Abstract
IL-10-competent subset within CD1dhiCD5+ B cells, also known as B10 cells, has been shown to regulate autoimmune diseases. Whether B10 cells can prevent or suppress the development of experimental autoimmune myasthenia gravis (EAMG) has not been studied. In this study, we investigated whether low dose granulocyte macrophage-colony stimulating factor (GM-CSF), which suppresses EAMG, can expand B10 cells in vivo, and whether adoptive transfer of CD1dhiCD5+ B cells would prevent or suppress EAMG. We found that treatment of EAMG mice with low-dose GM-CSF increased the proportion of CD1dhiCD5+ B cells and B10 cells. In vitro co-culture studies revealed that CD1dhiCD5+ B cells altered T cell cytokine profile but did not directly inhibit T cell proliferation. On the other hand, CD1dhiCD5+ B cells inhibited B cell proliferation and its autoantibody production in an IL-10-dependent manner. Adoptive transfer of CD1dhiCD5+ B cells to mice could prevent disease as well as suppress EAMG after disease onset. This was associated with downregulation of mature dendritic cell markers and expansion of regulatory T cells resulting in the suppression of acetylcholine receptor (AChR)-specific T cell and B cell responses. Thus, our data have provided significant insights into the mechanisms underlying the tolerogenic effects of B10 cells in EAMG. These observations suggest that in vivo or in vitro expansion of CD1dhiCD5+ B cells or B10 cells may represent an effective strategy in the treatment of human myasthenia gravis.
Keywords: Autoimmune disease, regulatory B cells, cytokines, acetylcholine receptor, immune tolerance, Tregs
Introduction
Myasthenia Gravis (MG) is a T cell-dependent, B cell-mediated autoimmune disease in which autoantibodies are produced targeting the skeletal muscle acetylcholine receptor (AChR) (1, 2). Experimental autoimmune MG (EAMG) can be induced in mice by immunization with AChR purified from the electric organs of the electric ray, Torpedo californica (3, 4). In both MG and EAMG, anti-AChR antibodies bind to the AChR at the neuromuscular junction, activate complement and accelerate AChR destruction, thus leading to neuromuscular transmission failure and fatigable muscle weakness (5–7).
EAMG has been used to study immune mechanisms and to develop new therapeutic strategies such as the use of granulocyte macrophage-colony stimulating factor (GM-CSF) to enhance tolerance (8–11). GM-CSF is capable of both stimulating the immune response and alternatively suppressing the immune response by favoring the development of immature dendritic cells (DCs) that induce / expand regulatory T cells (Tregs) (12–15). In experimental autoimmune encephalomyelitis (EAE), disease is augmented by local administration of GM-CSF, and is severely impaired in GM-CSF-deficient mice (16–18). In contrast, GM-CSF attenuates the severity of EAMG, which is accompanied by downregulation of AChR-specific T cell and humoral responses, and expansion of antigen-specific CD4+ Tregs (8, 11). Whether GM-CSF also expands other regulatory immune cells such as regulatory B cells or CD8+ Tregs has not been studied.
B cells are generally considered to positively regulate immune responses by producing autoantibodies and play a central role in the pathogenesis of MG. The regulatory role of B cells in autoimmune diseases was first reported by Janeway and colleagues in EAE (19). The existence of regulatory B cells was subsequently confirmed by other investigators (20–24). These studies indicate that, like their T cell counterparts, B cells can be divided into functionally distinct regulatory subsets capable of inducing immune tolerance (20, 25–29). One of the regulatory B cell subsets is the so called IL-10 producing B cells (B10 cells), which comprise 1–3% of splenic B cells in wild-type naive mice and are predominantly found within a phenotypically unique CD1dhiCD5+CD19+subset (20, 23, 30, 31).
The goal of the current study was to investigate the functional properties of CD1dhiCD5+ B cells / B10 cells in EAMG, and whether this regulatory B cell subset can be expanded by GM-CSF. B10 cells can be expanded in vitro by stimulation with LPS for 5 hrs or with CD40 agonists for 48 hrs (32). B10 cell function requires IL-10 expression and IL-21 signaling, as well as CD40 and MHCII interactions (26, 33–37). There is some evidence that susceptible mouse strains such as NOD mice (38–40) and MRL/lpr mice contain greater numbers of B10 cells than C57BL/6 mice (36, 38–42). However, strategies to expand B10 cells to suppress autoimmunity in vivo are limited at this time. Here, we have provided evidence that the expansion of CD1dhiCD5+ B cells / B10 cells by GM-CSF in vivo may represent an effective therapeutic approach to restore tolerance in an antibody-mediated disease like EAMG.
Materials and Methods
Mice and Purification of Torpedo AChR (tAChR)
Eight-week old female C57BL6/J mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice were housed and bred in the Animal Resources Center (ARC) at the University Chicago and were provided food and water ad libitum. All animal use procedures were conducted in strict accordance to the National Institutes of Health and University of Chicago institutional guidelines. AChR was purified from the electric organs of Torpedo californica by affinity chromatography using a conjugate of neurotoxin coupled to agarose, as previously described (9). Purified tAChR was used to induce EAMG and as antigen for in vitro studies of immune responses.
Induction and clinical scoring of EAMG
Eight-week old female C57BL6/J mice were immunized with 20 µg of tAChR/CFA in 100 µl subcutaneously, and boosted with 20 µg of tAChR emulsified in IFA in 100 µl injected in the flanks and tail base every 24–30 days. Mice were observed and scored daily or every other day after the first booster. For clinical examination, mice were evaluated for myasthenic weakness and assigned clinical scores as previously described (8, 9). Clinical weakness was graded as follows: grade 0, mouse with normal posture, muscle strength, and mobility at baseline and after exercise; grade 1, normal at rest but with muscle weakness post-exercise, as shown by a hunchback posture, restricted mobility, and difficulty in raising the head after exercise; grade 2, mild weakness at baseline, which worsens after exercise; grade 3, dehydrated and moribund with moderate weakness at baseline; and grade 4, dead. The evaluator was blinded to treatment status for all clinical evaluations.
GM-CSF treatment and adoptive transfer experiments
For adoptive transfer (AT) experiments, donor mice were immunized with tAChR (20µg of tAChR/CFA in 100 µl subcutaneously followed by one booster at 24–30 days later (day 0) and treatment with GM-CSF (2 µg daily IP for 10 days) or PBS. These donor mice were sacrificed 14 days after GM-CSF treatments (24 days after the booster immunization). Splenic CD19+ B cells were isolated from mice by positive selection using magnetic beads (Miltenyi Biotec, Auburn, CA) with obtained purity ≥ 95%. CD1dhiCD5+ and CD1dloCD5− B cells were purified (95–98%) using a FACSAria flow cytometer (BD Biosciences). After purification, CD1dhiCD5+ B cells (1 × 106) were immediately transferred into appropriate recipient mice by tail vein injection.
All recipient mice received booster immunizations every 24–30 days at least twice after the initial priming immunization with tAChR. The protocols, specifically the timing of AT and booster immunizations for EAMG prevention and suppression studies are summarized in Table S1. Clinical scores were followed daily starting at day 0. All AT experiments were repeated at least twice to ensure reproducibility.
Flow Cytometry
Single cell suspensions of splenocytes were prepared from mice sacrificed upon the completion of the GM-CSF/PBS treatment regimen for flow cytometric analysis. Cells were washed with PBS supplemented with 0.05% BSA, blocked with antiCD16/CD32 Fc-Block (BD PharMingen, San Jose, CA) on ice for 30 min. APC-conjugated anti-CD19, PB-conjugated anti-CD5 and PE-conjugated anti-CD1d (BioLegend), anti-IAb (MHC class II), anti-CD40, anti-CD80, anti-CD86, isotype control antibodies (Abs) (BD PharMingen) were used in flow cytometry and analyzed using FlowJo software (Treestar, Ashland, OR). Mouse regulatory T cell staining kit (w/ PE FoxP3 FJK-16s, FITC CD4, APC CD25 Abs) (eBioscience) was used for intracellular staining for FoxP3 expression. PE-conjugated antibodies against IL-10, IL-17A, and IFN-γ were used for intracellular cytokine staining of T or B cells. For B10 cells, isolated leukocytes or purified cells were resuspended (2 × 106 cells/ml) in complete medium (RPMI 1640 containing 10% FCS, 200 µg/ml penicillin, 200 U/ml streptomycin, 4 mM L-glutamine, and 5 × 10−5 M 2-ME; all from Invitrogen, Carlsbad, CA, USA) in the presence of lipopolysaccharide (LPS) (10 µg/ml; Sigma, St. Louis, MO, USA), phorbol 12-myristate 13 acetate (PMA) (50 ng/ml; BD PharMingen), ionomycin (500 ng/ml; BD PharMingen), and monensin (2 µM; BD PharMingen) in 48-well, flat-bottom plates for 5 h at 37 °C. Cells were fixed and permeabilized using the Cytofix/Cytoperm kit. Permeabilized cells were stained with PE-conjugated anti-IL10 antibody. For intracellular T cell cytokine analysis, the same protocol was used except LPS was omitted.
In vitro cell proliferation assay, antibody production, and cytokine detection
Splenic CD1dhiCD5+ B cells from donor mice in the PBS/EAMG (AChR-immunized mice receiving PBS) and in the GM-CSF/EAMG (AChR immunized mice receiving GM-CSF) groups were isolated and co-cultured with responder T or B cells from immunized mice at 1:1 ratio. For proliferation assay, responder CD4+ or CD19+cells were isolated from mice in untreated EAMG mice using magnetic cell sorting (Miltenyi Biotec, Auburn, CA) and were stained with CFSE at a concentration of 1 µM for 10 min at 37 oC. Cells were washed three times and plated into 96-well, flat-bottom plates at 5 × 105 cells/well. T cell-depleted enriched DCs (1 × 105 cells/well) (also accomplished by magnetic cell sorting) were used as feeder cells in these studies. Cells were stimulated with tAChR (5 µg/ml) for 72 hrs, and then harvested for CFSE dilution studies and intracellular cytokine expression using a FACS analyzer (BD Biosciences). For antibody production, total anti-AChR IgG concentrations in culture supernatants in B cell co-cultures were measured using a mouse IgG enzyme-linked immunosorbent assay (ELISA) set (Bethyl Laboratories, Montgomery, TX), according to the manufacturer’s specifications.
ELISA for mouse serum AChR antibody isotypes
Mice were bled via tail vein at day 0 prior to the adoptive transfer and at the end of study period. Affinity-purified mouse AChR (0.5 µg/ml) was used to coat 96-well microtiter plates (Corning Costar 96 wells plate, eBioscience, San Diego, CA) with 0.1 M carbonate bicarbonate buffer (pH 9.6) overnight at 4 oC. Serum samples diluted 1:5000 in blocking buffer were added and incubated at 37 oC for 90 min. After four washes, horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Caltag Laboratories, Burlingame, MA) or goat anti-mouse IgG2b (GeneTex, CA), diluted in 1:2000 in blocking buffer were added and incubated at 37 oC for 90 min. Subsequently, TMB substrate solution (eBioscience) was added, and color was allowed to develop at room temperature in the dark for 15 min. The reaction was stopped by adding 2 M H2SO4 and absorbance values were measured at a wavelength of 450 nm using a Bio-Rad microplate-reader (model 550). Results were expressed as OD values.
Determination of mouse muscle AChR content
Mice were sacrificed at end of study period and muscle AChR was extracted from limb muscles. Briefly, mouse muscle (20 mg) was homogenized in 4 volumes of Tris buffer (25 mM Tris HCl, 150 mM NaCl, pH 7.2) using a Polytron-equipped homogenizer (Model PT 3000, Kinematica, Littau, Switzerland) on ice. Mechanical homogenization was achieved using a 7 mm tip (generator). Each sample was processed for 1 min. at 10 rpm, and then centrifuged at 100,000 × g for 30 min (4 oC). Supernatants (100 µl) were used to coat 96-well microtiter plates with coating buffer overnight at 4 oC. Torpedo AChR (0.5 µg/ml) with double dilution was coated in the plates as the standard control. Biotin-conjugated alpha-bungarotoxin (Invitrogen, Carlsbad CA) (1 µg/ml) in blocking buffer was added and incubated at 37 oC for 90 min. After four washes, HRP-conjugated streptavidin (0.1 µg/ml) (Invitrogen, Carlsbad CA) in blocking buffer was added and incubated at 37 oC for 90 min. After addition of TMB substrate solution (eBioscience), color was allowed to develop at room temperature in the dark for 15 min. The reaction was stopped as described in the above section. The muscle content (ng/ml) was measured from the OD value according to the standard curve from Torpedo AChR. The percentage of loss of muscle AChR contents from test mice was calculated by comparison with values from control mice (only adjuvant) mice.
Statistical analysis
All statistical analyses were performed using the SPSS software application. Student’s t test and nonparametric tests such as Mann Whitney test were utilized as appropriate. Significance levels were set at p < 0.05. Experiments were repeated at least twice to ensure reproducibility, and results were pooled for statistical analysis. Unless otherwise specified, data are presented as mean ± standard error of the mean (SEM).
Results
GM-CSF treatment reduced clinical severity and expanded CD1dhiCD5+ B cells and B10 cells
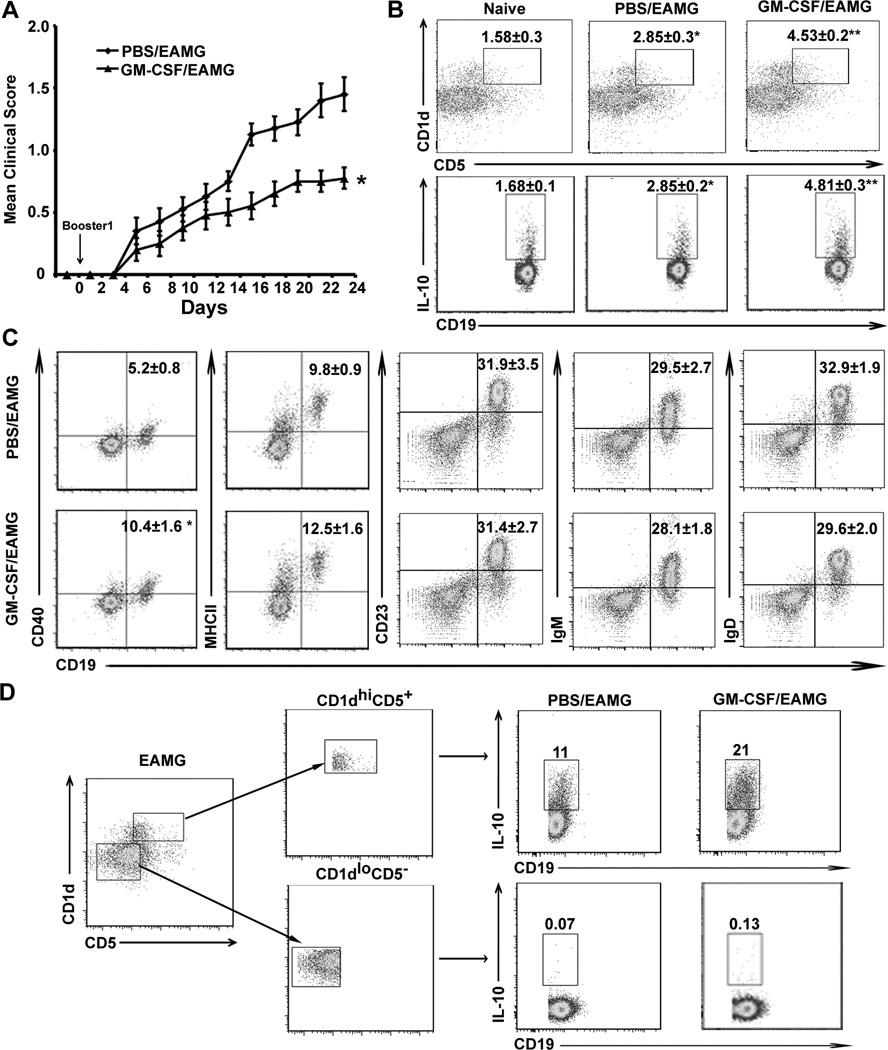
Prior to adoptive transfer studies, we first confirmed that donor EAMG mice receiving GM-CSF (designated as GM-CSF/EAMG group) exhibited less severe disease compared to donor EAMG mice treated with PBS (designated as PBS/EAMG group). Mice (8 weeks old) were treated i.p. with GM-CSF (2 µg daily for 10 days) starting on the day of a first booster immunization with tAChR (designated as Day 0). Animals were studied at Day 24. Figure 1A shows an example of pooled clinical data on donor mice used in prevention studies (Table S1). The effect of GM-CSF on clinical severity in donor EAMG mice was consistent (Fig. S1). Next, we investigated whether GM-CSF treatment could effectively expand B10 cells. The percentages of CD1dhiCD5+ and IL-10+ cells among the CD19+ B cells were increased to a greater extent in the spleen of EAMG animals that received GM-CSF compared to PBS/EAMG group and naïve mice (Fig. 1B). We also studied the expression of B cell markers (IgM, IgD) and surface molecules such as MHCII, co-stimulatory molecules (CD80, CD86), CD40 and CD23 in CD19+ B cells from GM-CSF-treated and PBS-treated EAMG animals by flow cytometric analysis (Fig. 1C). There was no difference in the percentage or the absolute number of total CD19+ cells, IgM+ B cells, IgD+ B cells or CD23+ B cells between the PBS/EAMG group and GM-CSF/EAMG group (Fig. 1C, Table S2). The expression of CD40 was significantly increased in GM-CSF/EAMG group, but the expression of MHCII, CD80 and CD86 of CD19+ was not different from PBS/ EAMG mice. We confirmed that GM-CSF expanded B10 cells were predominantly found within the sorted CD1dhiCD5+ B cell subset, but not detected within the sorted CD1dloCD5− B cell subset (Fig. 1D). These results indicate that GM-CSF can expand B10 cells in vivo.
Figure 1. Effects of GM-CSF treatment on the clinical severity and B cell subsets in donor EAMG mice.
A) Mean clinical score of donor EAMG mice during days 0–24. Day 0 corresponds to initiation of treatment (GM-CSF vs. PBS) for 10 days at the time of booster immunization. *p < 0.0002 starting at day 16 (n = 20). B) Percentage of CD1dhiCD5+ B cells within CD19+ B cells and IL10+ CD19+ cells (B10 cells) analyzed at day 24. Splenocytes were stimulated with PMA, ionomycin, monensin and LPS (PIM+L) for 5 hours prior to staining for cell surface CD19 and cytoplasmic IL-10 expression. Values within scatterplots represent mean (±SEM) percentages of CD1dhiCD5+ and IL-10+ B cells. *p < 0.02; **p < 0.0004 (a), p < 0.0001 (b) for CD1dhiCD5+ B cells and *p < 0.0007; **p < 0.000006 (a), p < 0.0005 (b) for B10 cells (n = 6). Data from PBS/EAMG were compared to naïve group, while data from GM-CSF/EAMG group were analyzed statistically vs. naïve group (a) and vs. PBS/EAMG (b). C) Expression of B cell markers IgM, IgD and surface molecules MHCII, CD40, and CD23 on CD19+ B cells isolated at day 24. Values in the scatterplots represent % positive cells (mean ± SEM) (n = 6). *p < 0.002 for CD40. D). Representative scatterplots showing that B10 cells are predominantly found within the CD1dhiCD5+ B cell subsets isolated from PBS/EAMG and GM-CSF/ EAMG mice. CD1dhiCD5+CD19+ and CD1dloCD5−CD19+ B cells were isolated from spleens of 5 mice each and sorted by FACS. Values in the scatterplots represent % positive cells from one representative experiment.
Effect of CD1dhiCD5+ B cells on T cell proliferation, Th cytokine profile and B cell proliferation in vitro
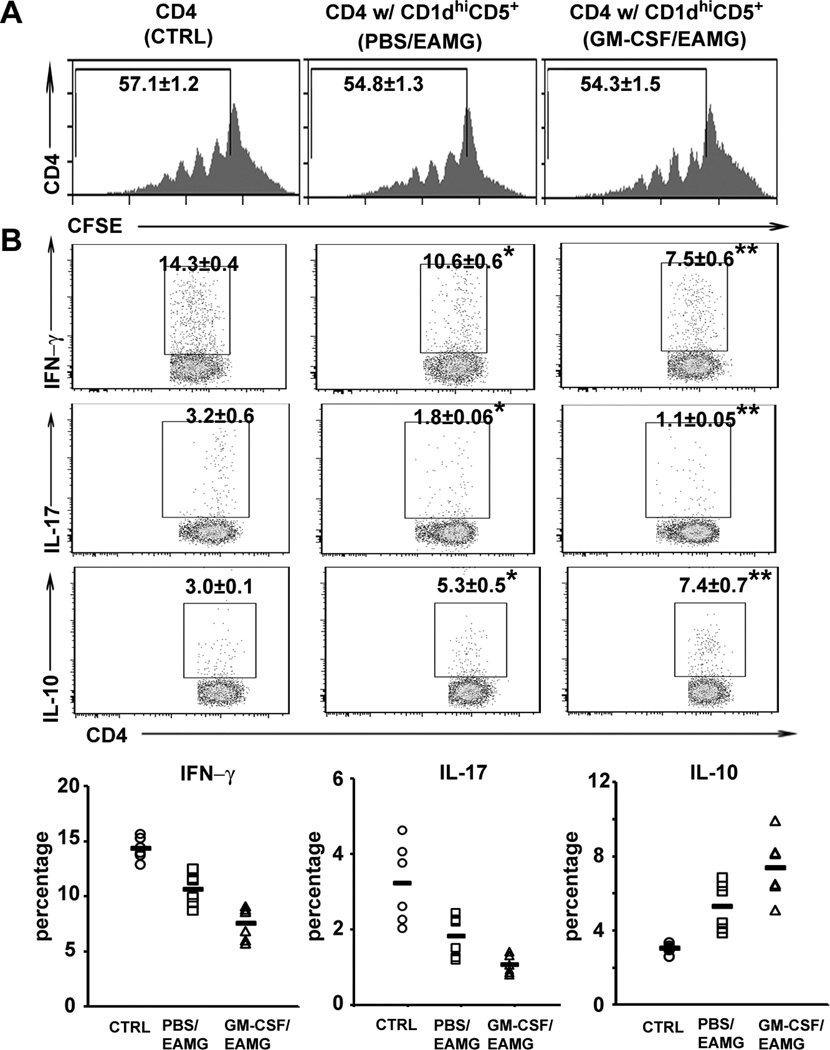
To investigate the functional properties of sorted CD1dhiCD5+ B cells, we examined their effects on in vitro T cell proliferation and cytokine response in the presence of AChR (5 µg/ml). We found that T cells co-cultured with CD1dhiCD5−, CD1dloCD5+ and CD1dloCD5− in vitro did not show any difference compared with T cells alone (n = 3, data not shown). As shown in Fig. 2A, GM-CSF/EAMG-expanded CD1dhiCD5+ B cells did not inhibit antigen-specific T cell proliferation in co-cultures compared to those sorted from PBS/EAMG group, or T cells alone (control). However, CD4 T cell cytokine profile was altered by sorted CD1dhiCD5+ B cells, resulting in decreased Th1 and Th17 CD4+ T cells and increased IL-10+ CD4+ T cells (Fig. 2B). CD1dhiCD5+ B cells from GM-CSF/EAMG mice exhibited a more potent modulatory effect on Th cytokine profile than those isolated from PBS/EAMG mice.
Figure 2. CD1dhiCD5+ B cells alter the cytokine profile, but not T cell proliferation in vitro.
A) Histograms showing T cell proliferative response to AChR stimulation, as determined by % cells with CSFE dilution. Responder CD4+ cells (CFSE-labeled) from EAMG mice were cultured alone [control (CTRL)] or with CD1dhiCD5+ B cells (1:1 ratio) from GM-CSF-treated or PBS-treated EAMG mice (as indicated in parenthesis) for 3 days with AChR (5 µg/ml) (n = 6, p > 0.05). B) Changes in cytokine profile corresponding to panel A experiments. A decrease in %Th1 and %Th17 cells and an increase in % IL-10+ T cells were induced by co-culture with CD1dhiCD5+ B cells for 3 days. For Th1 cells, *p < 0.0005; **p < 0.000004 (a), p < 0.005 (b); for Th17 cells, *p < 0.05; **p < 0.006 (a), p < 0.02 (b); for IL-10+ T cells, *p < 0.001; **p < 0.0001 (a), p < 0.04 (b); n = 6 each. The percentage of Th1, Th17 and IL-10+ T cells for each mouse was displayed in the lower panels. Data from PBS/EAMG were compared to CTRL, while data from GM-CSF/EAMG group were analyzed statistically vs. CTRL (a) and vs. PBS/EAMG (b) for this figure and subsequent figures unless otherwise specified.
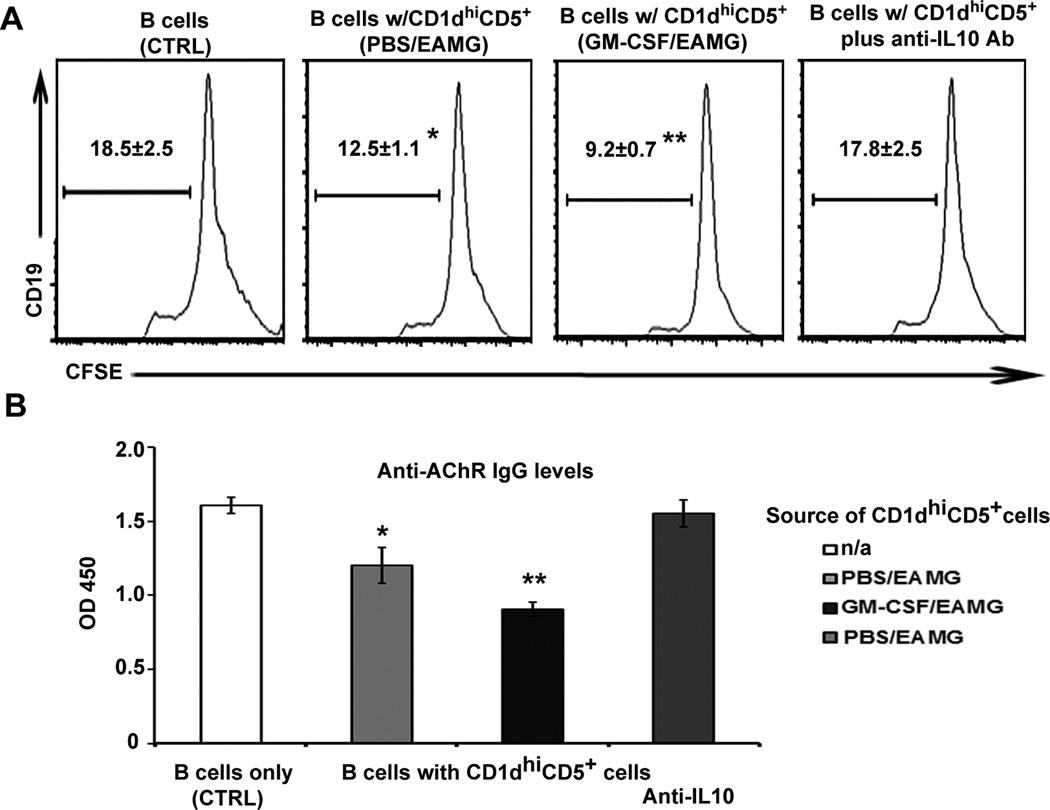
In contrast to the lack of effect of sorted CD1dhiCD5+ B cells on T cell proliferation, we found that CD1dhiCD5+ B cells attenuated B cell proliferation (also 1:1 ratio for 72 hrs). The extent of inhibition of B cell proliferation was greater with CD1dhiC5+ cells sorted from GM-CSF/EAMG mice than those from PBS/EAMG mice, and can be prevented by neutralizing anti-IL-10 Ab (20 µg/ml) (Fig. 3A). In addition, the production of anti-AChR IgG was reduced by CD1dhiCD5+ B cells from GM-CSF/EAMG and PBS/EAMG mice compared to B cells alone (Fig. 3B). Thus, CD1dhiCD5+ B cells regulate B cell function in vitro through IL-10 production.
Figure 3. Suppression of B cell proliferation and Ab production by CD1d hiCD5+ B cells via IL-10 in vitro.
A) Histograms of CFSE fluorescence. Responder CD19+ B cells labeled with CFSE were co-cultured with CD19+CD1dhiCD5+ cells from GM-CSF-treated and PBS-treated EAMG mice at 1:1 ratio in the presence or absence of neutralizing anti-IL-10 Ab. The percentage of B cells with diluted CFSE is indicated in the histograms. *p < 0.05; **p < 0.005 (a), p < 0.03 (b) (n = 6). B) CD19+CD1dhi B cells attenuated the production of anti-AChR Ab production expressed as OD450 levels, which was reversed by neutralizing anti-IL-10 Ab. *p < 0.01; **p < 0.002 (a), p < 0.04 (b) (n = 6).
GM-CSF/EAMG-expanded CD1dhiCD5+ B cells prevented the development of EAMG upon adoptive transfer (AT)
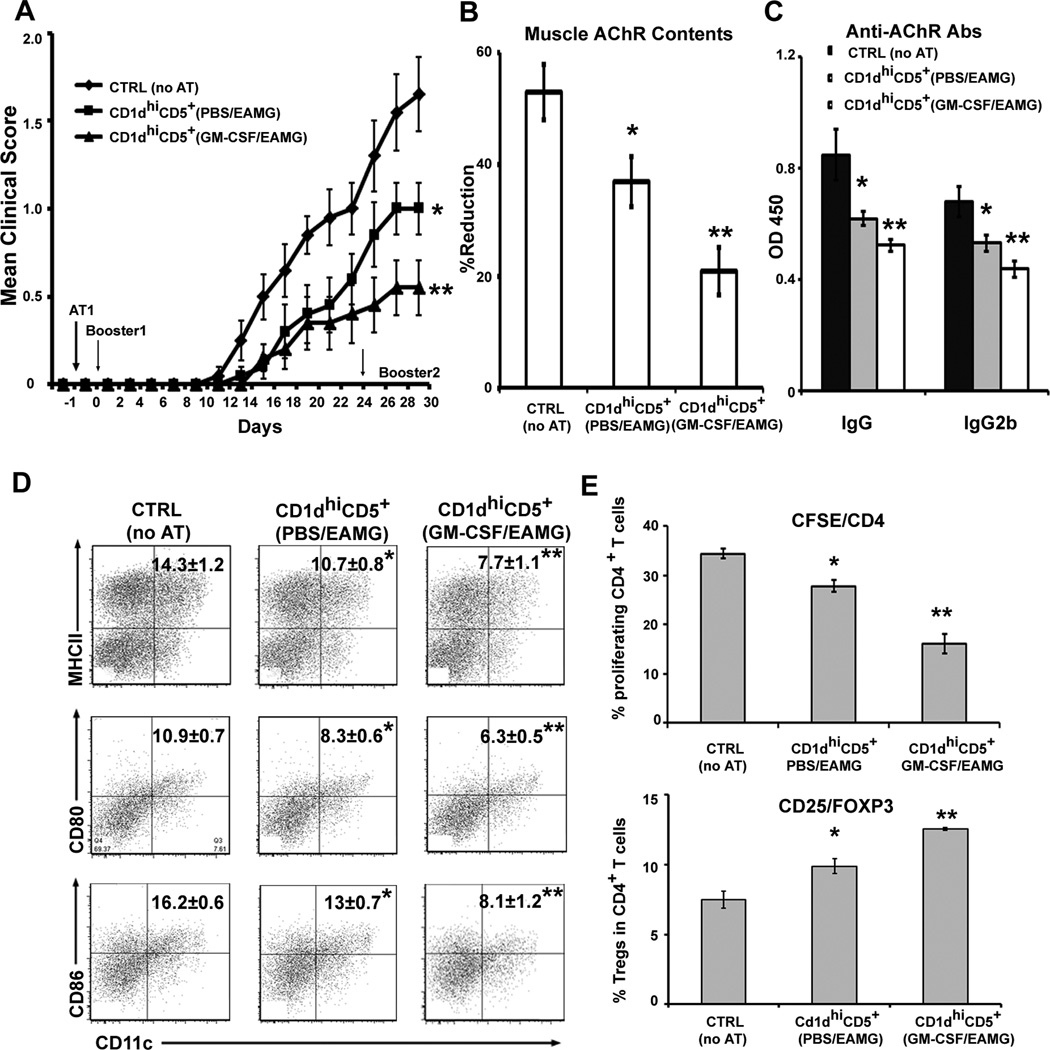
In view of the above in vitro findings, we proceeded to examine the in vivo preventive and suppressive effects of GM-CSF/ EAMG-expanded CD1dhiCD5+ B cells in AT experiments (Table S1). For prevention studies, AT was performed one day prior to first booster (day 0). Recipient animals were divided into three groups of 10 mice each: 1) ctrl group: no AT; animals received i.v. PBS; 2) AT with CD1dhiCD5+ B cells from donor PBS/EAMG mice; 3) AT with CD1dhiCD5+ B cells from donor GM-CSF/EAMG mice. The clinical severity was expressed as mean clinical score. As shown in Fig. 4A, mice receiving CD1dhiCD5+ B cells developed less severe disease than the ctrl group. In addition, CD1dhiCD5+ B cells from GM-CSF/EAMG mice had a more potent effect than those from PBS/EAMG mice. This was accompanied by preservation of muscle AChR contents, and decreased serum levels of anti-AChR Abs, as measured by ELISA (Fig. 4B, C).
Figure 4. Adoptive transfer (AT) of in vivo expanded CD19+CD1d hi B cells prior to immunization: clinical and immunological effects.
A) Clinical severity. Recipient mice were injected with 1 × 106 of CD1dhi CD5+ B cells from GM-CSF-treated or PBS treated donor EAMG group. Results are pooled from two independent experiments (n = 10, *p < 0.02; **p < 0.01 (a), p < 0.02 (b). Day -1 corresponds to the initiation of AT. Booster immunization was performed at day 0 and at day 24. Animals were sacrificed on day 30. B) Attenuated loss of muscle AChR contents by AT of CD1dhi CD5+ B cells. *p < 0.02; **p < 0.001 (a), p < 0.01 (b) (n = 10). C) Decreased serum anti-AChR Ab production by AT of CD1dhi CD5+ B cells. For IgG, *p < 0.04; **p < 0.006 (a), p < 0.01 (b); for IgG2b, *p < 0.03; **p < 0.001(a), p< 0.04 (b) (n = 10). D) Induction of tolerogenic state in DCs by AT of CD1d hiCD5+ B cells. A lower percentage of MHCII+, CD80+, and CD86+ DCs cells was observed in recipient mice. For MHCII, *p < 0.05; **p < 0.003 (a), p < 0.05 (b); for CD80, *p < 0.02; **p < 0.0008 (a), p < 0.04 (b); for CD86, *p < 0.008; **p < 0.0002 (a), p < 0.007 (b). E) Decreased T cell proliferation and increased percentage of Tregs in recipient mice. For T cell proliferation, *p < 0.002; **p < 0.00001 (a), p < 0.0006 (b); for Tregs, *p < 0.02; **p < 0.00001(a), p < 0.0007 (b). Data were derived from 6 animals in figure 4D & E.
To investigate possible mechanisms for the enhanced potency of GM-CSF/EAMG-expanded CD1dhiCD5+ B cells in vivo, we examined the immunophenotypic properties of splenic DCs, CD4+ T cell proliferative and cytokine response, and % CD4+ Tregs (CD25+Foxp3+) from all three groups of recipient mice. DCs from animals that had received AT of CD1dhiCD5+ B cells showed significant lower expression of MHCII, CD80 and CD86, which was more dramatic when donor EAMG animals were treated with GM-CSF than with PBS (Fig. 4D). Therefore, AT of CD1dhiCD5+ B cells led to altered DC phenotype from pathogenic to tolerogenic state. We also found that splenic CD4+ T cell proliferation was decreased by AT of CD1dhiCD5+ B cells, more significant when isolated from GM-CSF/EAMG group than from PBS/EAMG group. This was accompanied by corresponding increase in the percentage and absolute numbers of splenic CD4+ Tregs (Fig. 4E, Table S3).
GM-CSF/EAMG-expanded CD1dhiCD5+ B cells can suppress established EAMG
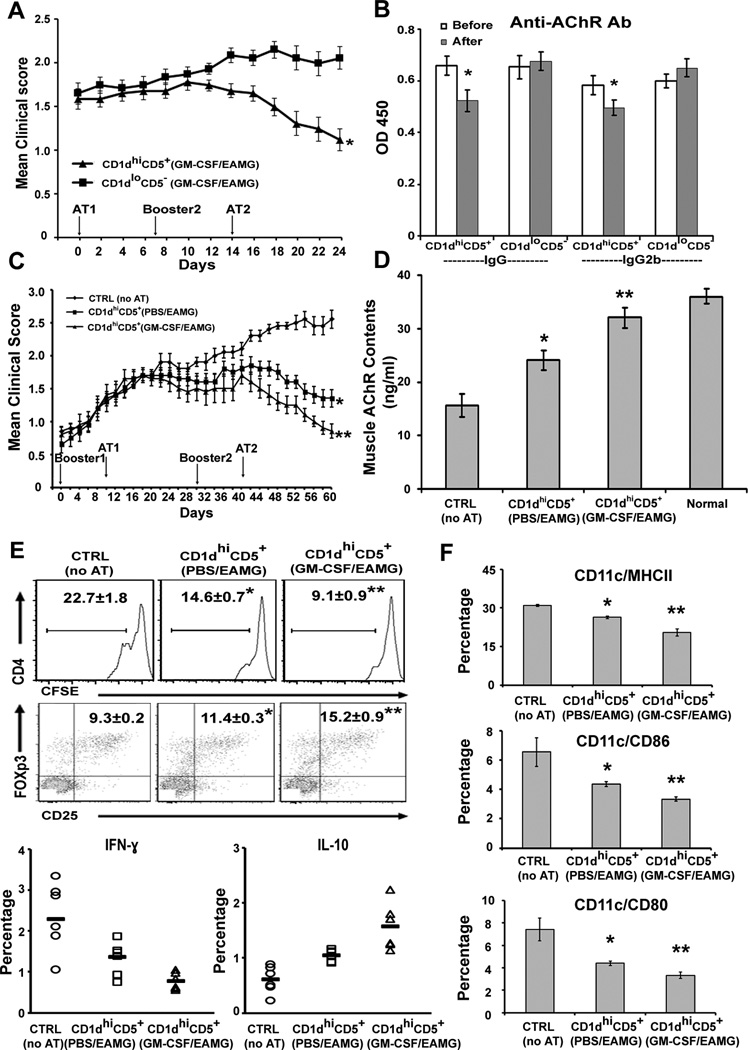
To investigate possible therapeutic effect of CD1dhiCD5+ B cells on established EAMG, we first compared the effect of CD1dhiCD5+ B cells and CD1dloCD5− B cells from GM-CSF-treated donor EAMG mice. Recipient mice received first AT at Day 0 (21 days after first booster), second booster at Day 7 and second AT at Day 14 (n = 16 each). The average clinical score was 1.6 ± 0.08 just prior to the first AT. The clinical severity of recipient EAMG mice was reduced after receiving 1 × 106 CD1dhiCD5+ B cells, but not after receiving CD1dloCD5− B cells (Fig. 5A). This was accompanied by reduction of serum mouse anti-AChR total IgG and IgG2b levels in mice receiving CD1dhiCD5+ B cells, but not in those receiving CD1dloCD5− B cells (Fig. 5B).
Figure 5. Adoptive transfer of in vivo expanded CD1d hiCD5+ B cells after onset of EAMG.
A) Amelioration of established EAMG by AT of CD1dhiCD5+ B cells, but not by AT of CD1dloCD5− B cells from GM-CSF/EAMG mice. Day 0 corresponds to first AT (AT1), which was 21 d post booster1; day 7: booster2; day 14: second AT (AT2). *p < 0.05 (n = 16). B) Reduction in serum anti-AChR Ab levels corresponding to experiments shown in A. *p < 0.05 (n = 10). C) Clinical score showing more potent suppressive effect of GM-CSF-expanded CD1dhiCD5+ B cells than those from PBS/EAMG mice. *p < 0.05, **p < 0.03 (a); p < 0.05 (b). Results are pooled from two separate experiments (n = 10). Day 0 corresponds to booster1; day 10: AT1; day30: booster2; day 40: AT2. D) Muscle AChR contents (ng/ml). AChR content was more significantly preserved in mice receiving CD1dhiCD5+ from GM-CSF/EAMG donor mice compared with mice receiving CD1dhiCD5+ from PBS/EAMG and CTRL (no AT), *p < 0.008; **p < 0.00002 (a), p < 0.007 (b) (n = 10). E) Effect of AT of CD1dhiCD5+ B cells on T cell proliferation, Tregs and cytokine profile. For T cell proliferation, *p < 0.002; **p < 0.00005 (a), p < 0.0006 (b); for Tregs, *p < 0.003; **p < 0.0007 (a), p < 0.004 (b). For Th1 and IL-10+ T cells, p < 0.05 comparing PBS/EAMG vs CTRL; p < 0.01 comparing GM-CSF/EAMG vs CTRL, p < 0.03 comparing GM-CSF/EAMG vs PBS/EAMG. F) Effect of AT of CD1dhiCD5+ B cells on DCs. For MHCII, *p < 0.002; **p < 0.001 (a), p < 0.002 (b); for CD86, *p < 0.04; **p <0.02 (a), p < 0.001 (b); for CD80, *p < 0.02; **p <0.001 (a), p < 0.01 (b). N = 6 for data in figure 5E & F.
Next, we compared the suppressive efficacy of GM-CSF/EAMG-expanded CD1dhiCD5+ B cells vs those isolated from PBS/EAMG mice on established EAMG. Three groups of recipient EAMG mice (n = 10 each) received booster injections at day 0 and day 30, with AT of CD1dhiCD5+ B cells (1 × 106) from donor EAMG mice performed at day 10 and day 40 (Table S1). The average clinical score was 1.4 ± 0.07 just prior to first AT. GM-CSF-expanded CD1dhiCD5+ B cells exhibited more potent suppressive action compared to those isolated from donor PBS/EAMG mice (Fig. 5C). The suppressive effect of CD1dhiCD5+ B cells was first detected on day 30 and maintained throughout the course of EAMG. There was a corresponding attenuation of loss of muscle AChR contents (Fig. 5D).
Flow cytometric studies on CD4+ T cells were performed at the end of study period (Day 60). CD4+ T cell proliferation and the percentage of Th1 cells were reduced by AT of CD1dhiCD5+ B cells from both donor groups, but the effect was greater with GM-CSF- expanded CD1dhiCD5+ B cells than those from PBS/EAMG mice (Fig 5E). Conversely, the percentages of IL-10+ CD4+ T cells and the proportion of Tregs were increased by AT of CD1dhiCD5+ B cells, which was more dramatic when expanded by GM-CSF in vivo. Effect on absolute numbers of proliferating CD4+ T cells and Tregs is summarized (Table S3). Studies on DC markers showed a decrease in the percentage of MHCII+, CD80+, and CD86+ cells (Fig. 5F). Overall, the consequence of AT of CD1dhiCD5+ B cells on immune function was similar in the prevention and suppression studies.
Discussion
One of the best characterized autoantibody-mediated diseases is myasthenia gravis where effector mechanisms mediated by anti-AChR Abs have been well-elucidated, but the triggering factors and regulatory mechanisms remain incompletely understood. Numerous T cell and B cell subpopulations have now been shown to exhibit regulatory activity. It is recognized that regulatory B cells (Bregs) are phenotypically diverse, though most recent studies have been focused on a rare IL-10-competent B cell subset found within CD19+ CD1dhiCD5+ population (20, 21, 26, 32, 37, 42, 43). Ag-specific B cell receptor signaling, CD40 ligation, Toll-like receptor (TLR) and B cell activating factor (BAFF) are crucial to the development and induction of B10 cells (32, 42, 44–48). Studies on B10 cells are limited to some extent by lack of a specific marker or master regulator, by low abundance in vivo and by paucity of defined methods to expand the B10 cells for adoptive cell therapy strategies. In this study, we found that treatment of EAMG mice with GM-CSF led to expansion of CD1dhiCD5+ B cell subset and B10 cells in the spleen of these animals. This was associated with an increase in the percentage of CD40+ B cells. It is possible that treatment with GM-CSF leads to an expansion of B10 progenitor cells, which mature to B10 cells through CD40 ligation.
Data from co-cultures revealed that CD1dhiCD5+ B cells from EAMG mice regulate the immune response by suppressing Th1 response without affecting Ag-specific T cell proliferation. Perhaps more importantly, they significantly attenuated B cell proliferation and production of anti-AChR Abs via an IL-10-dependent mechanism. GM-CSF treatment led to enhanced regulatory function of CD1dhiCD5+ B cells, most likely due to increased B10 cells within this cell population. While many B cell subsets have the capacity to produce IL-10 upon binding of TLR ligands, B10 cells are the major B cell source of IL-10 (20). Our in vitro findings are consistent with known anti-inflammatory actions of IL-10 such as downregulation of Th1 and Th17 responses, and suppression of activation and function of monocytes/macrophages (49–52). However, IL-10 has also been reported to exert immunostimulatory properties on human B cells resulting in enhanced proliferation and differentiation into Ab-secreting cells (53, 54). Under our experimental conditions, the immunoregulatory action of IL-10 predominates. Aside from B10 cells, IL-10 is produced by other immune cells, including DCs, T cells (Th1, Th2, Th3, Tregs, Tr1), NK cells, macrophages (52). IL-10 deficiency often leads to development of enterocolitis, and aggravates autoimmune pathology in many animal models such as EAE and experimental autoimmune neuritis (EAN) (49, 55, 56).
Experimental autoimmune disease is often worse in the absence of B10 cells and other Bregs, as occurs when B cells are depleted in contact hypersensitivity and in EAE (31, 35). B10 cells regulate autoimmunity in an Ag-restricted manner, implying a requirement for Ag-specific BCR signaling in addition to CD40 engagement (29, 31, 35, 45, 47). In EAE, B10 cells predominantly control disease initiation, whereas Tregs inhibit the late-phase of disease (57). While adoptive transfer of B10 cells at the time of induction of autoimmune disease has been shown to ameliorate disease severity in experimental models, this strategy has been less successful in suppressing established, chronic autoimmune diseases (23, 58–61).
We found that adoptive transfer of GM-CSF/EAMG-expanded B10 cells is an effective therapeutic approach in EAMG, in that it not only prevents but can also suppress established disease. This is associated with preservation of muscle AChR contents, and reduction in circulating levels of anti-AChR antibodies. A less potent effect was observed using B10 cells isolated from PBS/EAMG mice, which correlates with our in vitro findings on Ag-specific T cell and B cell responses. Note that EAMG has been shown to be aggravated by IL-10 administration and alleviated in IL-10 knockout mice (62, 63). Possible interpretations include: 1) IL-10-independent mechanisms (e.g. cell-cell contact) contribute to the beneficial effect of B10 cells in EAMG; 2). Cell-based targeted delivery of IL-10 to a specific anatomic site or during a specific time window is necessary to suppress disease severity or promote recovery in EAMG and other disease models.
In parallel to the clinical severity, the expression of MHCII, CD80 and CD86 on DCs cells was decreased and the frequency of CD4+ Tregs was increased by the adoptive transfer of CD1dhiCD5+ B cells in EAMG. These results indicate that B10 cells induce a tolerogenic state, and that the regulatory function of DCs and Tregs cells plays a critical role during the disease initiation and progression of EAMG. Consistent with our findings, B10 cells have been shown to regulate the Ag-presenting capability of DCs in vitro (57). We found that CD1dhiCD5+ B cells attenuated AChR-specific T cell proliferation in vivo but not in vitro. The apparent discrepancy of these findings may be due to: 1) B10 cells act indirectly via other cell types that are not present under our in vitro conditions; or 2) cytokine microenvironment differs in vivo from in vitro conditions. Our data also suggest that B10 cells may be important in the generation or maintenance of Tregs, similar to findings by other investigators (64–67). However, reports arguing against this concept also exist (23, 68).
In summary, we found that GM-CSF-expanded CD1dhiCD5+ B cells play a crucial role in the maintenance of immune homeostasis against self-antigens in EAMG. The protective effect of GM-CSF in EAMG has been previously postulated to involve mobilization of semi-mature or tolerogenic CD8α− DCs from bone marrow, which promotes the expansion of Tregs (8, 11, 69). We have now added another mechanism underlying the suppressive action of GM-CSF in EAMG, that is, by expansion of CD1dhiCD5+ B cell subset, which suppresses the immune response against AChRs. Interestingly, a fusokine (GM-CSF fused with IL-15) has been reported to expand B10 cells in vitro (70). That our findings are relevant to human MG is supported by data from two studies. One recent study found that MG patients had fewer B10 cells than controls, which correlated with disease activity and responsiveness to rituximab therapy (71). A case study had shown that treatment with GM-CSF was associated with clinical improvement, and expansion of circulating Tregs in a MG patient (72). Therefore, it appears feasible to translate our experimental findings to the clinical setting in human MG. Furthermore, autologous B10 cells can be expanded in vitro for subsequent use as cellular immunotherapy of MG.
Supplementary Material
Acknowledgements
We would like to thank Dr. M. Meriggioli and Dr. B. Prabhakar for their advice during the early stages of this project.
This work was supported by the Muscular Dystrophy Association (MDA 157286 and MDA 234223) to JRS, and by the NIH NINDS RO1 NS064059 to BS.
Abbreviations used in this paper
- MG
myasthenia gravis
- EAMG
experimental autoimmune myasthenia gravis
- Breg
regulatory B cell
- DC
dendritic cell
- AChR
acetylcholine receptor
- Treg
regulatory T cell
- Foxp3
forkhead box p3.
Footnotes
Disclosure
The authors have no financial conflict of interest.
References
- 1.Le Panse R, Berrih-Aknin S. Autoimmune myasthenia gravis: autoantibody mechanisms and new developments on immune regulation. Curr Opin Neurol. 2013;26:569–576. doi: 10.1097/WCO.0b013e328364d6cd. [DOI] [PubMed] [Google Scholar]
- 2.Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B, Goluszko E, Huda R, Tuzun E, Christadoss P. Experimental autoimmune myasthenia gravis in the mouse. Curr Protoc Immunol. 2013;Chapter 15(Unit 15):18. doi: 10.1002/0471142735.im1508s100. [DOI] [PubMed] [Google Scholar]
- 4.Christadoss P, Poussin M, Deng C. Animal models of myasthenia gravis. Clin Immunol. 2000;94:75–87. doi: 10.1006/clim.1999.4807. [DOI] [PubMed] [Google Scholar]
- 5.Cossins J, Belaya K, Zoltowska K, Koneczny I, Maxwell S, Jacobson L, Leite MI, Waters P, Vincent A, Beeson D. The search for new antigenic targets in myasthenia gravis. Ann N Y Acad Sci. 2012;1275:123–128. doi: 10.1111/j.1749-6632.2012.06833.x. [DOI] [PubMed] [Google Scholar]
- 6.Vincent A, Leite MI, Farrugia ME, Jacob S, Viegas S, Shiraishi H, Benveniste O, Morgan BP, Hilton-Jones D, Newsom-Davis J, Beeson D, Willcox N. Myasthenia gravis seronegative for acetylcholine receptor antibodies. Ann N Y Acad Sci. 2008;1132:84–92. doi: 10.1196/annals.1405.020. [DOI] [PubMed] [Google Scholar]
- 7.Vincent A. Immunology of disorders of neuromuscular transmission. Acta Neurol Scand Suppl. 2006;183:1–7. doi: 10.1111/j.1600-0404.2006.00605.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheng JR, Li LC, Ganesh BB, Prabhakar BS, Meriggioli MN. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia gravis. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheng JR, Li L, Ganesh BB, Vasu C, Prabhakar BS, Meriggioli MN. Suppression of experimental autoimmune myasthenia gravis by granulocyte-macrophage colony-stimulating factor is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 10.Meriggioli MN, Sheng JR, Li L, Prabhakar BS. Strategies for treating autoimmunity: novel insights from experimental myasthenia gravis. Ann N Y Acad Sci. 2008;1132:276–282. doi: 10.1196/annals.1405.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240–241:65–73. doi: 10.1016/j.jneuroim.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Keeffe M, Hochrein H, Vremec D, Pooley J, Evans R, Woulfe S, Shortman K. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood. 2002;99:2122–2130. doi: 10.1182/blood.v99.6.2122. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton JA, Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. 2013;34:81–89. doi: 10.1016/j.it.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–232. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 15.Pulendran B, Banchereau J, Burkeholder S, Kraus E, Guinet E, Chalouni C, Caron D, Maliszewski C, Davoust J, Fay J, Palucka K. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J Immunol. 2000;165:566–572. doi: 10.4049/jimmunol.165.1.566. [DOI] [PubMed] [Google Scholar]
- 16.Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 17.McQualter JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marusic S, Miyashiro JS, Douhan J, 3rd, Konz RF, Xuan D, Pelker JW, Ling V, Leonard JP, Jacobs KA. Local delivery of granulocyte macrophage colony-stimulating factor by retrovirally transduced antigen-specific T cells leads to severe, chronic experimental autoimmune encephalomyelitis in mice. Neurosci Lett. 2002;332:185–189. doi: 10.1016/s0304-3940(02)00947-3. [DOI] [PubMed] [Google Scholar]
- 19.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 21.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 22.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T. [Regulatory B cell and autoimmune disease] Nihon Rinsho Meneki Gakkai Kaishi. 2010;33:234–241. doi: 10.2177/jsci.33.234. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine S, Morva A, Youinou P, Jamin C. Regulatory B cells in autoimmune diseases: how do they work? Ann N Y Acad Sci. 2009;1173:260–267. doi: 10.1111/j.1749-6632.2009.04651.x. [DOI] [PubMed] [Google Scholar]
- 25.Lehmann-Horn K, Kronsbein HC, Weber MS. Targeting B cells in the treatment of multiple sclerosis: recent advances and remaining challenges. Ther Adv Neurol Disord. 2013;6:161–173. doi: 10.1177/1756285612474333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 Cells Restore Pregnancy Tolerance in a Mouse Model. Biol Reprod. 2013 doi: 10.1095/biolreprod.113.110791. [DOI] [PubMed] [Google Scholar]
- 28.Giannoukakis N, Trucco M. A role for tolerogenic dendritic cell-induced B-regulatory cells in type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2012;19:279–287. doi: 10.1097/MED.0b013e328355461b. [DOI] [PubMed] [Google Scholar]
- 29.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 30.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsushita T, Tedder TF. Identifying regulatory B cells (B10 cells) that produce IL-10 in mice. Methods Mol Biol. 2011;677:99–111. doi: 10.1007/978-1-60761-869-0_7. [DOI] [PubMed] [Google Scholar]
- 33.Mauri C. Regulation of immunity and autoimmunity by B cells. Curr Opin Immunol. 2010;22:761–767. doi: 10.1016/j.coi.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 34.Poe JC, Smith SH, Haas KM, Yanaba K, Tsubata T, Matsushita T, Tedder TF. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS One. 2011;6:e22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 36.Yang X, Yang J, Chu Y, Wang J, Guan M, Zhu X, Xue Y, Zou H. T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS One. 2013;8:e62855. doi: 10.1371/journal.pone.0062855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshizaki A, Miyagaki T, DiLillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature. 2012;491:264–268. doi: 10.1038/nature11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marino E, Grey ST. B cells as effectors and regulators of autoimmunity. Autoimmunity. 2012;45:377–387. doi: 10.3109/08916934.2012.665527. [DOI] [PubMed] [Google Scholar]
- 39.Marino E, Batten M, Groom J, Walters S, Liuwantara D, Mackay F, Grey ST. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes. 2008;57:395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 40.Rolf J, Motta V, Duarte N, Lundholm M, Berntman E, Bergman ML, Sorokin L, Cardell SL, Holmberg D. The enlarged population of marginal zone/CD1d(high) B lymphocytes in nonobese diabetic mice maps to diabetes susceptibility region Idd11. J Immunol. 2005;174:4821–4827. doi: 10.4049/jimmunol.174.8.4821. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, Sato S, Tedder TF, Fujimoto M. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol. 2013;10:122–132. doi: 10.1038/cmi.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 45.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauri C, Blair PA. Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- 47.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 48.Shen P, Lampropoulou V, Stervbo U, Hilgenberg E, Ries S, Mecqinion A, Fillatreau S. Intrinsic Toll-like receptor signalling drives regulatory function in B cells. Front Biosci (Elite Ed) 2013;5:78–86. doi: 10.2741/e597. [DOI] [PubMed] [Google Scholar]
- 49.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- 50.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 51.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 52.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9:447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Defrance T, Vanbervliet B, Briere F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992;89:1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bai XF, Zhu J, Zhang GX, Kaponides G, Hojeberg B, van der Meide PH, Link H. IL-10 suppresses experimental autoimmune neuritis and down-regulates TH1-type immune responses. Clin Immunol Immunopathol. 1997;83:117–126. doi: 10.1006/clin.1997.4331. [DOI] [PubMed] [Google Scholar]
- 56.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 57.Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, Poe JC, Tedder TF. Peritoneal Cavity Regulatory B Cells (B10 Cells) Modulate IFN-gamma+CD4+ T Cell Numbers during Colitis Development in Mice. J Immunol. 2013;191:2780–2795. doi: 10.4049/jimmunol.1300649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, Sugaya M, Kadono T, Tedder TF, Sato S. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J Leukoc Biol. 2013;94:563–573. doi: 10.1189/jlb.1112562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Huu D, Matsushita T, Jin G, Hamaguchi Y, Hasegawa M, Takehara K, Tedder TF, Fujimoto M. Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood. 2013;121:3274–3283. doi: 10.1182/blood-2012-11-465658. [DOI] [PubMed] [Google Scholar]
- 61.Horikawa M, Weimer ET, DiLillo DJ, Venturi GM, Spolski R, Leonard WJ, Heise MT, Tedder TF. Regulatory B cell (B10 Cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J Immunol. 2013;190:1158–1168. doi: 10.4049/jimmunol.1201427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang GX, Xiao BG, Yu LY, van der Meide PH, Link H. Interleukin 10 aggravates experimental autoimmune myasthenia gravis through inducing Th2 and B cell responses to AChR. J Neuroimmunol. 2001;113:10–18. doi: 10.1016/s0165-5728(00)00411-2. [DOI] [PubMed] [Google Scholar]
- 63.Poussin MA, Fuller CL, Goluszko E, Reyes VE, Braciale VL, Christadoss P. Suppressed clinical experimental autoimmune myasthenia gravis in bm12 mice is linked to reduced intracellular calcium mobilization and IL-10 and IFN-gamma release by acetylcholine receptor-specific T cells. J Neuroimmunol. 2003;134:104–110. doi: 10.1016/s0165-5728(02)00425-3. [DOI] [PubMed] [Google Scholar]
- 64.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–2015. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 66.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci U S A. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. e1118. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 68.Hoehlig K, Shen P, Lampropoulou V, Roch T, Malissen B, O'Connor R, Ries S, Hilgenberg E, Anderton SM, Fillatreau S. Activation of CD4(+) Foxp3(+) regulatory T cells proceeds normally in the absence of B cells during EAE. Eur J Immunol. 2012;42:1164–1173. doi: 10.1002/eji.201142242. [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharya P, Gopisetty A, Ganesh BB, Sheng JR, Prabhakar BS. GM-CSF-induced, bone-marrow-derived dendritic cells can expand natural Tregs and induce adaptive Tregs by different mechanisms. J Leukoc Biol. 2011;89:235–249. doi: 10.1189/jlb.0310154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, Boivin MN, Young YK, Perreault C, Galipeau J. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 71.Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, Bomprezzi R, Shi FD. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve. 2013 doi: 10.1002/mus.23951. [DOI] [PubMed] [Google Scholar]
- 72.Rowin J, Thiruppathi M, Arhebamen E, Sheng J, Prabhakar BS, Meriggioli MN. Granulocyte macrophage colony-stimulating factor treatment of a patient in myasthenic crisis: effects on regulatory T cells. Muscle Nerve. 2012;46:449–453. doi: 10.1002/mus.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.