Abstract
Genetic factors likely influence individual sensitivity to positive and negative effects of methamphetamine (MA) and risk for MA dependence. Genetic influence on MA consumption has been confirmed by selectively breeding mouse lines to consume high (MAHDR) or low (MALDR) amounts of MA, using a two-bottle choice MA drinking (MADR) procedure. Here, we employed a lickometer system to characterize the microstructure of MA (20, 40, and 80 mg/l) and water intake in MAHDR and MALDR mice in 4-h limited access sessions, during the initial 4 hours of the dark phase of their 12:12 h light:dark cycle. Licks at one-minute intervals and total volume consumed were recorded, and bout analysis was performed. MAHDR and MALDR mice consumed similar amounts of MA in mg/kg on the first day of access, but MAHDR mice consumed significantly more MA than MALDR mice during all subsequent sessions. The higher MA intake of MAHDR mice was associated with a larger number of MA bouts, longer bout duration, shorter interbout interval, and shorter latency to the first bout. In a separate 4-h limited access MA drinking study, MALDR and MAHDR mice had similar blood MA levels on the first day MA was offered, but MAHDR mice had higher blood MA levels on all subsequent days, which corresponded with MA intake. These data provide insight into the microstructure of MA intake in an animal model of differential genetic risk for MA consumption, which may be pertinent to MA use patterns relevant to genetic risk for MA dependence.
Keywords: amphetamine, genetics, lickometer, limited access, selective breeding, two-bottle choice drinking
1. INTRODUCTION
Genetic factors may influence who is and is not at risk for developing a pattern of methamphetamine (MA) use leading to dependence. Several genetic variants in human populations have been identified and associated with MA abuse, dependence, and psychosis [1]. We have examined the heritability of MA drinking (MADR) in mouse lines that were selectively bred for oral consumption of either high (MAHDR) or low (MALDR) amounts of MA [2, 3]. Selective breeding produced MAHDR lines that consume approximately 6 mg/kg of MA during an 18-h MA access period, compared to 0.5 mg/kg MA intake in MALDR mice [2, 3]. Calculated heritability was 0.34 in the replicate 1 set of lines and 0.35 in replicate 2, indicating that ~35% of the variance in intake could be attributed to heritable genetic factors. In addition to higher consumption of the drug, MAHDR mice are more sensitive to the conditioned rewarding and reinforcing effects of MA, whereas MALDR mice are more sensitive to the aversive effects of MA [2, 4]. The focus of the present study is on patterns of MA intake that may be informative with regard to genetic risk for further use.
Although we have examined the motivational drive for MA intake in our genetic model of high and low MA intake, we have not examined patterns of consumption during the time that MA drinking is established. Initial experiences are critical to further use and microstructural analysis of MA and water intake during this time period would provide information about the impact of differential genetic risk for intake on the acquisition of MA consumption. In previous work, MADR mice trained to perform an operant response to gain access to either a saccharin sweetened MA- or non-MA-containing tube did not differ in the amount of MA consumed during the first trial that it was offered. However, MALDR mice reduced their consumption during the next trial and the selected lines differed in MA consumption during all subsequent trials [5]. These data suggest that the MALDR line mice reduce their intake after experiencing pharmacological effects of MA that they perceive as aversive, rather than in response to taste or some other peripheral factor. Our previously published data, examining both taste factors and sensitivity to the aversive effects of MA, support this conclusion (Shabani et al., 2011; 2012b; Wheeler et al., 2009).
The microstructure of MA and water consumption was examined during 4-h limited access sessions using a lickometer system. The lickometer system provides precise time resolution of drinking behavior by continuously recording each lick of the sipper tube. By grouping these licks into bouts, several drinking measures can be obtained including number of drinking bouts, time between bouts, size of bout, and bout duration. Microstructural data using lickometer and similar systems have been commonly generated for ethanol and sucrose drinking [6–14]. In a separate study, we examined MA blood levels at time points that corresponded with the lickometer procedure. We hypothesized that similar to the operant oral self-administration data, the MADR lines would not differ in amount of MA consumed on the first day of MA access, but would diverge in amount consumed and in structural components (e.g., number and size of drinking bouts) of consumption from the MA-containing bottle, with subsequent access. We anticipated that blood MA levels would correspond with MA intake.
2. MATERIALS AND METHODS
2.1 Animals and husbandry
The MADR selected lines were created using mass and short-term selection. Mass selection was used to achieve a rapid response and selective breeding over few generations (i.e., short-term) was used to reduce the chance differential fixation of selection trait irrelevant genes within the lines [15]. Detailed selection procedures have previously been published [2, 3]. Briefly, offspring from the F2 cross of C57BL6/J and DBA2/J inbred strains of mice were phenotyped in an 18-h two-bottle choice MA vs water drinking procedure for their MA consumption from a 20 mg/l then 40 mg/l solution, for 4 days per concentration. The highest and lowest MA consuming (in mg/kg MA from the 40 mg/l concentration) animals were used to establish breeders for the MAHDR and MALDR lines, respectively. Their first litter offspring were similarly tested and breeders were again selected. This procedure was followed in each subsequent generation for a total of 5 selection generations. Mice used in the current study were MA- and experiment-naïve offspring of fifth selection generation (S5) parents. The MADR lines were replicated sequentially so that data obtained in one set of lines could be extended in the subsequent set of lines. Results for some common traits have been obtained in both sets of lines and have been in good agreement (Wheeler et al., 2009; Shabani et al., 2011). The current study used replicate 2 MADR mice (MAHDR-2 and MALDR-2; the MADR-2 lines), as the replicate 1 lines had been retired when the current work was performed. A total of 44 adult female mice, age 61–114 days were used for the lickometer study and 42 adult female mice aged 99–124 days were used for the study measuring blood MA levels. Mice were housed with dam and sire until they were weaned at 21–23 days of age and were then grouped in cages with 2–5 same sex littermates. Female mice were used because they were more readily available than males at the time of this study. Importantly, previous studies have not identified significant sex differences for the difference in MA intake for either replicate set of the MADR lines [2, 3]. Mice were housed in shoebox cages (28.5 × 17.5 × 12 cm) with Bed-O-Cob™ bedding (The Anderson Inc., Maumee, OH) and wire cage tops, and maintained at 21±1°C. They were placed on a reverse 12:12 h light:dark cycle (lights off at 0830 h and on at 2030 h) at least 2 weeks before the study began, and had free access to water and standard rodent diet (Purina 5001TM, Animal Specialties Inc., Hubbard, OR) at all times. The reverse light:dark cycle was used to allow drinking to be more conveniently assessed during the dark phase, when mice are more active and engage in more eating and drinking behavior. All procedures were performed in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of the Portland Veterans Affairs Medical Center.
2.2 Drinking solutions
(+) Methamphetamine hydrochloride was purchased from Sigma (St. Louis, MO) and dissolved in tap water to 20, 40, and 80 mg/l concentrations. MA solutions were made fresh every 4 days.
2.3 Drinking pattern analysis
Fluid intake was measured in 24, custom-made acrylic plastic lickometer chambers (17.8 × 10.2 × 10.2 cm) that have been used in our previous studies [6, 16]. The lickometer device was manufactured by MED Associates, Inc. (St Albans, VT). Each test chamber had a stainless steel wire grid floor (VWR; Tualatin, OR) and two small holes located in the back wall through which two metal sipper tubes were introduced. Tubes were secured to the chamber wall to reduce the potential for displacement by the mice and thus, reduce the recording of false intake volumes. A hinged acrylic plastic lid with ventilation holes covered each chamber. Stainless steel sippers (Anacore, Bellmore, NY) were attached to polystyrene serological pipettes (10 ml volume; VWR) to create drinking tubes. The pipettes were trimmed to a 6 ml capacity to allow them to fit properly behind the lickometer chambers. Tube volumes (0.1 ml accuracy) were recorded at the beginning and end of each 4-h drinking session.
The wire floor of the chamber and the metal sipper tubes form open electrical circuits connected to the lickometer device. A circuit is closed when an animal stands on the metal floor and makes contact by licking a sipper tube. A software program (MED-PC IV; MED Associates, Inc.) was used to automatically record cumulative sipper contacts. Individual animal cumulative lick records (total number of licks) were extracted using Soft CR version 4 (MED Associates, Inc.), and appetitive (latency to first bout) and consummatory (bout frequency, bout size, bout duration, interbout interval, bout lick rate) variables were extracted from the cumulative records using a custom data analysis program written for the online software R project for Statistical Computing (http://www.r-project.org). No previous data of this nature have been collected for MA, but based on multiple previous studies examining patterns of ethanol and sucrose drinking, a bout was defined as a series of at least 20 licks with less than 1 minute separating each lick [6–9].
2.4 MA and water intake
Data were collected in 2 equal size cohorts of mice. A 4-h limited access drinking procedure was used that was initiated at the beginning of the dark phase. This period was chosen as a time when consumption was expected to be relatively high, compared to other times within the 24-h day, similar to the approach that has been used to examine binge-like ethanol drinking [10, 17, 18]. Mice were acclimated to single housing for 2 days before the drinking procedure began. On study day 1, at lights out, mice were placed into individual lickometer chambers and two tubes, both containing tap water, were extended into the cage to allow for acclimation to the sipper tubes and collection of baseline water only data. This was repeated on day 2. On days 3–14, mice were offered a tap water tube and a tube containing 20, 40, and then 80 mg/l MA in tap water, with each MA concentration provided for 4 consecutive days. After each session, mice were returned to their home cages. Mice were weighed every other day and had ad libitum access to food and water in both the lickometer chambers and their home cages. Drug and water tube sides were randomized across subjects.
2.5 Blood MA levels
To examine blood MA levels resulting from MA consumption, a separate limited access drinking study was performed. Procedures were identical to those used for the lickometer study, except that the lickometer apparatus was not used and the drinking of individual mice was measured in their home cages. This allowed volumetric readings to be taken at multiple time points, which may have altered the drinking patterns of mice in a lickometer study. Mice were assigned to one of 3 groups based on when 20 µl lateral tail vein blood samples were collected. On days 3, 6, 10, and 14, a 20 µl blood sample was collected from the lateral tail vein of mice at either 2 h into the 4-h session or immediately after the 4-h session, depending on group assignment. Group 1 mice had blood samples taken on the first day of MA access (day 3 of the study), and immediately after the 4-h session on the final day of access to 20 and 80 mg/l MA (days 6 and 14). Groups 2 and 3 had blood samples taken on the final day of access to each MA concentration (days 6, 10, and 14) at either 2 h into the session or at the end of the 4-h session. Each sample was placed into a microcentrifuge tube that contained 80 µl of EIA buffer provided by Neogen (Lexington, KY) and MA levels from blood samples were assessed using the Neogen amphetamine group enzyme-linked immunosorbent assay (ELISA) kit. Samples were read with a Bio-Rad Benchmark Plus microplate spectrophotometer (Hercules, CA) equipped with a 450 nm filter. MA concentrations were determined using a calibration curve.
2.6 Statistical Analysis
All statistical analyses were conducted using Statistica version 6.1 software (StatSoft, Inc., Tulsa, OK). Repeated measures ANOVA, with selected line as the between-groups factor and MA concentration and time within the 4-h sessions as repeated factors, was used to analyze mg/kg MA consumed, ml/kg of total fluid consumed, cumulative licks, and drinking bout parameters. For some analyses, day within MA concentration was also used as a repeated measure. For analysis of the blood MA data, selected line and sampling time group were used as between-groups factors, with day of sample as a repeated measure. Significant two-way interactions were resolved using simple main effect analyses and post hoc mean comparisons were performed when appropriate, using the Newman-Keuls post hoc test. The criterion for significance was set at p≤0.05. Figures were created using Sigmaplot (Version 8.0; SPSS, Chicago, IL). Data from 9 mice (4 MALDR-2 and 5 MAHDR-2) were excluded due to technical difficulties with the lickometer equipment and interface.
3. RESULTS
Figure 1A shows MA consumed during the 4-h drinking sessions on days 6, 10, and 14, which were the final days that each concentration was offered. Data on other days were also examined and some of those results are presented below. These data are presented because they provide a simple summary of findings for a period after maximal access and acclimation to each concentration of MA. There was a significant concentration x line interaction (F[2,60]=6.6; p<0.01). As expected, MAHDR-2 mice consumed more MA than MALDR-2 mice at every concentration. In addition, MAHDR-2 mice significantly escalated their MA intake as the concentration was increased (p<0.001, for the comparison between MA intake at 80 mg/l and 20 mg/l MA concentrations), whereas MALDR-2 mice showed no change in their low levels of intake. Figure 1B shows water consumed during the same 4-h periods. There was a main effect of line (F[1,30]=14.2; p<0.001), but no effect of the MA concentration offered during the water access period, nor interaction of these two factors. MALDR-2 mice consumed more water compared to MAHDR-2 mice. Figure 1C shows total volume consumed in ml/kg. The MADR-2 lines did not differ in total fluid intake, nor were there MA concentration-dependent effects.
Figure 1. Consumption of MA and water in MADR mice, when offered in a 4-h limited access two-bottle choice study.
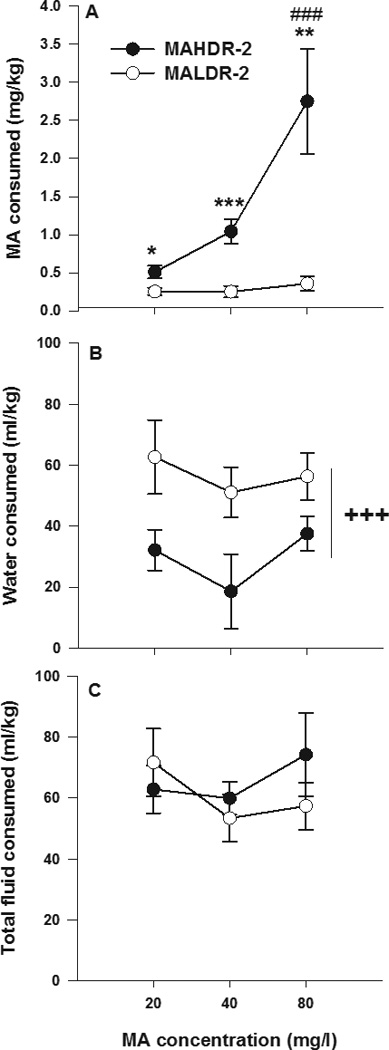
Shown is (a) mean ± SEM mg/kg MA consumed (20, 40, 80 and mg/l), (b) mean ± SEM ml/kg water consumed, and (c) mean ± SEM ml/kg total fluid intake. Each data point is the group average for day 4 consumption for each MA concentration offered. *p<0.05, **p<0.01, ***p<0.001 for the difference between the MAHDR-2 and MALDR-2 line mice at each MA concentration. ###p<0.001 for the difference between the amount of MA consumed at the 80, compared to 20 mg/l concentration within the MAHDR-2 line. +++p<0.001 for the main effect of selected line. N=17–18/line.
Lick data were next considered in 30-min blocks of time for the final 4-h drinking sessions for each MA concentration to determine whether there were periods of heightened MA drinking behavior (Figure 2). For the MA-containing tube (Figure 2A, B, and C), there was a main effect of line (F[1,30]=12.2; p<0.01) and time bin (F[7,210]=3.4; p<0.01), but no effect of MA concentration or any significant interactions. Figure 2D shows the total number of licks for each MA concentration. MAHDR-2 line mice took a greater number of licks from the MA-containing tube compared to MALDR-2 line mice, regardless of MA concentration (F[1,30]=12.4; p<0.01 for the main effect of line). For the water-containing tube, there were no statistically significant findings (Data not shown). Pearson’s r correlations were calculated to examine the relationship between total licks and total volume for each MA concentration, and all were significant (r=0.69–0.78; p<0.05).
Figure 2. Temporal pattern of licks taken from the MA-containing bottle.
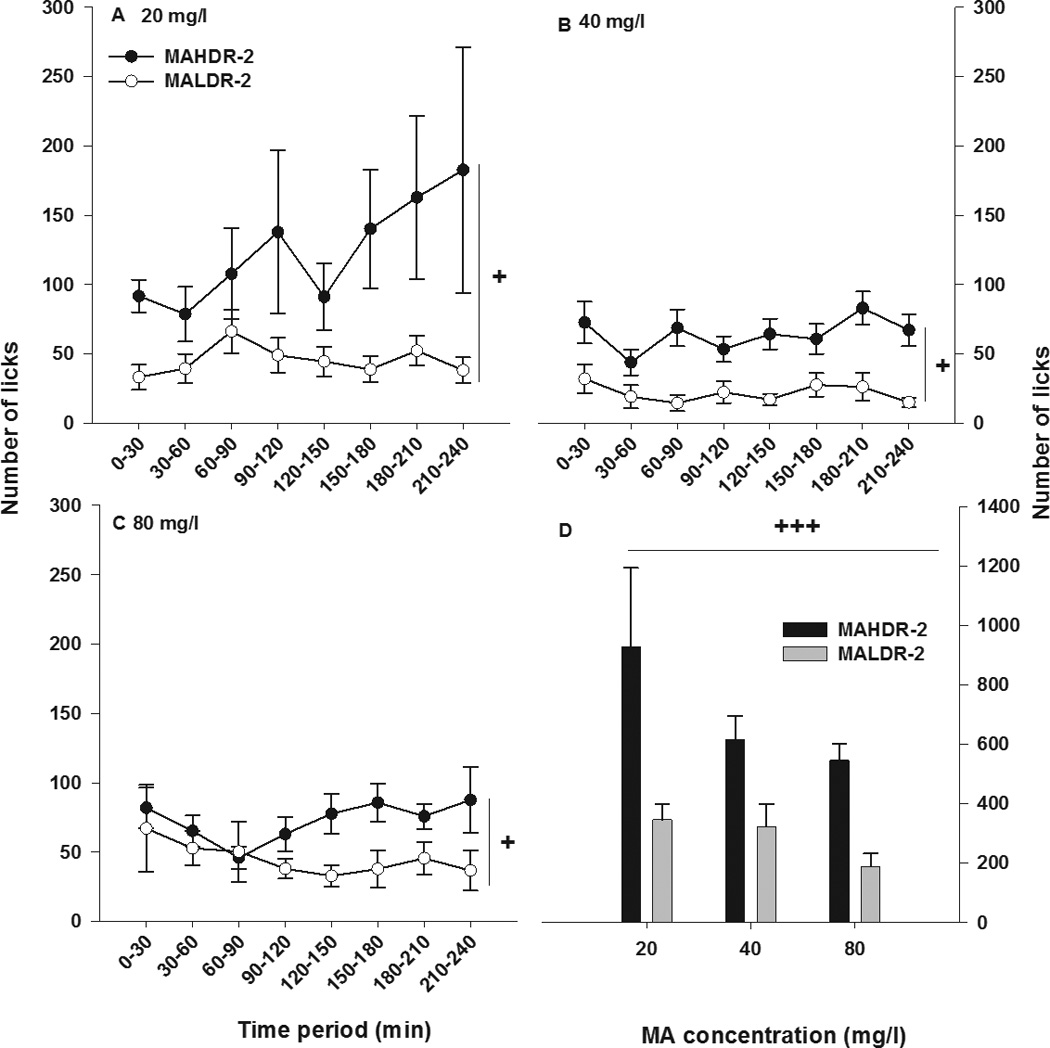
Shown is mean ± SEM number of licks across time taken from the MA-containing bottle at increasing concentrations of MA; (a) 20 mg/l, (b) 40 mg/l, and (c) 80 mg/l. Data are shown in 30-min increments for day 4 of each MA concentration. (d) Mean ± SEM total number of licks taken from the MA-containing bottle, accumulated for the entire 4-h session. +p<0.05, +++p<0.001 for the main effect of selected line. N=17–18/line.
Figure 3 shows bout parameters for MA intake during the final 4-h MA drinking sessions for each MA concentration. MAHDR-2 mice had significantly more MA bouts, compared to MALDR-2 mice (F[1,30]=17.8; p<0.001) and the number of bouts significantly decreased as the MA concentration increased, regardless of line (F[2,60]= 3.4; p<0.05; Figure 3A). However, MALDR-2 line mice had a significantly greater interbout interval, compared to MAHDR-2 line mice (F[1,30]=4.7; p<0.05; Figure 3E) and took significantly longer to complete their first drug bout (F[1,30]=6.5 p<0.05; Figure 3C). Overall, the latency to first bout increased as the concentration of MA was increased (F[2,60]=3.6; p<0.05; Figure 3F), regardless of line. Analysis of the same bout parameters for the water-containing tube did not identify any significant differences between the selected lines (data not shown).
Figure 3. MA drinking pattern characteristics.
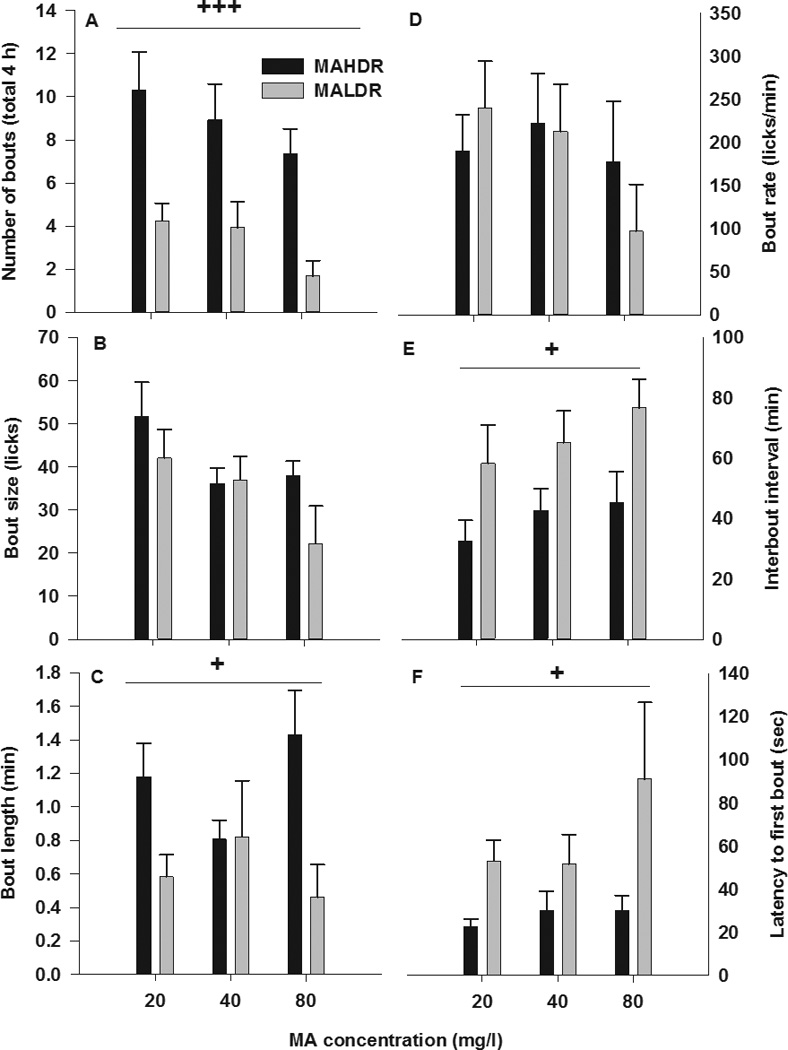
Shown is mean ± SEM (a) number of bouts, (b) bout size, (c) length of bout, (d) bout rate, (e) interbout interval, and (f) latency to first bout for MAHDR-2 and MALDR-2 mice for day 4 of each MA concentration. +p<0.05, +++p<0.001 for the main effect of selected line. N=17–18/line.
Figure 4 shows mg/kg MA consumed during the first four days of MA access, when MA was first offered (20 mg/l; days 3–6). This period was examined separately to detect changes in drinking patterns across initial access days, as previously examined in an oral operant MA self-administration procedure [5]. There was a significant line x day interaction (F[3,90]=2.6; p<0.05). Simple main effects analysis of the line difference on each day demonstrated that upon first access to MA, the two selected lines did not differ in MA intake, but a line difference emerged that was present on all subsequent days (all p<0.05). Simple main effects analysis of the difference across days within each line supported a significant increase in MA intake in MAHDR-2 mice (p<0.05 for the difference between day 6 and day 3), but no significant change across days in MALDR-2 mice, although there was a downward trend in MA intake. No differences in total volume consumed were identified during this 4-day period (data not shown).
Figure 4. MA intake across the first 4 days of MA access.
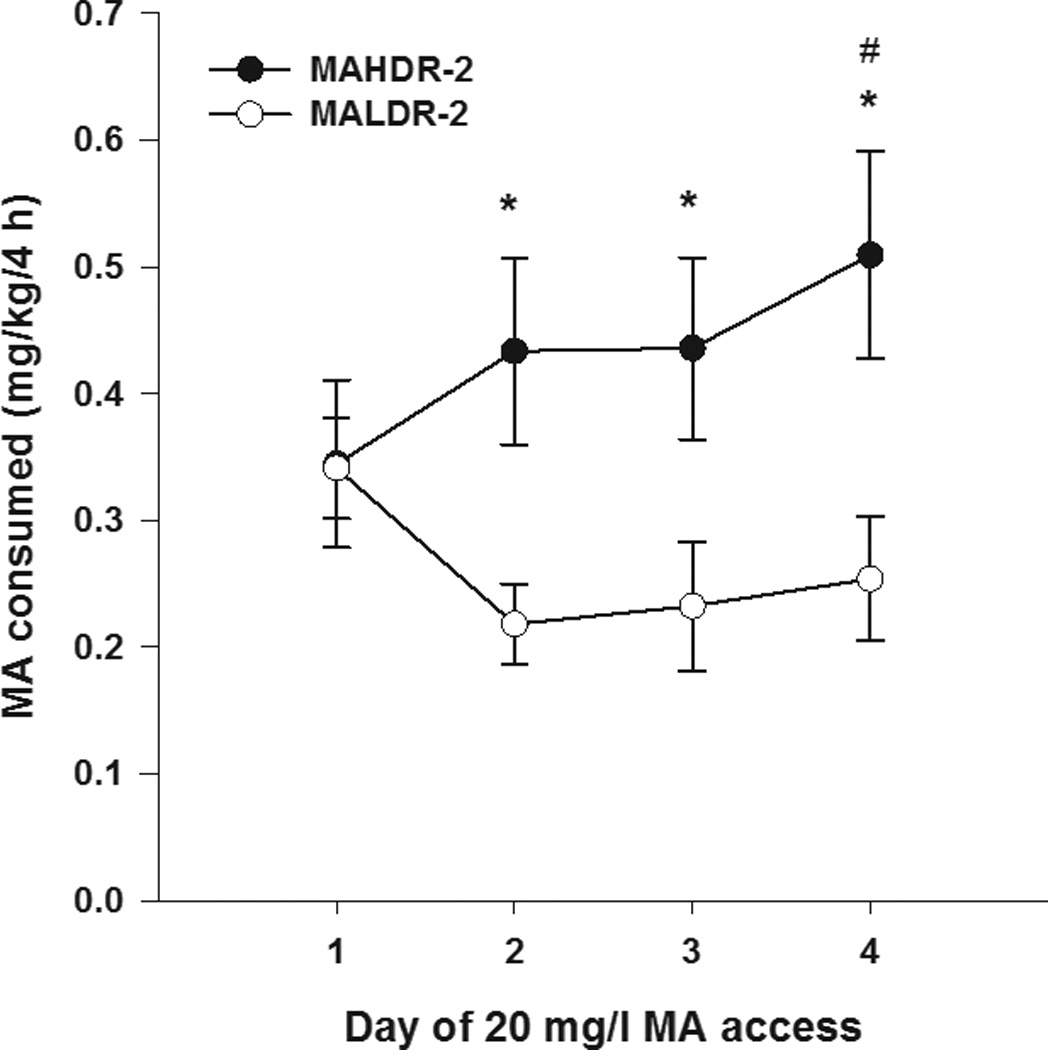
Shown is mean ± SEM mg/kg MA consumed during the first 4 days of MA (20 mg/l) access in MAHDR-2 and MALDR-2 mice. *p<0.05 for the difference between the MAHDR-2 and MALDR-2 line mice. #p<0.05 for the difference between day 4 and day 1 within the MAHDR-2 line. N=17–18/line.
Figure 5 shows bout measures for the first 4 days of MA access. Compared to MALDR-2 mice, MAHDR-2 mice had a greater number of MA bouts (F[1,30]=10.6; p<0.01; Figure 5A), longer bout length (F[1,30]=10.4; p<0.01; Figure 5C), shorter interbout interval (F[1,17]=10.0; p<0.01; Figure 5E) and shorter latency to first bout of MA drinking (F[1,24]=6.2; p<0.05; Figure 5F). The interaction of line x day was not statistically significant for any of these measures, although Figure 5 suggests changes in magnitude of the line difference over days. For MA bout size (Figure 5B) and bout rate (Figure 5D), there were significant line x day interactions (F[3,90]=3.1; p<0.5 and F[3,90]=4.2; p<0.01, respectively). MALDR-2 mice had a larger bout size, compared to MAHDR-2 mice, on day 1 of 20 mg/l MA access (p<0.05), but not on subsequent days of access to this concentration of MA. Additionally, on days 1 (p<0.001) and 2 (p<0.05) of 20 mg/l MA access, the MALDR-2 line had a greater bout rate, compared to the MAHDR-2 line, with this line difference disappearing by the third day of MA access. For water during the same time period, there were no differences in number of bouts, bout size, interbout interval, or latency to first bout (data not shown). However, MALDR-2 mice did have shorter length water bouts (F[1,30]=7.4; p<0.05 for the main effect of line; mean±SEM: 0.9±0.1 and 1.4±0.1 sec for MALDR-2 and MAHDR-2, respectively) and a quicker bout rate [F(1,30)=38.6; p<0.001 for the main effect of line; mean±SEM: 281.8±18.6 and 127.8±16.3 licks/min for MALDR-2 and MAHDR-2, respectively).
Figure 5. MA drinking pattern characteristics across the first 4 days of MA access.
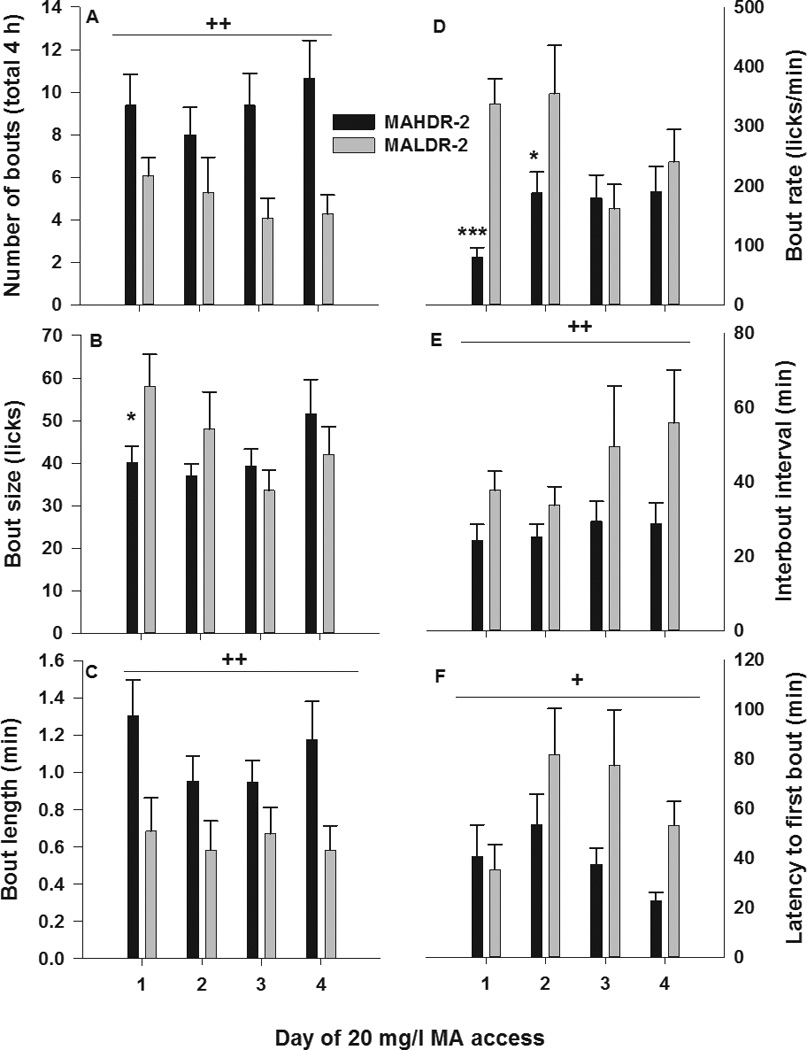
Shown are mean ± SEM (a) number of bouts, (b) bout size, (c) length of bout, (d) bout rate, (e) interbout interval, and (f) latency to first bout for MAHDR-2 and MALDR-2 mice during the first 4 days of MA (20 mg/l) access. *p<0.05, ***p<0.001 for the difference between the MAHDR-2 and MALDR-2 line mice. +p<0.05, ++p<0.01 for the main effect of selected line. N=17–18/line.
Figure 6 shows MA intake and corresponding blood MA level data for 3 groups of mice that each had 3 blood samples obtained at particular times. For group 1 mice, blood was taken after the first day of MA intake (day 3), because it was predicted that the MADR-2 line mice would consume similar amounts of MA on this day and would also have similar blood MA levels. This group was also sampled on the final day that the lowest concentration was offered and on the final day of the study, times when MA intake was predicted to be higher in the MAHDR-2 line than on the first day and lower in the MALDR-2 line. Data for this group were analyzed separately from groups 2 and 3, because mice in those groups they were not sampled at the same times. For MA intake (Figure 6A), there was a significant main effect of line (F[1,11]=4.9; p<0.05) and of day (F[2,22]=8.1, p<0.001), but the line x day interaction was not statistically significant (p=0.15). Thus, patterns of increasing intake in the two selected lines as the MA concentration was increased were not significantly different; however, greater MA intake in MAHDR-2 mice confirmed the trait for which they were bred. For blood MA levels in group 1 animals (Figure 6B), there was a significant line x day interaction [F(2,22)=8.6; p<0.01]. For the simple main effect of line at each of the sampling days, there was no significant difference between the lines on day 3, but the MAHDR-2 line mice had significantly greater blood MA concentrations than MALDR-2 line mice on days 6 and 14 (p<0.05 and p<0.01, respectively). With regard to differences across day within line, there was a significant simple main effect of day within the MAHDR-2 line; MAHDR-2 mice had significantly higher blood MA levels on day 14 compared to day 3 (p<0.001). There was no significant simple main effect of day within the MALDR-2 line, indicating that blood MA levels were comparable across days in these mice.
Figure 6. MA consumption and corresponding blood MA levels in MAHDR-2 and MALDR-2 mice.
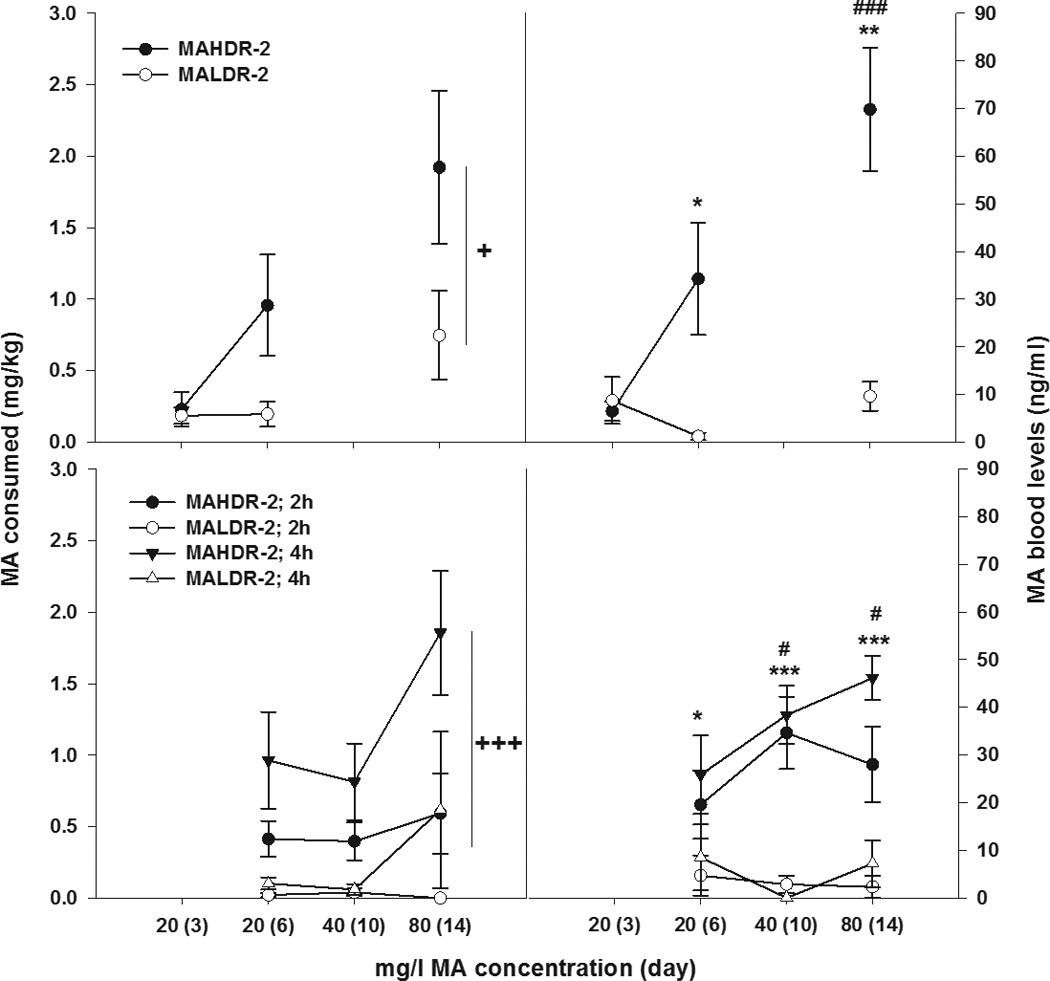
All data shown are means ± SEM. Group 1 data from the relevant study are shown in (a) for mg/kg MA consumed and (b) blood MA levels (ng/ml) on the first (day 3) and fourth (day 6) day of MA access (20 mg/l) and the fourth (day 14) day of MA at the highest concentration offered (80 mg/l). Group 2 and 3 data are shown in (c) for mg/kg MA and (d) blood MA levels (ng/ml) on the fourth day that each MA concentration was offered (day 6, 10 and 14), at either 2 or 4 h into the 4-h limited access procedure. *p<0.05,**p<0.01, ***p<0.001 for the MADR line difference. #p<0.05, ###p<0.001 for within-group difference between MAHDR-2 mice at the 40 and 80 mg/l MA concentrations and the 20 mg/l MA concentration. +++p<0.01 for the main effect of line. N=6–9/line/group.
Group 2 and 3 mice were sampled on the same 3 days at either 2 h or 4 h into the limited access session (Figure 6C and D). Data for these groups were statistically compared. For MA consumption, there was a significant main effect of line [F(1,25)=15.5; p<0.001] and of day [F(2,50)=3.5; p<0.05], but no significant line x day interaction. MAHDR-2 mice consumed significantly more MA than MALDR-2 mice, regardless of concentration, but more MA was consumed as the concentration of MA was increased (Figure 6C). There was also a significant main effect of sampling time [F(1,25)= 7.7; p<0.01]; more MA was consumed at the 4 h compared to the 2 h time point. For the corresponding blood MA level data (Figure 6D), there was no significant difference in amount of MA in samples obtained at 2 vs 4 h (group 2 compared to group 3). However, there was a significant line x day interaction [F(2,54)= 3.5; p<0.05]. The simple main effect of line at each day was significant; MAHDR-2 mice had significantly higher blood MA levels on each day (p<0.05, 0.001, and 0.001, respectively). In addition, the simple main effect of day within the MAHDR-2, but not MALDR-2 line, was significant; MAHDR-2 mice had significantly higher blood MA levels after consuming the 40 and 80 mg/l MA concentrations, compared to the 20 mg/l MA concentration (p<0.05 for both comparisons). A significant correlation (Pearson’s r; r=0.58; p<0.05) was found between established MA intake (days 6, 10 and 14) and blood MA levels obtained after consumption of each MA concentration. The correlation was comparable whether calculated using all data from days 6, 10 and 14, or for each MA concentration independently or for the 2-h vs. 4-h drinking period groups.
4. DISCUSSION
These data demonstrate that selective breeding for MA intake has resulted in differences in MA drinking characteristics between the high and low MADR mouse lines, some of which appear during the first drinking session. The MAHDR-2 line consumed more MA at each concentration of MA offered, compared to the MALDR-2 line, while the MALDR-2 line consumed more water compared to the MAHDR-2 line, resulting in comparable levels of total fluid consumed. MAHDR-2 line mice had a greater number of MA drinking bouts, longer bout duration, shorter interbout interval, shorter latency to first MA drinking bout and greater number of licks from the MA-containing bottle, compared to the MALDR-2 line. On the first day of MA access, the MADR-2 lines did not differ in MA consumption, but with subsequent access, the MAHDR-2 line escalated their MA intake, while the MALDR-2 line showed a pattern of decreasing MA intake, although this decrease was not statistically significantly. Although total intake did not differ on the first day, analysis of bout parameters during the initial 4-d MA access period showed that the MAHDR-2 line, regardless of day, had a greater number of and longer MA bouts, shorter interbout interval, and shorter latency to first MA bout, compared to the MALDR-2 line. In a separate study, MA consumption and blood levels were similar for the 2 lines on the first day of MA access, but the MAHDR-2 line mice had higher MA intake and significantly increased blood MA levels as the MA concentration in the drinking solution was increased. Results from the lickometer study, identified differences in intake parameters prior to MA intake differences. Significant differences between the lines across days of the initial access period were found only for bout size and rate.
A previous study demonstrated that the MADR-2 lines do not differ in rate of MA clearance from blood following a bolus 2 mg/kg MA injection [4]. Peak MA levels (~400 ng/ml) were observed 15 minutes following MA administration, with peak level somewhat higher in MAHDR-2 than MALDR-2 mice and almost complete clearance within 2 h later. In the current study, when MADR-2 mice were allowed to voluntarily consume MA during 4-h periods, MA blood levels for individual animals were between 0 and100 ng/ml. These levels were likely impacted by drinking pattern, concentration of the MA solution, and when during the session the samples were obtained.
A previous study examined locomotor activity following a 1 h operant MA self-administration study in MADR-2 mice [5]. On average, MAHDR-2 line mice consumed 0.4–0.7 mg/kg MA and MALDR-2 line mice consumed 0.1–0.2 mg/kg MA. These consumption values corresponded with level of activity, when locomotor activity data were collected on several days, within 15 min after the conclusion of the drinking sessions, suggesting that this level of MA intake was behaviorally relevant. In the current study, the MADR-2 lines consumed greater amounts of MA (up to 2 mg/kg on average for one group of MAHDR-2 mice and 0.75 mg/kg in one group of MALDR-2 mice), consistent with longer drinking sessions and the higher 80 mg/l MA concentration offered here that was not used in the previous study. In human subjects, a low to moderate dose of MA (5–30 mg or 0.06–0.4 mg/kg based in a 75 kg human) is known to produce euphoric subjective effects, whereas doses above 50 mg (or above 0.7 mg/kg in a 75 kg human) have been shown to induce euphoria followed by psychosis [19, 20]. We did not see large differences in blood MA levels in mice sampled at 2 vs 4 h, and the correlation between intake and MA levels was comparable for these groups. This may indicate that the mice consume MA in a pattern that titrates blood MA to a certain level and subjective effect.
It is of particular importance that the selected lines did not differ in MA drinking on their first day of MA access. In a previous examination of operant oral self-administration of MA, a similar outcome was obtained during a 1-h long trial [4]. However, in that study a saccharin fading procedure was used, so that MA was initially offered in a saccharin-sweetened solution. The current findings are of further interest, because the solutions offered were not sweetened to incentivize consumption, yet an almost identical outcome was obtained. In addition, the blood MA data, supported a lack of difference between the MADR-2 lines in MA level after this first MA drinking session. Using a two-bottle choice procedure, we have found no differences in taste preference for bitter or sweet solutions between the MADR lines [2, 3]. The current data suggest that the MALDR-2 line does not initially avoid consuming the MA-containing solution due to its bitter taste qualities. Rather, it appears that they must first experience the pharmacological effects of MA before choosing to avoid consumption. Findings that support extreme sensitivity of MALDR mice to aversive effects of MA, using conditioned place preference, conditioned place aversion and conditioned taste aversion (CTA) procedures [2–5], suggest the possibility that aversive effects of MA were experienced by the MALDR-2 mice during the first MA drinking access session.
It has been demonstrated that ethanol, under some conditions and particularly in low preferring strains, can produce conditioned aversion during acquisition of ethanol consumption [21, 22]. Cannon and Carrell (1994) examined several rat strains with high and low ethanol preference and obtained data suggesting that initial preference and pattern of initial consumption were related to subsequent patterns of intake. Strains that ultimately showed low ethanol preference tended to have consumed larger amounts of ethanol during their first access session, while those that ultimately exhibited higher preference had low to moderate initial ethanol intake, and continued to consume ethanol at about the same or at a higher level. Although the MADR lines initially consumed equivalent amounts of MA, due to the much higher sensitivity of the MALDR line to the aversive effects of MA, this amount could have induced taste aversion, resulting in reduced intake thereafter. Profound MA-induced CTA is seen at 1 mg/kg MA in MALDR mice, the lowest dose we have tested, and no CTA is seen in MAHDR mice even at a dose of 4 mg/kg). This difference between the MADR lines in sensitivity to MA-induced CTA appears to be specific to MA, as the lines show similar patterns of CTA development for cocaine [23] and ethanol (Phillips, unpublished). It is of interest to examine aversive effects in the MALDR mice at doses as low as those they voluntarily consume (0.3 – 0.75 mg/kg). The lines do not differ significantly in rate of MA clearance [4]. Further, the MADR lines do not differ in their oral preferences for salty (KCl; NaCl), bitter (qunine), or sweet (saccharin) drinking solutions [2, 3]. These findings for MA support the hypothesis that initial exposure to a drug is a particularly salient experience that influences future drug taking patterns.
To our knowledge, the microstructure of oral MA intake in an animal model has not been previously documented. However, such data have been generated for ethanol intake in some genotypes of rat and mouse [7–11, 13]. Those studies have supported a positive correlation between larger bout size and higher ethanol intake (g/kg). In addition, a similar finding has been demonstrated in a non-human primate model, in which classification of subjects as “sippers” or “gulpers” was predictive of later ethanol intake patterns [24]. We observed only a transient difference in MA bout size between our high and low MA consuming lines in the current study (day 1 only). Instead, a larger number of bouts, greater bout duration, and shorter interbout interval appeared to play a significant role in the greater MA consumption of MAHDR-2, compared to MALDR-2, mice. It has been suggested for ethanol that length of interbout interval and bout frequency indicate magnitude of “craving” [11]. Whether this is the case for MA will require additional study, perhaps using a model of extinguished use followed by relapse. Such studies in our genetic animal model of higher MA intake may provide insights into patterns of human MA use
We identified significant correlations between volume consumed and number of licks (r=0.69–0.78). Data were collected for all 4 consecutive days that each MA concentration was offered. However, stronger correlations were found when day 4 values alone were used, which led, in part, to our decision to focus on day 4 data for each MA concentration. Others have reported somewhat larger volume-lick correlations for ethanol drinking (e.g., r=0.87–0.97). However, in those studies, the rodents resided in isolate housing in the lickometer chambers, 24 h per day throughout the study, and were given a 7-d acclimation period in the lickometer chambers before beginning the experiment [7, 8, 10]. We wished to avoid isolate housing in the current limited access study, and placed the mice into the lickometer chambers for each daily drinking session and then returned them to home cages with same-sex littermates. Our selection protocol for MA consumption isolate houses mice for the 10-d selection procedure. The current data show that the MADR lines consume different amounts of MA when not chronically isolate housed and during a limited access procedure. This is consistent with other limited MA access data for two-bottle choice under isolate housing conditions [25] and for operant MA self-administration without chronic isolate housing [5], and indicates that the difference in MA intake between the lines is consistent across multiple procedures.
5. CONCLUSIONS
Our data illustrate that genetic susceptibility to MA consumption corresponds with a larger number of MA drinking bouts, a greater bout duration, a shorter latency to first MA bout, a shorter interbout interval, and higher blood MA levels. These MA drinking characteristics could be associated with greater genetic risk for MA dependence. A comparison of binge (MA use up to 22 times/day for 4–6 days) and non-binge patterned MA users that used an equivalent amount of MA over a 30-d period, demonstrated that binge patterned users were more likely to suffer from health, social, and behavioral consequences compared to non-binge patterned MA users [26, 27]. In the current study, we examined consumption of a more concentrated solution of MA (80 mg/l) than used previously (up to 40 mg/l). The MAHDR-2 line showed a marked increase in MA intake and blood MA levels, when MA was offered as an 80 mg/l solution, then when offered as a 20 or 40 mg/l solution. In part, this may be because a higher dose can be attained by consuming similar volume. However, because there was always a water choice, the mice could have chosen to reduce their intake if they had found the dose to be aversive. In this article, we show day 4 data for each concentration, indicating that the mice did not avoid consuming a high dose of MA on their final day of access. In an 18-h period (6 h during the light and 12 h during the dark), MAHDR mice consume ~6 mg/kg MA from a 40 mg/l solution. Here they consumed a dose of ~3 mg/kg in only 4 h (during the dark). Our future plans include further development of a binge-like model of MA intake over a more chronic period to better model human MA dependence.
Highlights.
Genetic differences influence the microstructure of methamphetamine drinking.
Breeding for high methamphetamine intake leads to greater and longer drinking bouts.
Breeding for low methamphetamine intake leads to longer time between drinking bouts.
Methamphetamine drinking patterns could predict genetic risk for higher intake.
Acknowledgements
This work was supported by the Department of Veterans Affairs, and NIH grants T32 DA07262, P50 DA018165, and R24 AA020245.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that they have no competing interests.
Authors’ contributions
ECE developed hypotheses, designed the studies, analyzed and interpreted the data, and wrote the manuscript. AMA consulted on data analysis and interpretation. TJP co-developed the hypotheses, assisted with study design, assisted with data analysis approach and interpretation, and edited the manuscript.
Contributor Information
Emily C. Eastwood, Email: eastwooe@ohsu.edu.
Amanda M. Barkley-Levenson, Email: barkleya@ohsu.edu.
Tamara J. Phillips, Email: phillipt@ohsu.edu.
References
- 1.Bousman CA, Glatt SJ, Everall IP, Tsuang MT. Genetic association studies of methamphetamine use disorders: A systematic review and synthesis. Am J Med Genet B Neuropsychiatr Genet. 2009;150B(8):1025–1049. doi: 10.1002/ajmg.b.30936. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler JM, Reed C, Burkhart-Kasch S, Li N, Cunningham CL, Janowsky A, et al. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8(8):758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shabani S, McKinnon CS, Reed C, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10(6):625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shabani S, McKinnon CS, Cunningham CL, Phillips TJ. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology. 2012;62(2):1134–1141. doi: 10.1016/j.neuropharm.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology. 2012;62(7):2169–2177. doi: 10.1016/j.neuropharm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pastor R, Kamens HM, McKinnon CS, Ford MM, Phillips TJ. Repeated ethanol administration modifies the temporal structure of sucrose intake patterns in mice: effects associated with behavioral sensitization. Addict Biol. 2010;15(3):324–335. doi: 10.1111/j.1369-1600.2010.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ford MM, Fretwell AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, et al. The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology. 2009;57(3):250–258. doi: 10.1016/j.neuropharm.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABA(A) receptors differentially modulate Ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29(9):1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barkley-Levenson AM, Crabbe JC. Ethanol drinking microstructure of a high drinking in the dark selected mouse line. Alcohol Clin Exp Res. 2012;36(8):1330–1339. doi: 10.1111/j.1530-0277.2012.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6(1):1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 11.Samson HH, Pfeffer AO, Tolliver GA. Oral ethanol self-administration in rats: models of alcohol-seeking behavior. Alcohol Clin Exp Res. 1988;12(5):591–598. doi: 10.1111/j.1530-0277.1988.tb00248.x. [DOI] [PubMed] [Google Scholar]
- 12.Samson H, Files F, Brice G. Patterns of ethanol consumption in a continuous access situation: the effect of adding a sweetener to the ethanol solution. Alcohol Clin Exp Res. 1996;20(1):101–109. doi: 10.1111/j.1530-0277.1996.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 13.Samson HH. The microstructure of ethanol drinking: genetic and behavioral factors in the control of drinking patterns. Addiction. 2000;95(Suppl 2):S61–S72. doi: 10.1080/09652140050111654. [DOI] [PubMed] [Google Scholar]
- 14.Cannon DS, Leeka JK, Block AK. Ethanol self-administration patterns and taste aversion learning across inbred rat strains. Pharmacol Biochem Behav. 1994;47(4):795–802. doi: 10.1016/0091-3057(94)90279-8. [DOI] [PubMed] [Google Scholar]
- 15.Belknap JK, Richards SP, O'Toole LA, Helms ML, Phillips TJ. Short-term selective breeding as a tool for QTL mapping: ethanol preference drinking in mice. Behav Genet. 1997;27(1):55–66. doi: 10.1023/a:1025615409383. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe AL, Phillips TJ. Central urocortin 3 administration decreases limited-access ethanol intake in nondependent mice. Behav Pharmacol. 2009;20(4):346–351. doi: 10.1097/FBP.0b013e32832f01ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL., 2nd GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88(1):105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84(1):53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Bell DS. The experimental reproduction of amphetamine psychosis. Arch Gen Psychiatry. 1973;29(1):35–40. doi: 10.1001/archpsyc.1973.04200010020003. [DOI] [PubMed] [Google Scholar]
- 20.Cruickshank CC, Dyer KR. A review of the clinical pharmacology of methamphetamine. Addiction. 2009;104(7):1085–1099. doi: 10.1111/j.1360-0443.2009.02564.x. [DOI] [PubMed] [Google Scholar]
- 21.Cannon DS, Carrell LE. Rat strain differences in ethanol self-administration and taste aversion learning. Pharmacol Biochem Behav. 1987;28(1):57–63. doi: 10.1016/0091-3057(87)90012-8. [DOI] [PubMed] [Google Scholar]
- 22.Cannon DS, Carrell LE. Effect of taste aversion learning on ethanol self-administration. Pharmacol Biochem Behav. 1987;28(1):53–56. doi: 10.1016/0091-3057(87)90011-6. [DOI] [PubMed] [Google Scholar]
- 23.Gubner NR, Reed C, McKinnon CS, Phillips TJ. Unique genetic factors influence sensitivity to the rewarding and aversive effects of methamphetamine versus cocaine. Behav Brain Res. 2013;256:420–427. doi: 10.1016/j.bbr.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW. Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res. 2008;32(10):1824–1838. doi: 10.1111/j.1530-0277.2008.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eastwood EC, Phillips TJ. Morphine intake and the effects of naltrexone and buprenorphine on the acquisition of methamphetamine intake. Genes Brain Behav. 2013;13(2):226–235. doi: 10.1111/gbb.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommers I, Baskin D, Baskin-Sommers A. Methamphetamine use among young adults: health and social consequences. Addict Behav. 2006;31(8):1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Semple SJ, Patterson TL, Grant I. Binge use of methamphetamine among HIV-positive men who have sex with men: pilot data and HIV prevention implications. AIDS Educ Prev. 2003;15(2):133–147. doi: 10.1521/aeap.15.3.133.23835. [DOI] [PubMed] [Google Scholar]


