Abstract
Importance
Solar ultraviolet (UV) irradiation causes photoaging, characterized by fragmentation and reduced production of type I collagen fibrils that provide strength to skin. UVB irradiation (280–320 nm) causes these changes by inducing matrix metalloproteinase (MMP)-1 and suppressing type I collagen synthesis. The role of UVA irradiation (320–400 nm) in promoting similar molecular alterations is less clear, yet important to consider, since it is 10–100 times more abundant in natural sunlight than UVB irradiation and penetrates deeper into the dermis than UVB irradiation. The majority (~75%) of solar UVA irradiation is comprised of UVA1 irradiation (340–400 nm), which is also the primary component of tanning beds.
Objective
To evaluate the effects of low levels of UVA1 irradiation, as might be encountered in daily life, on expression of MMP-1 and type I procollagen (the precursor of type I collagen).
Design
In vivo biochemical analyses after UVA1 irradiation of normal human skin.
Setting
Academic referral center.
Participants
Healthy human volunteers without skin disease.
Main Outcome(s) and Measure(s)
Skin pigmentation was measured by a color meter (chromameter) under the L* variable (luminescence), which ranges from 0 (black) to 100 (white). Gene expression in skin samples was assessed by real-time polymerase chain reaction.
Results
Lightly pigmented human skin (L*>65) was exposed up to four times (one exposure/day) to UVA1 irradiation at a low-dose (20 J/cm2), mimicking UVA levels from strong sun exposure lasting approximately two hours. A single exposure to low-dose UVA1 irradiation darkened skin slightly, and did not alter MMP-1 or type I procollagen gene expression. With repeated low-dose UVA1 irradiation, skin darkened incrementally with each exposure. Despite this darkening, two or more exposures to low-dose UVA1 irradiation significantly induced MMP-1 gene expression, which increased progressively with successive exposures. Repeated UVA1 exposures did not suppress type I procollagen expression.
Conclusions and Relevance
A limited number of low-dose UVA1 exposures, as commonly experienced in daily life, potentially promotes photoaging by affecting breakdown, rather than synthesis, of collagen. Progressive skin darkening in response to repeated low-dose UVA1 exposures in lightly pigmented individuals does not prevent UVA1-induced collagenolytic changes. Therefore, for optimal protection against skin damage, sunscreen formulations should filter all UV wavelengths, including UVA1 irradiation.
Introduction
Solar ultraviolet (UV) irradiation causes photoaging, which is characterized by skin wrinkling and laxity.1 This loss in structural support can be largely attributed to alterations in dermal connective tissue, including type I collagen fibrils, which provide strength and resiliency to skin.1
Type I collagen fibrils are synthesized as a soluble precursor (procollagen) by fibroblasts, and comprise most of the dermal extracellular matrix (ECM). Acute low-level UVB irradiation (280–320 nm), resulting in slight pinkness, but not sunburn, up-regulates matrix metalloproteinase (MMP)-1, which initiates type I collagen fibril degradation.2 UVB irradiation also up-regulates other MMPs.2, 3 Together, the actions of these enzymes can completely degrade type I collagen fibrils, impairing dermal ECM structure and function. UVB irradiation also suppresses type I procollagen synthesis, thereby promoting further loss of type I collagen fibrils.4
UVA (320–400 nm) irradiation is 10–100 times more abundant in natural sunlight than UVB irradiation and is not entirely filtered by window glass or clothing.5–7 Thus, compared with UVB irradiation, skin is exposed to more UVA irradiation, both daily and cumulatively over a lifetime.5, 8 Furthermore, UVA irradiation penetrates deeper into the dermis than UVB irradiation and, therefore, potentially causes more widespread alterations in the dermis.5
Despite these observations, the specific role of UVA irradiation in the molecular pathogenesis of photoaging remains unclear. Here, we evaluated the effects of repeated UVA irradiation on the disruption of dermal ECM integrity. We exposed skin to UVA1 irradiation (340–400 nm), as these wavelengths penetrate deeper into the dermis than UVB or UVA2 (320–340 nm). Additionally, UVA1 irradiation comprises the majority (~75%) of solar UVA irradiation and is the primary component of tanning beds.5, 9 We used low-dose UVA1 irradiation (20 J/cm2), mimicking UVA levels from strong sun exposure lasting approximately two hours.10–12 We exposed subjects up to four times (one exposure per day), so that irradiation plus tissue procurement could be completed in one week. We found that repeated low-level exposure to UVA1 irradiation promotes damage to the dermal ECM in spite of skin darkening (tanning).
Methods
The University of Michigan Institutional Review Board approved this study. Subjects were age 18 years or older (range 22–61 years, mean 43.4 ± 2.5 years), provided written informed consent, and were irradiated using a Sellamed 2000 (Sellas Medizinische Gerate GmbH, Gevelsberg-Vogelsang, Germany), with a peak output 360–390 nm, and power output distribution 94.4% UVA1 and 5.6% visible to near-infrared (400–800 nm) (Dr. Robert Sayre, Rapid Precision Testing Laboratories, Cordova, TN). To deliver a particular dose, the light source was held 20 cm from the target site and exposed to skin for an appropriate duration of time, based on the lamp’s irradiance, which was measured by a spectroradiometer (typical irradiance 55.6 mW/cm2; Sola-Scope 2000, Solatell Ltd, Croydon, England) and independently verified (Dr. Sayre, Rapid Precision Testing Laboratories). Irradiation intensity was monitored before each session by a multimeter (model 8060A, Fluke Corporation, Everett, WA). Skin pigmentation was measured by a chromameter (Minolta CR200; Minolta, Osaka, Japan) under the L* variable (luminescence).13 Gene expression was measured by real-time polymerase chain reaction, as described elsewhere.14 One-way repeated measures ANOVA with Tukey adjustment for multiple comparisons were used in data analysis. When appropriate, logarithmic transformation was applied to achieve normality. Logarithmic transformations were performed for all gene expression data. Data were analyzed using SAS v9.1 (SAS Institute Inc, Cary, NC), with statistical significance if p<0.05 for a 2-tailed hypothesis.
Results
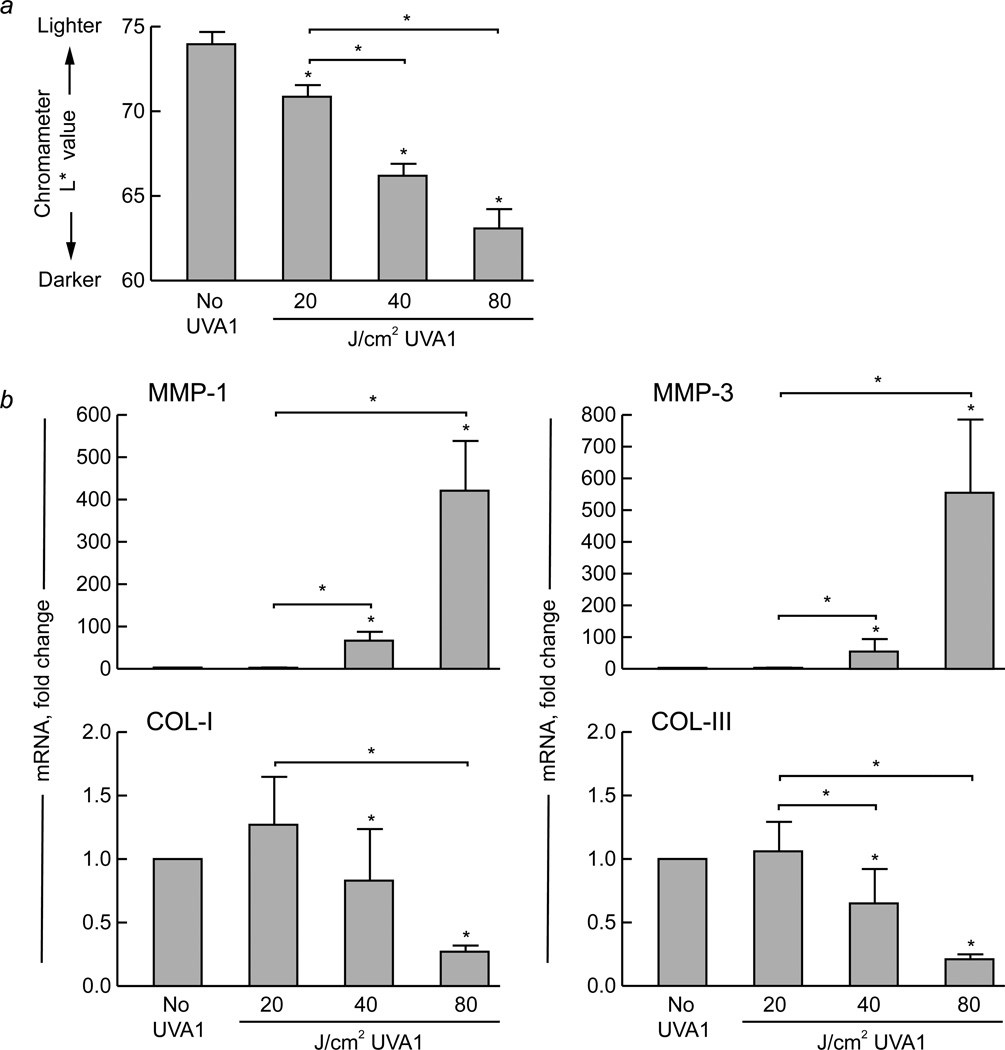
Subjects were exposed to a light source that emitted nearly pure UVA1 irradiation. There were no adverse events. In lightly pigmented human skin (defined as L*>65)14, a single exposure to low-dose UVA1 irradiation (20 J/cm2) caused slight, but significant, skin darkening at 24 hours post-exposure (p<0.05, n=10) (Figure 1a). Exposure to higher UVA1 doses (40 or 80 J/cm2) caused more skin darkening than 20 J/cm2 (p<0.05, n=10) (Figure 1a). Mild erythema occurred after low-dose UVA1 exposure, and was more prominent after exposure to higher doses.
Figure 1. Exposure of lightly pigmented human skin to UVA1 irradiation causes skin darkening, induction of matrix metalloproteinases, and suppression of procollagens in a dose-dependent fashion.
Skin pigmentation was measured using a color meter (chromameter) under the L* variable (luminescence), which ranges from 0 (black) to 100 (white). Lightly pigmented (L*>65) buttock skin of healthy human subjects (n=10) was exposed to the indicated doses of UVA1 irradiation. (a) Changes in skin pigmentation were measured 24 hours later (L* value, n=10). (b) Additionally, skin samples (4 mm) were obtained 24 hours following exposure, and real-time polymerase chain reaction was performed to assess gene expression of matrix metalloproteinase (MMP)-1, MMP-3, type I procollagen (COL-I), and type III procollagen (COL-III) (all n=10). The housekeeping gene acidic ribosomal phosphoprotein P0 (36B4) was used as an internal control. Data are presented as mean fold change + SEM. Asterisk (*) over bars, p<0.05 compared with no UVA1 irradiation. Asterisk (*) over bracket, p<0.05 when comparing response to different UVA1 doses.
Under these conditions, gene expression of MMP-1 was induced in a dose-dependent fashion, with no significant change following 20 J/cm2, and substantial up-regulation following higher doses (p<0.05, n=10) (Figure 1b). MMP-3 (stromelysin) exhibited a similar induction pattern (n=10) (Figure 1b). Additionally, type I and III procollagen expression displayed a dose-dependent response, with no down-regulation following 20 J/cm2, and significant suppression following higher doses (p<0.05, n=10) (Figure 1b).
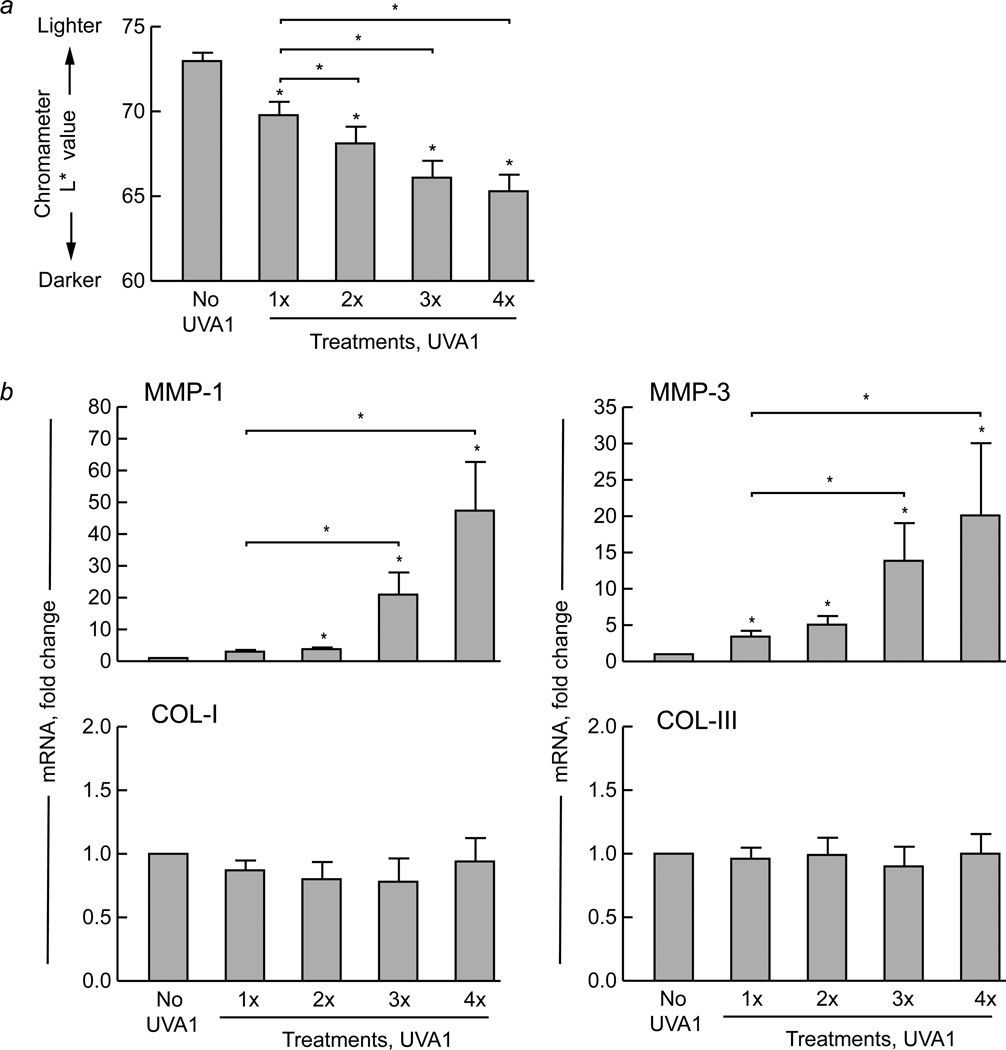
Next, we exposed lightly pigmented human skin repetitively at daily intervals to 20 J/cm2 UVA1 irradiation. Skin darkening occurred after one exposure and increased incrementally with successive exposures (p<0.05, n=12) (Figure 2a). Additionally, mild erythema occurred after the first exposure, and increased with subsequent exposures.
Figure 2. Repeated daily exposure of lightly pigmented human skin to low-dose UVA1 irradiation causes incremental darkening and progressive induction of matrix metalloproteinases.
Skin pigmentation was measured using a color meter (chromameter) under the L* variable (luminescence). Lightly pigmented (L*>65) buttock skin of healthy human subjects (n=12) was exposed to low-dose UVA1 irradiation (20 J/cm2) one, two, three, or four times at daily intervals. (a) Changes in skin pigmentation were measured 24 hours after each exposure (L* value, n=12). (b) Skin samples (4 mm) were also obtained 24 hours following each exposure, and real-time polymerase chain reaction was performed to assess gene expression of matrix metalloproteinase (MMP)-1, MMP-3, type I procollagen (COL-I), and type III procollagen (COL-III) (all n=12). The housekeeping gene acidic ribosomal phosphoprotein P0 (36B4) was used as an internal control. Data are presented as mean fold change + SEM. Asterisk (*) over bars, p<0.05 compared with no UVA1 irradiation. Asterisk (*) over bracket, p<0.05 when comparing response to different UVA1 exposures.
Under the same conditions, gene expression of MMP-1 was not significantly induced after one exposure, but exhibited significant and progressive up-regulation after two or more exposures (p<0.05, n=12) (Figure 2b). Furthermore, three or four exposures caused significantly greater MMP-1 induction than one exposure (p<0.05). MMP-3 exhibited a similar induction pattern (n=12) (Figure 2b). Induction of MMP-1 or 3 was not due to a delayed response to the first exposure, as no induction of these MMPs was observed four days after a single exposure to 20 J/cm2 UVA1 irradiation (data not shown). In contrast to MMP expression, gene expression of type I procollagen was not significantly altered after multiple exposures to low-dose UVA1 irradiation (n=12) (Figure 2b). Additionally, multiple exposures did not alter expression of type III procollagen (n=12) (Figure 2b).
Comment
In this study, we exposed lightly pigmented human skin to UVA1 irradiation at a low dose, simulating UVA levels from strong sun exposure lasting approximately two hours.10–12 We found that despite skin darkening the effects of repeated, daily low-dose UVA1 irradiation include progressive mRNA induction of MMPs that break down the dermal ECM. Of note, the mRNA induction of MMPs correlates with protein expression and enzymatic activity.3 Interestingly, this induction does not occur after a single exposure to low-dose UVA1 irradiation. Further investigation is needed to determine why MMP induction requires two or more low-dose UVA1 exposures. Apparently, the first exposure initiates cellular responses that facilitate MMP mRNA induction following subsequent exposures. Indeed, prior UVA1 exposure may boost response to the next exposure, as seen not only with the second exposure, but also with subsequent exposures beyond the second.
Furthermore, while the magnitude of MMP mRNA induction in response to low-dose UVA1 irradiation is less than that following mildly erythemogenic doses of UVB irradiation,2 our data suggest that repetitive exposure to low levels of UVA1 irradiation, as commonly experienced in daily life, could promote photoaging. Indeed, accumulation of dermal damage from repeated low-level UVA1 exposures over a lifetime likely compromises structural support of the skin. As such, regular use of sunscreen may reduce the signs of photoaging.15 Additionally, we suggest that sunscreens need to filter all UV wavelengths, including UVA1 irradiation, to protect against skin damage. Currently, the Food and Drug Administration (FDA) has approved many sunscreen ingredients that filter UVB irradiation and several that filter UVA2 irradiation, but only one chemical sunscreen ingredient (avobenzone) and one physical sunscreen ingredient (zinc oxide) are approved to provide UVA1 protection.16
Based on previous studies, in which human skin was exposed to UV wavelengths that include the UVA1 range, we suggest that the MMPs induced by low-dose UVA1 irradiation may be derived from the epidermis, and to a lesser extent the dermis.3, 19, 20 Additionally, we speculate that one of the chromophores that mediate this induction is DNA.5, 9
Interestingly, in lightly pigmented individuals, we also found that progressive skin darkening (tanning) in response to repeated low-dose UVA1 exposures does not prevent UVA1-induced collagenolytic changes. Thus, prior exposure to low levels of UVA1 irradiation from natural sunlight or tanning lamps is unlikely to protect against dermal damage caused by subsequent exposures. One reason for this observation may be that melanin absorbs long wavelengths (e.g., UVA1 irradiation) less effectively than shorter wavelengths (e.g., UVB irradiation).17 This property of melanin further indicates that optimal sunscreen formulations should provide protection against UVA1 wavelengths. This property of melanin also suggests that darkly pigmented individuals, who may have adequate UVB protection, might still benefit from protection against UVA1 irradiation.8, 18
In addition to MMP induction, we considered whether repeated low-dose UVA1 irradiation may promote photoaging by suppressing type I or III procollagen expression, as seen with UVB irradiation. Type III procollagen is the precursor to type III collagen fibrils, which associate with type I collagen fibrils in the dermal ECM and can be cleaved by MMP-1.21 In contrast with higher doses, we found no suppression of type I or III procollagen expression after a single or repeated exposures to low-dose UVA1 irradiation, suggesting that a limited number of such exposures primarily affects breakdown, rather than synthesis, of collagen fibrils. It is possible, however, that more low-dose exposures (beyond the number done here) may suppress type I or III procollagen expression. Additionally, more low-dose exposures may maintain or further induce MMP-1 and -3, while continuing to make skin darker.
Finally, our observations have implications for UVA1 phototherapy regimens used to treat excessive cutaneous collagen deposition.22 Previously, we reported that substantial skin darkening following high-dose UVA1 exposures (120–150 J/cm2) prevents sustained MMP induction, potentially limiting the efficacy of high-dose UVA1 phototherapy for fibrotic skin.14 Here, we found that repeated low-dose exposures allow sustained up-regulation of MMP-1 and -3, likely because of less skin darkening than higher doses. Thus, our observations provide a rationale for using low-dose UVA1 phototherapy, which is reported to soften fibrotic skin.23, 24
Acknowledgements
We are indebted to Heather Chubb for statistical analyses; Laura VanGoor for assistance with graphical material; and Suzan Rehbine, LPN, for tissue procurement.
Funding Sources: This study was supported by grants AG025186 (GF) and AR048077 (SK).
Abbreviations
- UV
ultraviolet
- MMP
matrix metalloproteinase
Footnotes
Author Contributions: Drs. Wang and Fisher had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wang, Smith, Kang, and Fisher. Acquisition of data: Wang, Smith, Kang, and Fisher. Analysis and interpretation of data: Wang, Tran, Smith, Kang, Voorhees, and Fisher. Drafting of the manuscript: Wang, Tran, and Fisher. Critical revision of the manuscript for important intellectual content: Wang, Smith, Tran, Kang, Voorhees, and Fisher. Statistical analysis: Wang. Obtaining funding: Kang, Voorhees, and Fisher. Administrative, technical, or material support: Wang, Smith, Kang, Voorhees, and Fisher. Supervision: Wang, Kang, Voorhees, and Fisher.
Financial disclosure: None reported.
Conflict of interest: The authors state no conflict of interest.
Contributor Information
Noah R. Smith, Email: noahsmit@med.umich.edu.
Bao Anh Patrick Tran, Email: docpat@med.umich.edu.
Sewon Kang, Email: swk@jhmi.edu.
John J. Voorhees, Email: voorhees@med.umich.edu.
Gary J. Fisher, Email: gjfisher@med.umich.edu.
References
- 1.Fisher GJ, Varani J, Voorhees JJ. Looking older: fibroblast collapse and therapeutic implications. Arch Dermatol. 2008 May;144(5):666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996 Jan 25;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 3.Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997 Nov 13;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 4.Fisher GJ, Datta S, Wang Z, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest. 2000 Sep;106(5):663–670. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tewari A, Grage MM, Harrison GI, Sarkany R, Young AR. UVA1 is skin deep: molecular and clinical implications. Photochem Photobiol Sci. 2013 Jan;12(1):95–103. doi: 10.1039/c2pp25323b. [DOI] [PubMed] [Google Scholar]
- 6.Tuchinda C, Srivannaboon S, Lim HW. Photoprotection by window glass, automobile glass, and sunglasses. J Am Acad Dermatol. 2006 May;54(5):845–854. doi: 10.1016/j.jaad.2005.11.1082. [DOI] [PubMed] [Google Scholar]
- 7.Menter JM, Hatch KL. Clothing as solar radiation protection. Curr Probl Dermatol. 2003;31:50–63. doi: 10.1159/000072237. [DOI] [PubMed] [Google Scholar]
- 8.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: a review. Photochem Photobiol Sci. 2013 Jan;12(1):54–64. doi: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 9.Tewari A, Sarkany RP, Young AR. UVA1 induces cyclobutane pyrimidine dimers but not 6-4 photoproducts in human skin in vivo. J Invest Dermatol. 2012 Feb;132(2):394–400. doi: 10.1038/jid.2011.283. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization: International Agency for Research on Cancer. [Accessed July 30, 2013];Exposure to Artificial UV Radiation and Skin Cancer. 2006. 2013 http://www.iarc.fr/en/publications/pdfsonline/wrk/wrk1/ArtificialUVRad&SkinCancer.pdf.
- 11.Halliday GM, Rana S. Waveband and dose dependency of sunlight-induced immunomodulation and cellular changes. Photochem Photobiol. 2008 Jan-Feb;84(1):35–46. doi: 10.1111/j.1751-1097.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 12.Kimlin MG, Parisi AV, Sabburg J, Downs NJ. Understanding the UVA environment at a sub-tropical site and its consequent impact on human UVA exposure. Photochem Photobiol Sci. 2002 Jul;1(7):478–482. doi: 10.1039/b200844k. [DOI] [PubMed] [Google Scholar]
- 13.Westerhof W, Estevez-Uscanga O, Meens J, Kammeyer A, Durocq M, Cario I. The relation between constitutional skin color and photosensitivity estimated from UV-induced erythema and pigmentation dose-response curves. J Invest Dermatol. 1990 Jun;94(6):812–816. doi: 10.1111/1523-1747.ep12874671. [DOI] [PubMed] [Google Scholar]
- 14.Wang F, Garza LA, Cho S, et al. Effect of increased pigmentation on the antifibrotic response of human skin to UV-A1 phototherapy. Arch Dermatol. 2008 Jul;144(7):851–858. doi: 10.1001/archderm.144.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes MC, Williams GM, Baker P, Green AC. Sunscreen and prevention of skin aging: a randomized trial. Ann Intern Med. 2013 Jun 4;158(11):781–790. doi: 10.7326/0003-4819-158-11-201306040-00002. [DOI] [PubMed] [Google Scholar]
- 16.Beasley DG, Meyer TA. Characterization of the UVA protection provided by avobenzone, zinc oxide, and titanium dioxide in broad-spectrum sunscreen products. Am J Clin Dermatol. 2010 Dec 1;11(6):413–421. doi: 10.2165/11537050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J Photochem Photobiol B. 1991 May;9(2):135–160. doi: 10.1016/1011-1344(91)80147-a. [DOI] [PubMed] [Google Scholar]
- 18.Moyal D. Need for a well-balanced sunscreen to protect human skin from both Ultraviolet A and Ultraviolet B damage. Indian J Dermatol Venereol Leprol. 2012 Jun;78(Suppl 1):S24–S30. doi: 10.4103/0378-6323.97352. [DOI] [PubMed] [Google Scholar]
- 19.Brennan M, Bhatti H, Nerusu KC, et al. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UVirradiated human skin. Photochem Photobiol. 2003 Jul;78(1):43–48. doi: 10.1562/0031-8655(2003)078<0043:mmitmc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009 Aug;14(1):20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams KE, Olsen DR. Matrix metalloproteinase-1 cleavage site recognition and binding in full-length human type III collagen. Matrix Biol. 2009 Jul;28(6):373–379. doi: 10.1016/j.matbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Fisher GJ, Kang S. Phototherapy for scleroderma: biologic rationale, results, and promise. Curr Opin Rheumatol. 2002 Nov;14(6):723–726. doi: 10.1097/00002281-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Pereira N, Santiago F, Oliveira H, Figueiredo A. Low-dose UVA(1) phototherapy for scleroderma: what benefit can we expect? J Eur Acad Dermatol Venereol. 2012 May;26(5):619–626. doi: 10.1111/j.1468-3083.2011.04137.x. [DOI] [PubMed] [Google Scholar]
- 24.Rose RF, Turner D, Goodfield MJ, Goulden V. Low-dose UVA1 phototherapy for proximal and acral scleroderma in systemic sclerosis. Photodermatol Photoimmunol Photomed. 2009 Jun;25(3):153–155. doi: 10.1111/j.1600-0781.2009.00422.x. [DOI] [PubMed] [Google Scholar]