Abstract
During the development of the nervous system, neurons encounter signals that inform their outgrowth and polarization. Understanding how these signals combinatorially function to pattern the nervous system is of considerable interest to developmental neurobiologists. The Wnt ligands and their receptors have been well characterized in polarizing cells during asymmetric cell division. The planar cell polarity (PCP) pathway is also critical for cell polarization in the plane of an epithelium. The core set of PCP genes include members of the conserved Wnt signaling pathway, such as Frizzled and Disheveled, but also the cadherin-domain protein Flamingo. In Drosophila, the Fat and Dachsous cadherins also function in PCP, but in parallel to the core PCP components. C. elegans also have two Fat-like and one Dachsous-like cadherins, at least one of which, cdh-4, contributes to neural development. In C. elegans Wnt ligands and the conserved PCP genes have been shown to regulate a number of different events, including embryonic cell polarity, vulval morphogenesis and cell migration. As is also observed in vertebrates, the Wnt and PCP genes appear to function to primarily provide information about the anterior to posterior axis of development. Here we review the recent work describing how mutations in the Wnt and core PCP genes affect axon guidance and synaptogenesis in C. elegans.
Keywords: Disheveled, Flamingo, Frizzled, Planar cell polarity, Wnt
Introduction
Neural networks are dependent on connections made between neurons and their targets. Neurons use hierarchical sets of cues to interpret the body axes in order to migrate to their final position and subsequently extend axons and/or dendrites to target fields. Secreted factors like the Wnt family of glycoproteins have been shown in multiple contexts to provide polarizing signals to guide asymmetric cell division, directional cell migration, axon outgrowth and synaptogenesis (Cadigan and Nusse, 1997; Eisenmann, 2005; Park and Shen, 2012). Wnts are secreted from discrete sets of cells during development, and can diffuse away from the source in a graded fashion. Thus, positional information can be sensed by cells as they interact with the Wnt gradient. Wnts have been shown to function as both attractants and repellents, indicating cells can respond differentially to Wnts (Lyuksyutova et al., 2003; Liu et al., 2005; Li et al., 2009; Vivancos et al., 2009; Morton et al., 2012).
Cells sense and transduce Wnt signals via three overlapping sets of pathways including canonical Wnt signaling, non-canonical and the Wnt-Ca2+ pathways (Eisenmann, 2005; De, 2011; Sakaki et al., 2012). The canonical Wnt signaling pathway uses the Frizzled family of seven transmembrane receptors to activate Disheveled, a cytoplasmic adaptor protein, to ultimately regulate the stability of β-catenin, which can translocate to the nucleus and regulate target gene expression. The non-canonical pathway is typically β-catenin independent, whereas the Wnt-Ca2+ pathway uses calcium-dependent secondary messengers, e.g. calmodulin kinase II (CaMKII).
Frizzled and Disheveled are also core components of the Planar Cell Polarity (PCP) pathway (Usui et al., 1999; Shimada et al., 2001). PCP was originally described in insects because mutations in these genes caused disorganization of epithelial cells and their appendages (for an excellent introduction to PCP see (Vladar et al., 2009)). Most of our understanding of PCP has emerged from work in the wing epithelium as cells are polarized along the proximal-distal axis and in the retina as the ommatidia are polarized in the dorsal-ventral axis. PCP-like events have since been described in other tissues and systems, including vertebrates (Liebersbach and Sanderson, 1994; Kreidberg, 2002; Wang et al., 2004; Kuriyama and Mayor, 2009; Noguer et al., 2009; Simon et al., 2010; Sugiyama et al., 2011).
Two interacting sets of PCP protein have been described (see Table 1). The first involves Flamingo (Fmi), Frizzled (Fz), Disheveled (Dsh), Van Gogh (Vang), Prickle (Pk) and Diego (Dgo) (Kreidberg, 2002). These proteins form two discrete complexes, one containing Fmi/Fz/Dsh/Dgo and the other containing Fmi/Vang/Pk. Ultimately the complexes become asymmetrically localized, with the Fz group on the distal side of cells and the Vang complex on the proximal side, via a process that is not completely understood. However, the asymmetrical localization of these complexes can be transmitted to adjacent cells, leading to a field of polarized cells.
Table 1.
Core PCP Genes in C. elegans
| Gene Family | Abbreviation | Description C. elegans Homolog(s) |
||
|---|---|---|---|---|
| Frizzled/Flamingo Core Molecules | ||||
| Diego | Dgo | Ankyrin-repeat protein, scaffold for PCP complex | ||
| unc-44 a | ||||
| Dishevelled | Dsh | Cytoplasmic scaffolding protein, central hub for multiple signaling pathways | ||
| dsh-1 | ||||
| dsh-2 | ||||
| mig-5 | ||||
| Flamingo | Fmi | Seven-pass TM non-classical cadherin with cadherin, LamG, EGF domains | ||
| fmi-1 | ||||
| Frizzled | Fz | Seven-pass TM protein, binds Dsh at plasma membrane | ||
| cfz-2 | ||||
| lin-17 | ||||
| mig-1 | ||||
| mom-5 | ||||
| Prickle | Pk | Cytoplasmic LIM domain protein, binds Dsh, Vang | ||
| prkl-1 | ||||
| Van Gogh | Vang | Four-pass TM protein, binds Pk, Dgo and Dsh | ||
| vang-1 | ||||
| Fat/Dachsous Core Molecules | ||||
| Fat | Ft | Cadherin, LamG, EGF domains, binds Dach | ||
| cdh-3 | ||||
| cdh-4 | ||||
| Dachsous | Ds | Cadherin-domains, functions with Fat, often in parallel to Fmi-Fz pathway | ||
| cdh-1 | ||||
| Four-jointed | Fj | Type II TM protein, localized to Golgi, has kinase activity | ||
| none | ||||
| Wingless Signaling Components c | ||||
| Wingless b | Wg | Secreted growth factor, involved in many developmental patterning events | ||
| cwn-1 | ||||
| cwn-2 | ||||
| egl-20 | ||||
| lin-44 | ||||
| mom-2 | ||||
| Armadillo | Arm | β-catenin, functions in cell adhesion and transcriptional activation downstream of Wnt signaling | ||
| bar-1 | ||||
| hmp-2 | ||||
| wrm-1 | ||||
| Ryk/Derailed | Ryk | Receptor tyrosine kinase | ||
| lin-18 | ||||
| Ror kinase | Ror | Receptor tyrosine kinase orphan related, can act as Wnt receptor and Wnt antagonist | ||
| cam-1 | ||||
| Axin | Axin | Part of the β-catenin destruction complex | ||
| axl-1 | ||||
| pry-1 | ||||
| Glycogen Synthase Kinase | GSK3β | Part of the β-catenin destruction complex | ||
| gsk-3 | ||||
| SRFP Secreted frizzled related protein | SRFP | Secreted Wnt antagonist, can bind Wnts extracellularly | ||
| sfrp-1 | ||||
Not a direct homolog, but has high sequence identity in functional domains
Wingless seems not to function in Drosophila PCP, but can in some vertebrates.
A second set of PCP genes that function in an intersecting pathway include another set of cadherin-domain containing proteins, Fat and Dachsous, and Four-jointed a type II membrane protein resident in the Golgi that can phosphorylate Fat and Dachsous (Matakatsu and Blair, 2004; Simon, 2004). There is evidence that the Fat/Ds/Fj group is asymmetrically localized within cells (Brittle et al., 2012), and there is also evidence for Fat signaling through tissues in a graded fashion (Matakatsu and Blair, 2004; Ambegaonkar et al., 2012). The exact pathways by which Fat/Ds/Fj signal to cells remains a work in progress, although it uses the Dachs myosin protein, and interacts with the Hippo signaling pathway (Matakatsu and Blair, 2008; Kuriyama and Mayor, 2009).
It is only recently that Wnt ligands, Wg and dWnt4, have been linked to PCP in Drosophila (Wu et al., 2013), while Wnts have been found in PCP-like events in vertebrates (Witzel et al., 2006; Vivancos et al., 2009; Blakely et al., 2011). Many of the genes and proteins identified to function in PCP and Wnt signaling have since been shown to contribute to neuronal development in both vertebrates and invertebrates (Lindwall et al., 2007; Salinas and Zou, 2008; Yang and Luo, 2011; Park and Shen, 2012; Salinas, 2012; Zou, 2012). Understanding the exact contribution of these molecules and their interactions has been facilitated by the use of model systems that permit a molecular genetic analysis.
Wnt signaling has been well characterized in C. elegans. The first Wnt ligand functionally characterized in C. elegans was lin-44, when it was demonstrated that it affected the specification of a set of asymmetric daughter cells (Herman et al., 1995). Since then Wnt signaling has been shown to regulate different asymmetric cell division events during both embryonic and larval development (Whangbo et al., 2000; Park et al., 2004; Chang et al., 2005; Wu and Herman, 2006; Sawa, 2012), vulval morphogenesis (Eisenmann et al., 1998; Sternberg, 2005; Seetharaman et al., 2010), intestinal development (Fukushige et al., 2005) and neuroblast migration (Herman et al., 1999; Herman, 2001; Ch'ng et al., 2003; Forrester et al., 2004; Cabello et al., 2010; Harterink et al., 2011). In C. elegans the five Wnt ligands are expressed in overlapping domains along the anterior-posterior axis during the time when axon outgrowth is occurring (Harterink et al., 2011).
Wnts/PCP contribute to neural patterning in C. elegans
The C. elegans nervous system provides an excellent platform to study the molecular mechanisms of neural development. In wild-type hermaphrodites there are 302 neurons, and the complete cell lineage for each is known. Cell-type specific markers provide a simple method to visualize neurons during development. Finally, neurons are in highly reproducible positions in the animal and the pattern of axon outgrowth and synapse formation is stereotyped. Thus, even subtle changes to the organization of the nervous system can be detected. Recent work using in situ hybridization has provide a highly detailed spatial map of Wnt ligand expression during embryogenesis and the first larval stage (L1) (Harterink et al., 2011) (Fig. 1A, B). It is in these stages that most of the axon patterning is occurring. Mutations in Wnt and/or PCP core genes cause defects in the formation of the C. elegans nervous system, affecting cell migration, axon guidance and synaptogenesis. Unless otherwise indicated the defects discussed were identified in backgrounds using cell-specific promoters driving fluorescent proteins, suggesting that cell identity/specification of these neurons is grossly normal in the mutant animals being examined.
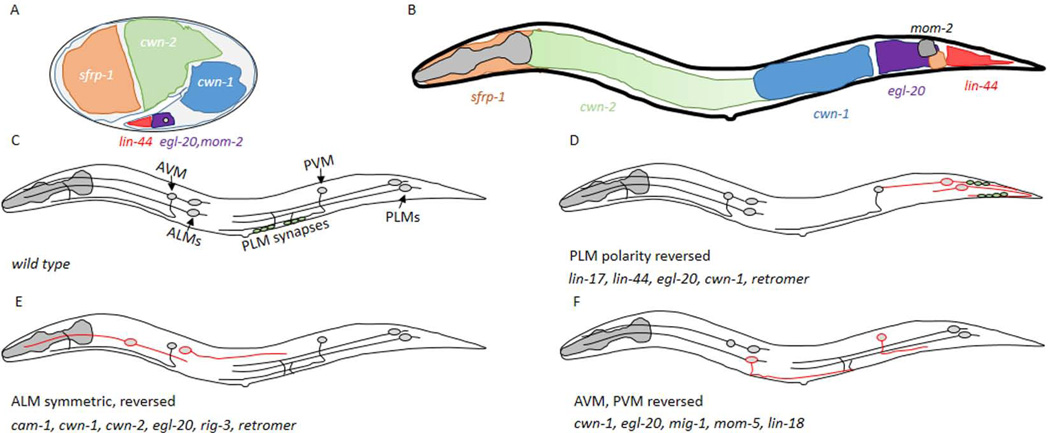
Figure 1. The mechanosensory neurons are dependent on Wnt signaling.
A,B) A depiction of the areas that express the five different Wnt ligands during embryogenesis (A) and the L1 stage (B), based on in situ data from (Harterink et al., 2011). The grey circle in (A) indicates the approximate position of the PLM when axon outgrowth begins. Anterior is to the left and dorsal is up in all panels of this schematic. C) A cartoon of the six mechanosensory neurons present in C. elegans. The ALM neurons are in the anterior of the animal, the PLM neurons are posterior and the AVM and PVM cells are more centrally located. The position where PLM neurons make synapses is indicated by the green circles. D) In lin-17, lin-44, egl-20 mutants, or when the retromer complex was inactive, the PLM axons were polarized posteriorly. The axons were either symmetric or the posterior process was longer. The presynaptic protein, SNB-1 (green circles) was not aberrantly found posterior to the cell body. E) ALM neurons have migration and/or axon outgrowth defects in different Wnt signaling mutants. F) AVM and PVM make normal ventral projections, but have polarized growth errors in Wnt signaling mutants.
Multiple Wnts and Frizzled regulate polarized growth in mechanosensory axons
The first observation that Wnt signaling affects axon patterning was that loss of function (LOF) mutations in several Wnt ligands affect the patterning of the six mechanosensory neurons (Fig. 1) (Hilliard and Bargmann, 2006; Pan et al., 2006; Prasad and Clark, 2006). PLM neurons, normally extend a long axon anteriorly and short posterior dendrite. In lin-44 mutants ~80% of PLM neurons have long posteriorly-directed processes. In approximately half of the affected PLM neurons, two roughly symmetrically sized anterior and posterior processes were present, while in the other half the cell appeared completely reversed with a long posterior process and a short anterior one (Fig 1D). In addition, a presynaptic marker, SNB-1∷GFP, which is normally found in the ventral branch of the anterior axonal compartment, was mislocalized to the posterior process in lin-44 mutants, consistent with a complete reversal of cell polarity (Hilliard and Bargmann, 2006).
Since lin-44 is normally expressed in the posterior of the animal, LIN-44 could simply be a repellant for the PLM processes. However, the polarity phenotype is rescued when LIN-44 is expressed in regions posterior or anterior to the PLM, even if expressed throughout the animal using a heat-shock promoter. This suggested that LIN-44 was unlikely to be simply providing spatial information to guide the PLM axon anteriorly. Rather LIN-44 seems to be permissive for PLM anterior outgrowth and for preventing presynaptic components to accumulate in the posterior compartment.
To understand how PLM axons receive Wnt signals mutations in the different Frizzled receptors and the Ryk kinase (lin-18) were analyzed. Only one Frizzled gene, lin-17, affected PLM in a fashion similar to lin-44. In lin-17 mutants the PLM cells extended long posterior processes and contained presynaptic components. Unlike the LIN-44 ligand, the localization of LIN-17 is required asymmetrically. A cell autonomously rescuing LIN-17∷mRFP chimera was enriched in the posterior process. However, in lin-44 mutant LIN-17 is no longer asymmetrically localized, suggesting LIN-44 polarizes LIN-17 accumulation in the cell. When LIN-17 was overexpressed, such that it was symmetrically localized throughout the cell, defects in PLM polarization were observed, including elongated processes in both directions and ectopic neurites projecting from the cell body or other processes. This data suggests that in normal conditions LIN-17 functions to inhibit neurite outgrowth, but that at high concentrations LIN-17 can promote axon outgrowth.
Subsequent analysis demonstrated that two additional Wnt ligands, egl-20 and cwn-1, function in a partially redundant fashion with lin-44, to influence PLM development. Double or triple mutants of lin-44 with egl-20 and cwn-1 strongly enhance the PLM polarity defects. Since egl-20 and cwn-1 are expressed in regions anterior to the PLM, this also argues against a simple model whereby Wnts are directly binding LIN-17 to signal positional information. One possibility is that LIN-44 function is required early to polarize LIN-17 to the posterior of the PLM cell body and that this permits EGL-20 and CWN-1 to impart spatial information to drive axon growth anteriorly, perhaps in a LIN-17-dependent fashion, although this remains to be determined.
The other mechanosensory neurons (ALMs, AVM and PVM) also depend on overlapping sets of Wnt ligands and Frizzled receptors for axon patterning (Fig. 1E, F). The ALM neurons are located in the anterior of the animal, and have a long anterior process and a short posterior one. Unlike the PLMs, the ALMs undergo long-range migrations during embryogenesis to a position just anterior to the midbody. Animals where egl-20, cwn-1 or cwn-2 are functionally impaired in any combination, but not alone, leads to ALM neurons with apparent migration defects, but also polarity defects in correctly positioned cells (Pan et al., 2006; Prasad and Clark, 2006).
Mutations in lin-17 do not grossly affect ALM polarity, nor do two of the other Frizzleds, mig-1 or cfz-2. It is possible that these Frizzleds function redundantly, thus requiring a combination of mutations to observe an effect. Alternatively, another Frizzled, mom-5, could contribute to ALM outgrowth. However, assessing the contribution of MOM-5 to embryonic neural development is complicated because embryos lacking mom-5 die early in development (Park et al., 2004). Thus, it is not entirely clear how egl-20, cwn-1 and cwn-2 affect ALM neurons, however, there is evidence that the Ror kinase, cam-1, and an Ig-superfamily member protein, rig-3, regulate Wnt signaling to ALM neurons (Babu et al., 2011).
The AVM and PVM neurons, which form during the first larval stage (L1) also exhibit axon guidance errors in Wnt mutant backgrounds (Pan et al., 2006). Both AVM and PVM are positioned in the midbody and have axons that first project toward the ventral midline, and then turn and grow anteriorly. LOF in Wnt ligands did not appear to grossly affect ventral outgrowth, but in the cwn-1;egl-20 double mutants both AVM and PVM demonstrated posteriorly directed axons at the ventral midline (Fig. 1F). These defects are also found in double mutant animals lacking both mig-1 and mom-5, suggesting these two Frizzled-like receptors function redundantly in AVM and PVM guidance. Because the AVM and PVM neurons form during the L1 stage, it was possible to examine the contribution of mom-5, where the embryonic development was maternally rescued. Double mutants of mig-1;lin-18 also caused defects in the PVM, but not the AVM neuron (Pan et al., 2006).
In contrast to LIN-44, the spatial presentation of EGL-20 is critical for ALM, AVM and PVM outgrowth. When egl-20 is expressed broadly from a heat shock promoter, the LOF phenotypes cannot be rescued, but ectopic cell migration and axon outgrowth defects are observed. Using a combination of cell-specific promoters to vary the location of egl-20 production strongly indicate that axons are repelled by EGL-20 (Hilliard and Bargmann, 2006; Pan et al., 2006). The effects of the ectopic egl-20 expression are dependent on mig-1 and mom-5 consistent with these acting as EGL-20 receptors. It is also supportive of a model whereby early LIN-44 signaling polarizes LIN-17 so that EGL-20 can repel axons from the posterior. It remains to be identified how the more anterior cells (ALMs, AVM, and PVM) that are not exposed to a LIN-44 gradient become polarized to appropriately respond to the other Wnt ligands.
lin-44 and egl-20 regulate axon termination in the posterior of the animal via canonical Wnt signaling
LIN-44 normally functions to prevent the PLM axons from growing into the posterior of the animal. However, neurons other than the mechanosensory neurons are also sensitive to perturbations in Wnt signaling. The DD and VD GABAergic motoneurons provide inhibitory input into the body wall muscles (Fig. 2A). The embryonically derived DD neurons innervate dorsal muscles, while the larval-born VD neurons innervate the ventral musculature (Jorgensen, 2005). Although the neurons are born at separate times and derived from different lineages they share an H-shaped morphology (Fig 2C). The cell bodies are positioned along the ventral midline and the neurons extend a single process anteriorly which bifurcates to form a dorsally-directed commissural process, which bifurcates a second time at the dorsal nerve cord and forms both an anterior and posterior branch. Ultimately, the individual D-type neurons of each class become tiled such that the process from each terminate at the next cell of the same class (i.e. the VD5 neuron axons terminate anteriorly at VD4 and posteriorly at VD6).
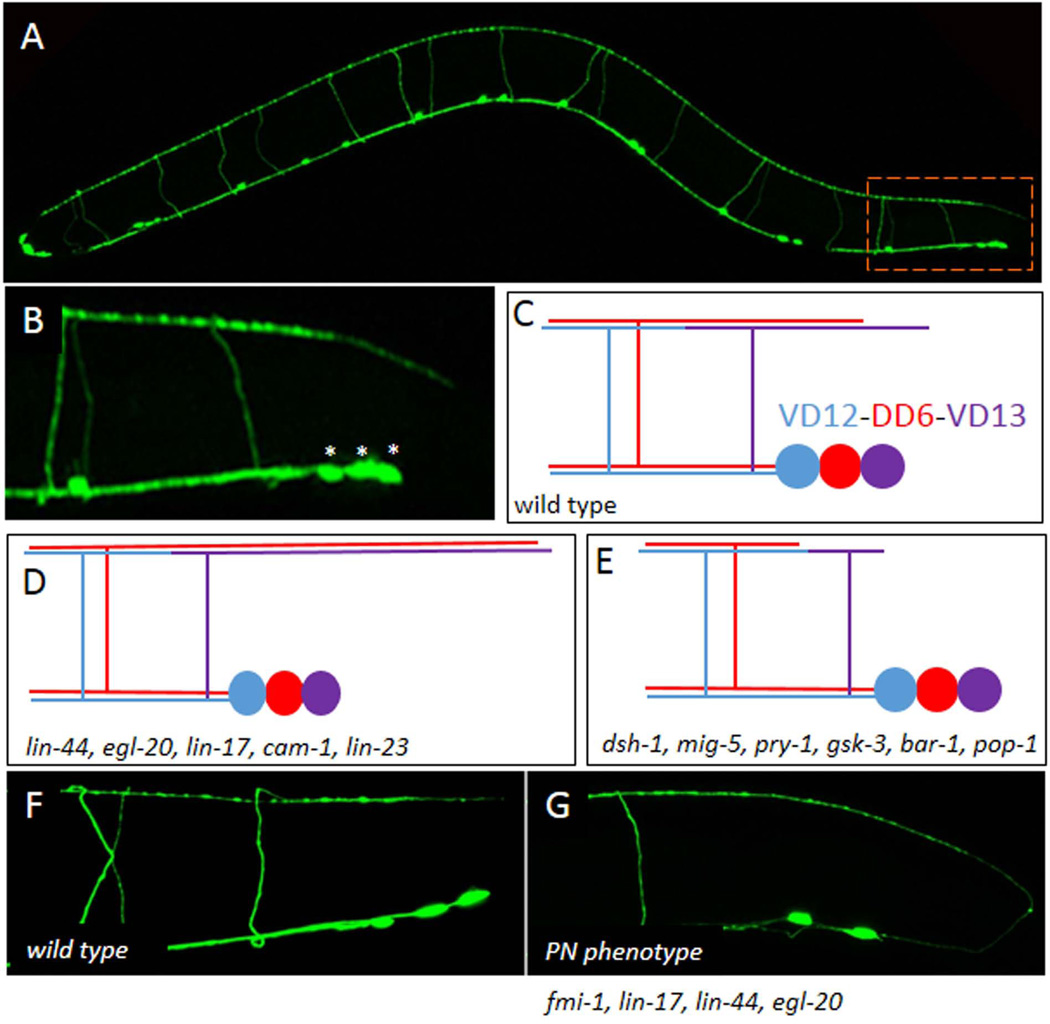
Figure 2. The GABAergic motoneurons use Wnts and FMI-1 to regulate A/P growth.
A) The Punc-25∷gfp marker illuminates the four RME neurons in the head and the six DD and 13 VD motoneurons which are organized along the ventral midline. All 19 DD and VD neurons form commissures that project to the dorsal side of the animal resulting in a ladder-like appearance. B) In the tail the most posterior neurons VD12, DD6 and VD13 (asterisks) form a cluster. The dorsal branch of the VD13 axon projects to a point approximately even with the posterior edge of the cell cluster. C) A schematic of the VD12-DD6-VD13 cluster. D) Dorsal cord overgrowth due to mutations in the Wnt genes and receptors. E) Dorsal cord undergrowth was found in the downstream signaling proteins. F) In wild-type animals all the DD and VD neurons project anteriorly. G) An example of the PN defect present in the fmi-1 and Wnt signaling backgrounds.
The most posterior of these cells, the DD6 and VD13 neurons have dorsal posterior branches that terminate at discrete points along the dorsal nerve cord (Fig. 2B). Work from Kang Shen’s lab has found that in lin-44 mutants the DD6 and/or VD13 axons terminate at a more posterior point, demonstrating an overgrowth phenotype, suggesting LIN-44 is acting as a repellent (Maro et al., 2009) (Fig. 2D). Mutations in egl-20 exacerbated the phenotype causing a more significant overgrowth. Interestingly, null mutations in three different receptors, lin-17, lin-18 or cam-1, result in an overgrowth of these processes well, with lin-17 having the biggest effect. This suggests that LIN-44 may be functioning through multiple receptors to regulate axon outgrowth in the DD and VD neurons.
Ectopic expression of LIN-44 from the egl-20 promoter, or a promoter active specifically in the dorsal muscles causes an under extension of DD processes in the L1 stage, suggesting LIN-44 can act locally to inhibit axon growth. Similarly, over expression of LIN-44 from the endogenous promoter is sufficient to induce undergrowth in DD6, and this effect depends on lin-17, suggesting it is the Frizzled mediating this activity of LIN-44.
Analysis of potential downstream signaling components suggests that signaling functions through the Disheveled homolog, mig-5. In animals lacking mig-5 the axons actually displayed an undergrowth phenotype, suggesting that LIN-44 signaling through LIN-17 normally antagonizes MIG-5 activity. It may be slightly more complicated than that, however, as lin-17 null mutants were also observed to occasionally exhibit an undergrowth phenotype as well (Fig 2E).
Genetic epistasis suggests that MIG-5 is inhibiting gsk-3/GSK3β and pry-1/Axin to regulate BAR-1 activity. bar-1 LOF results in undergrowth of the DD6 process, while increasing BAR-1 levels using mutations in lin-23 caused process overgrowth. LIN-23 is a SCFβTrCP-like E3 ubiquitin ligase that targets BAR-1 for degradation (Moghal and Sternberg, 2003). Cell-specific expression of lin-17, bar-1 and lin-23 all were consistent with a cell-autonomous function, suggesting LIN-44 and EGL-20 are acting instructively on these cells to regulate axon growth.
In vertebrates β-catenin has a dual role in cell adhesion and transcription. In C. elegans these roles appear to have been largely distributed between the different β-catenin orthologs, with bar-1 primarily affecting transcription and hmp-2 mediating cell adhesion, although some evidence exists for compensation between these two genes, suggesting each retains the ability to contribute to both functions. Mutations in pop-1, a TCF-like transcription factor cause a bar-1-like undergrowth phenotype, suggesting that BAR-1 is functioning as a transcriptional activator to regulate posterior axon growth, and not a cell adhesion protein.
FMI-1/Flamingo and Wnts function redundantly to direct initial anterior outgrowth of VD neurons
In our lab we have found that the single fmi-1/Flamingo ortholog also regulates anteroposterior axon growth in the VD neurons (Huarcaya Najarro and Ackley, 2013). Animals with mutations in fmi-1 have defects in the direction and extent of outgrowth of the VD neurites, not in the dorsal cord, but rather as they emanate from the cell body (Fig 2G), a defect we have termed posterior neurite (PN). Mutations in the Wnt signaling pathway, including lin-44, egl-20, lin-17, dsh-1, mig-5 or bar-1 all enhance the penetrance of defects in fmi-1 mutants, suggesting that these work in two parallel signaling pathways. In those experiments we observe dorsal cord under extension in dsh-1 mutants, although it is not clear if this is contributing to the canonical signaling pathway.
We do not observe PNs forming in DD neurons when using a DD-specific marker (Pflp-13∷gfp). In contrast, with a VD-selective marker (Punc-55∷gfp) we could demonstrate that the PN phenotype was prevalent in the VD neurons, and were distributed throughout the animal, i.e., not just in the posterior. This suggests that either the DD neurons are less sensitive to mutations in fmi-1 or that other proteins promote the anterior outgrowth of these neurons during embryogenesis.
We watched as the VD neurons began forming process at the end of the L1 stage. We observed that after the division of the precursor neuroblast the cell extended a lamellipodia-like process away from the midline toward the major axon bundle. This process began to extend anterior and posterior processes along the nerve bundle. In wild-type animals the posterior process was shorter than the anterior process, or not present. In contrast, at this stage of development we found that fmi-1 mutants had equivalent sized neurites or longer posterior ones. This indicates that FMI-1 functions in the earliest stages of neurite outgrowth.
Interestingly, mutations in lin-17 and lin-44 cause a dramatic increase in the penetrance of the PN phenotype in the fmi-1 mutant background, but only in the posterior most part of the animal, consistent with a local effect of LIN-44, and with LIN-44 signaling primarily through LIN-17. In contrast, removing dsh-1 from the fmi-1 mutant animals resulted in an enhancement of the PN phenotype throughout the animal. This suggests other Wnt ligands are likely to be involved in locally promoting anterior outgrowth of the VD motoneurons. In contrast, no PN phenotype is present in vang-1 or prkl-1 mutants, nor do those mutants enhance the penetrance of the PN phenotype in fmi-1, indicating that the PN phenotype arises from only a subset of the core PCP genes.
In addition to the posterior neurite phenotype we find that in fmi-1 mutants the VD neurites often fail to fully extend to their expected axon termination point (the next VD cell body anterior) in the ventral cord. This phenotype was observed in the DD neurons, indicating that these neurons can be impacted by the loss of fmi-1. As with the PN phenotype we also observe a significant enhancement of the under extended axon phenotype in fmi-1;dsh-1 double mutants. Because dsh-1 mutations result in incomplete extension of DD and VD neurons in both anterior and posterior directions in, it is likely that DSH-1 has a critical role in axon outgrowth, independent of directionality. Consistent with this, when DSH-1 is overexpressed, using the endogenous promoter, we find that the dorsal branch of the GABAergic neurons is overextended, similar to what is observed in lin-17 mutants.
The FMI-1 protein does not appear to be expressed in the VD cells, and cell rescue experiments suggest that FMI-1 is functioning cell non-autonomously to instruct VD outgrowth. The temporal and spatial requirement for FMI-1 in regulating VD outgrowth appears to be quite strict. Although the phenotype can be completely rescued using the endogenous promoter, no promoters that are active in a subset of fmi-1 expressing cells can even partially rescue the phenotype. That is true even for promoters that can rescue other phenotypes associated with the fmi-1 mutants (Steimel et al., 2010; Najarro et al., 2012; Huarcaya Najarro and Ackley, 2013).
FMI-1 has also been shown to regulate the guidance of two pairs of neurons, the PVP left and right (L/R) and PVQ (L/R) interneurons, are located in the tail and extend axons all the way to the nerve ring (Steimel et al., 2010). The PVP neurons are positioned just adjacent to the major (right) and minor (left) axon bundles of the ventral nerve cord. The axons of the PVP neurons cross over just after exiting the cell bodies, and project along the contralateral side of the animal. The PVPR axon actually pioneers the left axon tract during embryogenesis. The PVQ neurons are more posteriorly located and they extend axons posteriorly, which immediately turn ventrally and then back toward the midline, but then join the ipsilateral bundle. In wild-type animals once the axons are within the nerve fascicle they do not cross back over the midline. However, in fmi-1 mutants both PVP and PVQ axons are observed to cross over multiple times along their trek to the nerve ring. In addition, as was seen in the DD and VD motoneurons, the PVQ neurons often stopped short along their migration route. Misguided axons and short stop phenotypes were also observed in hermaphrodite specific neurons (HSNs) and the cholinergic motoneurons (DA, DB).
The requirement for FMI-1 appears to be cell autonomous in the PVP neurons, and at least partially autonomous in the PVQ neurons. The navigation errors of the PVQ follower neurons is partially rescued when FMI-1 is replaced specifically in the PVP neurons, but the short stop phenotype is not. Structure function analysis of the FMI-1 protein indicates that the intracellular domain of FMI-1 is necessary in the PVP pioneer neurons, but dispensable in the PVQ followers. Curiously, removal of parts of the extracellular domain, either the cadherin repeats, or the EGF domains, did not affect the rescue of the PVP navigation phenotypes. Overall the structure function analysis concluded that FMI-1 is unlikely to act in a homophilic binding manner to regulate pioneer-follower guidance.
Finally, to understand how FMI-1 might be functioning in PVP and PVQ guidance, Steimel et al., tested for interactions with other components of the Wnt and PCP pathways. No fmi-1-like defects were observed in cfz-2, mig-1, vang-1, prkl-1 or cdh-1 mutant animals. However, lin-17 mutants have PVP pioneer neuron guidance errors, but the effects were enhanced when both lin-17 and fmi-1 were mutated, suggesting these molecules function in parallel pathways. The story was slightly different in PVQ neurons, where only mild defects were caused by lin-17 in PVQL navigation. In PVQL, the double mutants of lin-17;fmi-1 were equivalent to fmi-1 single mutants, indicating a common genetic pathway. Overall these results indicate that are both PCP-like and novel interactions between Flamingo and Frizzleds in the development of the C. elegans nervous system.
Vang and Prickle negatively regulate neurite formation
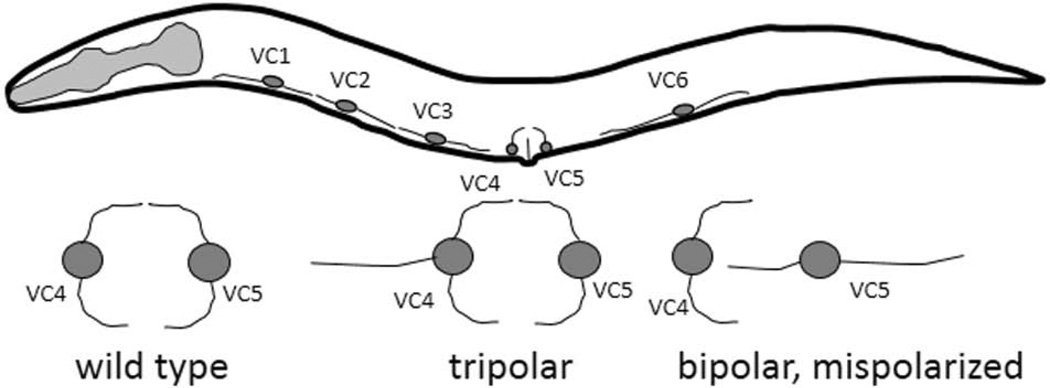
Despite the contribution of FMI-1 and multiple Frizzleds to the outgrowth of different neuron types, no role could be identified for vang-1 or prkl-1 in those contexts. It was unclear whether these core PCP components retained the ability to regulate neuronal development in C. elegans. However using the VC4 and 5 motoneurons Sanchez-Alvarez, et al., have found that VANG-1 and PRKL-1 function with DSH-1 to inhibit neurite formation. The VC neurons innervate the vulval muscles to control egg laying. Unlike VC1-3 and VC6, which extend processes along the A/P axis, VC4 and VC5 project orthogonal left-right processes in a mirror image of one another and encircle the vulva (Fig. 3).
Figure 3. The VC motoneurons have distinct morphologies.
The six VC motoneurons are organized along the ventral midline, and normally have a bipolar appearance, with VC1, 2, 3 and 6 extending process anterior/posterior, while VC4 and 5 are rotated ~90 degrees to send process out left and right. Below, are examples of the phenotypes seen in the VC4 and VC5 neurons in the vang-1, prkl-1 and dsh-1 mutants, including extra neurites (tripolar) and a reversion to a more VC1-like bipolar appearance.
In the dsh-1, vang-1 or prkl-1 mutant backgrounds the VC4 and VC5 neurons send out an extra process along the ventral midline in either an anterior (VC4) or posterior (VC5) direction, resulting in a tripolar appearance (Fig.3). In a smaller percentage of the animals, the left and right neurites were absent, but the cells formed both an anterior and posterior process, presenting a bipolar appearance oriented along the incorrect axis. The prkl-1;vang-1 double mutants were similar to either single mutant, suggesting these molecules function in the same pathway. Removing either vang-1 or prkl-1 from the dsh-1 mutants resulted in fewer tripolar neurons but more bipolar neurons with an incorrect orientation.
The test when VANG-1 and PRKL-is required the experimenters analyzed the phenotypes at different stages of development. The percentage of animals with polarity defects increased during the L4 stage, going from ~30% in early L4, when axon outgrowth begins, to 70–90% of animals in adults. Thus, rather than specifying the orientation of outgrowth, VANG-1 and PRKL-1 are required for maintenance of the acquired polarity.
Rescue experiments suggest a complex pattern of function for these genes however. To identify the tissues in which these genes were functioning they used promoters active in the VC4 and VC5 neurons (Pcat-1), a broader subset of neurons, including all the VC neurons (Punc-4) or the hypodermis (Pajm-1 or Pcol-10). The phenotype could be partially rescued from multiple promoters, with different efficiencies for each gene. For example, prkl-1 was most efficiently rescued by expression throughout the VCs, or in just VC4 and 5, but not when it was expressed in the hypodermis. Conversely, vang-1 was only partially rescued when expressed throughout the VCs or in the hypodermis, but was not rescued when expressed just in VC4 and 5. The dsh-1 rescue was most efficient when expressed hypodermally, but expression in the VC neurons or VC4 and 5 could partially repair the lesion. These results suggest overall both autonomous and non-autonomous functions for these proteins. More interestingly, it may suggest that one, VANG-1, is required earlier than PRKL-1 and DSH-1, despite the observation that the proteins are required later in development. Finally, in lines where these proteins were over-expressed the VC neurons often had only one observable process. Combining the LOF and over-expression analysis indicates that the VANG-1, PRKL-1 and DSH-1, in the VC neurons, inhibit neurite formation. This is different from what we and others have observed for DSH-1 in the adjacent VD neurons, possibly because of the interaction with VANG-1 and PRKL-1 in the VCs.
Consistent with our results suggesting that fmi-1 is required for efficient neurite outgrowth, when double mutants of vang-1 or prkl-1 were made with fmi-1 a partial suppression occurred such that fewer supernumerary processes were observed in the VC4 and VC5 neurons. This suggests that FMI-1 may function downstream of VANG-1 and PRKL-1, providing evidence that perhaps the core PCP pathway may be functioning together in axon development in C. elegans, although more work is necessary to define how these genes are interacting.
cwn-2 and cfz-2 interact to position an anterior neural structure
Neurons in the anterior of the animal are also responsive to Wnt signaling. The C. elegans nerve ring is a bundle of axons and dendrites that encircle the isthmus of the pharynx positioned in the anterior of the animal. In cwn-2 mutants the nerve ring is displaced anteriorly such that it is often found around the more anterior metacorpus of the pharynx (Kennerdell et al., 2009). Two classes of interneurons, the SIA and SIB contribute axons to the nerve ring, and these neurons appear to respond to CWN-2 to influence nerve ring position.
In the posterior of the animal LIN-44 functions to polarize the PLMs, rather than specifically repelling the axons. To understand how CWN-2 affects the nerve ring, the gene was expressed from different tissues both anterior to and posterior to the forming nerve ring. Like LIN-44 the exact source of CWN-2 was not important as ectopic expression was able to rescue the defects. This is somewhat interesting since in situ hybridization data suggests that, at the comma stage, when the nerve ring is beginning to form, cwn-2 expression appears to be posterior to the nerve ring region, while the putative Wnt antagonist, sfrp-1, is expressed in the anterior of the animal, where the nerve ring is forming. One explanation for the results of the ectopic expression is that the presence of SFRP-1 is sufficient to overcome the ectopic CWN-2, or possibly prevent it from accumulating in the anterior of the animal.
To respond to the CWN-2 signal, the SIA and SIB neurons appear to use a trio of cell-surface receptors. LOF in cam-1, mig-1 and cfz-2 all had displaced axons, and double mutant combinations gave ~100% penetrant defects, suggesting partial redundancy. The kinase domain of the CAM-1 receptor was unnecessary for its function, suggesting it may be part of a Wnt receptor complex. If so, it would seem that it is critical for the formation of that complex, as the loss of cam-1 has a highly penetrant phenotype. CAM-1 has been shown to function cell non-autonomously as a Wnt antagonist in other contexts, but CAM-1 also appears to be required cell autonomously in the SIA and SIB neurons.
The position of the nerve ring is also affected by the single Robo-like receptor in C. elegans, sax-3 (Zallen et al., 1999). LOF in sax-3 causes a highly penetrant anterior displacement of the nerve ring, although loss of the Slit ligand, slt-1, does not have an equivalent effect (Hao et al., 2001). Mutations in unc-44, a Diego-like molecule, also affects the position of the nerve ring, although it is unclear whether this is related to any PCP-like function for this molecule.
CWN-2 also appears to influence the length of neurites migrating posteriorly from the nerve ring. An allele of cwn-2 was isolated in a screen for molecules that affect the outgrowth of the RME motoneurons (Song et al., 2010). The four RME cells are positioned just posterior to the nerve ring, and two of them, RMEV and RMED extend processes toward the posterior along the ventral and dorsal midline, respectively. In cwn-2 mutants the projections of the RMED/V were either completely absent or shortened. Similar defects were observed in mutants for cfz-2 and mig-1, with the cfz-2;mig-1 double approximately as penetrant as mutations in cwn-2. The source of the CWN-2 was directionally required as expression from regions either anterior or posterior to the RME cell bodies could partially rescue the phenotype, but when expressed from anterior sources RME neurons could project anteriorly. Also, the cwn-2 phenotype could be partially rescued by expression of either CWN-1, MOM-2 or EGL-20 from the cwn-2 promoter. This suggests a flexibility in these cells to respond to the Wnt ligands during outgrowth.
In addition to the requirement for cfz-2 and mig-1, the CAM-1 and DSH-1 proteins are critical for RME outgrowth. In animals lacking cam-1 neurites were approximately half the length of wild type, and in the dsh-1 mutants or cam-1;dsh-1 double mutants neurites were <10% of the wild-type length. CAM-1 was identified to work cell autonomously in the RME neurons to respond to CWN-2, and was partially dependent on the kinase domain. By yeast two hybrid assays, CAM-1 interacts with DSH-1, with the kinase region of CAM-1 able to bind either the PDZ or DEP, but not the DAX, domain of DSH-1. By the same assay, no interaction between DSH-1 or CAM-1 was observed with the intracellular domains of either MIG-1 or CFZ-2. This suggests a model whereby CAM-1 binds to CWN-2, probably with either CFZ-2 or MIG-1 acting as co-receptors, and that this then activates DSH-1 signaling. Since the kinase domain of CAM-1 was not fully required, it is likely that other means of activating DSH-1 via this complex exist.
Dendrite outgrowth is Wnt-signaling dependent
In addition to axon guidance LIN-17 and LIN-44 also affect dendritic outgrowth in the PQR neuron, an oxygen sensing neuron that is located in the posterior of the animal. PQR has a morphology that is similar a PLM, in that it has a long anterior axon and a short posterior dendrite. In lin-44 and lin17 mutants the axon appeared grossly normal, and was directed anteriorly. However the dendrite was often absent, or projected anteriorly. A small percentage of the dendrites (<10%) contain presynaptic proteins, but not all, suggesting the effect is on the guidance of the dendrite, rather than the cell polarity.
Interestingly, lin-17 is required very early in the development of the PQR. A promoter that becomes active in PQR just prior to dendritic outgrowth (Pgcy-36) does not rescue the phenotype where as a promoter (Pegl-17) that is active in the PQR precursor cell (QR) can rescue the lin-17 defect. This might suggest an early patterning event establishes an asymmetry that is later manifested in the direction of dendritic outgrowth. Consistent with this effect, heat shock expression of LIN-44 was necessary early, around the time of hatching, to rescue the loss of lin-44. When supplied at the time of dendrite outgrowth the phenotype was not efficiently rescued.
Unlike axons, which were repelled from LIN-44, dendrites appear to be attracted toward the source of the ligand. When LIN-44 was ectopically expressed anterior to the PQR the dendrite was attracted toward the anterior. Finally, although no dendritic misdirection was observed in egl-20, cwn-1 or cwn-2 mutants, in cwn-1;lin-44 double mutants the directional defects were enhanced. In addition, cwn-1;cwn-2 mutants presented with ectopic branching from the PQR cell body and in the dendrite, independent of any directional growth errors. Overall, when integrated with the findings using the PLMs, suggest a hierarchical order of Wnt signaling where lin-44 is required early and egl-20, cwn-1 and cwn-2 function in later developmental events.
Wnts and FMI-1 regulate synaptic development
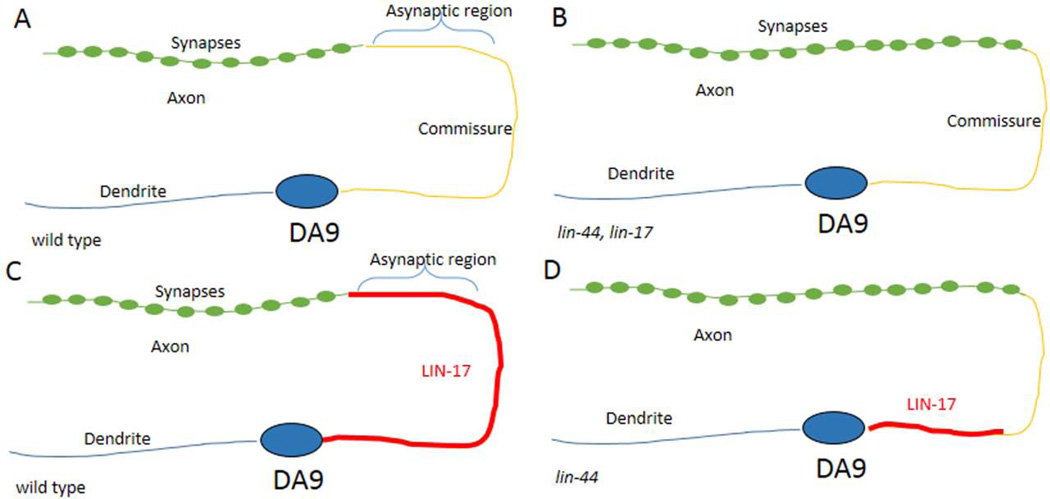
In addition to directing axon outgrowth, the Wnt signaling pathway and fmi-1 can regulate synaptogenesis, and can do so independent of their effects on axon guidance. The first evidence of this came from the observation that bar-1 regulates the abundance of the glutamate receptor, GLR-1 (Dreier et al., 2005). A more thorough examination of the Wnt pathway’s contribution to synaptogenesis was demonstrated in C. elegans using the DA9 motoneuron. DA9 is the most posterior of this class of neurons, and forms an axonal process that extends posteriorly from the cell body, then turns dorsally and then back anteriorly, to form an inverted “C” shape. The dendritic process of the DA9 extends anteriorly from the cell, but does not branch, staying along the ventral midline (Fig. 4A). The DA9 motoneurons innervate the dorsal musculature, and thus, synaptic proteins accumulate specifically along the distal aspect of the dorsal branch of the axon but are restricted from the proximal part and the commissural region. The proximal aspect of the dorsal branch is in the region of the dorsal nerve cord, which includes parts of the DA9, DA7, DD6 and VD13 axons, and is known to be asynaptic in wild-type animals based on ultrastructural reconstruction of the nervous system (Hall and Russell, 1991).
Figure 4. DA9 NMJs form in a LIN-17-dependent fashion.
A) The DA9 neuron forms in the posterior of the animal, just anterior to the domain of LIN-44 expression (not shown). The cell body extends and anterior dendrite and a posterior process that forms an inverted C. Along the dorsal cord there is an asynaptic region, followed by a more anterior axonal process that forms NMJs. B) In the lin-17 or lin-44 mutants the asynaptic domain is shortened and synapses are formed closer to the commissure. C) In wild type the LIN-17 protein decorates the posterior process along the commissure and into the asynaptic domain, but not where synapses form. D) The LIN-17 accumulation to the commissure is lin-44 dependent.
In lin-44 mutants multiple presynaptic components, including SNB-1/synaptobrevin, RAB-3, SYD-2/α-liprin and CCB-2, a presynaptic calcium channel β subunit, are displaced into the asynaptic region of the axon, toward the DA9 commissure, and similarly, presynaptic components were found in the asynaptic region of the adjacent DB7 axon, which has a different morphology. The presynaptic proteins are not present in the dendritic compartment, nor is a dendritic protein, CAM-1, found in the axon, indicating that the apico-basal polarity is not inverted in these neurons. Further, only a small fraction (<1%) of the DA9 neurons had any visible guidance defects. Overall, the data suggest that LIN-44 is preventing the formation of synapses in a discrete region of axons, one that is closest to the LIN-44 source.
In DA9 the LIN-17 receptor appears to be specifically localized to the non-synaptic regions of the commissure and the asynaptic region of the axon (Fig. 4C). In lin-44 mutants LIN-17 is mislocalized, and does not extend into the commissure, rather it remains along the ventral aspect of the posterior process (Fig. 4D). This suggests that the function of LIN-44 is to recruit LIN-17 to the commissural process. Consistent with this, expression of LIN-44 from body wall muscles recruits LIN-17 to regions where it is normally absent, and there it can locally inhibit the accumulation of synaptic components. In vertebrates there is evidence for Disheveled regulating the transport and localization of Frizzled (Shafer et al., 2011). However, in the DA9 neuron no significant difference in the LIN-17 distribution was seen in dsh-1 mutants. In those mutants there was an overlap between the LIN-17 domain and that of the presynaptic components, suggesting that, in part, LIN-17 is acting via DSH-1 to suppress synapse formation.
Multiple alleles of fmi-1 were isolated in a Synapse defective (Syd) screen (Najarro et al., 2012). These mutations result in a significant morphological defects in the GABAergic neuromuscular junctions (NMJs). By ultrastructural analysis, we found that presynaptic regions of both the cholinergic and GABAergic neurons had abnormal accumulations of electron dense material. Genetic experiments suggest that cdh-4 functions with fmi-1 to pattern the GABAergic NMJs because loss of cdh-4 caused an equivalent defect, but did not exacerbate the defects found in the fmi-1 mutants. It is not clear whether lin-44 and/or egl-20 or other Wnt signaling components can enhance fmi-1 NMJ defects like they do the A/P guidance of the VD neurons. cdh-4 was found, independently, to be necessary for multiple axon guidance and cell migration events in development (Schmitz et al., 2008).
As was found for fmi-1 in VD axon guidance the NMJ morphology defects are unlikely to occur cell autonomously. Expression of FMI-1 lacking the intracellular domain, or expression of FMI-1 from a promoter active in the cholinergic motoneurons (Pacr-2) are able to, at least partially, rescue the phenotype. The cholinergic motoneurons are adjacent to the VD neurons in the ventral nerve cord fascicle, and some make NMJs at positions very close to where the VDs make theirs. The difference between the requirement for FMI-1 broadly to affect VD outgrowth at its initial stages, but in a more limited fashion later for synaptogenesis may be simply a matter of time. The window in which the VD neuron makes its decision to grow anterior or posterior is likely to be narrow. Conversely, GABA neurons are adding synapses throughout development.
Finally, canonical Wnt signaling has been shown to regulate the pattern of synaptic partners formed in the ventral nerve cord by the VA and VB motoneurons (Schneider et al., 2012). These neurons regulate backward and forward locomotion, respectively, by making cholinergic synapses on the body wall muscles and by receiving gap junctions from specific interneurons (AVA>VA and AVB>VB) (for review see (Hobert, 2005)). The unc-4 homeodomain transcription factor functions with the unc-37/Groucho repressor to specifically promote VA-specific synaptic connectivity. In the absence of either unc-4 or unc-37, the VA neurons are converted to a VB-like fate, in terms of their choice of synaptic partners. Recent work has shown that the EGL-20 ligand signals through MIG-1 and MOM-5 Frizzleds to regulate BAR-1 levels which cell-autonomously induce VB-type inputs (Schneider et al., 2012). UNC-4 and UNC-37 function to limit the expression of MIG-1/MOM-5 in the VA neurons, inhibiting this signaling. Conversely, LIN-17 appears to be required for the proper acquisition of VA-specific synapses in those neurons, but does not appear to be via the canonical signaling pathway.
There are 12 VA neurons and 11 VB, and, with some exceptions, the individual pairs (i.e. VA2 and VB3) are generated from the same precursor neuroblast, and have cell bodies positioned in close proximity along the ventral midline. Thus, even sharing a lineage and very similar microenvironment, they respond differentially to local cues, including different ligands, using a specific set of Frizzled receptors to activate both canonical and non-canonical signaling to ensure cell-type specific synaptic partners. The AVA interneuron forms gap junctions to the VA neurons, and the AVB forms gap junctions on the VB neurons. The ability of the AVA able to “ignore” the VB axons and vice versa for the AVB and VA neurons decisions must be made along the length of the entire animal as the VA/VB cells are distributed throughout the animal on the ventral midline along A/P axis. This suggest a local control over synaptogenesis that must be discretely reiterated. Understanding how this is functioning on a local level will be fascinating to uncover.
Conclusions and Perspectives
I have summarized here the contributions of different core PCP proteins and multiple Wnt ligands, receptors and downstream signaling proteins to axon guidance and synapse development in C. elegans. The relationships between the molecules tell a complicated story of cell-specific effects that, in some cases, appear contradictory. For example, LIN-44 and LIN-17 function to prevent PLM axons from projecting into the posterior, but are required for PQR dendrites to do so.
How might Wnts be coordinately regulated to provide temporally and spatially distinct signals? Obviously, the promoter is regulated in a spatial and temporal fashion proving a first order of control. However, subsequent handling of Wnt ligands is also important. In multiple contexts the retromer complex has been shown to regulate the secretion of Wnt ligands (Coudreuse, 2006; Prasad and Clark, 2006; Pan et al., 2008; Harterink et al., 2011). Mutations in the retromer complex affect both cell migration and axon guidance, at least in part, by regulating Wnt distribution. Also, the C. elegans genome encodes a homolog of the secreted frizzled-related protein (SFRP), sfrp-1. Other molecules are also present in C. elegans that are known to affect Wnt distribution and/or activity including cam-1/Ror (Green et al., 2007), mig-14/Wntless (Eisenmann and Kim, 2000; Pan et al., 2008; Yang et al., 2008), the heparan-sulfate proteoglycans sdn-1/Syndecan, and two glypicans gpn-1 and lon-2. In addition, post-translational modification is known to affect Wnt signaling, and the myotubularin phosphatases MTM-6 and MTM-9 affect Wnt-dependent cell migration (Silhankova et al., 2010). Thus, Wnt signaling is likely to be regulated spatially and temporally on multiple levels. When coupled with the different Wnt receptors and potential downstream signaling opportunities, it is possible for the animals to use these ligands iteratively to achieve different outcomes.
It is important to note that while I have ascribed function of molecules involved in PCP in other systems, there does not appear to be a PCP-like decision occurring. Unlike a single cell present in an epithelium acquiring polarity, what appears to be occurring in the events described here are cells receiving information about body axes. It is interesting that the development of these neurons may have co-opted this system. Alternatively, the PCP pathway may have aggregated the function of multiple polarizing signals. At this point, either would be speculative, but it is an area that deserves additional experimental inquiry.
Wnts and PCP genes regulate asymmetry in tissues. In asymmetric cell division, Wnt signaling is known to affect the polarity of centrosomes and/or the mitotic spindle (Schlesinger et al., 1999; Sawa, 2012; Kim et al., 2013). Because these serve as microtubule organizing centers, and axon outgrowth is microtubule-dependent, it has been compelling to wonder if Wnt signaling is impacting axon outgrowth via positioning of the centrosome (Baas and Joshi, 1992; Ahmad et al., 1994). There appears to be a discrepancy in the literature about whether the position of the centrosome does in fact regulate axon outgrowth, although the most current evidence from in vivo studies suggest it may not be necessary (de Anda et al., 2010; Distel et al., 2010; Stiess et al., 2010; Nguyen et al., 2011; Randlett et al., 2011; Stiess and Bradke, 2011; Andersen and Halloran, 2012; Roth et al., 2012; Holcomb et al., 2013; Sakakibara et al., 2013). This, in concert with the observation that at least some of the axon phenotypes present in Wnt mutants in C. elegans requires downstream transcription, suggests a model whereby Wnt information is processed by the neurons, probably in concert with other signals, to make an informed choice about directional growth. The recent advent of live imaging techniques for watching C. elegans growth cone development should assist in a better understanding of how Wnts and PCP genes affect neurite growth (Norris and Lundquist, 2011).
What is most fascinating about the work done in C. elegans is that despite having a simple body plan, with a relatively small number of neurons, there is an incredible amount of heterogeneity in how cells respond to Wnt and PCP signals. The experiments reviewed here drive home the point of how a few ligands and receptors can be used varied spatially and temporally to create a complex neural network. Overall the mechanics of positional information provided by components of the PCP pathway and Wnts appears to be conserved in other organisms. But, like C. elegans the norm for these molecules is the spectrum of events and contributions of individual proteins is variable. However, by identifying all the different mechanisms that occur in simple models systems, we can better understand how Wnt signaling and PCP genes may contribute to vertebrate development.
Acknowledgements
BDA was supported in part by funds from The University of Kansas, P20 RR016475 from the INBRE Program of the NCRR and RC1 GM091086 from the NIH. I thank members of the Ackley lab for their critical reading of this manuscript. I regret being unable to cover the contributions of some of my colleagues due to space restrictions.
Footnotes
Competing Interests
The author declares no competing interests
References
- Ahmad FJ, Joshi HC, Centonze VE, Baas PW. Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron. 1994;12:271–280. doi: 10.1016/0896-6273(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-Fat planar cell polarity. Curr Biol. 2012;22:1302–1308. doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen EF, Halloran MC. Centrosome movements in vivo correlate with specific neurite formation downstream of LIM homeodomain transcription factor activity. Development. 2012;139:3590–3599. doi: 10.1242/dev.081513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Joshi HC. Gamma-tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J Cell Biol. 1992;119:171–178. doi: 10.1083/jcb.119.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu K, Hu Z, Chien SC, Garriga G, Kaplan JM. The immunoglobulin super family protein RIG-3 prevents synaptic potentiation and regulates Wnt signaling. Neuron. 2011;71:103–116. doi: 10.1016/j.neuron.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely BD, Bye CR, Fernando CV, Horne MK, Macheda ML, Stacker SA, Arenas E, Parish CL. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS One. 2011;6:e18373. doi: 10.1371/journal.pone.0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar polarity specification through asymmetric subcellular localization of Fat and Dachsous. Curr Biol. 2012;22:907–914. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello J, Neukomm LJ, Gunesdogan U, Burkart K, Charette SJ, Lochnit G, Hengartner MO, Schnabel R. The Wnt pathway controls cell death engulfment, spindle orientation, and migration through CED-10/Rac. PLoS Biol. 2010;8:e1000297. doi: 10.1371/journal.pbio.1000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Ch'ng Q, Williams L, Lie YS, Sym M, Whangbo J, Kenyon C. Identification of genes that regulate a left-right asymmetric neuronal migration in Caenorhabditis elegans. Genetics. 2003;164:1355–1367. doi: 10.1093/genetics/164.4.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W, Lloyd CE, Zarkower D. DSH-2 regulates asymmetric cell division in the early C. elegans somatic gonad. Mech Dev. 2005;122:781–789. doi: 10.1016/j.mod.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Coudreuse DYM. Wnt Gradient Formation Requires Retromer Function in Wnt-Producing Cells. Science. 2006;312:921–924. [Google Scholar]
- De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai) 2011;43:745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- de Anda FC, Meletis K, Ge X, Rei D, Tsai LH. Centrosome motility is essential for initial axon formation in the neocortex. J Neurosci. 2010;30:10391–10406. doi: 10.1523/JNEUROSCI.0381-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel M, Hocking JC, Volkmann K, Koster RW. The centrosome neither persistently leads migration nor determines the site of axonogenesis in migrating neurons in vivo. J Cell Biol. 2010;191:875–890. doi: 10.1083/jcb.201004154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier L, Burbea M, Kaplan JM. LIN-23-mediated degradation of beta-catenin regulates the abundance of GLR-1 glutamate receptors in the ventral nerve cord of C. elegans. Neuron. 2005;46:51–64. doi: 10.1016/j.neuron.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Eisenmann DM. Wnt signaling. WormBook. 2005:1–17. doi: 10.1895/wormbook.1.7.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann DM, Kim SK. Protruding vulva mutants identify novel loci and Wnt signaling factors that function during Caenorhabditis elegans vulva development. Genetics. 2000;156:1097–1116. doi: 10.1093/genetics/156.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann DM, Maloof JN, Simske JS, Kenyon C, Kim SK. The beta-catenin homolog BAR-1 and LET-60 Ras coordinately regulate the Hox gene lin-39 during Caenorhabditis elegans vulval development. Development. 1998;125:3667–3680. doi: 10.1242/dev.125.18.3667. [DOI] [PubMed] [Google Scholar]
- Forrester WC, Kim C, Garriga G. The Caenorhabditis elegans Ror RTK CAM-1 inhibits EGL-20/Wnt signaling in cell migration. Genetics. 2004;168:1951–1962. doi: 10.1534/genetics.104.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushige T, Goszczynski B, Yan J, McGhee JD. Transcriptional control and patterning of the pho-1 gene, an essential acid phosphatase expressed in the C. elegans intestine. Dev Biol. 2005;279:446–461. doi: 10.1016/j.ydbio.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Green JL, Inoue T, Sternberg PW. The C. elegans ROR receptor tyrosine kinase, CAM-1, non-autonomously inhibits the Wnt pathway. Development. 2007;134:4053–4062. doi: 10.1242/dev.005363. [DOI] [PubMed] [Google Scholar]
- Hall DH, Russell RL. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao JC, Yu TW, Fujisawa K, Culotti JG, Gengyo-Ando K, Mitani S, Moulder G, Barstead R, Tessier-Lavigne M, Bargmann CI. C. elegans Slit Acts in Midline, Dorsal-Ventral, and Anterior-Posterior Guidance via the SAX-3/Robo Receptor. Neuron. 2001;32:25–38. doi: 10.1016/s0896-6273(01)00448-2. [DOI] [PubMed] [Google Scholar]
- Harterink M, Kim DH, Middelkoop TC, Doan TD, van Oudenaarden A, Korswagen HC. Neuroblast migration along the anteroposterior axis of C. elegans is controlled by opposing gradients of Wnts and a secreted Frizzled-related protein. Development. 2011;138:2915–2924. doi: 10.1242/dev.064733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harterink M, Port F, Lorenowicz MJ, McGough IJ, Silhankova M, Betist MC, van Weering JR, van Heesbeen RG, Middelkoop TC, Basler K, Cullen PJ, Korswagen HC. A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol. 2011;13:914–923. doi: 10.1038/ncb2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M. C. elegans POP-1/TCF functions in a canonical Wnt pathway that controls cell migration and in a noncanonical Wnt pathway that controls cell polarity. Development. 2001;128:581–590. doi: 10.1242/dev.128.4.581. [DOI] [PubMed] [Google Scholar]
- Herman MA, Ch'ng Q, Hettenbach SM, Ratliff TM, Kenyon C, Herman RK. EGL-27 is similar to a metastasis-associated factor and controls cell polarity and cell migration in C. elegans. Development. 1999;126:1055–1064. doi: 10.1242/dev.126.5.1055. [DOI] [PubMed] [Google Scholar]
- Herman MA, Vassilieva LL, Horvitz HR, Shaw JE, Herman RK. The C. elegans gene lin-44, which controls the polarity of certain asymmetric cell divisions, encodes a Wnt protein and acts cell nonautonomously. Cell. 1995;83:101–110. doi: 10.1016/0092-8674(95)90238-4. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI. Wnt signals and frizzled activity orient anterior-posterior axon outgrowth in C. elegans. Dev Cell. 2006;10:379–390. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Hobert O. Specification of the nervous system. WormBook. 2005:1–19. doi: 10.1895/wormbook.1.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb PS, Deerinck T, Ellisman MH, Spirou GA. Construction of a Polarized Neuron. J Physiol. 2013 doi: 10.1113/jphysiol.2012.248542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huarcaya Najarro E, Ackley BD. C. elegans fmi-1/flamingo and Wnt pathway components interact genetically to control the anteroposterior neurite growth of the VD GABAergic neurons. Dev Biol. 2013 doi: 10.1016/j.ydbio.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen EM. Gaba. WormBook. 2005:1–13. doi: 10.1895/wormbook.1.14.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell JR, Fetter RD, Bargmann CI. Wnt-Ror signaling to SIA and SIB neurons directs anterior axon guidance and nerve ring placement in C. elegans. Development. 2009;136:3801–3810. doi: 10.1242/dev.038109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ishidate T, Sharma R, Soto MC, Conte D, Jr, Mello CC, Shirayama M. Wnt and CDK-1 regulate cortical release of WRM-1/beta-catenin to control cell division orientation in early Caenorhabditis elegans embryos. Proc Natl Acad Sci U S A. 2013;110:E918–E927. doi: 10.1073/pnas.1300769110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA. Applications of adhesion molecule gene knockout cell lines. Methods Cell Biol. 2002;69:309–324. doi: 10.1016/s0091-679x(02)69020-x. [DOI] [PubMed] [Google Scholar]
- Kuriyama S, Mayor R. A role for Syndecan-4 in neural induction involving ERK- and PKC-dependent pathways. Development. 2009;136:575–584. doi: 10.1242/dev.027334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebersbach BF, Sanderson RD. Expression of syndecan-1 inhibits cell invasion into type I collagen. J Biol Chem. 1994;269:20013–20019. [PubMed] [Google Scholar]
- Lindwall C, Fothergill T, Richards LJ. Commissure formation in the mammalian forebrain. Curr Opin Neurobiol. 2007;17:3–14. doi: 10.1016/j.conb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shi J, Lu CC, Wang ZB, Lyuksyutova AI, Song XJ, Zou Y. Ryk-mediated Wnt repulsion regulates posterior-directed growth of corticospinal tract. Nat Neurosci. 2005;8:1151–1159. doi: 10.1038/nn1520. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Maro GS, Klassen MP, Shen K. A beta-catenin-dependent Wnt pathway mediates anteroposterior axon guidance in C. elegans motor neurons. PLoS ONE. 2009;4:e4690. doi: 10.1371/journal.pone.0004690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. The DHHC palmitoyltransferase approximated regulates Fat signaling and Dachs localization and activity. Curr Biol. 2008;18:1390–1395. doi: 10.1016/j.cub.2008.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghal N, Sternberg PW. A component of the transcriptional mediator complex inhibits RAS-dependent vulval fate specification in C. elegans. Development. 2003;130:57–69. doi: 10.1242/dev.00189. [DOI] [PubMed] [Google Scholar]
- Morton DG, Hoose WA, Kemphues KJ. A genome-wide RNAi screen for enhancers of par mutants reveals new contributors to early embryonic polarity in Caenorhabditis elegans. Genetics. 2012;192:929–942. doi: 10.1534/genetics.112.143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarro EH, Wong L, Zhen M, Carpio EP, Goncharov A, Garriga G, Lundquist EA, Jin Y, Ackley BD. Caenorhabditis elegans flamingo cadherin fmi-1 regulates GABAergic neuronal development. J Neurosci. 2012;32:4196–4211. doi: 10.1523/JNEUROSCI.3094-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Stone MC, Rolls MM. Microtubules are organized independently of the centrosome in Drosophila neurons. Neural Dev. 2011;6:38. doi: 10.1186/1749-8104-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguer O, Villena J, Lorita J, Vilaro S, Reina M. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp Cell Res. 2009;315:795–808. doi: 10.1016/j.yexcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- Norris AD, Lundquist EA. UNC-6/netrin and its receptors UNC-5 and UNC-40/DCC modulate growth cone protrusion in vivo in C. elegans. Development. 2011;138:4433–4442. doi: 10.1242/dev.068841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Baum PD, Gu M, Jorgensen EM, Clark SG, Garriga G. C. elegans AP-2 and retromer control Wnt signaling by regulating mig-14/Wntless. Dev Cell. 2008;14:132–139. doi: 10.1016/j.devcel.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan CL, Howell JE, Clark SG, Hilliard M, Cordes S, Bargmann CI, Garriga G. Multiple Wnts and frizzled receptors regulate anteriorly directed cell and growth cone migrations in Caenorhabditis elegans. Dev Cell. 2006;10:367–377. doi: 10.1016/j.devcel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Park FD, Tenlen JR, Priess JR. C. elegans MOM-5/frizzled functions in MOM-2/Wnt-independent cell polarity and is localized asymmetrically prior to cell division. Curr Biol. 2004;14:2252–2258. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Park M, Shen K. WNTs in synapse formation and neuronal circuitry. EMBO J. 2012 doi: 10.1038/emboj.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BC, Clark SG. Wnt signaling establishes anteroposterior neuronal polarity and requires retromer in C. elegans. Development. 2006;133:1757–1766. doi: 10.1242/dev.02357. [DOI] [PubMed] [Google Scholar]
- Randlett O, Poggi L, Zolessi FR, Harris WA. The oriented emergence of axons from retinal ganglion cells is directed by laminin contact in vivo. Neuron. 2011;70:266–280. doi: 10.1016/j.neuron.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S, Bisbal M, Brocard J, Bugnicourt G, Saoudi Y, Andrieux A, Gory-Faure S, Villard C. How morphological constraints affect axonal polarity in mouse neurons. PLoS One. 2012;7:e33623. doi: 10.1371/journal.pone.0033623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki K, Yoshina S, Shen X, Han J, DeSantis MR, Xiong M, Mitani S, Kaufman RJ. RNA surveillance is required for endoplasmic reticulum homeostasis. Proc Natl Acad Sci U S A. 2012;109:8079–8084. doi: 10.1073/pnas.1110589109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara A, Sato T, Ando R, Noguchi N, Masaoka M, Miyata T. Dynamics of Centrosome Translocation and Microtubule Organization in Neocortical Neurons during Distinct Modes of Polarization. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs411. [DOI] [PubMed] [Google Scholar]
- Salinas PC. Wnt signaling in the vertebrate central nervous system: from axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas PC, Zou Y. Wnt signaling in neural circuit assembly. Annu Rev Neurosci. 2008;31:339–358. doi: 10.1146/annurev.neuro.31.060407.125649. [DOI] [PubMed] [Google Scholar]
- Sawa H. Control of cell polarity and asymmetric division in C. elegans. Curr Top Dev Biol. 2012;101:55–76. doi: 10.1016/B978-0-12-394592-1.00003-X. [DOI] [PubMed] [Google Scholar]
- Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Wacker I, Hutter H. The Fat-like cadherin CDH-4 controls axon fasciculation, cell migration and hypodermis and pharynx development in Caenorhabditis elegans. Dev Biol. 2008;316:249–259. doi: 10.1016/j.ydbio.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Schneider J, Skelton RL, Von Stetina SE, Middelkoop TC, van Oudenaarden A, Korswagen HC, Miller DM., 3rd UNC-4 antagonizes Wnt signaling to regulate synaptic choice in the C. elegans motor circuit. Development. 2012;139:2234–2245. doi: 10.1242/dev.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharaman A, Cumbo P, Bojanala N, Gupta BP. Conserved mechanism of Wnt signaling function in the specification of vulval precursor fates in C. elegans and C. briggsae. Dev Biol. 2010;346:128–139. doi: 10.1016/j.ydbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Shafer B, Onishi K, Lo C, Colakoglu G, Zou Y. Vangl2 promotes Wnt/planar cell polarity-like signaling by antagonizing Dvl1-mediated feedback inhibition in growth cone guidance. Dev Cell. 2011;20:177–191. doi: 10.1016/j.devcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–863. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- Silhankova M, Port F, Harterink M, Basler K, Korswagen HC. Wnt signalling requires MTM-6 and MTM-9 myotubularin lipid-phosphatase function in Wnt-producing cells. Embo J. 2010;29:4094–4105. doi: 10.1038/emboj.2010.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Zhang B, Sun H, Li X, Xiang Y, Liu Z, Huang X, Ding M. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Genet. 2010;6 doi: 10.1371/journal.pgen.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimel A, Wong L, Najarro EH, Ackley BD, Garriga G, Hutter H. The Flamingo ortholog FMI-1 controls pioneer-dependent navigation of follower axons in C. elegans. Development. 2010;137:3663–3673. doi: 10.1242/dev.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg PW. Vulval development. WormBook. 2005:1–28. doi: 10.1895/wormbook.1.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiess M, Bradke F. Neuronal polarization: the cytoskeleton leads the way. Dev Neurobiol. 2011;71:430–444. doi: 10.1002/dneu.20849. [DOI] [PubMed] [Google Scholar]
- Stiess M, Maghelli N, Kapitein LC, Gomis-Ruth S, Wilsch-Brauninger M, Hoogenraad CC, Tolic-Norrelykke IM, Bradke F. Axon extension occurs independently of centrosomal microtubule nucleation. Science. 2010;327:704–707. doi: 10.1126/science.1182179. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Lovicu FJ, McAvoy JW. Planar cell polarity in the mammalian eye lens. Organogenesis. 2011;7:191–201. doi: 10.4161/org.7.3.18421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, Takeichi M, Uemura T. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, Studer M, Guthrie S. Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Dev. 2009;4:7. doi: 10.1186/1749-8104-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar EK, Antic D, Axelrod JD. Planar cell polarity signaling: the developing cell's compass. Cold Spring Harb Perspect Biol. 2009;1:a002964. doi: 10.1101/cshperspect.a002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shen D, Parsons DW, Bardelli A, Sager J, Szabo S, Ptak J, Silliman N, Peters BA, van der Heijden MS, Parmigiani G, Yan H, Wang TL, Riggins G, Powell SM, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. Mutational analysis of the tyrosine phosphatome in colorectal cancers. Science. 2004;304:1164–1166. doi: 10.1126/science.1096096. [DOI] [PubMed] [Google Scholar]
- Whangbo J, Harris J, Kenyon C. Multiple levels of regulation specify the polarity of an asymmetric cell division in C. elegans. Development. 2000;127:4587–4598. doi: 10.1242/dev.127.21.4587. [DOI] [PubMed] [Google Scholar]
- Witzel S, Zimyanin V, Carreira-Barbosa F, Tada M, Heisenberg CP. Wnt11 controls cell contact persistence by local accumulation of Frizzled 7 at the plasma membrane. J Cell Biol. 2006;175:791–802. doi: 10.1083/jcb.200606017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Roman AC, Carvajal-Gonzalez JM, Mlodzik M. Wg and Wnt4 provide long-range directional input to planar cell polarity orientation in Drosophila. Nat Cell Biol. 2013;15:1045–1055. doi: 10.1038/ncb2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Herman MA. A novel noncanonical Wnt pathway is involved in the regulation of the asymmetric B cell division in C. elegans. Dev Biol. 2006;293:316–329. doi: 10.1016/j.ydbio.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Yang GY, Luo ZG. Implication of Wnt signaling in neuronal polarization. Dev Neurobiol. 2011;71:495–507. doi: 10.1002/dneu.20851. [DOI] [PubMed] [Google Scholar]
- Yang PT, Lorenowicz MJ, Silhankova M, Coudreuse DY, Betist MC, Korswagen HC. Wnt signaling requires retromer-dependent recycling of MIG-14/Wntless in Wnt-producing cells. Dev Cell. 2008;14:140–147. doi: 10.1016/j.devcel.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Kirch SA, Bargmann CI. Genes required for axon pathfinding and extension in the C. elegans nerve ring. Development. 1999;126:3679–3692. doi: 10.1242/dev.126.16.3679. [DOI] [PubMed] [Google Scholar]
- Zou Y. Does planar cell polarity signaling steer growth cones? Curr Top Dev Biol. 2012;101:141–160. doi: 10.1016/B978-0-12-394592-1.00009-0. [DOI] [PubMed] [Google Scholar]