full AChR stabilization to develop.
Supplementary Material
Neural agrin promotes the recycling of internalized AChRs at nerve-free AChR clusters in vivo.
The maintenance of a high AChR density depends on the strength of agrin stimulus
Agrin stabilizes AChR clusters in chronically denervated muscles through stimulating AChR recycling.
Acknowledgments
This work was supported by the Swiss National Science Foundation, The Swiss Foundation for Research on Muscle Diseases (HRB), and by NIH (MA). We would like to thank Dr. Richard Hume for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bezakova G, Helm JP, Francolini M, Lomo T. Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. Journal of Cell Biology. 2001a;153:1441–1452. doi: 10.1083/jcb.153.7.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezakova G, Lomo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AChR) aggregates in skeletal muscle fibers. Journal of Cell Biology. 2001;153:1453–1463. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezakova G, Rabben I, Sefland I, Fumagalli G, Lomo T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. P Natl Acad Sci USA. 2001b;98:9924–9929. doi: 10.1073/pnas.171539698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner HR, Rotzler S, Kues WA, Witzemann V, Sakmann B. Nerve-Dependent Induction of Achr Epsilon-Subunit Gene-Expression in Muscle Is Independent of State of Differentiation. Developmental Biology. 1994;165:527–536. doi: 10.1006/dbio.1994.1272. [DOI] [PubMed] [Google Scholar]
- Bruneau E, Sutter D, Hume RI, Akaaboune M. Identification of nicotinic acetylcholine receptor recycling and its role in maintaining receptor density at the neuromuscular junction in vivo. J Neurosci. 2005;25:9949–9959. doi: 10.1523/JNEUROSCI.3169-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau EG, Akaaboune M. The dynamics of recycled acetylcholine receptors at the neuromuscular junction in vivo. Development. 2006;133:4485–4493. doi: 10.1242/dev.02619. [DOI] [PubMed] [Google Scholar]
- Caroni P, Rotzler S, Britt JC, Brenner HR. Calcium Influx and Protein-Phosphorylation Mediate the Metabolic Stabilization of Synaptic Acetylcholine-Receptors in Muscle. Journal of Neuroscience. 1993;13:1315–1325. doi: 10.1523/JNEUROSCI.13-03-01315.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Rimer M, Lomo T, McMahan UJ. Agrin-induced postsynaptic-like apparatus in skeletal muscle fibers in vivo. Molecular and Cellular Neuroscience. 1997;9:237–253. doi: 10.1006/mcne.1997.0623. [DOI] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, Smith C, DiStefano PS, Glass DJ, Burden SJ, Yancopoulos GD. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Schumacher B, Ruegg MA. An Amino-Terminal Extension Is Required for the Secretion of Chick Agrin and Its Binding to Extracellular- Matrix. Journal of Cell Biology. 1995;131:1547–1560. doi: 10.1083/jcb.131.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na+ Channels and Ankyrin in Neuromuscular-Junctions Is Complementary to That of Acetylcholine-Receptors and the 43 Kd Protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Jones G, Meier T, Lichtsteiner M, Witzemann V, Sakmann B, Brenner HR. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci U S A. 1997;94:2654–2659. doi: 10.1073/pnas.94.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kim N, Stiegler AL, Cameron TO, Hallock PT, Gomez AM, Huang JH, Hubbard SR, Dustin ML, Burden SJ. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell. 2008;135:334–342. doi: 10.1016/j.cell.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle GB, Friedenwald JS. A Histochemical Method for Localizing Cholinesterase Activity. P Soc Exp Biol Med. 1949;70:617–622. doi: 10.3181/00379727-70-17013. [DOI] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR, Lee KF. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Lomo T, Rosentha J. Control of Ach Sensitivity by Muscle Activity in Rat. J Physiol-London. 1972;221:493-&. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, Lichtman JW. From plaque to pretzel: Fold formation and acetylcholine receptor loss at the developing neuromuscular junction. Journal of Neuroscience. 2000;20:3663–3675. doi: 10.1523/JNEUROSCI.20-10-03663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Pena y Valenzuela I, Mouslim C, Akaaboune M. Calcium/calmodulin kinase II-dependent acetylcholine receptor cycling at the mammalian neuromuscular junction in vivo. J Neurosci. 2010;30:12455–12465. doi: 10.1523/JNEUROSCI.3309-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Hauser DM, Chiquet M, Landmann L, Ruegg MA, Brenner HR. Neural agrin induces ectopic postsynaptic specializations in innervated muscle fibers. J Neurosci. 1997;17:6534–6544. doi: 10.1523/JNEUROSCI.17-17-06534.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier T, Masciulli F, Moore C, Schoumacher F, Eppenberger U, Denzer AJ, Jones G, Brenner HR. Agrin can mediate acetylcholine receptor gene expression in muscle by aggregation of muscle-derived neuregulins. Journal of Cell Biology. 1998;141:715–726. doi: 10.1083/jcb.141.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzler S, Brenner HR. Metabolic stabilization of acetylcholine receptors in vertebrate neuromuscular junction by muscle activity. J Cell Biol. 1990;111:655–661. doi: 10.1083/jcb.111.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzler S, Schramek H, Brenner HR. Metabolic stabilization of endplate acetylcholine receptors regulated by Ca2+ influx associated with muscle activity. Nature. 1991;349:337–339. doi: 10.1038/349337a0. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Salpeter MM. Degradation Rate of Acetylcholine-Receptors Inserted into Denervated Vertebrate Neuromuscular-Junctions. Journal of Cell Biology. 1989;108:647–651. doi: 10.1083/jcb.108.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137:1017–1033. doi: 10.1242/dev.038711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron. 2008;60:285–297. doi: 10.1016/j.neuron.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.