Abstract
While the Odor Span Task (OST) was developed to assess working memory in rodents, it appears that odor (“What”) and time since an odor was last reinforced (“When”) jointly control responding in the OST. The OST uses an incrementing non-match to sample procedure such that the number of stimuli to remember increases during the session; the rodent is trained to remember stimuli within a session but not between sessions. We used a variation of the OST to add a “Where” dimension to the task to examine whether rodents could learn to respond to scents based on contextual cues as well. In Experiment 1, 6 rats well-trained on the OST procedure were exposed to four target scents in a holding cage before the OST session began [What-Where-When (WWW) condition]. When these target scents appeared in the OST, rats treated them as novel scents despite their being previously encountered that day; WWW responding was comparable to baseline (BL) responding. Controls were implemented to account for relative familiarity: frequency of target presentation and time since the target odor was presented. On both types of control probes, rats typically responded to target scents less than during WWW or BL conditions, took longer to make a response, and visited more comparison stimuli. In Experiment 2, the study was replicated adding reinforcement delivery for responding to pre-session presentation of target stimuli. Subjects were the same 6 rats plus 2 additional rats also well-trained on the OST. Results were similar to those from Experiment 1. These data indicate that the variables controlling performance on the OST task include What stimulus is presented, Where (i.e., in which location) it was presented, and When it was presented. Thus, the OST-probe methodology may provide a useful vehicle for the study of episodic-like memory processes in non-humans.
Keywords: episodic-like memory, Odor Span Task, rats, olfactory scents
1. Introduction
Tulving (1972) originally coined the term episodic memory to refer to an individual's ability to distinguish between events based on their spatiotemporal relations; in other words, it is an individual's ability to recall a personal past event based on what event occurred, where it occurred, and when it occurred. In this way, episodic memory was distinguished from other memory processes such as semantic memory or an individual's factual knowledge. Many of the insights about episodic memory were based on observations with patients who suffered from brain trauma leading to strange cases of amnesia; in particular, Tulving's work with patient K. C. suggested a neurological difference between episodic memory and other memory systems. Tulving (1985; 2001) noted that while patient K. C. could remember facts and events about his past (which Tulving termed noetic consciousness or “knowing”), he could not recall any of his personal experiences (which Tulving termed autonoetic consciousness or “self-knowing”). Patient K. C.'s lack of autonoetic consciousness seemed to render him “stuck in time;” leading Tulving to further suggest that episodic memory involves chronesthesia or the ability to mentally travel back in time. Importantly, Alzheimer's disease is characterized by particularly pronounced deficits in functions that appear to reflect episodic memory loss (cf. Crystal, 2012).
Tulving (1983, 2002) initially claimed that episodic memory was likely unique to humans. Mental time travel and autonoetic consciousness, or an individual's ability to claim that they recall a specific event, not simply know that it occurred, are difficult to operationalize at best and can only be assessed through self-report using language. Perhaps because of the translational importance with respect to disorders such as Alzheimer's disease, researchers have attempted to develop animal models of episodic memory based on more concrete behavioral measures. Indeed, a number of studies have now demonstrated that several nonhuman species are able to discriminate items based on what the item is, where it is located, and when it was encountered; this is referred to as episodic-like memory or what, where, when (WWW) memory (Clayton & Dickinson, 1998). Clayton and Dickinson showed that Western scrub jays were able to discriminate their caching experiences based on what food item they cached, where they cached it, and when they cached it. Using similar procedures, control by what-where-when stimulus features has also been demonstrated to varying degrees in rats (Babb & Crystal, 2005; 2006a; 2006b), rhesus macaques (Hoffman, Beran, & Washburn, 2009), chickadees (Feeney, Roberts, & Sherry, 2009), magpies (Zinkivskay, Nazir, & Smulders, 2009) and several great apes (Martin-Ordas, Haun, Colmenares, & Call, 2010).
A variety of other approaches have been developed in the study of episodic-like remembering in nonhumans. For example, Eacott, Easton and Zinkivskay (2005) substituted a which or contextual component in place of the when component and found that rats were able to discriminate a less familiar (preferred) item based on what object (what) was in which location (where) during which context.
Olfactory procedures have been used to study episodic memory in rodents by Eichenbaum and colleagues (e.g., DeVito & Eichenbaum, 2011; Ergorul & Eichenbaum, 2004; Fortin, Wright & Eichenbaum, 2004; Sauvage, Fortin, Owens, Yonelinas, & Eichenbaum, 2008; Sauvage, Beer, & Eichenbaum, 2010). A procedure that shows particular promise is the Odor Span Task (OST) originally developed by Dudchenko, Wood, and Eichenbaum (2000) as a model for assessing working memory in rodents. It can be described as an incrementing non-match-to-sample task, such that a rat is trained to respond to the new stimulus in each trial, and as trials increase, the number of stimuli for the rat to remember increases. The premise of the OST is that rats are required to remember odor stimuli within a testing session but not between sessions (Dudchenko et al., 2000). In the OST, as the trials in a session progress, the number of stimuli for the rat to remember increases, but this is also true for the number of comparison or distractor stimuli present in the testing arena.
However, using the OST procedure, April, Bruce and Galizio (2013) found that if the number of distracting stimuli is controlled and held constant throughout the testing session, the number of stimuli that rats can remember does not appear to be limited; indeed, rats showed accurate performance on the task with over 70 odor stimuli to remember. This finding is inconsistent with a limited capacity, working memory account.
If working memory is not the primary process involved in the OST, then what is the basis for stimulus control in the OST and how can this task be used to assess other memory processes? April et al. (2013) used a probe procedure in which no new stimuli were presented on certain trials to assess the relative importance of recent familiarity with odor stimuli. They found that rats tended to select the odor that had been encountered least recently on such trials, but that number of stimuli visited and latencies to respond were much longer. Indeed, rats failed to respond at all on a number of these probe trials. This result suggests that rats' responding is under “what-when” control, and these findings suggest that stimulus control in the OST may be better characterized as a model of episodic-like memory rather than working memory.
Further, by using probe procedures, the April et al. (2013) approach also addresses a concern voiced by Zentall and colleagues. Zentall, Clement, Bhatt, and Allen (2001) argued that what, where, when memory tasks may be interpreted as inflexible trained relations and therefore, may not be sufficient to meet the criteria for episodic-like memory. Thus, they developed a task with pigeons to probe the subject that more closely resembled human episodic memory tests. Pigeons were probed with an “unexpected question” which required the subject to respond to a stimulus complex not previously reinforced in training. The probes used in April et al. (2013), and in the current study, may similarly be interpreted as “unexpected questions,” because at unpredictable points during a session, the rat is probed with an unfamiliar stimulus arrangement.
In the current study we extended the April et al. (2013) findings to examine what-where-when remembering in rats using a variation of the OST. Rats were presented with four odors in an alternate context, and then were presented with those scents again during the OST. We were interested in whether rats could respond to the odor stimuli based on what stimuli are present, where they have been presented (holding cage versus the testing arena), and when they have been presented (today's session or only in previous sessions). We predicted that rats will be able to learn to choose the target scent that was presented in the holding cage when it is presented again in the testing arena, despite their extensive and well-learned training of always responding to the new scent for that day's session in the OST. Their performance should be similar to baseline levels of responding and not under the control of possible confounds such as familiarity with the target scents or time since they were encountered. If so, the OST may be a useful procedure to study episodic-like or WWW processes in rodents. This is an important possibility, because the OST is an affordable procedure that rats learn rapidly and accurately.
2. Experiment 1
3. Method
3.1 Subjects
Six male Sprague-Dawley albino rats began testing between 90-150 days old. They were individually housed on a reversed 12 hour light-dark cycle. The rats were kept at 85% of their free feeding weight and received ad libitum access to water in their home cages. Rats used in this study received extensive training in the OST as a part of other experiments during which most were exposed to acute doses of drugs (see Table 1 for each rat's experimental history).
Table 1.
Subjects' experimental histories including drug exposure and prior OST training.
| Subject | Drug Exposure | OST sessions prior to current study | Sessions to meet Probe criteria | Experiment 1 sessions | Experiment 2 sessions |
|---|---|---|---|---|---|
| Y21 | MDMA flunitrazepam | 157 | 17 | 28 | 8 |
| D6 | none | 58 | 9 | 16 | 8 |
| D2 | methamphetamine | 124 | 14 | 16 | 8 |
| E5 | ketamine MDMA | 97 | 5 | 16 | 8 |
| E13 | ketamine MDMA | 111 | 5 | 16 | 8 |
| E18 | none | 30 | 5 | 16 | 8 |
| E12 | flunitrazepam MDMA | 156 | 6 | - | 12 |
| E22 | ketamine methylphenidate | 144 | 8 | - | 12 |
Y2 was the first rat tested. He had already received four WWW, BL and FC probes before the FTC probe was added to the procedure. He received additional sessions with all four probe types to include FTC probes. Only the first four probes of WWW, BL, FC and FTC were used in the analyses.
E1 and E2 actually received three blocks of probe types in Experiment 2; however, only the first two blocks were analyzed to match the number of sessions for the other subjects.
3.2 Apparatus and Stimuli
Testing sessions were conducted in an open-field arena made from a circular table, 94 cm in diameter, standing 29.2 cm high. The table was surrounded by 32 cm high walls made from sheet metal baffling. The table top contained 18 circular holes, 5.5 cm in diameter, positioned equidistant from one another (13.3 cm apart). The holes were in a two ring configuration; the outer ring consisted of 12 holes positioned 2.5 cm from the wall and the inner ring contained six holes, 21.5 cm from the outer wall. One 2 oz plastic cup was placed in each of the 18 holes during a session. Each session was recorded using a Logitech, Inc. web cam.
The experimental stimuli were plastic lids placed on top of the cups. These lids were odorized by storing them in airtight plastic containers with one of the following 35 odorants: allspice, almond, anise, bay, caraway, carob, celery, clove, cinnamon, coriander, cumin, dill, fennel, fenugreek, garlic, ginger, grape, lime, marjoram, mustard, nutmeg, onion, orange, oregano, paprika, peach, rosemary, sage, savory, spinach, sumac, thyme, turmeric, vanilla and Worcestershire (Great American Spice Co). Odorants in the plastic containers were refreshed every three weeks to ensure the consistency of the scent.
3.3 Procedure
3.3.1. OST Baseline Training
Rats were trained on the OST procedure described in Galizio, Deal, Hawkey, and April (2013). Rats were first habituated to the testing arena and stimuli (plastic cups with unscented lids). Cups were filled with 1 cm of white play sand on which a 45-mg sugar pellet was placed. Habituation continued until the rat was reliably removing the plastic lid and eating sugar pellets. After habituation, baseline training began with the introduction of scented lids. On the first trial of each baseline session, a cup with a scented lid was placed in a randomly selected location in the arena. The rat was placed in the center of the arena and permitted to locate the cup, remove the scented lid, and consume the sugar pellet. The rat was then removed from the testing arena and placed in a plastic holding cage beside the arena for an inter-trial interval of approximately 60 s. Trial 2 presented two cups with scented lids. One lid was the same scent as Trial 1 but, in order to prevent scent marking, a different lid of the same scent was used for each presentation; the cup it covered was unbaited. The other was a new scent for that day and this cup was baited. Trial 3 consisted of three cups with scented lids, two with odors previously presented in Trials 1 and 2 and one that was new for that session. Once again the cup with the new odor was baited and the previously presented odors were not. Thus, the procedure resembles a non-matching-to-sample arrangement but with the number of samples to remember incrementing with each successive trial. On all trials in the session, if the rat removed the lid of an incorrect stimulus, the trial continued until the rat responded to the correct stimulus (the new scent for that session).
Trials continued in the same manner up to Trial 5. After the fifth trial, the number of comparisons in the arena was held constant at five: four previously presented scents (randomly selected) and one new odor for that session. For example, in Trial 8, there would be one new scent that the rat had not been presented with that day and four other scents randomly chosen from the seven presented earlier in the session. Thus, the number of stimuli to remember continued to increment by one as each trial progressed, but the number of comparison stimuli in the arena remained constant after Trial 5; this eliminates the confound between number of stimuli to remember and number of comparisons present in other OST procedures (see Galizio et al., 2013).
Each rat was tested in one session per day (five sessions/week) consisting of 30 total trials; six simple discrimination (SD) trials (as a performance control) were interpolated with 24 OST trials. The OST trials were with 24 different scents (arbitrarily selected for each rat from among the 35 available odors). The 24 scents chosen for each rat remained the same throughout the duration of testing, as did the two different scents used for the SD trials. The order in which the OST scents were presented and the location of the S+ and S- odors were randomly determined before each session. After 30-60 training sessions, most of the rats were then studied in an experiment designed to determine the effects of drugs on OST performance (see Table 1 for training history). Thus, all rats received extensive OST training (between 30 and 158 sessions) prior to the present study.
In order to verify that rats' responding was not guided by the scent of the reward, during selected sessions, six trials interspersed through the session were designated as unbaited trials. On these trials, none of the cups contained a sugar pellet, but the experimenter dropped a pellet into the correct cup after the rat removed the lid. As in previous studies (Galizio et al., 2013; MacQueen, Bullard, & Galizio, 2011) accuracy on unbaited trials was comparable to that seen on normal trials indicating that responding was under the control of the lid odor and not the sugar pellet. Following the completion of other OST studies, in order to move on to the Probe phase of the present experiment, rats were required to meet a criterion of five consecutive sessions at 18/24 (75%) correct or better and a mean of 19/24 (79.17%) correct or better across those five sessions (see Table 2 for accuracy on normal and unbaited trials and span prior to the probe phase). OST sessions were conducted as described above, with the exception that SD trials were removed from the program.
Table 2.
Performance data from the final five OST sessions prior to the Probe phase.
| Subject | % Correct | Mean Span Length1 | % Correct Unbaited2 |
|---|---|---|---|
| Y2 | 79.2 | 5.8 | 80 |
| D6 | 85 | 8.2 | 73.3 |
| D2 | 89.2 | 10.4 | 90 |
| E5 | 81.7 | 7.8 | 83.3 |
| E13 | 90.8 | 5.2 | 96.7 |
| E18 | 88.3 | 10 | -- |
| E1 | 83.3 | 4 | 83.3 |
| E2 | 86.7 | 8.8 | 96.7 |
Percent correct and span length represent means of the final five sessions prior to the beginning of the Probe phase. Span is defined by the number of correct responses before the first error, excluding trial 1.
Percent correct unbaited indicates the mean accuracy on unbaited trials during the final five unbaited sessions prior to the beginning of the Probe phase.
3.3.2. Probe Phase
In the Probe phase, sessions consisted of 24 OST trials as described above, but probe trials were included within the session. Four different types of probe trials were arranged, and sessions containing these probe trials formed the four conditions of the experiment: What-Where-When (WWW), Baseline (BL), Frequency Control (FC) and Frequency-Time Control (FTC). Each session contained only one of these probe types. There were four replicates of each probe session type; these were arranged in blocks such that each probe type was represented before moving on to the next block. One block was completed in four days (e.g. Monday, WWW; Tuesday, BL; Wednesday, FC; Thursday, FTC). The order of probe types was counterbalanced within and across subjects to account for order effects.
WWW sessions proceeded as described above; however, before the session began, rats were presented with four target scents in an alternative context (the holding cage beside the OST arena). Target scents were chosen from the rat's typical OST odor list. Target scents were presented one at a time for 10 s each using the same plastic lids as in the OST task and were handled with a pair of metal pliers. The scented lid was lowered into the holding cage and when the rat's snout had come within 1 cm of the lid, the 10 s presentation period was initiated. Once 10 s had elapsed, the next scent was presented (approximately 5 s between scented lid presentations). The OST session began approximately 60 s after the last scent presentation in the holding cage. On each of four probe trials during the OST session, one target scent was presented along with four odor comparisons that had previously been presented during the session. Thus, on WWW probe trials all five stimuli had been previously presented that day (“when”), but the target stimulus (“what”) presentation was in the holding cage, not the arena (“where”). On these probe trials, reinforcement was provided for responding to the target scent; in other words, rats were trained to respond to the scent presented in the holding cage as new when they encountered them again in the testing arena. For example, if oregano was one of the four target scents presented in the holding cage before the OST session, when the rat was presented with oregano and four previously presented scents during the OST session, removing the oregano lid would result in a reinforcer. As above, the rat remained in the arena until it responded to the target scent and received the reinforcer. Probe trials were selected randomly prior to each session, with the constraint that the first probe occurred on Trials 6, 7, or 8, the last probe occurred on Trials 21, 22, or 23, and the other two probes were selected from remaining trials. There were always at least two trials separating probe trials.
Several dependent variables were measured: percent target lid removal, number of visits to incorrect stimuli and latency to respond. The percentage of first responses to the target or new scent and other stimuli in each session type was recorded. The number of times the rat visited each scented lid before making a response was defined as pausing and approaching within 1 cm of a lid without removing the lid. Latency to respond was defined as the amount of time in seconds from placement in the arena until the rat made its first lid removal response. Each trial continued until the rat removed the lid of the new or target stimulus or until 120 s elapsed (response omission) from placement in the arena.
Baseline (BL) probe sessions were conducted using the basic OST procedure, but four trials within the session were designated as probe trials for comparison and analysis. These probe trials matched the trial numbers for probes in the WWW condition for that block (e.g., if WWW probe trials for Block 1 were Trials 7, 13, 17, and 24, then the same trials in the BL condition would be used for statistical analysis). This equated number of stimuli to remember in the OST across the two conditions. If responding on WWW probes is under “what-where-when” control, then percent target selection, visits and latencies should be comparable between BL and WWW sessions. Even though the target stimulus had been presented prior to the session on WWW probes, it should still be selected if the location of stimulus presentation is one of the controlling stimuli (holding cage vs. arena).
However, comparable responding on BL and WWW probe trials could be based on relative familiarity with the scents. On both BL and WWW trial types, the target stimulus is also the least recently presented of all the comparisons in the arena; for BL, the target had not been presented since the last session (on a previous day) and for WWW, the target had been presented just prior to that session's onset). Also, not only had the WWW targets been presented in a different place than the other four comparison stimuli, but also, by virtue of being presented before the session began, the WWW target was always the most temporally remote of the five comparisons. Thus, two control conditions (FC and FTC) were conducted to assess the contribution of relative familiarity as the basis for target selection.
Frequency Control (FC) probes were used to control for the relative unfamiliarity of the target scents presented in the WWW sessions. In the FC condition, there were four probe trials within the session in which all five scents in the arena had been previously presented in that day's session (probe trial numbers were the same as the WWW and BL probe trials in that block). The target scent used for analysis and comparison in these trials was the one scent that had only been presented once in the session before being presented again as the target scent. This equated the frequency of exposure to the target scent that day across WWW and FC conditions. Each of the other four comparison scents in the four FC probe trials had been presented more than once already in the session. As the rats had been trained to choose the new stimulus on each trial to receive a reinforcer in the OST, on the FC probe trials there was no ‘correct response’- all of the scents had already been presented at least once in the arena in that session. The rat did not receive a reinforcer for removing any of the lids during these probe trials; once the animal made its first response, it was removed from the arena and placed back into the holding cage until the next trial began. If the rat's responding to the target scent in a WWW probe was the result of its being the least frequently encountered of the five comparisons in the arena, then we would expect responding in the FC probes to be similar to that in the WWW probes.
Although FC probe stimuli had been presented only once prior to the probe trial (similar to the WWW target stimuli), the rat had encountered them more recently than the WWW targets. Because initial presentations of WWW target scents were done immediately before the OST began, there was a greater amount of time between those initial presentations in the holding cage and the subsequent probe trial presentation in the arena. In fact, the time between initial and probe presentations varied for each of the WWW scents. Thus, we added Frequency-Time Control (FTC) probes to control for the relative amounts of time since the rat had been presented with the WWW target scents in the holding cage. Probe trials in FTC sessions were linked to the WWW trials according to the time/number of intervening trials between stimulus presentations. To do this, designated target scents were “held back” after they had been presented once as an S+ in the OST; each of these scents was then presented on later trials that approximated the inter-stimulus intervals that had occurred in the WWW trial type. (Because it took approximately 35 min to complete 24 trials in a daily session, this meant that there could only be three probe trials in the FTC condition- that is, only three scents could be “held back” long enough to match the WWW probes). Overall, intervening trials for the FTC probes were similar to the WWW probes (M= 12.2 for WWW probes and M= 10.3 for FTC probes).
Similar to the FC probes, there was no reinforcement for any response on an FTC probe; as soon as the rat made its first lid removal response, it was removed from the testing arena and the next trial began. If the longer amount of time since the rats had smelled the WWW target scents compared to the time since the comparison scents were encountered is what is controlling responding, then we should see similar responding in the FTC probes compared to the WWW probes.
3.3.3. Reliability
All sessions were video recorded. Approximately 10% of the sessions were randomly selected for review by an independent observer, blind to the experimental conditions, and two dependent measures were examined for reliability- number of visits and target selection. Agreement for number of visits before making a first response was 78.79% (11 of the 96 total sessions for a total of 264 trials). Agreement for target selection was 100%, based on 3 trials of the above 11 sessions (33 trials total).
4. Results and Discussion
4.1. OST Baseline Training
Overall accuracy and span length for the final five sessions of OST training before the Probe Phase began are summarized in Table 2 for each rat. Mean percent correct for the six rats was 85.7 across the 24-trial session and ranged from 79.2 to 90.1. The mean span (number of trials before an error occurred, omitting Trial 1) length was 7.9 odors. Both of these values are similar to those obtained in previous studies and indicate that OST performance was characterized by high levels of accuracy at the onset of the Probe Phase. That is, animals were highly accurate in selecting which of up to five comparison stimuli on a given trial had not yet been presented during that particular session.
4.2 OST Probe Performance
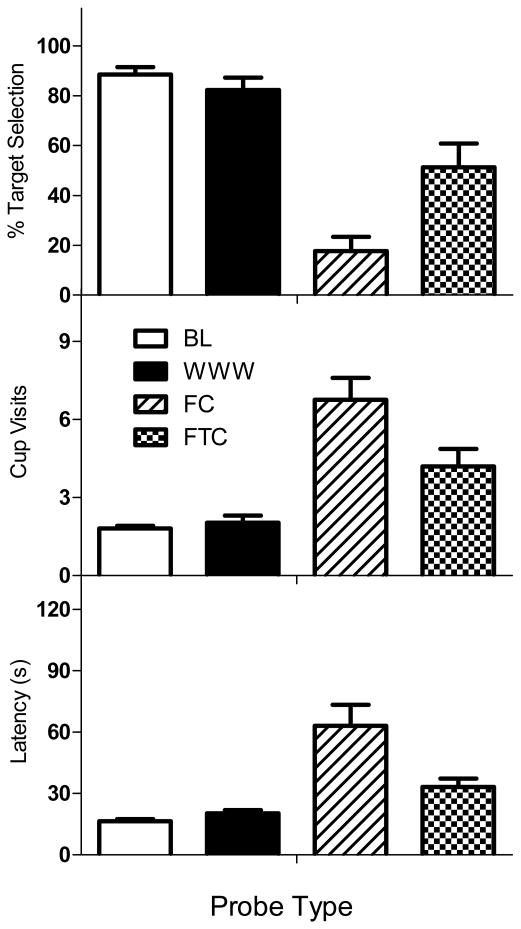
Rats' performance on probe trials was summarized by three critical dependent variables (percent target chosen, number of cup visits and latency to the first response) and Figure 1 shows the outcomes averaged across session blocks. One-way within subjects ANOVA confirmed clear and statistically significant differences between the conditions on each measure: percent target selections (top panel), F (3, 15) = 22.60, p < .05; cup visits (middle panel), F (3, 15) = 24.87, p < .05; and latency (bottom panel), F (3, 15) = 14.35, p < .05. The leftmost columns of Figure 1 (white) show percent target selections, cup visits, and latency on BL probe trials. As on typical baseline trials, percent target selection was high (> 80%) and number of visits per trial and latency to respond were relatively low (slightly more than 2 cups visited and < 20 s, respectively). A striking feature of Figure 1 is that the WWW probe data (black bars, second column) were virtually identical to the BL probes (post hoc HSD tests: p > .05 in each case). Even though rats had smelled these target odors in their home cage just before the session, they still consistently selected the targets over the other four odors which had been previously presented in the arena during the session. Number of cups visited and latency to respond on WWW probes were also virtually the same as BL trials.
Figure 1.
Responding for each probe type is depicted for percentage of target scents chosen (top), number of cups visited (middle), and latency to a first response (bottom). Data were computed based on the 3 or 4 target probe trials within the probe types: Baseline (white), WWW (black), Frequency Control (diagonal lines), and Frequency-Time Control (checkered). Error bars represent standard errors of the mean.
In the FC condition, all five comparison stimuli had previously been presented during the current OST session; however, there was one comparison that had only appeared once before in that day's OST and it was designated as the target stimulus. As Figure 1 shows, on these FC probe trials (striped bars, 3rd column) rats were much less likely to select the target stimulus than in BL or WWW conditions (p < .05 in both cases). Indeed, target scent selection was quite close to 20%, which corresponds to chance levels on these 5-comparison tests. Also of interest, the number of cup visits was higher and latency to select any cup was much longer than was observed on the BL and WWW trials (both p < .05). Further, on a number of trials in this condition, rats failed to select any cup during the 120-s trial and individual cups were often revisited, events that were extremely rare under BL conditions. These marked differences between behavior on FC and WWW probe trials suggest that rats discriminate the context in which a particular odor was present even if it was only presented once before.
The FC condition does not rule out a relative familiarity account based on time. In the FC condition, the time between the first and second presentation of the target condition was not controlled, but was always shorter than the time difference between target scent presentations for the WWW condition. So although rats did not select the least frequently encountered stimulus in the FC control condition, perhaps this was because the differences in familiarity based on time were not as large as those in the WWW condition. The FTC condition addressed this concern by approximating the time differential between stimulus presentations.
The rightmost bars of Figure 1 (stippled) reveal an interesting pattern: the FTC conditions resulted in performances that were intermediate between the WWW and FC conditions on all three measures. For example, percent target selection for FTC probes was just over 50% which was significantly lower than on BL and WWW probes (p < .05 for both) and higher than on FC probes (p < .05). The same pattern was evident for number of visits; the rats made more visits in the FTC probes compared to WWW and BL (p < .05 for both), but fewer visits compared to FC (p < .01). Finally, latency to respond was longer in the FC compared to the FTC (p < .05), but the latencies in the FTC were not statistically different from latencies in the WWW and BL probes. Although latency to respond did not differ significantly between WWW and FTC probe trials, the differences in both of the other dependent variables suggest that rats were not responding equivalently on these probes. On FTC probe trials, rats did select the least familiar (based on time and frequency) comparison stimulus about 50% of the time, but even so, they visited nearly every comparison cup before responding. This outcome suggests that both familiarity and WWW stimulus control may be involved in OST performance.2
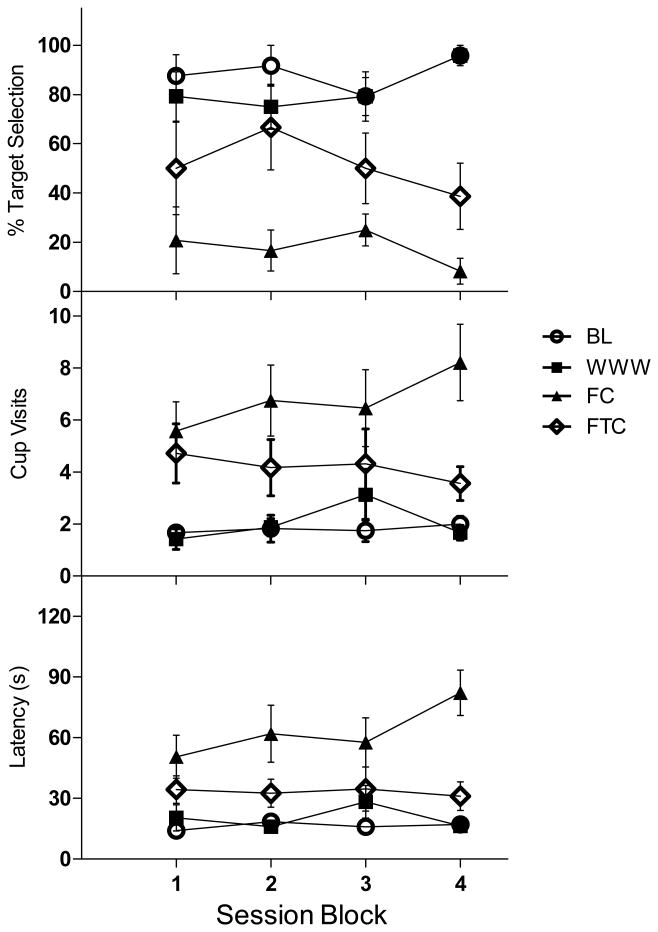
However, another possible explanation for these results must be considered: target selection on the BL and WWW probe trials was always reinforced, but none of the five cups was baited on either the FC or FTC probe trials. Perhaps the differences between WWW and the control probe trials emerged during the four sessions of probe exposure and reflect the formation of a discrimination between WWW and the two control probe trials. In order to evaluate this possibility, Figure 2 breaks down all three dependent measures as a function of the four determinations of each probe type. As Figure 2 shows, there was some trend of decreased target selection in both control conditions over the course of the four probes, and a small increase in target selection across WWW probes. As might be expected, target selection in BL probes was unchanged across the four determinations. There was also a trend toward increased latency and number of visits on FC probes, but interestingly, no changes on either index were apparent in the other three groups. A two-way within subjects ANOVA reconfirmed the main effect of probe type, but did not show an effect of determinations on percent target chosen [F (3, 15) = .142, p = .932], number of visits [F (3, 15) = .306, p = .820] or latency to respond [F (3, 15) = .636, p = .604]. Neither was there a significant interaction of determination number on probe type for percent target chosen [F (9, 45) = .930, p = .509], number of visits [F (9, 45) = .811, p = .609], nor latency to respond [F (9, 45) = 1.739, p = .108].
Figure 2.
Responding across the four blocks of sessions for each probe type are depicted for percentage of target scents chosen (top), number of cups visited (middle), and latency to a first response (bottom). Data were computed based on the 3 or 4 target probe trials within the probe types: Baseline (white circles), WWW (black squares), Frequency Control (black triangles), and Frequency-Time Control (white diamonds). Error bars represent standard errors of the mean.
It is worth emphasizing that differences between the conditions were evident even during the initial probe session. That is, BL and WWW performances were similar on all three dependent measures, and FC and FTC probes were characterized by fewer target selections, more cup visits and longer latencies. One-way within subjects ANOVAs confirmed the effect of probe type on percent target chosen [F (3, 15) = 4.29, p <.05], number of visits [F (3, 15) = 8.81, p <. 05], and latency to respond [F (3, 15) = 4.36, p <.05]. HSD post hoc comparisons indicated that for all three dependent measures, WWW performance was similar to BL (see Figure 2; first session block) and FC scores were significantly different from BL (p < .05). For all measures, FTC scores were intermediate, but were only significantly different from WWW and BL for number of visits, and were not statistically different from FC, likely a result of the high variability in individual responding.
Overall the results of this study support the hypothesis that during OST training rats come under the control of several stimulus properties including the odor (what), the location in which the odor was initially presented that day (where) and the time of presentation (when- today versus a previous session). However, another hypothesis might be developed to account for the outcomes of Experiment 1. When odors were presented to rats in the holding cage in the WWW condition, the odorized lid was inserted into the holding cage without a cup, and importantly, no lid removal was required and no reward was presented. In contrast, on regular OST trials, exposure to the target stimulus was consistently followed by lid removal and reinforcement. Thus, scents that were presented as target scents in the FC and FTC probes had all been previously responded to by lid removal and reinforcement, but this was not the case for the target scents presented in the holding cage for the WWW probes. Perhaps it is this difference between target stimuli that resulted in the differing performances between WWW and control probes. Indeed, reinforcement of lid removal is likely to enhance attention to lid odor; rats in the WWW condition may simply have not attended sufficiently to odors presented in the home cage. Experiment 2 provides a test of this hypothesis by replicating the conditions of Experiment 1 with the exception that WWW probes were presented by inserting a baited cup with scented lid into the holding cage prior to the onset of the OST session. Rats were required to remove the scented lid and obtain the sugar pellet which equated the response/reinforcement condition with target stimuli on control probe trials. Further, using this lid removal and reinforcement procedure similar to the standard OST procedure assured that the rat made contact with the lid and increased the likelihood of attention to the odor stimuli presented in the holding cages.
5. Experiment 2
6. Method
6.1. Subjects
The same six rats studied in Experiment 1 were used in Experiment 2. In addition, two rats that were naïve to the Probe conditions of Experiment 1 were tested (see Table 1 for experimental histories) for a total of eight subjects in Experiment 2.
6.2. Apparatus and Stimuli
The apparatus and stimuli were identical to those in Experiment 1.
6.3. Procedure
The two new rats began the probe phase of Experiment 2 after meeting the OST mastery criterion as described in Experiment 1. The other six rats were exposed to another series of probe sessions immediately following Experiment 1. Three of the Experiment 2 probe session types were identical to those studied in Experiment 1 (BL, FC, and FTC). A fourth type of probe trial (RWWW, Reinforced What-Where-When probes) was introduced to control for differences in reinforcement history for target scents in the WWW condition. Thus in this phase, the four target scents were again presented prior to OST session, but with reinforcement for removing the scented lids. Four baited cups with scented lids were sequentially inserted in the holding cage and the rat was required to remove the lid and obtain the sucrose pellet before moving on to the next target scent. The OST session began immediately after the fourth target scent had been presented. As in Experiment 1, number of intervening trials for the FTC condition (M=10. 6) approximately matched the number of intervening trials for the RWWW probes (M=11.7). Two determinations of each type of probe session were conducted (see Table 1).
7. Results and Discussion
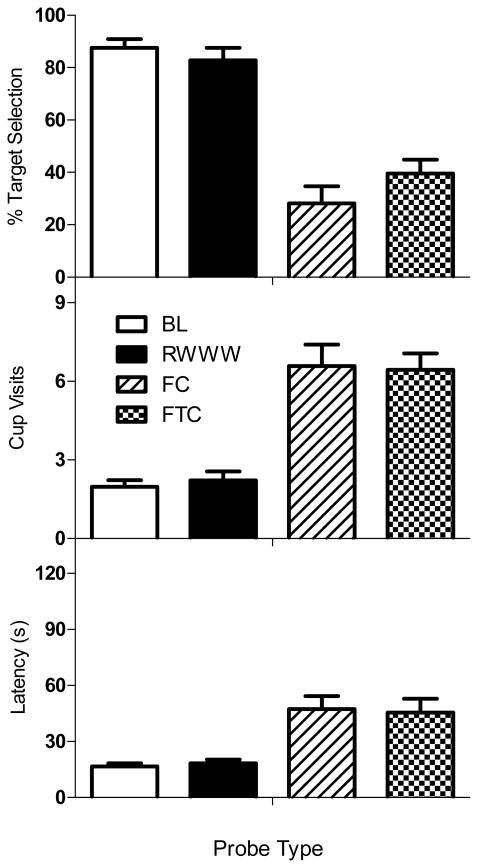
The outcomes of Experiment 2 are shown in Figure 3, and the pattern of results is similar to that seen in Experiment 1. Even when rats were permitted to remove the lid to receive a sucrose pellet in the holding cage on RWWW probe trials, their performances were very similar to those during BL. Target selection was generally quite high on both BL and RWWW probe trials, and latency to respond and number of cups visited were generally low. In contrast, both control conditions (FC and FTC) showed low rates of target selection and relatively longer latencies with more cup visits. One-way within subjects ANOVAs provided statistical confirmation of these observations: percent target selections, F (3, 21) = 30.73, p < .05; cup visits, F (3, 21) = 26.38, p < .05; and latency F (3, 21) = 15.11, p < .05. Post hoc HSD tests showed that BL and RWWW did not differ significantly from one another on any of the three dependent variables (p > .05), but that both were significantly different from FC and FTC performances on all three measures (p < .05). Performances on FC and FTC probes for all dependent measures were statistically similar (p > .05).
Figure 3.
Responding for each probe type is depicted for percentage of target scents chosen (top), number of cups visited (middle), and latency to a first response (bottom). Data were computed based on the 3 or 4 target probe trials within the probe types: Baseline (white), RWWW (black), Frequency Control (diagonal lines), and Frequency-Time Control (checkered). Error bars represent standard errors of the mean.
Thus, even when rats made reinforced responses to odors presented in the holding cage on RWWW probe trials, they later respond to those same odors as if they had not been previously presented when they encountered them again in the arena. This effect is not simply due to an increased amount of time that passed since the presentation of the scent as responding was quite different on RWWW probes compared to FTC probe trials, which approximated the time differences of first and second presentations of the RWWW target scents.
8. General Discussion
Taken together, Experiments 1 and 2 provide evidence that training rats in the OST results in control by a combination of stimulus properties including the specific odors presented (What), the context in which the stimulus was presented- holding cage versus the testing arena (Where), and when they were presented, today versus any other day (When). Thus, these data appear to meet criteria developed by a number of researchers (e.g., Clayton & Dickinson, 1998; Crystal, 2010) to define episodic-like remembering in rats. Further, these data support and extend the findings of April et al. (2013). They tested conditions similar to the frequency and frequency-time control groups studied here and obtained similar results that suggested that relative familiarity could not fully explain OST performance and that “What-How long ago” remembering was involved. The WWW and RWWW probe conditions of the present study extend the analysis by showing that the context in which odors were encountered (arena vs. holding cage) is a critical feature determining OST responding. Thus, during an OST session rats remember the specific odors they have encountered, where they encountered them, and at least something about when they were encountered.
Whether temporal control in the present study may be viewed as meeting the “When” criterion for episodic-like memory is worth more detailed consideration. It could be argued that the results of the present study might be better interpreted in the context of “What-Which” control (Eacott et al., 2005) rather than “What-Where-When” control. In the current study, “Where” and “Which” would both characterize the location in which the scents were first encountered that day (the holding cage or the OST arena), and thus our results are consistent with the predictions of Eacott et al. for “What-Which” memory. As Roberts, Feeney, MacPherson, Petter, McMillan, and Musolino (2008) have noted, it is often difficult to determine the specificity of temporal control in studies of episodic-like memory (i.e., differentiating “What-When” vs. “What-How long ago”). In the present study, rats reliably and rapidly selected a stimulus odor that had not yet been previously presented in the arena during the current session (BL conditions), but had been presented in previous sessions on different days. They also reliably and rapidly selected a stimulus that had quite recently been presented, if the presentation was in the holding cage (WWW and RWWW probes), but not if the presentation was in the arena (FC and FTC probes). These findings confirm a highly specific “What-Where” control, but the extent of temporal specificity will require additional research perhaps using a time-of-day variable (cf. Babb & Crystal, 2006b; Zhou & Crystal, 2009).
One possible confound in the present study design is that target cups were always baited on BL and WWW (and RWWW) probe trials, but were never baited on FC or FTC trials. This difference raises a concern that perhaps differential behavior across these probe trials is based on the animal smelling the sucrose pellet on BL and WWW probes. However, performances were quite comparable on unbaited and baited trials both in the present study (Table 2) and in previous studies using this methodology (April et al., 2013; Galizio et al., 2013; MacQueen et al., 2011), so this concern seems unfounded. A related concern might be that differential reinforcement enhanced responding on WWW and RWWW probe trials and extinguished responding on FC and FTC trials. However there were no significant trends toward increased or decreased responding across blocks of probes as would be expected if differential probe responding was a factor (Figure 2) and indeed differences between probe conditions were apparent as early as the initial block of probe sessions. Thus it would appear that control by What-Where-When stimulus properties may develop during the ordinary course of OST training.
An alternative interpretation of the present results is that the rats simply failed to attend to the WWW stimuli presented in the holding cage. Such an account may be plausible for Experiment 1 in which the lid stimuli were simply inserted into the home cage. However, Experiment 2 was designed to ensure that rats made tactile contact with all RWWW target scents using precisely the same stimulus presentation, response requirement and response consequences as during an ordinary OST trial. Still, it would be useful in future research to develop a procedure that demonstrates a different form of stimulus control by WWW stimuli to eliminate this alternative interpretation.
There are other features of the present procedure that may be advantageous in the study of What-Where-When remembering. For example, the use of intermittent probes in the context of an ongoing OST makes it possible to use a within-subject experimental design. This may prove advantageous in the study of the effects of drugs and other reversible neurobiological manipulations (cf. Zhou, Hohmann, & Crystal, 2012) that are of interest to researchers studying episodic memory. A more theoretical advantage is that the use of the probe procedure means that the presentation of critical trials is unpredictable and, because each probe trial presents the subject with a relatively unique array of stimuli, it might also be said to meet Zentall and colleagues' criterion of “surprisingness” (Zentall, 2006; Zentall, et al., 2001; Zentall, Singer, & Stagner, 2008). They note that episodic memory in humans is defined by the ability to remember details in response to a surprising or unexpected question, and have argued that similar properties should be sought in animal models. The current study presents “unexpected” stimuli during the WWW probe sessions because on unpredictable trials, responding to a scent that has already been presented today is reinforced, the opposite of the typical OST procedure.
One puzzling feature of the present study is worth particular attention. Studies of episodic memory in humans and animals have emphasized the importance of hippocampal involvement (Ergorul and Eichenbaum, 2004; Fortin et al., 2004; Hasselmo, 2012; Zhou et al., 2012). Indeed, the similarity of animal and human hippocampal involvement in comparable types of memory tasks has led some researchers to reconsider the notion that episodic memory is unique to humans (see e.g., Corballis, 2013; Suddendorf & Corballis, 2007). In contrast, Dudchenko et al. (2000) reported that hippocampectomized rats could perform the OST (but not a similar spatial span task), a finding that may be viewed as inconsistent with an episodic memory account of OST performance. However, as noted earlier, accurate OST performances can be based on relative familiarity as well as on more detailed episodic-like memory. It may be that procedural factors determine which type of stimulus control will emerge. April et al. (2013) showed that the number of distractor comparison stimuli negatively affected performance on the OST, and the Dudchenko et al. (2000) procedure included incrementing comparison stimuli. Further, the extensive experience with the OST before episodic-like probes were presented may be a factor (see Table 1) as animals in the Dudchenko et al. study had relatively minimal training prior to hippocampal lesions. It will be useful in future work to examine the contribution of training experience and number of comparison stimuli on the episodic-like memory observed in this study.
In summary, the OST has historically been viewed as a “working memory” task because it requires rats to remember specific odors within a given session, but not between sessions (Dudchenko, 2004; Dudchenko, Talpos, Young, & Baxter, 2013). However, perhaps it should not be surprising that the OST also appears to involve stimulus control processes that may be better characterized as episodic-like in nature. As noted above, April et al. (2013) showed that the number of odors that rats can remember within a session can exceed 70. Such a high memory capacity certainly suggests that this task does not provide a model of human working memory (with capacity limitations of approximately 4 items, see Cowan, 2001). In addition, Eichenbaum and his colleagues have found evidence of episodic-like memory in a number of studies using odor memory tasks that are very similar in form to the OST (DeVito & Eichenbaum, 2011; Ergorul & Eichenbaum, 2004; Fortin et al, 2004; Sauvage et al., 2008, 2010). However, most of these studies have used signal detection methodologies to dissociate familiarity from more specific recollection processes (see Sauvage, 2010) and the validity of these methods in non-humans has been questioned (Wixted & Squire, 2008). Developing tasks that are equivalent for humans and nonhumans is a challenge. Interestingly, recent work extending WWW tasks based on nonhuman studies to humans (e.g. Cheke & Clayton, 2013; Holland & Smulders, 2011) indicates that humans do not solve all WWW tasks similarly, but that stimulus control varies across different tests. Thus, it is critical to develop a battery of tests that may be useful to study this type of remembering. It may then be possible to increase the translational value of nonhuman models. The present OST methodology may offer an alternative way of studying episodic-like processes in rats.
Acknowledgments
This research was supported by DA029252 to Mark Galizio. The authors thank Brooke April, Melissa Deal, Andrew Hawkey, and Kevin Jacobs for their assistance with OST training of the subjects, and Danielle Panoz-Brown for comments on the manuscript.
Footnotes
Each data point in the analysis represents mean performance on probes during a session. Because only three probes contributed to each mean in the FTC condition compared to the others, we verified that the pattern of results was the same for the critical comparisons between FTC and WWW (and RWWW in Experiment 2) probes by comparing mean FTC scores to the mean WWW (and RWWW) scores based only on the first three probes in the sessions. All statistical conclusions were the same with one exception; in Experiment 1, the difference in FTC latencies (M = 33.2) only approached significance compared to WWW latencies (M = 24.2; p = .10, correlated t-test).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- April LB, Bruce K, Galizio M. The magic number 70 (plus or minus 20): Variables determining performance in the Rodent Odor Span Task. Learning and Motivation. 2013;44:143–158. doi: 10.1016/j.lmot.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Discrimination of what, when, and where: Implications for episodic-like memory in rats. Learning and Motivation. 2005;36:177–189. [Google Scholar]
- Babb SJ, Crystal JD. Episodic-like memory in the rat. Current Biology. 2006a;16:1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Babb SJ, Crystal JD. Discrimination of what, where, when, where is not based on time of day. Learning & Behavior. 2006b;34:124–130. doi: 10.3758/bf03193188. [DOI] [PubMed] [Google Scholar]
- Cheke LG, Clayton NS. Do different tests of episodic memory produce consistent results in human adults? Learning and Memory. 2013;20:491–498. doi: 10.1101/lm.030502.113. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395:272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Corballis MC. Mental time travel: a case for evolutionary continuity. Trends in Cognitive Sciences. 2013;17:5–6. doi: 10.1016/j.tics.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behavioral and Brain Sciences. 2001;24:87–114. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Episodic-like memory in animals. Behavioural Brain Research. 2010;215:235–243. doi: 10.1016/j.bbr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD. Animal models of human cognition. In: Vonk J, Shackelford TK, editors. The Oxford handbook of comparative evolutionary psychology. New York: Oxford University Press; 2012. pp. 261–270. [Google Scholar]
- DeVito LM, Eichenbaum H. Memory for the order of events in specific sequences: Contributions of the hippocampus and medial prefrontal cortex. The Journal of Neuroscience. 2011;31:3169–3175. doi: 10.1523/JNEUROSCI.4202-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neuroscience and Biobehavioral Reviews. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: A review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neuroscience and Biobehavioral Reviews. 2013;37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span recognition, and alternation. Journal of Neuroscience. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learning and Memory. 2005;12:221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learning and Memory. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney MC, Roberts WA, Sherry DF. Memory for what, where, and when in the black-capped chickadee (Poecile atricapillus) Animal Cognition. 2009;12:767–777. doi: 10.1007/s10071-009-0236-x. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;43:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Deal M, Hawkey A, April B. Working memory in the odor span task: effects of chlordiazepoxide, dizocilpine (MK801), morphine, and scopolamine. Psychopharmacology. 2013;225:397–406. doi: 10.1007/s00213-012-2825-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. How we remember: Brain mechanisms of episodic memory. Cambridge, MA: MIT Press; 2012. [Google Scholar]
- Hoffman ML, Beran MJ, Washburn DA. Memory for “what”, “where”, and “when” information in rhesus monkeys (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. 2009;35(2):143–152. doi: 10.1037/a0013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM, Smulders TV. Do humans use episodic memory to solve a What-Where-When memory task? Animal Cognition. 2011;14:95–102. doi: 10.1007/s10071-010-0346-5. [DOI] [PubMed] [Google Scholar]
- MacQueen DA, Bullard L, Galizio M. Effects of dizocilpine (MK801) on span in rats. Neurobiology of Learning and Memory. 2011;95:57–63. doi: 10.1016/j.nlm.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Ordas G, Haun D, Colmenares F, Call J. Keeping track of time: Ecidence for episodic-like memory in great apes. Animal Cognition. 2010;13:331–340. doi: 10.1007/s10071-009-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WA, Feeney MA, MacPherson K, Petter M, McMillan N, Musolino E. Episodic-like memory in rats: Is it based on when or how long ago? Science. 2008;320:113–115. doi: 10.1126/science.1152709. [DOI] [PubMed] [Google Scholar]
- Sauvage MM. ROC in animals: Uncovering the neural substrates of recollection and familiartiy in episodic recognition memory. Consciousness and Cognition. 2010;19:816–828. doi: 10.1016/j.concog.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Beer Z, Eichenbaum H. Recognition memory: Adding a response deadline eliminates recollection but spares familiarity. Learning and Memory. 2010;17:104–108. doi: 10.1101/lm.1647710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvage MM, Fortin NJ, Owens CB, Yonelinas AP, Eichenbaum H. Recognition memory: Opposite effects of hippocampal damage on recollection and familiarity. Nature Neuroscience. 2008;11:16–18. doi: 10.1038/nn2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: what is mental time travel, and is it unique to humans? Behavioral and Brain Science. 2007;30:299–351. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: American Press; 1972. pp. 381–402. [Google Scholar]
- Tulving E. Elements of episodic memory. Oxford, England: Clarendon Press; 1983. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–10. [Google Scholar]
- Tulving E. Origin of Autonoesis in Episodic Memory. In: Roediger H III, Nairne J, Neath I, Surprenant A, editors. The nature of remembering: Essays in honor of Robert G Crowder. Washington, DC: American Psychological Association; 2001. pp. 17–34. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Squire LR. Constructing receiver operating characteristics (ROCs) With experimental animals: Cautionary notes. Learning and Memory. 2008;15:687–690. doi: 10.1101/lm.1077708. [DOI] [PubMed] [Google Scholar]
- Zentall TR. Mental time travel in animals: A challenging question. Behavioural Processes. 2006;72:173–183. doi: 10.1016/j.beproc.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Clement TS, Bhatt RS, Allen J. Episodic-like memory in pigeons. Psychonomic Bulletin & Review. 2001;8:685–690. doi: 10.3758/bf03196204. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Singer RA, Stagner JP. Episodic-like memory: Pigeons can report location pecked when unexpectedly asked. Behavioural Processes. 2008;79:93–98. doi: 10.1016/j.beproc.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Zhou W, Crystal JD. Evidence for remembering when events occurred in a rodent model of episodic memory. Proceedings of the National Academy of Sciences USA. 2009;106:9525–9529. doi: 10.1073/pnas.0904360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hohmann AG, Crystal JD. Rats answer an unexpected question after incidental encoding. Current Biology. 2012;22:1149–1153. doi: 10.1016/j.cub.2012.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkivskay A, Nazir F, Smulders TV. What-Where-When memory in magpies (Pica pica) Animal Cognition. 2009;12:119–125. doi: 10.1007/s10071-008-0176-x. [DOI] [PubMed] [Google Scholar]