Abstract
Objectives
Recent studies have pointed to neuroinflammation, oxidative stress and neurotrophic factors as key mediators in the pathophysiology of mood disorders. Little is however known about the cascade of biological episodes underlying the cognitive deficits observed during the acute and euthymic phases of bipolar disorder (BD). The aim of this review is to assess the potential association between cognitive impairment and biomarkers of inflammation, oxidative stress and neurotrophic activity in BD.
Methods
Scopus (all databases), Pubmed and Ovid Medline were systematically searched with no language or year restrictions, up to November 2013, for human studies that collected both inflammatory markers and cognitive data in BD. Selected search terms were bipolar disorder, depression, mania, psychosis, inflammatory, cognitive and neurotrophic.
Results
Ten human studies satisfied the criteria for consideration. The findings showed that high levels of peripheral inflammatory-cytokine, oxidative stress and reduced brain derived neurotrophic factor (BDNF) levels were associated with poor cognitive performance. The BDNF val66met polymorphism is a potential vulnerability factor for cognitive impairment in BD.
Conclusions
Current data provide preliminary evidence of a link between the cognitive decline observed in BD and mechanisms of neuroinflammation and neuroprotection. The identification of BD specific inflammatory markers and polymorphisms in inflammatory response genes may be of assistance for therapeutic intervention.
Keywords: neuroinflammation, oxidative stress, neurotrophin, cognitive functioning, bipolar disorder
Introduction
The mood symptoms of bipolar disorder (BD) are more often than not accompanied by verbal and working memory deficits (1, 2), poor sustained attention (3) and reduced executive functioning (4–6). Cognitive deficits persist during the euthymic phase of BD (7, 8) which suggests that cognitive dysfunction may not be attributable to mood disturbance. In the last decade an increasing number of papers have emphasized the roles of inflammation, oxidative stress and related cellular degeneration in the pathophysiology of mood disorders (9–11). It is however still unclear whether these mechanisms are associated with the risk of developing cognitive impairment in patients diagnosed with BD.
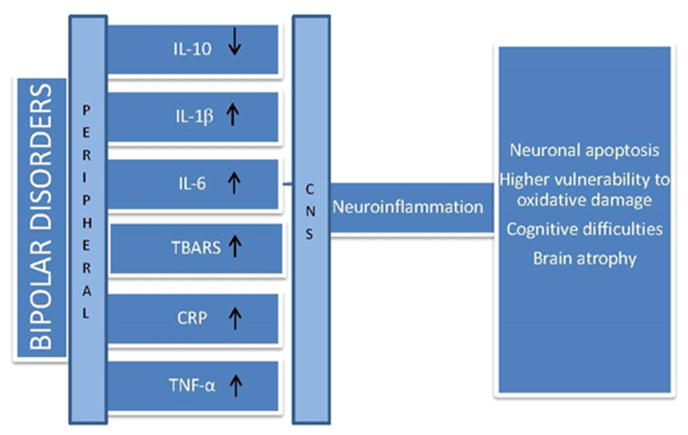
BD is characterized by high peripheral levels of pro-inflammatory agents, such as interleukins (in particular IL-6, IL-2R, IL-1beta), tumour necrosis factor (TNF-α) and cellular TNF-α receptors (TNFR1) (12), and elevated pro-oxidative C-reactive protein (CRP) concentrations (13–16). This increase in the peripheral inflammation is likely to be associated with elevated neuroinflammation. Indeed cytokines penetrate the brain via leaky regions (e.g. choroid plexus) and are associated with the increased expression of pro-inflammatory eicosanoids (prostaglandin 2 - PGE2), nitric oxide (NO) (17), TNF-α, IL-1β, reactive oxygen species as well as monocytes and macrophages in the brain (17–19) (Figure 1). Alongside the increase in peripheral inflammation, BD has been associated with a decrease in brain-derived neurotrophic factor (BDNF) levels (20, 21). Neurotrophins, such as BDNF, are a group of secreted proteins that are essential for neuron survival and synaptic functioning (22–25).
Figure 1.
Bipolar disorders are characterized by elevated levels of peripheral pro-inflammatory cytokines such as interleukins (IL-6, IL-2R, IL-1beta), tumour necrosis factor (TNF-α) and oxidative stress (Thiobarbituric acid reactive substances -TBARS and C-reactive protein - CRP). Pro-inflammatory agents enter the central nervous system (CNS) via the blood brain barrier, activate the brain inflammatory signal and release inflammatory agents, monocytes and macrophages in the brain. Exposure to pro-inflammatory substances and reactive oxidative substances is associated with neuronal damage and loss of brain function.
Clinical and preclinical evidence suggest that multiple mood episodes disrupt the homeostasis between inflammatory mechanisms, oxidative processes, and neuroprotective mechanisms, such as BDNF, and lead to neuronal death (apoptosis) (26, 27). This cycle of events is defined as “neuroprogression” and has been linked to an increase in the individual’s vulnerability to psychological stress, brain atrophy and ultimately cognitive impairment (28, 29). The concept of “staging” has been applied to the pathophysiology of BD to explain the progressive decline in mental health, psychosocial functioning and cognitive performance over the course of the disease (30–32).
Accordingly, neuroimaging studies show that individuals diagnosed with BD exhibit a significant loss of gray matter volume and white matter integrity, which is likely related to inflammatory processes such as apoptosis, cellular shrinkage, alterations in neurogenesis and reduced gliogenesis (33). Recent neuroimaging studies have also identified a significant cortical atrophy and enlargement of the ventricles in individuals who experienced multiple mood episodes as compared to gender and age-matched healthy individuals (34, 35). Furthermore, an inverse relationship between gray matter volumes and length of illness has also been reported (36, 37).
In summary, chronic inflammation may lead to structural brain abnormalities and cognitive deficits in individuals diagnosed with BD. However, to date, this hypothesis has not been systematically reviewed. Thus, the purpose of this review is to assess the potential association between cognitive impairment and biomarkers of inflammation, oxidative stress and neurotrophic activity in individuals diagnosed with BD.
Literature search
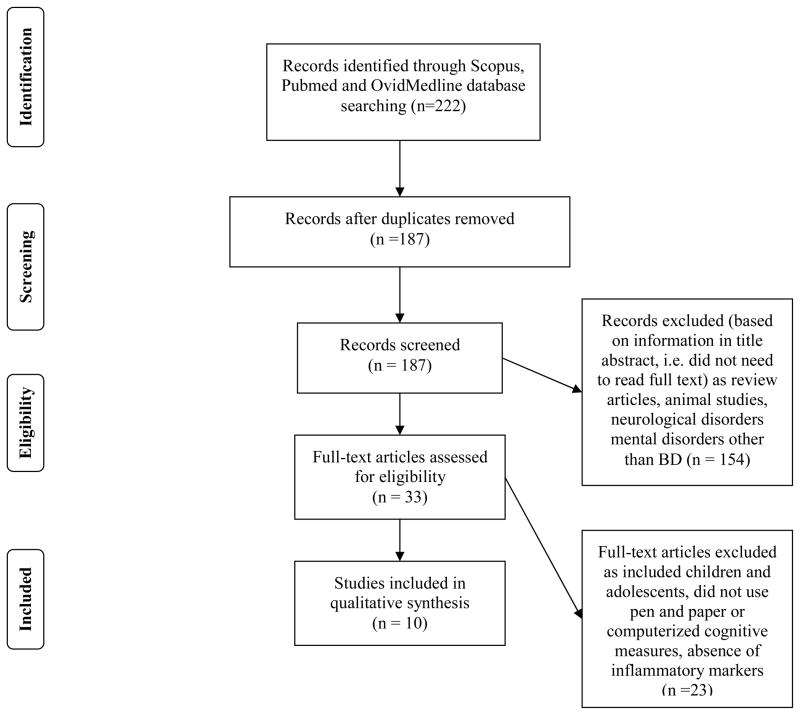
Scopus (all databases), Pubmed and Ovid Medline were systematically searched with no language or year restrictions, up to November 2013, for research articles addressing the relationship between bipolar disorder, inflammation and cognition. Selected search terms were ‘bipolar disorder’, ‘depression’, ‘mania’, ‘psychosis’, ‘inflammatory’, ‘cognitive’ and ‘neurotrophic’ as occurring either anywhere in the article (for Pubmed and Ovid Medline) or in the case of PUBMED, in the title, abstract or keywords only. The search engines listed above were chosen because of their well-established accuracy and exhaustive search across multidisciplinary fields such as psychology, nutrition, biochemistry and medicine (38). Inclusion was restricted to studies with clinical populations with a diagnosis of BD, studies were cognitive functioning was assessed using pen and paper or computerized cognitive batteries, and where inflammatory markers or polymorphisms of inflammatory genes using blood or other tissues were quantified. Excluded studies included those using animal models, clinical populations with neurological and cardiovascular diseases, children, adolescents, pregnant or lactating mothers,. Exclusion criteria was defined by the following considerations. Cognition is affected by a range of neurochemical mechanisms and cardiovascular parameters. During pregnancy and lactation, a number of physiological changes take place and this physiological state could affect cognitive performance. Finally, since the nervous system of children, and adolescents is still developing, the relationship between inflammation and cognition in children cannot be equated to that observed in a mature central nervous system. All data were extracted by a single, non-blinded, reviewer (IB) to determine if studies met inclusion criteria and, in cases where this information was not provided in abstracts, full texts were obtained. All papers identified were published in English. No papers were identified prior to 2003. Duplicates, review articles and articles not fulfilling the search criteria were removed (Figure 2).
Figure 2.
PRISMA flowchart (38) showing the filtering process used to select the 10 studies included in the systematic review of studies investigating inflammatory markers and cognition in bipolar disorder
Quality evaluation
Since there is no official instrument for the evaluation of observational studies in psychiatry and inflammation we conducted a quality evaluation based on the Centre for Reviews and Dissemination (CRD) Hierarchy of evidence (39) and a revised version of Ibrahim and colleague’s quality evaluation scale (40). The CRD Hierarchy of evidence ranks study designs in descending order of strength: 1. Experimental studies, 2. Quasi experimental studies, 3. Controlled observational studies, 3a. Cohort studies, 3b. Case control studies, 4. Observational studies without control groups, 5. Expert opinion based on theory, laboratory research or consensus. The quality evaluation scale was composed of 6 items: 1) The clinical sample was representative of the target population, 2) The control group was appropriately matched (e.g. by age, gender) to the clinical sample 3) The authors conducted sample size calculations and/or power analyses 4) The study used well-established measures of inflammation 5) The study used well-established measures of cognitive functioning 6) The authors reported confidence intervals and/or effect sizes of their findings. Each item was scored one point if the criterion was satisfied. The overall quality score was calculated by adding the scores of all items.
Study Characteristics
We identified ten published clinical studies exploring the association between peripheral pro-inflammatory cytokines, oxidative markers, neurotrophins and cognitive performance in individuals diagnosed with BD. Two studies collected peripheral measures of oxidative stress (CRP, RBANS) and peripheral pro-inflammatory cytokine measurements (IL-18, TNF). Eight studies examined the relationship between BDNF levels or polymorphism and cognitive functioning (see Table 1). All studies were observational in nature and did not involve any anti-inflammatory and/or antioxidant treatments. Cognitive performance in all studies comprised of traditional pen-and-paper tests and computerized cognitive batteries. Table 1 summarizes the study characteristics.
Table 1.
Summary of studies that explored changes in inflammatory and oxidative stress biomarkers, and cognitive measures in individuals with bipolar disorder; BD = Bipolar disorder, HC = Healthy control
| Citation | Subject description (diagnosis, gender (M/F) |
Age (Years) | Design | Duration of illness (years) |
Antipsychotic/mood stabilizer medication (Yes or No) |
Cognitive measures | Inflammatory biomarker |
Statistics | Outcome + Evidence of a link between inflammation and cognition −no evidence of a link between inflammation and cognition |
|---|---|---|---|---|---|---|---|---|---|
| Oxidative stress and cytokines | |||||||||
| Dickerson et al. (2013) Journal of Affective Disorders |
107 BD (31/76) | 36.3±13.4 | Observational study | ≈ 20 years | Yes | Repeatable Battery for the Assessment of Neuropsychological status (RBANS) Wechsler Adult Intelligence Scale (WAIS III), Trail Making Test |
Serum CRP | Logistic regression | +Lower RBANS scores in the High CRP group compared to the Low CRP group |
| Doganavsargil-Baysal et al. (2013) Human Psychopharmacology |
54 euthymic BDI (18/36) 18 HC (5/13) |
BD: 39.46±11.62 HC 38.33±10.80 |
Observational study | ≈ 13 years | Yes | Wisconsin Card Sorting Test (WCST), Rey’s Auditory Verbal Leaning Test (RAVLT) | Serum TNF | Spearman’s correlation, t-test | +BD have higher levels of sTNFr1 and sTNFr2 than HC +BD exhibit lower cognitive performance on WCST and RAVLT than HC +sTNFr2 levels correlate positively with illness duration |
| Neurotrophin | |||||||||
| Aas et al. (2013) Progress in Neuropsychopharmacology |
249 BD (122/127) and 476 HC | BD: 20–40 HC: 34.79±10.25 |
Observational study | Not provided | Yes | California Verbal Learning test, letter number sequencing, digit span forwards/backwards, Color Word Interference test, verbal fluency, block design, matrix reasoning, vocabulary | BDNF gene (not specified whether serum or plasma) | Regression model | +val/met BDNF gene carriers are more vulnerable to childhood trauma sequels, have smaller hippocampal volumes and larger ventricles |
| Barbosa et al. (2012) Journal of Affective Disorders |
25 euthymic BD (8/17) vs 25 HC (11/14) | BD: 50.88±9.11 HC: 48.04±7.08 |
Observational study | 27.88±11.8 | Yes | Mini-Mental State Examination and Frontal Assessment Battery | Plasma BDNF | Mann-Whitney U test, Spearman’s correlation | +Higher BDNF in the BD than HC −No correlation between BDNF and executive functioning |
| Chou et al. (2012) Journal of affective disorders |
23 BD (6/17) and 33 HC (12/21) | HC: 37.6±7.8 BD: 36.5±8.9 |
Observational study | 5.7±4.68 | Yes | Go/No-Go task of the test for attentional performance, Memory (Wechsler Memory scale – III), Word list, Face test, Color trail test, Wisconsin card sorting test | Plasma BDNF | t-test, ANCOVA, correlation | −no difference in BDNF levels between BD and HC +reduced face recognition and accuracy on the WCST in BD compared to HC |
| Dias et al. (2009) Bipolar disorders |
65 euthymic BD (24/41), 50 HC | BD: 37.8±10.51 HC:33.6±9.66 |
Observational study | 13.3±8.78 | Yes | Wechsler Memory Scale, Wechsler Intelligence Scale for adults revised | Serum BDNF | t-test, ANOVA, | −No difference in BDNF levels between BD and HC +Positive correlation between BDNF levels and verbal fluency in BD and HC |
| Rybarkowski et al. (2003) Bipolar disorder |
54 BD I (18/36) | BD: 18–72 (mean 46 years) | Observational study | 16±12 | Yes | Wisconsin Card Sorting Test | Whole blood BDNF gene | t-test | +the val/val BDNF polymorphism exhibits better cognitive functioning than the val/met genotype +the val allele was associated with an earlier onset of illness |
| Rybarkowski et al. (2006) Psychiatry and Clinical Neurosciences |
111 BD (37/74) 160 HC (gender ratio N/A) |
BD: 43.4±13.7 HC: 32.9±11.5 |
Observational study | N/A | N/A | Wisconsin Card Sorting Test, N-back test | Whole blood BDNF gene | t-test, ANOVA | +The percentage of correct reactions to the N-back test and accuracy on WCST was higher in the val/val group compared with val/met and met/met |
| Rybarkowski et al. (2010) International Journal of Neuropsychopharmacology |
60 BD (25/35) 60 HC (25/35) |
BD: 52.6±10.2 HC:52.1±13.6 |
Observational study | 22.2±10.8 | Yes | CANTAB | Plasma BDNF level | Mann-Whitney/Kruskal-Wallis ANOVA | +BD have lower BD have poorer cognitive performance and lower BDNF plasma levels than HC +No difference in plasma BDNF levels between ELR and HC |
| Tramontina et al. (2009) Revista Brasileira de Psiquiatria |
64 BD I (14/50) | BD I: 42.3±11.1 | Observational study | N/A | Yes | Wisconsin Card Sorting Test | BDNF gene (not specified whether serum or plasma) | Mann-Whitney’s U analysis, ANCOVA | −The percentage of non-perseverative errors was higher in the val/val group −No association between met allele and cognitive functioning |
Results of identified Studies
Pro-inflammatory cytokines, markers of oxidative stress and cognitive functioning
Peripheral serum CRP expression was negatively correlated with performance scores of immediate memory, language, and attention, on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in a study involving 107 individuals diagnosed with BD. The authors interpreted these results as indicating that oxidative damage negatively affects cognitive functioning in BD patients. However, as this study did not include a control population it is unknown whether the relationship between CRP expression and cognitive performance is specific to individuals diagnosed with BD, or whether it may also be observed in healthy controls (41).
Peripheral serum expression of the pro-inflammatory cytokine, TNF-α, was found to be negatively correlated with accuracy on the delayed memory component on the Rey Auditory Verbal Learning Test (RAVLT), in a study consisting of 54 medicated individuals diagnosed with euthymic (absence of a depressive or manic cycle) BD type I. Furthermore, the expression of two soluble TNF receptors (sTNFr1 and sTNFr2) was higher in euthymic BD individuals as compared to healthy controls. (42). It is noteworthy that BD patients and healthy individuals did not differ in terms of TNF-α levels. The authors concluded that this result may have been related to the fast degradation of TNF-α in peripheral tissues (42). Further, the elevated production of sTNF receptors may explain why cognitive deficits persist during the euthymic phase of BD (43, 44). Given that previous research shows that the production of sTNF receptors is catalyzed by TNF-α (45), it is however unclear why the levels of sTNF receptors did not correlate with cognitive performance in Doganavsargil Baysal et al.’s study (42).
At present, research regarding the relationship between inflammatory response and cognitive performance in BD is extremely limited. The above studies however provide preliminary evidence of the negative effects of pro-inflammatory and oxidative processes on high-order cognitive abilities such as memory, attention and executive functioning.
Neurotrophins and cognitive functioning
In one study, middle-aged euthymic BD patients were found to have higher peripheral BDNF expression than gender-matched healthy individuals. However, there was no significant correlation between BDNF expression and the Mini-Mental State Examination (MMSE) and Frontal Assessment Battery (FAB) scores (46). This negative finding may be due to the type of tests used to measure cognitive functioning. Indeed the MMSE and the FAB provide a short and generic assessment of age-related cognitive decline (e.g. in Alzheimer’s and fronto-temporal dementia) but are not sufficiently sensitive to detect mood-related cognitive changes (47, 48). Furthermore, since the participants of this study were relatively young (M±SD: 50.88±9.11 years), they likely exhibited a high level of accuracy on these tests.
Dias et al. (2009) found that serum BDNF levels positively correlated with accuracy on a verbal fluency task in individuals diagnosed with BD. It is important to emphasize that, contrary to the findings of Barbosa et al., Dias et al. found no difference in BDNF expression between euthymic BD and healthy individuals, in peripheral blood samples (49). Participants in this study were medicated, which may have influenced BDNF expression and confounded results. In particular, valproate-treated participants had higher BDNF levels, and lithium-treated participants lower BDNF levels, when compared with non-medicated healthy volunteers (49). Additionally, this study involved individuals with euthymic BD. While previous research shows that BDNF expression can fluctuate during manic and depressive episodes (20, 50), previous research demonstrated that, during the euthymic phase of BD, BDNF expression is comparable to that of healthy volunteers (51).
Consistent with Dias et al.’s study (2009), Chou et al. did not find any difference in plasma BDNF expression between euthymic BD and healthy controls. Furthermore, in the clinical sample there was no significant correlation between BDNF expression and cognitive performance (52). Since the mean illness duration was shorter (6 years) than that in Dias et al.’s study (13 years) it could be hypothesized that illness duration counteracts the beneficial effects of BDNF on cognitive performance. In another study poor lithium responders were found to have lower BDNF levels compared to healthy controls. By contrast, excellent lithium responders (ELR) exhibited BDNF levels comparable to those of healthy controls, and performed better than non-ELR on all tasks of the Cambridge Neuropsychological Test Automated Battery (CANTAB), in particular the spatial working memory task (53). Thus, it could be hypothesized that high BDNF levels counteract the cognitive decline observed in BD.
Previous research has focused on the relationship between the genotype of BDNF and cognitive functioning. In particular, studies have associated the BDNF val66met polymorphism with BD symptomatology. In this BDNF gene variation the valine (val) allele is replaced by the methionine (met) allele at codon 66 (54). In the present review, we identified one study showing that met carriers diagnosed with BD have smaller hippocampi volumes and larger ventricles than val/val carriers. Moreover, met carriers were seen to encounter more difficulties in verbal fluency and working memory tasks than the val/val group (55).
Additionally, Rybakowski et al. found that individuals with a BDNF val66met polymorphism developed BD type 1 approximately 11 years earlier than val/val carriers and performed more poorly on a test of executive functioning (Wisconsin Card Sorting Test - WCST) (56). A few years later Rybakowski et al. found that the val/val genotype was associated with higher accuracy on the N-back and WCST tests when compared with val/met and met/met genotypes (57).
By contrast, another identified study, by Tramontina et al. found that val/val carriers diagnosed with BD type I made more perseverative errors than val/met and met/met participants. Thus the met allele was not seen to be associated with cognitive impairment in this study (58), possibly due to the heterogeneous ancestry of Tramontina et al.’s participants (European, Amerindian and African) as compared to the more homogenous European ancestry of participants involved in Rybakowski’s study. Alternatively, other factors such as the age of onset of the disease and the severity of BD may have blunted the differences between met and val carriers, however the influence of these variables were not explored.
Overall, current findings provide initial evidence of an association between decreased BDNF levels and a high risk of cognitive decline in BD. Furthermore, the BDNF val66met polymorphism appears to be a potential risk factor for cognitive impairment in BD.
Quality evaluation: findings
The quality and reliability of the 10 studies included in this review are shown in Table 2. The CDR hierarchy of evidence was estimated to be 3–4, as the current studies are not randomized cross-sectional studies with and without a control group. Given the observational nature of the studies, the current findings provide little information on trends over time and do not investigate possible causality link between inflammation and cognitive impairment in bipolar disorder. Further, investigators were not blinded to the case/control status of their participants. This raises the possibility that the knowledge of the diagnosis may have affected their testing style and cognitive evaluation. The clinical populations were recruited in hospital settings and their diagnosis was based on well-established clinical scales such as the SCID (59) and the Mini International Neuropsychiatric inventory, (60) which indicates that the clinical profile of the samples is a reliable representation of the bipolar illness. All studies used well-accepted techniques to estimate inflammatory markers (e.g. ELISA assays) and widely used measures of cognitive functioning (e.g. Repeatable Battery for the Assessment of Neuropsychological Status). It could therefore be concluded that the current results provide an accurate description of the cognitive functioning and inflammatory response in BD patients. While the average sample size was satisfactory as it ranged from medium to large (N > 30), only two studies reported estimating the sample size based on power analyses. As a result, some of the studies may be underpowered and report misleading findings. In addition, the lack of information on the effect sizes and the confidence intervals of the statistical analyses limit the evaluation of the size of the experimental effects of the findings. Other methodological flaws include the absence of a control population in three studies and inadequate matching of the control population to the clinical population in six studies. Moreover, given the current trend for overrepresentation of positive studies in medicine and hard sciences (11), it is possible that a number of unpublished studies did not find any link between inflammation and cognition in bipolar disorder.
Table 2.
Quality assessment of the 10 studies included in the review. The Overall Quality Score was calculated by adding scores of items 1 to 6; CRD levels are: 1. Experimental studies, 2. Quasi-experimental studies, 3. Controlled observational studies, 3a. Cohort studies, 3b. Case control studies, 4. Observational studies without control groups, 5. Expert opinion based on theory, laboratory research or consensus; BD = Bipolar disorder, HC = healthy control
| Citation | Overall Quality Score (1–6) | CRD Hierarchy of evidence (Levels 1–5) | Sample size N < 30 small N = 30–50 medium N > 50 large |
Item 1 Representative sample |
Item 2 Matched control groups |
Item 3 Power analysis/Sample size calculation |
Item 4 Adequate assessment of inflammation |
Item 5 Adequate measure of cognition |
Item 6 Confidence intervals (CI) or or effect size (ES) |
|---|---|---|---|---|---|---|---|---|---|
| Criterion is fulfilled = 1 Criterion is not fulfilled = 0 |
|||||||||
| Dickerson et al. (2013) | 4 | 4 | Large, 107 BD | 1, patients were recruited in a psychiatric health care program, diagnosis was based on SCID | 0, No HC | 0 | 1 | 1 | 1, CI, no ES |
| Doganavsargil-Baysal et al. (2013) | 6 | 3 | Small/Medium 54 BDI 18 HC |
1, patients were recruited in an outpatient psychiatric clinic, diagnosis was based on DSM-IV TR | 1, HC matched by age, gender, educational level | 1 | 1 | 1 | 0, No CI, Estimated ES = 1 |
| Aas et al. (2013) | 3 | 3 | Large 249 BD 476 HC |
1: Patients recruited in hospitals, diagnosis based on DSM-IV criteria. | 0, HC not demographically matched | 0 | 1 | 1 | 0, No CI/ES |
| Barbosa et al. (2012) | 4 | 3 | Small, 25 BD 25 HC |
1, patients recruited in an out/inpatient psychiatric clinic, diagnosis based on Mini International Psychiatric inventory | 1, HC matched by age and gender | 0 | 1 | 1 | 0, No CI/ES |
| Chou et al. (2012) | 4 | 3 | Small 23 BD 33 HC |
1: patients were diagnosed based on DSM-IV criteria. | 1, age-matched HC | 0 | 1 | 1 | 0, No CI/ES |
| Dias et al. (2009) | 3 | 3 | Medium 65 BD 50 HC |
1, patients diagnosed based on DSM-IV criteria and Mini International Psychiatric inventory | 0, HC not demographically matched | 0 | 1 | 1 | 0, No CI/ES |
| Rybakowski et al. (2003) | 3 | 4 | Medium 54 BD I |
1: Outpatients recruited in hospitals, diagnosis based on DSM-IV criteria. | 0, No HC | 0 | 1 | 1 | 0, No CI/ES |
| Rybakowski et al. (2006) | 3 | 3 | Large 111 BD 160 HC |
1: inpatients recruited in hospitals, diagnosis based on DSM-IV criteria. | 0, Not matched | 0 | 1 | 1 | 0, No CI/ES |
| Rybakowski et al. (2010) | 4 | 3 | Large 60 BD 60 HC |
1: Patients attending an outpatient lithium clinic, diagnosis based on DSM-IV criteria - SCID | 1, age and gender-matched HC | 0 | 1 | 1 | 0, No CI/ES |
| Tramontina et al. (2009) | 4 | 4 | Medium 64 BD I |
1: outpatients recruited in a hospital setting, diagnosis based on DSM-IV criteria. | 0, No HC | 1 | 1 | 1 | 0, No CI/ES |
Discussion
This review aimed to examine the literature exploring the relationship between the cognitive deficits seen among individuals diagnosed with bipolar disorder, and markers of inflammation, neurotrophins and oxidative damage. Thus, we conducted a systematic search of the human literature on inflammation and cognition in BD. It is important to emphasize that this is a novel approach as previous reviews have linked inflammation with mood symptoms, but have not explored the relationship between peripheral markers of inflammation and cognitive impairment. We identified 10 observational studies. Of these studies 2 investigated the relationship between pro-inflammatory cytokines (TNF-a) and markers of oxidative stress (CRP) and 8 investigated the relationship between the neurotrophin BDNF, and cognitive functioning. The results of these studies indicate that the cognitive deficits observed in individuals diagnosed with BD appear to be associated with an increased inflammatory state and a decrease in the neurotropic factor, BDNF. Despite the limited number of studies in this field and their heterogeneity in terms of cognitive outcome measures, these studies provide preliminary evidence that an elevated inflammatory state negatively affects frontotemporal cognitive abilities such as memory, attention and executive functions, and indicate a need for further investigation.
Consistent with previous research (28), we identified one study showing that individuals diagnosed with BD have reduced hippocampi volumes and larger ventricles. Importantly, this was associated with more difficulties in verbal fluency and working memory tasks (55). Previous authors have speculated that systemic toxicity and cognitive dysfunction are directly related to the number of episodes suffered by the patient (32) and that peripheral cytokine and BDNF expression could be potential markers of illness progression, thus corroborating the “staging” hypothesis of BD (61). However, as all the studies identified in the present systematic review were observational in nature, there appears to be no research regarding the relationship between inflammatory markers and changes in cognitive measures over the course of the BD. A longitudinal design study could be a suitable approach to explore the relationship between peripheral biomarkers of inflammation, oxidative stress and neurotrophic activity. This type of design may also help clarify the relationship between biomarkers and cognitive impairment at different phases of the disease.
None of the reviewed studies investigate the relationship between oxidative stress, mitochondrial dysfunction and neuroprogression. However, a number of studies using animal models of mania have observed increased levels of reactive oxygen species (ROS), a marker of mitochondrial dysfunction (62). One study demonstrated increased lipid peroxidation, and a high number of free radical, superoxide in submitochondrial particles of the prefrontal cortex and hippocampus (2). A second study showed that repeated amphetamine exposure, which induces manic symptomatology in animal models, increases levels of anti-oxidant enzymes, superoxide dismutase (SOD) and catalase (CAT), in regional specific manner, in the prefrontal cortex, hippocampus and striatum (3). The authors interpreted these results to reflect an imbalance between SOD and CAT expression, potentially indicative of a predisposition to the generation of ROS (63, 64). Increased oxidative stress has been associated with abnormalities in the glutamatergic system and neuronal apoptosis. Neuronal apoptosis has been hypothesized to be a progressive process that begins at synaptic terminals and dendrites and continues to the cell body via apoptotic cascades (65, 66). The dynamic of neuronal cell death mechanisms may underlie the decline in neurocognitive function observed over the course of the bipolar illness. Additionally, clinical research indicates that BD is characterized by low levels of brain energy metabolites such as creatine and high lactate and glutamate-related metabolite concentrations, which are clinical markers of mitochondrial dysfunction (67), as assessed using magnetic resonance spectroscopy (MRS)(68). Lactate accumulation may indicate a shift to anaerobic glycolytic mechanisms, possibly due to inadequate energy production within the mitochondria (68, 69). Anaerobic glycolysis produces less adenosine triphosphate (ATP) molecules than aerobic glycosis, and reduced ATP production could lead to cerebral hypometabolism, brain dysfunction and eventually cognitive impairment (70).
A further limitation of the studies reviewed here is that they differ with respect to the estimation of the peripheral levels of inflammatory biomarkers. Indeed, as illustrated in Table 1, the majority of the studies measured the expression of cytokines, BDNF and antioxidants in serum, plasma, or whole blood cells (a combination of serum, plasma and erythrocytes). For instance, since plasma cells have a short turnover (71), they may be ideal to measure acute inflammation (e.g. infection), but may not reflect a state of chronic inflammation. Erythrocytes and whole blood cells (which have a turnover of approximately 12 weeks) (72) may therefore be better indices of inflammatory markers in cell membranes and possibly the brain tissue. Further, previous studies have shown that aminoacids and carbohydrate levels differ significantly between plasma and serum (8, 73). In particular, BDNF levels are higher in serum compared to plasma (40, 73, 74). The latter result is probably due to the release of BDNF from platelets to serum during the coagulation process (40). Hence, finding of studies using serum BDNF levels may not be comparable to those of studies using plasma BDNF levels. Quality evaluation of the current studies reveals some methodological concerns in terms of research design and statistical analyses, as earlier discussed. Hence, caution should be taken in the interpretation of the data presented in this systematic review, regarding the relationship between inflammation and cognition in bipolar disorder.
Surprisingly, none of the studies identified investigated the relationship between the inflammatory response and the hypothalamic-pituitary-adrenal (HPA) axis activation. Indeed a number of mood disorders present with abnormalities in the HPA axis, such as increased levels of cortisol and corticotropin-releasing factor (CRF)(75). A potential explanation for the abnormal HPA axis activity is that pro-inflammatory cytokines disrupt the glucocorticoid function and lead to glucorticoid resistance, characterized by cortisol and CRF hypersecretion. In turn, glucocorticoid resistance initiates pro-inflammatory mechanisms and reduces peripheral BDNF levels (73, 76). Both glucocorticoid resistance and inflammation have been associated with depressed mood and cognitive difficulties (73). It is notable that in medicated BD patients the glucocorticoid receptor antagonist, mifepristone improves mood and spatial working memory, and to a lesser extent, verbal fluency and spatial recognition (74, 77). In particular, the improvement in spatial memory appear to be related to the cortisol response to mifiprestone, not mood changes (74). It could be speculated that mifiprestone inhibits the proinflammatory response by regulating the HPA-axis activity, however this remains unknown as these studies did not collect inflammatory markers. Taken together these findings provide a strong rationale for the development of trials investigating the synergistic role of the inflammatory response and the HPA-axis function in the pathophysiology of BD.
In conclusion, research in the field of cognition and inflammation in BD is still in its infancy and additional work is needed to understand how pro-inflammatory processes affect brain function and induce cognitive impairment. The identification of inflammatory markers and polymorphisms in inflammatory response genes underlying cognitive decline will have important implications for the development of new therapies targeting chronic inflammatory conditions such as BD.
Acknowledgments
This work was partly supported by the Stanley Medical Research Institute, NIH grant MH 085667 (JCS), and by the Pat Rutherford Jr. Chair in Psychiatry (UTHealth).
Glossary
- ACC
Anterior cingulate cortex
- AMPH
d-amphetamine
- ATP
adenosine triphosphate
- BBB
Brain blood barrier
- BD
Bipolar disorder
- CAT
Catalase
- CANTAB
Cambridge Neuropsychological Test Automated Battery
- CPT
Continuous performance test
- CRF
Corticotropin-releasing factor
- CRP
C-reactive protein
- DNA
Deoxyribonucleic acid
- ELR
excellent lithium responders
- ERK
Extracellular signal-regulated kinase
- FAB
Frontal Assessment Battery
- FEP
First episode psychosis
- FTT
Finger tapping test
- GABA
Gamma-Aminobutyric acid
- GPx
Glutathione peroxidase
- GR
Glutathione reductase
- GSH
Glutathione (GSH)
- HC
Healthy control
- HPA
Hypothalamic-pituitary-adrenal
- IL
Interleukin
- INF-α
Interferon-α
- LPS
Lipopolysaccharide
- MDA
Malondialdehyde
- MDD
Major Depression Disorder
- MINI
Mini International Neuropsychiatric Interview
- MMSE
Mini-Mental State Examination
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- Na+K+ATPase
Sodium-potassium adenosine triphosphatase pump
- NFkB
Nuclear factor-kappa B
- NGF
Nerve growth factor
- NO
Nitric oxide
- NMDA
N-methyl-D-aspartic acid
- NOS
Reactive nitrogen species
- NPSH
Non-protein thiols
- NT-3/NT-4
Neurotrophin 3 or 4
- O&NS
Oxidative and nitrosative stress
- PANSS
Positive and negative syndrome scale
- PET
Positron emission tomography
- PG
Prostaglandins
- PhSe2
Diphenyldiselenide
- RAVLT
Rey’s Auditory Verbal Leaning Test
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- ROS
Reactive oxygen species
- sACC
Subgenual anterior cingulate cortex
- SB
Sodium butyrate
- fMRI
functional magnetic resonance imaging
- SCID
Structured Clinical Interview for DSM-IV Axis I Disorders
- sMRI
structural magnetic resonance imaging
- SOD
Superoxide dismutase
- SSRI
Selective serotonin reuptake inhibitors
- TAS
Total anti-oxidant status
- TBARS
Thiobarbituric acid reactive substances
- TNF
Tumour necrosis factor
- WAIS
Wechsler Adult Intelligence Scale
- WCST
Wisconsin Card Sorting Test
Footnotes
Contributors
Isabelle Bauer managed and searched the literature and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Declaration of interest
Dr Bauer, Dr Pascoe and Dr Wollenhaupt-Aguiar have no conflicts of interest
Professor Kapczinski has received grants/research support from Astra-Zeneca, Eli Lilly, Janssen-Cilag, Servier, CNPq, CAPES, NARSAD and Stanley Medical Research Institute; has been a member of the board of speakers for Astra-Zeneca, Eli Lilly, Janssen and Servier; and has served as a consultant for Servier.
Professor J. C. Soares has received grants/research support from Forrest, BMS, Merck, Stanley Medical Research Institute, NIH and has been a speaker for Pfizer and Abbott.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Isabelle E. Bauer, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
Michaela C. Pascoe, Department of Clinical Neuroscience and Rehabilitation, Sahlgrenska Academy at University of Gothenburg, Box 440, 40530 Gothenburg, Sweden
Bianca Wollenhaupt-Aguiar, Laboratório de Psiquiatria Molecular, Instituto Nacional de Ciência e Tecnologia – Translacional em Medicina (INCT), Hospital de Clínicas de Porto Alegre, Programa de Pós-Graduação em Ciências Biológicas: Bioquímica, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Flavio Kapczinski, Laboratório de Psiquiatria Molecular, Instituto Nacional de Ciência e Tecnologia – Translacional em Medicina (INCT), Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil.
Jair C. Soares, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
References
- 1.Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, et al. Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry research. 2006;142(2):139–50. doi: 10.1016/j.psychres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Diwadkar VA, Goradia D, Hosanagar A, Mermon D, Montrose DM, Birmaher B, et al. Working memory and attention deficits in adolescent offspring of schizophrenia or bipolar patients: comparing vulnerability markers. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35(5):1349–54. doi: 10.1016/j.pnpbp.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, et al. The neurocognitive signature of psychotic bipolar disorder. Biological psychiatry. 2007;62(8):910–6. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72(3):209. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 5.Najt P, Perez J, Sanches M, Peluso M, Glahn D, Soares JC. Impulsivity and bipolar disorder. European neuropsychopharmacology. 2007;17(5):313–20. doi: 10.1016/j.euroneuro.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ancin I, Santos JL, Teijeira C, Sanchez-Morla EM, Bescos MJ, Argudo I, et al. Sustained attention as a potential endophenotype for bipolar disorder. Acta Psychiatr Scand. 2010;122(3):235–45. doi: 10.1111/j.1600-0447.2009.01532.x. [DOI] [PubMed] [Google Scholar]
- 7.Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. Journal of Affective Disorders. 2006;93(1–3):105–15. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Malhi GS, Ivanovski B, Hadzi-Pavlovic D, Mitchell PB, Vieta E, Sachdev P. Neuropsychological deficits and functional impairment in bipolar depression, hypomania and euthymia. Bipolar disorders. 2007;9(1–2):114–25. doi: 10.1111/j.1399-5618.2007.00324.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biological psychiatry. 2011;70(7):663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey AN, Fisher DR, Joseph JA, Shukitt-Hale B. The ability of walnut extract and fatty acids to protect against the deleterious effects of oxidative stress and inflammation in hippocampal cells. Nutr Neurosci. 2013;16(1):13–20. doi: 10.1179/1476830512Y.0000000023. [DOI] [PubMed] [Google Scholar]
- 11.Turner EH. Publication Bias, with a Focus on Psychiatry: Causes and Solutions. CNS drugs. 2013;27(6):457–68. doi: 10.1007/s40263-013-0067-9. [DOI] [PubMed] [Google Scholar]
- 12.Barbosa IG, Huguet RB, Sousa LP, Abreu MNS, Rocha NP, Bauer ME, et al. Circulating levels of GDNF in bipolar disorder. Neuroscience letters. 2011;502(2):103–6. doi: 10.1016/j.neulet.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant’Anna M, Klamt F, et al. Peripheral biomarkers and illness activity in bipolar disorder. Journal of psychiatric research. 2011;45(2):156–61. doi: 10.1016/j.jpsychires.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 14.Kunz M, Ceresér KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, et al. Serum levels of IL-6, IL-10 and TNF-α in patients with bipolar disorder and schizophrenia: differences in pro-and anti-inflammatory balance. Revista Brasileira de Psiquiatria. 2011;33(3):268–74. doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- 15.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological medicine. 2009;39(3):413. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Myint AM, Schwarz MJ, Steinbusch HW, Leonard BE. Neuropsychiatric disorders related to interferon and interleukins treatment. Metabolic brain disease. 2009;24(1):55–68. doi: 10.1007/s11011-008-9114-5. [DOI] [PubMed] [Google Scholar]
- 17.Capuron L, Dantzer R. Cytokines and depression: the need for a new paradigm. Brain Behav Immun. 2003;17(1):119–24. doi: 10.1016/s0889-1591(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 18.Minagar A, Alexander JS. Inflammatory Disorders of the Nervous System: Pathogenesis, Immunology, and Clinical Management. Springer; 2005. [Google Scholar]
- 19.Blank T, Prinz M. Microglia as modulators of cognition and neuropsychiatric disorders. Glia. 2013;61(1):62–70. doi: 10.1002/glia.22372. [DOI] [PubMed] [Google Scholar]
- 20.Cunha A, Frey BN, Andreazza AC, Goi JD, Rosa AR, Gonçalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neuroscience letters. 2006;398(3):215–9. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 21.Bourne C, Aydemir Ö, Balanzá-Martínez V, Bora E, Brissos S, Cavanagh J, et al. Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatrica Scandinavica. 2013;128(3):149–62. doi: 10.1111/acps.12133. [DOI] [PubMed] [Google Scholar]
- 22.Ichim G, Tauszig-Delamasure S, Mehlen P. Neurotrophins and cell death. Experimental Cell Research. 2012;318(11):1221–8. doi: 10.1016/j.yexcr.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophrenia research. 2007;91(1):1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang L, Shi Y, Wang L, Yang Z. The influence of orally administered docosahexaenoic acid on cognitive ability in aged mice. The Journal of Nutritional Biochemistry. 2009;20(9):735–41. doi: 10.1016/j.jnutbio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Fries GR, Pfaffenseller B, Stertz L, Paz AVC, Dargél AA, Kunz M, et al. Staging and neuroprogression in bipolar disorder. Current psychiatry reports. 2012;14(6):667–75. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 27.Berk M, Kapczinski F, Andreazza A, Dean O, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neuroscience & biobehavioral reviews. 2011;35(3):804–17. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Berk M, Conus P, Kapczinski F, Andreazza AC, Yücel M, Wood SJ, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(4 Suppl):S36–40. [PubMed] [Google Scholar]
- 29.Kapczinski F, Vieta E, Andreazza AC, Frey BN, Gomes FA, Tramontina J, et al. Allostatic load in bipolar disorder: implications for pathophysiology and treatment. Neuroscience & Biobehavioral Reviews. 2008;32(4):675–92. doi: 10.1016/j.neubiorev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Gama CS, Kunz M, Magalhães PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Revista Brasileira de Psiquiatria. 2013;35(1):70–4. doi: 10.1016/j.rbp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Frodl T, Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;3(48):295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Kapczinski F, Dias VV, Kauer-Sant’Anna M, Frey BN, Grassi-Oliveira R, Colom F, et al. Clinical implications of a staging model for bipolar disorders. Expert review of neurotherapeutics. 2009;9(7):957–66. doi: 10.1586/ern.09.31. [DOI] [PubMed] [Google Scholar]
- 33.Czéh B, Lucassen PJ. What causes the hippocampal volume decrease in depression? European archives of psychiatry and clinical neuroscience. 2007;257(5):250–60. doi: 10.1007/s00406-007-0728-0. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffenseller B, Gama CS, Kapczinski F, Duarte JA, Kunz M. Anatomical faces of neuroprogression in bipolar disorder. Neuropsychiatry. 2012;2(4):279–80. [Google Scholar]
- 35.Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14(4):313–25. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology. 2001;43(4):242–7. doi: 10.1159/000054897. [DOI] [PubMed] [Google Scholar]
- 37.Frey BN, Zunta-Soares GB, Caetano SC, Nicoletti MA, Hatch JP, Brambilla P, et al. Illness duration and total brain gray matter in bipolar disorder: evidence for neurodegeneration? Eur Neuropsychopharmacol. 2008;18(10):717–22. doi: 10.1016/j.euroneuro.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 38.Moher DLA, Tetzlaff J, Altman DG The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(6):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cochrane, Collaboration. Cochrane Reviewers’ Handbook. Version 4.2. 1. 2003 [Google Scholar]
- 40.Ibrahim AK, Kelly SJ, Adams CE, Glazebrook C. A systematic review of studies of depression prevalence in university students. Journal of psychiatric research. 2012;47(3):391–400. doi: 10.1016/j.jpsychires.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. Journal of Affective Disorders. 2013;150(2):456–59. doi: 10.1016/j.jad.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 42.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas H, Metin O, Fettahoglu C, et al. Levels of TNF-α, soluble TNF receptors (sTNFR1, sTNFR2), and cognition in bipolar disorder. Human Psychopharmacology: Clinical and Experimental. 2013;28(2):160–7. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 43.Pattanayak RD, Sagar R, Mehta M. Neuropsychological performance in euthymic Indian patients with bipolar disorder type I: Correlation between quality of life and global functioning. Psychiatry and clinical neurosciences. 2012;66(7):553–63. doi: 10.1111/j.1440-1819.2012.02400.x. [DOI] [PubMed] [Google Scholar]
- 44.King DA, Caine ED. Cognitive impairment and major depression: beyond the pseudodementia syndrome. In: Grant KMA, editor. Neuropsychological Assessment of Neuropsychiatric Disorders. I. New York: Oxford University Press; 1996. [Google Scholar]
- 45.Grassi-Oliveira R, Brietzke E, Pezzi JC, Lopes RP, Teixeira AL, Bauer ME. Increased soluble tumor necrosis factor-α receptors in patients with major depressive disorder. Psychiatry and clinical neurosciences. 2009;63(2):202–8. doi: 10.1111/j.1440-1819.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 46.Barbosa IG, Rocha NP, Huguet RB, Ferreira RA, Salgado JV, Carvalho LA, et al. Executive dysfunction in euthymic bipolar disorder patients and its association with plasma biomarkers. Journal of affective disorders. 2012;137(1):151. doi: 10.1016/j.jad.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Gluhm S, Goldstein J, Loc K, Colt A, Van Liew C, Corey-Bloom J. Cognitive Performance on the Mini-Mental State Examination and the Montreal Cognitive Assessment Across the Healthy Adult Lifespan. Cognitive and Behavioral Neurology. 2013;26(1):1–5. doi: 10.1097/WNN.0b013e31828b7d26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boban M, Malojčić B, Mimica N, Vuković S, Zrilić I. The Frontal Assessment Battery in the Differential Diagnosis of Dementia. Journal of geriatric psychiatry and neurology. 2012;25(4):201–7. doi: 10.1177/0891988712464821. [DOI] [PubMed] [Google Scholar]
- 49.Dias VV, Brissos S, Frey BN, Andreazza AC, Cardoso C, Kapczinski F. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar disorders. 2009;11(6):663–71. doi: 10.1111/j.1399-5618.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 50.Goldstein BI, Collinger KA, Lotrich F, Marsland AL, Gill M-K, Axelson DA, et al. Preliminary findings regarding proinflammatory markers and brain-derived neurotrophic factor among adolescents with bipolar spectrum disorders. Journal of Child and Adolescent Psychopharmacology. 2011;21(5):479–84. doi: 10.1089/cap.2011.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandes BS, Gama CS, Maria Ceresér K, Yatham LN, Fries GR, Colpo G, et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. Journal of psychiatric research. 2011;45(8):995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Chou Y-H, Wang S-J, Lirng J-F, Lin C-L, Yang K-C, Chen C-K, et al. Impaired cognition in bipolar I disorder: The roles of the serotonin transporter and brain-derived neurotrophic factor. Journal of affective disorders. 2012;143:131–7. doi: 10.1016/j.jad.2012.05.043. [DOI] [PubMed] [Google Scholar]
- 53.Rybakowski JK, Suwalska A. Excellent lithium responders have normal cognitive functions and plasma BDNF levels. The International Journal of Neuropsychopharmacology. 2010;13(05):617–22. doi: 10.1017/S1461145710000404. [DOI] [PubMed] [Google Scholar]
- 54.Craddock N, O’donovan M, Owen M. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. Journal of Medical Genetics. 2005;42(3):193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aas M, Haukvik UK, Djurovic S, Bergmann Ø, Athanasiu L, Tesli MS, et al. BDNF val66met modulates the association between childhood trauma, cognitive and brain abnormalities in psychoses. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;46:181–8. doi: 10.1016/j.pnpbp.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 56.Rybakowski JK, Borkowska A, Czerski PM, Skibińska M, Hauser J. Polymorphism of the brain-derived neurotrophic factor gene and performance on a cognitive prefrontal test in bipolar patients. Bipolar disorders. 2003;5(6):468–72. doi: 10.1046/j.1399-5618.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 57.Rybakowski JK, Borkowska A, Skibinska M, Szczepankiewicz A, Kapelski P, Leszczynska-Rodziewicz A, et al. Prefrontal cognition in schizophrenia and bipolar illness in relation to Val66Met polymorphism of the brain-derived neurotrophic factor gene. Psychiatry and clinical neurosciences. 2006;60(1):70–6. doi: 10.1111/j.1440-1819.2006.01462.x. [DOI] [PubMed] [Google Scholar]
- 58.Tramontina JF, Yates D, Magalhães PVdS, Trentini C, Sant’Anna MK, Fries GR, et al. Brain-derived neurotrophic factor gene val66met polymorphism and executive functioning in patients with bipolar disorder. Revista Brasileira de Psiquiatria. 2009;31(2):136–40. doi: 10.1590/s1516-44462009000200010. [DOI] [PubMed] [Google Scholar]
- 59.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- 60.Sheehan DV, Lecrubier Y, Harnett Sheehan K, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12(5):232–41. [Google Scholar]
- 61.Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, et al. Brain-derived neurotrophic factor and inflammatory markers in patients with early-vs. late-stage bipolar disorder. The International Journal of Neuropsychopharmacology. 2009;12(04):447–58. doi: 10.1017/S1461145708009310. [DOI] [PubMed] [Google Scholar]
- 62.Murphy MP. Mitochondrial dysfunction indirectly elevates ROS production by the endoplasmic reticulum. Cell metabolism. 2013;18(2):145–6. doi: 10.1016/j.cmet.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 63.Frey BN, Valvassori SS, Gomes KM, Martins MR, Dal-Pizzol F, Kapczinski F, et al. Increased oxidative stress in submitochondrial particles after chronic amphetamine exposure. Brain Research. 2006;1097(1):224–9. doi: 10.1016/j.brainres.2006.04.076. [DOI] [PubMed] [Google Scholar]
- 64.Frey BN, Valvassori SS, Réus GZ, Martins MR, Petronilho FC, Bardini K, et al. Changes in antioxidant defense enzymes after d-amphetamine exposure: implications as an animal model of mania. Neurochemical research. 2006;31(5):699–703. doi: 10.1007/s11064-006-9070-6. [DOI] [PubMed] [Google Scholar]
- 65.Mattson MP. Apoptosis in neurodegenerative disorders. Nature Reviews Molecular Cell Biology. 2000;1(2):120–30. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 66.Mattson MP, Keller JN, Begley JG. Evidence for synaptic apoptosis. Experimental neurology. 1998;153(1):35–48. doi: 10.1006/exnr.1998.6863. [DOI] [PubMed] [Google Scholar]
- 67.Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120(6):1326–33. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 68.Strakowski S. The bipolar brain: integrating neuroimaging and genetics. Oxford University Press; 2012. [Google Scholar]
- 69.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar disorders. 2012;14(5):478–87. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 70.Minuzzi L, Antônio G, Fonseca Moreira JC, Frey BN. Mitochondrial Dysfunction in Bipolar Disorder: Lessons from Brain Imaging and Molecular Markers; Disfunción mitocondrial en el trastorno bipolar: lecciones de las imágenes cerebrales y los marcadores moleculares. Rev colomb psiquiatr. 2011;40(supl 1):166–82. [Google Scholar]
- 71.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral Immunity Due to Long-Lived Plasma Cells. Immunity. 1998;8(3):363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 72.Katan M, Deslypere J, Van Birgelen A, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. Journal of lipid research. 1997;38(10):2012–22. [PubMed] [Google Scholar]
- 73.Pace TWW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watson S, Gallagher P, Porter RJ, Smith MS, Herron LJ, Bulmer S, et al. A randomized trial to examine the effect of mifepristone on neuropsychological performance and mood in patients with bipolar depression. Biological psychiatry. 2012;72(11):943–9. doi: 10.1016/j.biopsych.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 75.DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends in Endocrinology & Metabolism. 2006;17(3):117–21. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Mackin P, Gallagher P, Watson S, Young AH, Ferrier IN. Changes in brain-derived neurotrophic factor following treatment with mifepristone in bipolar disorder and schizophrenia. Australian and New Zealand journal of psychiatry. 2007;41(4):321–6. doi: 10.1080/00048670701213211. [DOI] [PubMed] [Google Scholar]
- 77.Young AH, Gallagher P, Watson S, Del-Estal D, Owen BM, Ferrier IN. Improvements in neurocognitive function and mood following adjunctive treatment with mifepristone (RU-486) in bipolar disorder. Neuropsychopharmacology. 2004;8(29):1538–45. doi: 10.1038/sj.npp.1300471. [DOI] [PubMed] [Google Scholar]