Abstract
Background
In three previously published works (Brumback et al., 2007; King et al., 2011a; Roche and King 2010), our group characterized acute alcohol responses in a large group of young, heavy binge drinkers (n = 104) across a variety of subjective, eye tracking, and psychometric performance measures.
Methods
The primary goal of the current study was to directly replicate prior findings of alcohol response in heavy social drinkers in a second independent cohort (n = 104) using identical methodology. A secondary goal was to examine the effects of family history of alcohol use disorders on acute alcohol response in both samples. Participants attended two randomized laboratory sessions in which they consumed 0.8 g/kg alcohol or a taste-masked placebo. At preand post-drink time points, participants completed subjective scales, psychomotor performance and eye movement tasks, and provided salivary samples for cortisol determination.
Results
Results showed that the second cohort of heavy drinkers exhibited a nearly identical pattern of alcohol responses to the original cohort, including sensitivity to alcohol’s stimulating and hedonically rewarding effects during the rising BrAC limb, increases sedation during the declining BrAC limb, a lack of cortisol response, and psychomotor and eye tracking impairment that was most evident at peak BrAC. The magnitude and temporal pattern of these acute effects of alcohol in the second cohort were similar to the first cohort across all measures, with the exception of three eye movement measures: pro- and anti-saccade accuracy and anti-saccade velocity. Family history of alcohol use disorders did not affect alcohol response in the first cohort and this was replicated in the second cohort.
Conclusions
In sum, in two independent samples, we have demonstrated that heavy social drinkers display a consistent and reliable sensitivity to alcohol’s subjective effects and impairment of eye tracking and psychomotor performance, which is not affected by family history status. This acute alcohol response phenotype in heavy, frequent binge drinkers appears to be robust and reproducible.
INTRODUCTION
Replication is a fundamental component of the scientific method, and while it adds credence to the validity of original results, the majority of scientists devote most of their resources to producing new work rather than replicating their own or that of others (Collaboration, 2012; Nosek et al., 2012). Accordingly, there is a paucity of psychology studies that attempt to directly replicate previously published results using the same subject population and methodology as the original study (Ioannidis, 2005; Koole and Lakens, 2013; Makel et al., 2012; Roediger, 2012). Due to some recent large-scale failures to replicate in the medical field, with only 11% of high-impact findings in preclinical cancer research successfully replicated, there has been a renewed focus on the importance of reproducibility of novel and influential results in various fields of science (Begley and Ellis, 2012). The pharmaceutical industry also has shown difficulty in reproducing previous results, with one company replicating only 25 % of its previously published findings (Prinz et al., 2011). In light of such problems, researchers from the fields of psychology, oncology, and biomedicine have urged other scientists to designate the time and resources needed to attempt to directly replicate their own findings, as well as those from other laboratories.
In the field of human psychopharmacology, alcohol challenge studies in particular stand to benefit from direct replication attempts, as laboratory studies measuring acute alcohol response traditionally suffer from a number of methodological issues that limit impact and generalizability. For example, such studies often have a small sample size, consist of heterogeneous samples, and use alcohol administration routes and dependent measures that may not be psychometrically sound and can often be quite disparate across studies. As observed in other fields (Ioannidis, 2005; Schmidt, 2009), most alcohol challenge studies have focused on conceptual replication, i.e., trying to replicate the existence of a concept using different methodologies or sample characteristics (for example, see Corbin et al., 2013; Duranceaux et al., 2008; Schuckit et al., 2004). However, conceptual replication studies can only confirm a prior finding; if they present results that differ from the original study, the failure to replicate is often ascribed to the methodological differences between the studies and as such, may lead to publication bias (Ioannidis, 2005; Nosek et al., 2012; Pashler and Harris, 2012). In contrast, direct replication studies, which use the same experimental conditions, subject population, and methodology to examine the reproducibility of previously reported results, have been rare, with some exceptions (see Newlin and Thomson, 1999). The lack of direct replication studies in alcohol research may have contributed to an incomplete understanding of the relationship between risk factors for the development of alcohol use disorders (AUD) and acute alcohol response.
There is an ongoing debate regarding how acute alcohol response may convey risk for future drinking problems. Both higher and lower responses to alcohol have been purported to play a role in the development of future alcohol dependence among young adults with family history (FH) of the disorder or who engage in frequent binge drinking (Schuckit 1980; Schuckit and Smith 1996; Newlin and Renton, 2010; Quinn and Fromme, 2011; Schuckit, 2011; King et al., 2011a; Kerfoot 2013 in press; King et al., 2013 in press). For example, while FH has been the most commonly studied risk factor for the development of AUD, findings have varied considerably on how FH may relate to acute response to alcohol (Newlin and Renton 2010). Before drawing firm conclusions on the relationship between acute response and risk for the development of AUD, a consistent and reproducible pattern of acute response in at risk drinkers must be established. Direct replication of reliable subjective and objective alcohol responses across the breath alcohol curve may be crucial to achieving this goal.
In a series of papers by our group, we have attempted to characterize the pattern of acute subjective and objective response to an oral alcohol challenge (vs. placebo) in heavy social drinkers (HD) compared with light drinking (LD) controls. After alcohol challenge, HD, relative to LD, exhibited greater stimulant, liking, and wanting responses, and lower sedative and cortisol responses (King et al., 2011a). Importantly HD’s sensitivity to the stimulating and rewarding (i.e., liking and wanting) effects of alcohol predicted future alcohol problems, both at two years (King et al., 2011a) and six years after the initial alcohol challenge (King et al., in press) . Further, relative to LD, HD showed lower perception of impairment despite largely similar decrements in smooth pursuit and saccadic eye movements (Roche and King, 2010) and psychomotor performance (Brumback et al., 2007). While the within-subjects and placebo-controlled design was robust, the measures were psychometrically sound, and the sample size was large with 104 HD, reproducing these findings in an independent sample would ensure the results are generalizable and not the product of an “over fit” to one specific sample.
The primary goal of the current study was to ascertain identical subjective, objective, and performance responses to alcohol in a placebo-controlled laboratory study in an independent sample of HD and compare them to our previously published findings (Brumback et al., 2007; King et al., 2011a; Roche and King, 2010). The main experimental question was whether alcohol responses in a second HD cohort would directly replicate those previously observed in the original HD cohort. If the prior results were reproducible, this would provide confirmation that alcohol-induced increases in stimulation, liking, and wanting during the rising limb and peak breath alcohol content (BrAC), increases in sedation from peak BrAC through the declining limb, and marked performance impairments at peak BrAC characterize the alcohol response phenotype in young adult binge drinkers. Furthermore, in our original cohort, we have found little evidence that FH related to acute response in a combined sample of HD and LD (Brumback et al., 2007; King et al., 2011a; Roche and King, 2010), but we have yet to examine how FH relates to alcohol response in only HD. Therefore, the secondary goal was to examine whether FH affected acute response to alcohol in the original cohort of HD and to then attempt to replicate this pattern of response in a second cohort.
METHODS
Participants & Screening
Participants in the second HD cohort were enrolled between 2009 - 2011 and all study procedures were approved by the University of Chicago Institutional Review Board. Recruitment methods, eligibility determination, and experimental procedures were identical to those employed for the original cohort (2004 – 2006; for details, see King et al., 2011a). In-person screening included written informed consent and completion of several questionnaires to determine demographics, health history, and drinking patterns. Candidates completed the Timeline Follow-Back Interview (Sobell et al., 1979); Quantity Frequency Interview (Cahalan et al., 1969) Beck depression inventory (Beck et al. 1961) and Spielberger Trait Anxiety Inventory (Spielberger et al., 1970) as well as a modified Structured Clinical Interview for DSM-IV (First et al., 2002) to assess lifetime mood, alcohol, and substance use disorders. Standard cut-off thresholds were utilized to exclude subjects with significant major current or past psychiatric illness (i.e., lifetime history of psychotic disorder, alcohol and other substance dependence, or a past year history of other Axis I disorders). Participants also underwent a brief physical examination, urine toxicology screen and pregnancy test (females), and a blood draw for liver enzyme levels.
Inclusion criteria were age of 21-29 years, a body mass index between 19-30 kg/m2, and liver function test within 2 SD of normal limits. In addition, as used in our first cohort, candidates had to meet the study definition of a heavy social drinker [engaging in weekly binge drinking (5 or more drinks for males, 4 for females) 1-5 times/ week] and as defined by both SAMSHA (SAMHSA 2005) and NIAAA (NIAAA 2005). A weekly total drinks criterion (10-40 drinks/week) was included to ensure regular alcohol consumption.
During the screening session, participants completed a family tree of AUD for all primary and secondary biological relatives. If a subject identified a family member as having an AUD, follow-up questions consistent with FH-RDC for drinking consequences were included (Andreasen et al., 1977). Participants were enrolled regardless of FH status. Subjects were defined as FH+ if they reported at least one primary relative or two or more secondary relatives with history of AUD and as FH− if they reported at least two generations without AUD based on estimates of at least half of their secondary relatives. In cohorts 1 and 2, n = 81 (78%) and n = 83 (80%) subjects, respectively, could be identified as either FH+ or FH− (Table 1). Those who were not classified into FH groups reported one secondary relative with AUD, were unable to estimate at least half of secondary relatives, or were adopted.
Table 1.
Participant Characteristics
| Baseline General Characteristics | Cohort 1 (n=104) |
Cohort 2 (n=104) |
|---|---|---|
| Age (years) | 25.28 (0.30) | 24.88 (0.22) |
| Education (years) | 15.70 (0.14) | 15.57 (0.15) |
| Race – Caucasian | 87 (83.6%) | 80 (76.9%) |
| Sex – male | 61 (58.7%) | 65 (62.5%) |
| BMI (kg/m2) | 24.84 (0.30) | 25.04 (0.35) |
| FH+ / FH− | 43 / 38 | 47 / 36 |
|
| ||
| Baseline Alcohol-Related Variables a | ||
|
| ||
| Average drinking days per month | 14.32 (0.51) | 14.79 (0.46) |
| Average standard drinks per drinking day | 5.16 (0.18) | 6.10 (0.27) ** |
| Average binge days per month b | 7.90 (0.33) | 8.93 (0.29) * |
| Maximum # drinks consumed on one occasion | 9.69 (0.40) | 11.25 (0.50) * |
|
| ||
| Breath Alcohol Concentration (mg/L) | ||
|
| ||
| 30 minutes c | 0.083 (0.002) | 0.080 (0.002) |
| 45 minutes | 0.089 (0.002) | 0.087 (0.002) |
| 60 minutes | 0.094 (0.002) | 0.093 (0.002) |
| 120 minutes | 0.075 (0.001) | 0.075 (0.001) |
| 180 minutes | 0.058 (0.001) | 0.058 (0.001) |
Note. Data are Mean (SEM) or N (%)
Drink based on standard definition of one drink = 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor, and monthly average taken from Timeline Follow-Back Interview for the month preceding study enrollment.
Binge defined as ≥5 drinks per occasion for males and ≥4 drinks for females.
Minutes after the initiation of beverage consumption during the high dose session.
p < 0.05,
p < 0.01
Experimental Procedures
Participants completed two 4 - 5 hour randomized sessions in which they received a beverage containing either a placebo (1% alcohol as taste mask) or a high alcohol dose (0.8 g/kg). Sessions took place between 3:00-5:00 PM and were separated by at least 48 hours. Participants were instructed to abstain from alcohol and other drugs (including over the counter medication) for 48 hours and food and cigarettes for 3 hours prior to the experiment. Upon arrival, participants completed a questionnaire of last substance use, and a breathalyzer test (Intoximeters, St. Louis, Missouri, Alco-sensor IV) and a urine test to verify non-pregnancy. Following this, participants consumed a light snack at 20% of daily kilocalorie needs, with 55% of kilocalories from carbohydrates, 10% from protein, and 35% from fat.
Approximately 30 minutes after arrival the participant completed various subjective and objective baseline measures and then consumed their allocated beverage. The beverage consisted of a mixture of the appropriate amount of 190-proof ethanol, water, grape flavored drink mix, and a sucralose-based sugar substitute based upon body weight (women received a 15% smaller dose adjustment to account for differences in body water). The beverages were served in two equal parts in clear, plastic cups with lids and straws to help reduce olfactory cues and consumed within a 15 minute period (5 minutes for each portion with a 5 minute rest in between) in the presence of the research assistant who engaged the participant in light conversation. The study employed the Alternative Substance Paradigm (Conrad et al., 2012) in which minimized expectancy by informing the participant that they would be drinking a beverage that would contain a stimulant, sedative, alcohol, placebo, or a combination of two substances. The participant then completed study measures at 30, 60, 120, and 180 minutes after the initiation of beverage consumption and BrAC was collected at each time point.
To ensure safety, the participant was not allowed to drive after each session and was given a car service or other arranged transportation home. Participants were compensated $50 for each session and given a $50 bonus if they completed both sessions. These procedures were identical to those used with the original cohort, with the exception that the original sample also participated in a third randomized session that included a low dose of alcohol (0.4 g / kg). This dose was subthreshold to produce changes on most subjective and objective measures, so it was not administered in the second cohort. There were six and three participants in the first and second cohorts, respectively, who enrolled but did not complete all sessions, which resulted in N=104 for each cohort.
Experimental Dependent Measures
The measures given were identical to those described in King et al. (2011a), and included the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) and the Drug Effects Questionnaire (DEQ; (Johanson and Uhlenhuth, 1980). The BAES was given at each time point and is a 14-item unipolar questionnaire involving ratings of adjectives describing either the stimulating (i.e. “Energized”, “Excited”, “Up”, etc.) and sedating (i.e. “Sedated”, “Slow Thoughts”, “Sluggish”, etc.) effects of alcohol. The DEQ was given at all post-drink time points and includes several visual analogue scale ratings of post-beverage effects including the extent that the participant liked the beverage effects (“Do you LIKE the effects you are feeling now?”) and wanted more of the beverage (“Would you like MORE of what you consumed right now?”).
Objective measures taken at each time point included salivary cortisol, the Digit-Symbol Substitution Test (DSST; (Wechsler, 1955), and the Grooved Pegboard (Lafeyette Industries, Lafeyette, Indiana). Salivary cortisol was obtained via a plain cotton swab (Sarstedt, Sarstedt, Germany) and later stored in a −80 °C freezer until assayed using a high sensitivity salivary cortisol enzyme immunoassay kit (Salimetrics, State College, PA). The DSST and Grooved Pegboard are psychomotor performance tasks that measure cognitive-motor processing speed and fine motor coordination, respectively, and were previously described in detail in (Brumback et al., 2007).
Finally, at baseline, peak BrAC (60 minute time point), and the 180 minute time point, eye movements were measured and analyzed using the VisualEyes™ VNG system (Micromedical Technologies, Chatham, IL), a non-invasive oculographic device. As previously detailed in (Roche and King 2010), participants performed smooth pursuit, saccade, and anti-saccade tasks, which resulted in dependent measures of gain (smooth pursuit) and velocity, latency, and accuracy (pro- and anti-saccade).
Statistical Analyses
The second cohort of HD was compared to the first cohort on background characteristics, drinking behavior, and BrAC by t-tests, repeated measures ANOVAs, and χ2 tests as appropriate. In order to compare alcohol response between the cohorts, three sets of analyses were made to test whether our original published results were replicated. First, as prior analyses of the original cohort focused on HD vs. LD comparisons, the first cohort data was reanalyzed independently including HD only on all measures using a repeated measures ANOVA with dose (two levels) and time (three to five levels, depending on the dependent measure being examined) entered as within subject factors. Results for the reanalysis are presented in Table 2.
Table 2.
Summary of first and second cohort replication results in HD
| Study |
Outcome
Measure |
Original Cohort (Dose × Time) |
Second Cohort (Dose × Time) |
Between Cohort Comparison (Dose × Time × Cohort) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N a | F | P | f b | N c | F | P | f | F | P | ||
| King et al., 2011a | BAES | ||||||||||
| Stimulation | 104 | 24.5 | < 0.0001 | 0.45 | 104 | 17.2 | < 0.0001 | 0.39 | 1.8 | 0.13 | |
| Sedation | 104 | 17.7 | < 0.0001 | 0.39 | 104 | 8.2 | < 0.0001 | 0.28 | 0.5 | 0.46 | |
| DEQ | |||||||||||
| Like | 104 | 34.5 | < 0.0001 | 0.51 | 104 | 41.4 | < 0.0001 | 0.55 | 0.6 | 0.64 | |
| Want More | 104 | 10.4 | < 0.0001 | 0.31 | 104 | 8.8 | < 0.0001 | 0.29 | 0.5 | 0.72 | |
| Salivary Cortisol | 104 | 0.7 | 0.62 | 0.08 | 103 | 1.0 | 0.42 | 0.1 | 0.2 | 0.94 | |
| Roche and King 2010 | Smooth Pursuit | ||||||||||
| Gain | 75 | 28.4 | < 0.0001 | 0.54 | 103 | 34.7 | < 0.0001 | 0.51 | 0.5 | 0.76 | |
| Pro-saccade | |||||||||||
| Latency | 77 | 47.6 | < 0.0001 | 0.63 | 103 | 35.9 | < 0.0001 | 0.52 | 1.9 | 0.21 | |
| Velocity | 77 | 6.4 | 0.002 | 0.28 | 103 | 23.7 | < 0.0001 | 0.44 | 1.3 | 0.24 | |
| Accuracy | 77 | 4.3 | 0.015 | 0.24 | 103 | 2.3 | 0.107 | 0.15 | 0.2 | 0.87 | |
| Anti-saccade | |||||||||||
| Latency | 77 | 13.4 | < 0.0001 | 0.39 | 100 | 11.6 | < 0.0001 | 0.33 | 0.4 | 0.70 | |
| Velocity | 77 | 4.9 | 0.009 | 0.25 | 100 | 0.6 | 0.527 | 0.08 | 1.6 | 0.21 | |
| Accuracy | 77 | 6.4 | 0.002 | 0.28 | 100 | 4.4 | 0.013 | 0.21 | 0.3 | 0.74 | |
| Brumback et al., 2007 | DSST | 77 | 26.5 | < 0.0001 | 0.52 | 104 | 26.3 | < 0.0001 | 0.46 | 0.5 | 0.72 |
| Pegboard | 77 | 13.7 | < 0.0001 | 0.40 | 104 | 17.6 | < 0.0001 | 0.39 | 1.4 | 0.22 | |
Roche and King 2010 and Brumback et al., 2007 only included subjects who had positive or negative family history of alcohol disorders and, therefore, involved fewer subjects included than King et al., 2007.
Effect size as measured by Cohen’s f.
Eyetracking measures had fewer subjects compared to other measures in both the original and second cohorts due to instrumentation problems.
Next, alcohol response in the new cohort was determined by a repeated measures ANOVA with dose (two levels) and time (three to five levels) entered as within subject factors. Given the large number of comparisons, in order to reduce Type I error a family-wise error correction (Dar et al., 1994) was used to determine significance with α = 0.004 (0.05/14 main dependent measures = 0.004). To examine if alcohol responses differed between cohorts, a final set of repeated measures ANOVAs was also employed for all measures with cohort (first, second) entered as a between-subject factor and dose and time as within-subject factors. For the between-cohort comparison statistical comparison (e.g., dose × time × cohort), α = 0.05 was used due to our concern that a corrected α may be too conservative to detect differences between groups. The results of both analyses are presented in Table 2. In short, replication was supported if the independent analysis of cohort 2 produced a significant dose × time interaction (p ≤ 0.004) in the first model and the cohorts did not significantly differ in the second model 2 (i.e., dose × time × cohort, p > 0.05).
In order to explore whether acute response to alcohol differed by FH status, analyses were repeated with FH entered as a between subject factor. A dose × FH or dose × time × FH interaction was considered significant at the corrected p < 0.004 threshold (Dar et al., 1994) and a difference between cohorts was significant at p < 0.05.
Results
Background Characteristics and Session BrAC
The second cohort was similar to the original cohort on most background characteristics (Table 1) with the exception that the second cohort reported significantly more drinks per drinking day (p < 0.01), binges per month (p < 0.05), and maximum number of drinks in one drinking occasion within the past six months (p < 0.05). Because these drinking variables were highly intercorrelated (r’s > 0.25, p’s < 0.05), binges per month was selected as a covariate to avoid collinearity and included in the analyses comparing cohorts. Alcohol produced a similar BrAC curve in both cohorts (time × cohort. F(1,4) = 1.84, p = 0.12; Table 1). Acute response to alcohol did not significantly differ by sex in either cohort.
Subjective effects and cortisol response
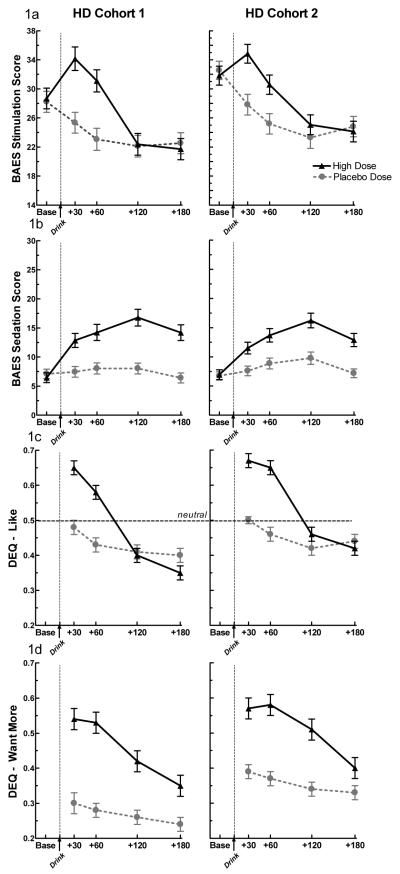
As shown in Table 2, the second cohort responded to alcohol in an identical pattern as the original cohort across all measures. In the second cohort, alcohol significantly increased reports of stimulation (p < 0.0001), liking (p < 0.0001), wanting more (p < 0.0001), and sedation (p < 0.0001) in a limb-specific fashion without affecting cortisol secretion. There were no differences between the cohorts on subjective measures (Table 2 and Figure 1a – c) or salivary cortisol response. These findings provide support for a full replication of all the results presented in King et al. (2011a).
Figure 1. Subjective Measures.
Subjective response to alcohol and placebo in Cohort 1 and Cohort 2 heavy drinkers as measured by (a) BAES Stimulation, (b) BAES Sedation, (c) DEQ Like, & (d) DEQ Want More. Alcohol significantly increased all subjective measures in both cohorts (dose × time, p’s < 0.0001 for both cohorts on stimulation, sedation, like, and want more). The data presented from Cohort 1 was previously published in King et al., (2011a).
Saccadic and Smooth Pursuit Eve Movements
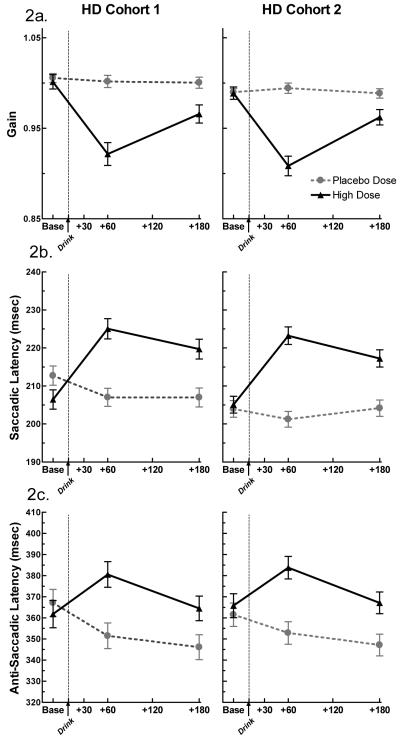
Similar to the original cohort (Table 2), alcohol significantly impaired smooth pursuit gain (p < 0.0001), pro-saccade latency (p < 0.0001) and velocity (p < 0.0001), and anti-saccade latency in the second cohort (p < 0.0001). Impairment was most evident at peak BrAC (60 min) for these measures. While alcohol did marginally affect anti-saccade accuracy in the second cohort (p = 0.013), this effect did not reach the significance threshold after correction for multiple testing and was therefore not considered replicated. In contrast to results observed in the original cohort, alcohol did not significantly decrease pro-saccade accuracy in the second cohort. Anti-saccade velocity was not significantly affected by alcohol in either cohort, though there was a trend in the original cohort (p = 0.009). The second set of analyses directly comparing both cohorts revealed no differences of the seven eye tracking variables (Table 2 and Figure 2a - c). These results provide evidence for a full replication of the majority of the eye movement findings reported in Roche and King (2010).
Figure 2. Eye Tracking Results.
The effect of alcohol and placebo on (a) smooth pursuit gain, (b) pro-saccade latency (msec.), & (c) anti-saccade latency (msec.) in Cohort 1 and Cohort 2 HD. Alcohol significantly decreased smooth pursuit gain and increased pro- and anti-saccade latency in both cohorts (dose × time, p’s < 0.0001 for gain, pro-saccade latency, and anti-saccade latency). The data presented from Cohort 1 was previously published in Roche and King (2010).
Psychomotor Effects
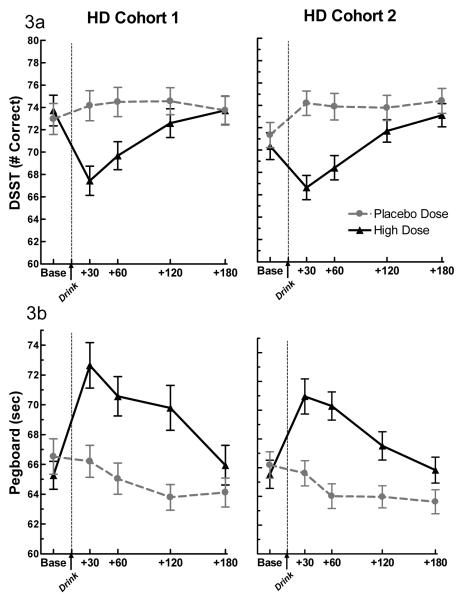
As observed in the original cohort, alcohol, vs. placebo, significantly decreased the number of correct responses on the DSST (p <0.0001) and an increased the time to completion of the Grooved Pegboard (p <0.0001) in the second cohort (Table 2). Both effects were most evident at peak BrAC. In the direct comparison between cohorts, no significant differences were detected on the DSST and Grooved Pegboard (Figure 3a & b). The observed results provide evidence for a full replication of all the findings presented in Brumback et al. (2007).
Figure 3. Performance Measures.
The effect of alcohol and placebo on (a) DSST & (b) Grooved Pegboard performance in Cohort 1 and Cohort 2 heavy drinkers. Alcohol significantly decreased DSST (# correct) and Grooved Pegboard (sec.) performance in both cohorts (dose × time, p’s < 0.0001 for DSST and Pegboard). The data presented from Cohort 1 was previously published in Brumback et al., (2007).
Effects of FH status
Acute response to alcohol in both cohorts did not significantly differ by FH status (Table 3). No measures examined met the corrected p < 0.004 threshold for significant differences in either cohort.
Table 3.
Summary of first and second cohort results based on family history of AUD
|
Outcome
Measure |
Original Cohort (Dose × Time × FH) |
Second Cohort (Dose × Time × FH) |
Between Cohort Comparison (Dose × Time × FH × Cohort) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Na (FH+) | F | P | f b | N (FH+) | F | P | f | F | P | |
| BAES | ||||||||||
| Stimulation | 81 (43) | 0.4 | 0.84 | 0.07 | 83 (47) | 1.0 | 0.41 | 0.11 | 0.5 | 0.77 |
| Sedation | 81 (43) | 0.3 | 0.89 | 0.06 | 83 (47) | 0.3 | 0.85 | 0.07 | 0.1 | 0.99 |
| DEQ | ||||||||||
| Like | 81 (43) | 0.3 | 0.80 | 0.07 | 83 (47) | 0.2 | 0.89 | 0.05 | 0.5 | |
| Want More | 81 (43) | 0.2 | 0.88 | 0.05 | 83 (47) | 0.1 | 0.96 | 0.03 | 0.1 | 0.97 |
| Salivary Cortisol | 81 (43) | 3.5 | 0.008 | 0.21 | 82 (46) | 1.2 | 0.33 | 0.12 | 0.6 | 0.70 |
| Smooth Pursuit | ||||||||||
| Gain | 75 (40) | 4.5 | 0.01 | 0.25 | 83 (47) | 1.7 | 0.19 | 0.14 | 0.3 | 0.75 |
| Pro-saccade | ||||||||||
| Latency | 77 (42) | 0.5 | 0.62 | 0.08 | 82 (47) | 0.8 | 0.48 | 0.10 | 1.2 | 0.30 |
| Velocity | 77 (42) | 1.9 | 0.16 | 0.16 | 82 (47) | 0.1 | 0.90 | 0.04 | 0.7 | 0.51 |
| Accuracy | 77 (42) | 2.8 | 0.06 | 0.20 | 82 (47) | 0.3 | 0.78 | 0.06 | 1.9 | 0.15 |
| Anti-saccade | ||||||||||
| Latency | 77 (42) | 0.6 | 0.57 | 0.09 | 79 (46) | 0 | 0.96 | 0.02 | 0.2 | 0.85 |
| Velocity | 77 (42) | 1.1 | 0.33 | 0.12 | 79 (46) | 1.3 | 0.28 | 0.13 | 0.8 | 0.45 |
| Accuracy | 77 (42) | 3.7 | 0.03 | 0.22 | 79 (46) | 1.8 | 0.18 | 0.15 | 1.1 | 0.32 |
| DSST | 81 (43) | 0.3 | 0.85 | 0.07 | 83 (47) | 1.4 | 0.22 | 0.13 | 0.9 | 0.50 |
| Pegboarda | 81 (43) | 0.4 | 0.79 | 0.07 | 83 (47) | 0.9 | 0.48 | 0.11 | 0.3 | 0.91 |
Number of participants identified as either FH+ or FH−.
Effect size as measured by Cohen’s f
Discussion
In this paper, the majority of our previously reported alcohol response findings across a variety of subjective and objective measures in HD were replicated in an independent second sample. Of the fourteen measures being reported, we were able to fully replicate the results of eleven, including all four subjective effects, cortisol response, and performance impairment measures. These findings confirm that in response to an intoxicating dose of alcohol, HD report escalations in the stimulating and hedonically rewarding effects during rising and at peak BrAC, with increases in sedative effects primarily during declining BrAC. Furthermore, HD are acutely impaired by alcohol on most measures of psychomotor and eye tracking performance at peak BrAC without displaying a discernible cortisol response at any point. As a whole, this acute alcohol response phenotype in HD appears to be robust and reproducible.
In the past, effectively measuring subjective response to alcohol has been difficult due in part to a lack of both psychometrically sound measures of positive response and paradigms that reliably ascertain subjective effects without confounds of invasive measures. Because of this, objective measures were often viewed as more desirable and robust than subjective measures. However, the subjective responses obtained in the current study were highly reproducible between cohorts and demonstrate a biphasic alcohol response in HD, which also conceptually support the results of others (Quinn and Fromme, 2011; Ray et al., 2009; Thomas et al., 2004). We believe that these results are due to employing reliable and valid scales to ascertain both positive (stimulating, liking, wanting) and negative (sedation) responses across the rising and declining limbs of the BAC curve (Martin et al., 1993; Morean et al., 2013). Importantly, because HD’s sensitivity to the stimulating and hedonically rewarding effects of alcohol predict future alcohol problems (King et al., 2011a; King et al., 2013 in press), these results suggest that subjective response to alcohol challenge may be a sensitive, cost-effective, and non-invasive method of identifying individuals at risk for the development of AUD.
Several objective responses to alcohol were also found to be highly reproducible between HD cohorts. Psychometric (Grooved Pegboard and DSST) and eye tracking performance impairment was most evident at peak BrAC in both cohorts, suggesting HD’s cognitive and psychomotor function are impaired at the same time they are reporting highest stimulating and hedonically rewarding effects. While the effect of alcohol on smooth pursuit gain, pro- and anti-saccade latency, and pro-saccade velocity was highly similar between samples, other eye movement measures failed to meet the standards for replication. Anti-saccade accuracy was significantly impaired by alcohol in the original cohort and would have been in the second cohort as well under normal statistical standards (p < 0.05), but did not meet the corrected alpha (p = 0.004) used in the current study. It is not necessarily surprising that pro- and anti-saccade accuracy were either not affected by alcohol or not reproducible in both cohorts, as several studies have failed to find an effect of alcohol on saccade accuracy (Blekher et al. 2002a, b; Lehtinen et al. 1979; Moser et al. 1998; Vassallo and Abel 2002; Vorstius et al. 2008) and HD may display some tolerance on this measure (Roche and King 2010). Therefore, saccade accuracy may not be a sensitive measure for detecting impairment in HD. Complicating the matter, anti-saccade measures had larger variability than pro-saccade measures, which may partially account for the lack of replication for anti-saccade velocity.
Family history status was not significantly related to acute response to alcohol in either cohort. These results support a recent null finding of FH on acute response to an intravenous alcohol challenge in moderate social drinkers (Kerfoot et al., 2013 in press). Although FH has been identified as a predictor of future alcohol-related problems, it is still unclear how it conveys risk. While persons with FH+ have been theorized to be need to drink more to feel the effects of alcohol, support for this relationship in laboratory findings has been inconsistent (Newlin and Thomson 1990; Newlin et al., 2010; Morean and Corbin 2011). That FH status was not related to acute alcohol response in HD may suggest that FH and HD are two risk factors that represent distinct and non-overlapping pathways to early alcohol misuse and eventual AUD (Quinn & Fromme, 2011; King et al., 2011b), or that engaging in frequent binge drinking in late adolescence and young adulthood, regardless of FH status, produces numerous systemic changes that ultimately result in the distinct alcohol response phenotype observed in our two independent samples. Future studies should attempt to clarify the relationship between FH and HD in order to determine whether these risk factors are synergistic or separate, as to better understand how these factors convey risk for AUD.
This study had several strengths, including a large sample size, identical measures and participant characteristics to the first cohort, and measures that were repeatedly collected across the BrAC curve. However, some limitations should also be noted. Because the main purpose of the study was to examine alcohol response in a high-risk population, the second cohort of subjects only included HD. Therefore, it is unclear whether LD’s subjective and objective responses to alcohol are also reproducible. Since this replication study was performed within the same laboratory as the original (though with a different research staff), unintentional experimenter biases may have influenced the results in both studies. Therefore, a direct replication attempt by an outside lab should be performed in order to remove such potential confounds. Finally, as previously described, one notable difference between the two studies was the lack of a third session administering a subthreshold dose of alcohol to participants of the second sample. While we found no significant order effects in our previous papers, future studies should attempt to characterize acute responses to various doses of alcohol in HD, including those resulting in BrAC below, and even exceeding the legal limit for intoxicated driving. The FH results should be interpreted with some caution, as the criteria for meeting FH+ was, by including secondary relatives, somewhat broader than in studies examining children of alcoholics. However, both FH groups were evenly represented in the two cohorts (Table 1), increasing confidence in our negative findings in HD.
In sum, despite these potential limitations, the present study effectively demonstrated that HD have a distinct and reproducible pattern of subjective and objective response to an intoxicating dose of alcohol and provide confirmation of prior findings in an independent sample. As several seminal findings in biological and medical sciences have failed to be replicated (Begley and Ellis 2012; Prinz et al. 2011), the importance of direct replication cannot be overstated.
Acknowledgments
This study was supported by grant R01-AA013746 from the National Institute on Alcohol Abuse and Alcoholism, the University of Chicago Comprehensive Cancer Center (#P30-CA14599), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research (#UL1 RR024999).
References
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–671. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Begley CG, Ellis LM. Drug development: Raise standards for preclinical cancer research. Nature. 2012;483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend. 2007;91:10–17. doi: 10.1016/j.drugalcdep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan D, Cisin IH, Crossley HM. American drinking practices: A national study of drinking behavior and patterns. In: Studies RCoA, editor. Monograph Rutgers Center of Alcohol Studies. New Brunswick, N. J: 1969. [Google Scholar]
- Collaboration OS An open, large-scale, collaborative effort to estimate the reproducibility of psychological science. Perspect Psychol Sci. 2012;7:657–660. doi: 10.1177/1745691612462588. [DOI] [PubMed] [Google Scholar]
- Conrad M, McNamara P, King A. Alternative Substance Paradigm: Effectiveness of Beverage Blinding and Effects on Acute Alcohol Responses. Exp Clin Psychopharmacol. 2012;20:382–389. doi: 10.1037/a0029261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin WR, Scott C, Leeman RF, Fucito LM, Toll BA, O’Malley SS. Early Subjective Response and Acquired Tolerance as Predictors of Alcohol Use and Related Problems in a Clinical Sample. Alcohol Clin Exp Res. 2013 doi: 10.1111/j.1530-0277.2012.01956.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar R, Serlin RC, Omer H. Misuse of statistical tests in three decades of psychotherapy research. J consulting and clinical psychology. 1994;62:75–81. doi: 10.1037//0022-006x.62.1.75. [DOI] [PubMed] [Google Scholar]
- Duranceaux NC, Schuckit MA, Luczak SE, Eng MY, Carr LG, Wall TL. Ethnic differences in level of response to alcohol between Chinese Americans and Korean Americans. J Stud Alcohol Drugs. 2008;69:227–234. doi: 10.15288/jsad.2008.69.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM IV Axis I Disorders, Research version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Ioannidis JP. Why most published research findings are false. PLoS Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Kerfoot K, Pittman B, Ralevski E, Limoncelli D, Koretski J, Newcomb J, Arias AJ, Petrakis IL. Effects of Family History of Alcohol Dependence on the Subjective Response to Alcohol using the Intravenous Alcohol Clamp. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12199. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiatry. 2011a;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Roche DJO, Rueger SY. Subjective responses to alcohol: a paradigm shift may be brewing. Alcohol Clin Exp Res. 2011b;35:1726–8. doi: 10.1111/j.1530-0277.2011.01629.x. [DOI] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin D, Cao D. Rewarding alcohol responses predict future alcohol use disorder and binge drinking frequency: A six-year prospective study. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.08.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koole SL, Lakens D. Rewarding Replications: A Sure and Simple Way to Improve Psychological Science. Perspect Psychol Sci. 2013 doi: 10.1177/1745691612462586. [DOI] [PubMed] [Google Scholar]
- Makel MC, Plucker JA, Hegarty B. Replications in Psychology Research How Often Do They Really Occur? Perspect Psychol Sci. 2012;7:537–542. doi: 10.1177/1745691612460688. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013 doi: 10.1007/s00213-012-2954-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Chronic tolerance and sensitization to alcohol in sons of alcoholics: II. Replication and reanalysis. Exp Clin Psychopharmacol. 1999;7:234–243. doi: 10.1037//1064-1297.7.3.234. [DOI] [PubMed] [Google Scholar]
- NIAAA . Helping patients who drink too much: A clinician’s guide NIH Publication No 05–3769. National Institutes of Health; Bethesda, MD: 2005. [Google Scholar]
- Nosek BA, Spies JR, Motyl M. Scientific Utopia: II. Restructuring Incentives and Practices to Promote Truth Over Publishability. Perspect Psychol Sci. 2012;7:615–631. doi: 10.1177/1745691612459058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashler H, Harris CR. Is the replicability crisis overblown? Three arguments examined. Perspect Psychol Sci. 2012;7:531–536. doi: 10.1177/1745691612463401. [DOI] [PubMed] [Google Scholar]
- Prinz F, Schlange T, Asadullah K. Believe it or not: how much can we rely on published data on potential drug targets? Nat Rev Drug Discov. 2011;10:712–712. doi: 10.1038/nrd3439-c1. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, MacKillop J, Leventhal A, Hutchison KE. Catching the alcohol buzz: an examination of the latent factor structure of subjective intoxication. Alcohol Clin Exp Res. 2009;33:2154–2161. doi: 10.1111/j.1530-0277.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche DJ, King AC. Alcohol impairment of saccadic and smooth pursuit eye movements: impact of risk factors for alcohol dependence. Psychopharmacology (Berl) 2010;212:33–44. doi: 10.1007/s00213-010-1906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger H. Psychology’s woes and a partial cure: The value of replication. APS Observer. 2012;25 [Google Scholar]
- SAMHSA . National survey on drug use and health. Office of Applied Studies; Bethesda, MD: 2005. [Google Scholar]
- Schmidt S. Shall we really do it again? The powerful concept of replication is neglected in the social sciences. Rev Gen Psychol. 2009;13:90–100. [Google Scholar]
- Schuckit MA. Comment on the paper by Quinn and Fromme entitled subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1723–5. doi: 10.1111/j.1530-0277.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. Findings across subgroups regarding the level of response to alcohol as a risk factor for alcohol use disorders: a college population of women and latinos. Alcohol Clin Exp Res. 2004;28:1499–1508. doi: 10.1097/01.alc.0000141814.80716.32. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. J Stud Alcohol. 1980;41:242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behav Res Ther. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Luchene RE. Test Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1970. [Google Scholar]
- Thomas SE, Drobes DJ, Voronin K, Anton RF. Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol. 2004;65:330–335. doi: 10.15288/jsa.2004.65.330. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Psychological Corp.; Oxford: 1955. [Google Scholar]