Summary
Broadly neutralizing antibodies (bNAbs) against HIV-1 provide both effective pre-exposure prophylaxis and treatment of HIV-1 infection in murine and non-human primate models, suggesting their potential use in humans. While much is known about the role of variable domains in the neutralization breadth and potency of these bNAbs, the contribution of Fc domains to their activities is, by contrast, poorly characterized. Assessment of the in vivo activity of several bNAbs revealed that FcγR-mediated effector function contributes substantially to their capacity to block viral entry, suppress viremia and confer therapeutic activity. Enhanced in vivo potency of anti-HIV-1 bNAbs was associated with preferential engagement of activating, but not inhibitory FcγRs and Fc domain-engineered bNAb variants with selective binding capacity for activating FcγRs displayed augmented protective activity. These findings reveal key roles for Fc effector function in the in vivo activity of anti-HIV-1 bNAbs and provide novel strategies for generating bNAbs with improved efficacy.
Introduction
Passive administration of antibodies directed against the envelope protein (Env) of HIV-1 represents a promising mode of clinical intervention for this disease as a result of the recent isolation and characterization of human antibodies with exceptionally broad and potent neutralizing activity (Klein et al., 2013; Scheid et al., 2009; Walker et al., 2011). Passive administration of these anti-HIV-1 broadly neutralizing antibodies (bNAbs) has been shown to confer sterilizing immunity against SHIV challenge in macaques and HIV-1 infection in humanized mouse models (Balazs et al., 2012; Hessell et al., 2007; Mascola et al., 2000). Furthermore, effective control of viral replication in HIV-1-infected humanized mice and in SHIV-infected non-human primates by bNAbs clearly suggested their potential use as a therapeutic modality for human HIV-1 infection (Barouch et al., 2013; Horwitz et al., 2013; Klein et al., 2012b; Shingai et al., 2013).
While the neutralizing activity of an antibody has been commonly considered to be the result of Fab-antigen interactions that block viral entry, substantial evidence suggests that the in vivo activity of an antibody is highly dependent upon interactions of the IgG Fc domain with its cognate receptors, Fcγ receptors (FcγR) expressed on the surface of effector leukocytes (Abboud et al., 2010; Bournazos et al., 2014; Corti et al., 2011; DiLillo et al., 2014; Li and Ravetch, 2011; Nimmerjahn and Ravetch, 2005; Smith et al., 2012). The FcγR system represents a balance of activating and inhibitory receptors that transduce immunostimulatory or immunomodulatory signals following engagement with IgG immune complexes, determining thereby the outcome of IgG-mediated inflammation and immunity (Pincetic et al., 2014). Indeed, selective engagement of activating or inhibitory FcγRs by IgG has been shown to determine the in vivo outcome of antibody treatment, suggesting that Fc-mediated pathways play a key role in modulating the effector activity of a mAb (Li and Ravetch, 2011; Nimmerjahn and Ravetch, 2005). For example, the neutralizing activity of anti-toxin and anti-viral antibodies has been demonstrated to require selective FcγR engagement to mediate their in vivo protective activities (Akiyoshi et al., 2010; Bournazos et al., 2014; Corti et al., 2011; DiLillo et al., 2014).
Although the potential involvement of FcγR effector pathways in antibody-mediated protection against HIV-1 has been previously discussed in numerous studies (Ackerman et al., 2013; Forthal et al., 2010; Forthal et al., 2011; Lai et al., 2014; Perez et al., 2013), there is still very limited evidence on the exact contribution of Fc effector function in the protective activity of anti-HIV-1 mAbs, stemming from the lack of an in vivo model system that recapitulates FcγR functional and structural diversity. Indeed, the use of artificial in vitro systems for the study of Fc effector function poorly reflect the diversity and complexity of in vivo FcγR-mediated pathways, providing thereby limited information on the precise Fc effector mechanisms that mediate viral neutralization in vivo. To assess the contribution of Fc-mediated interactions in the in vivo activity of anti-HIV-1 bNAbs, we utilized recently developed mouse models for HIV-1 entry (Pietzsch et al., 2012) and infection (Baenziger et al., 2006; Klein et al., 2012b; Traggiai et al., 2004). These models have been previously characterized extensively and used to successfully assess the capacity of several anti-HIV-1 bNAbs to inhibit HIV-1 entry (Pietzsch et al., 2012) or suppress viremia in vivo (Horwitz et al., 2013; Klein et al., 2012b). Furthermore, the immunotherapeutic potential of anti-HIV-1 bNAbs against HIV-1 infection in these murine infection models has been shown to correlate with non-human primates (Barouch et al., 2013; Shingai et al., 2013), supporting their use for the pre-clinical evaluation of anti-HIV-1 bNAbs intended for human HIV-1 prevention or therapy.
In the present study, we assessed the role of Fc effector function in the in vivo protective activity of several anti-HIV-1 bNAbs targeting different epitopes on the HIV-1 Env protein. We found that FcγR-mediated effector function contributes significantly to their in vivo activity and clearly demonstrated that preferential engagement of activating FcγRs substantially augmented the in vivo protective activity of anti-HIV-1 bNAbs. Fc domain engineering of anti-HIV-1 bNAbs to selectively enhance their capacity to interact with activating FcγRs resulted in a substantial augmentation of their in vivo activity, when assessed in an in vivo model of HIV-1 entry (Pietzsch et al., 2012), as well as in models of mAb-mediated therapy using HIV-1-infected humanized mice.
Results
Generation and characterization of mouse-human chimeric anti-HIV-1 bNAbs for the study of in vivo Fc effector activity
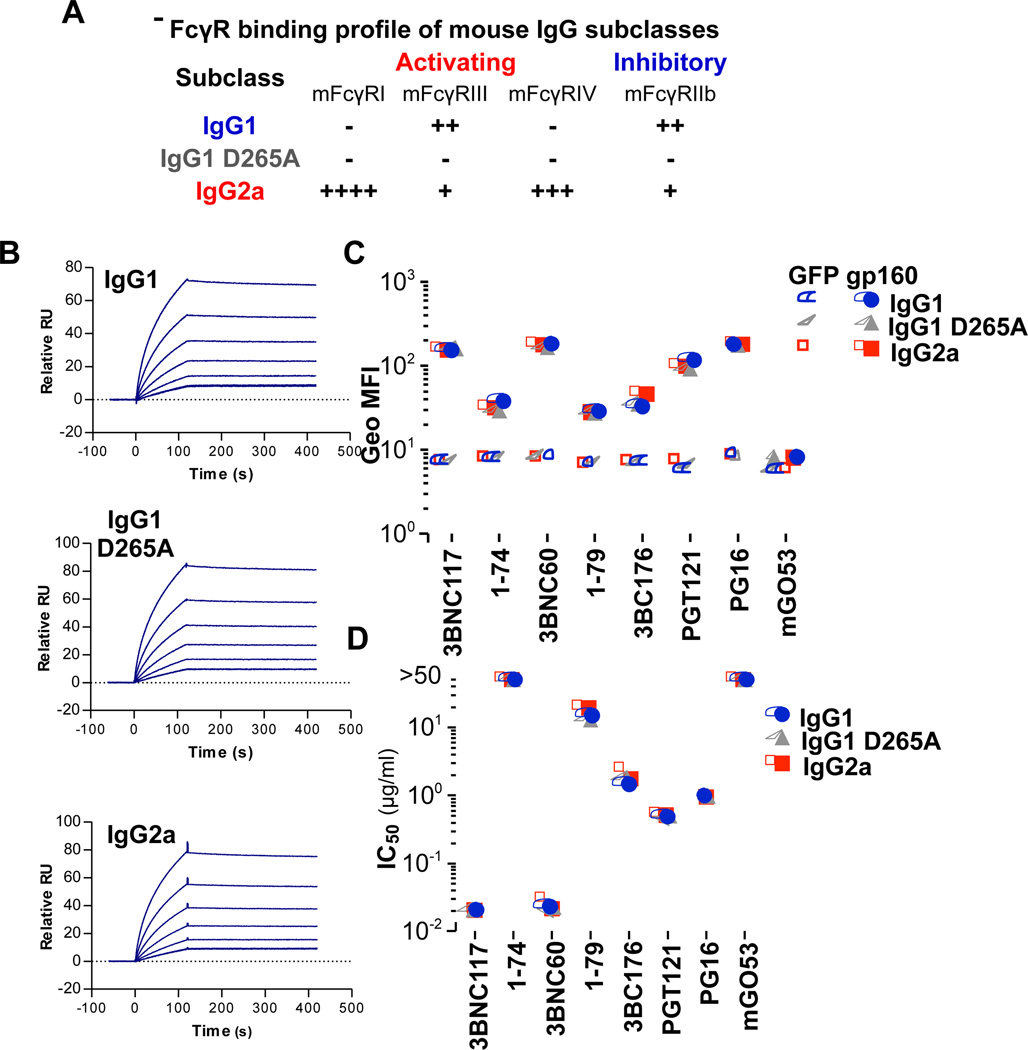
Since the binding specificity of the Fc region to the respective FcγRs is species specific, we generated mouse-human chimeric versions of several anti-HIV-1 bNAbs in which the constant region of the heavy chain (CH1–3; human IgG1) was replaced with that of different mouse IgG Fc subclass variants that exhibit differential and selective binding capacity for activating or inhibitory mouse FcγRs (Table S1; Figure 1A)(Nimmerjahn and Ravetch, 2005). This approach has been previously exploited to dissect the individual contribution of activating or inhibitory FcγR-mediated pathways in the in vivo activity of protective and immunomodulatory antibodies (DiLillo et al., 2014; Li and Ravetch, 2011). We have additionally generated a mouse IgG1 variant (D265A) with diminished binding to all classes of FcγRs for the assessment of Fc effector function in the in vivo anti-HIV-1 bNAb activity.
Figure 1. Generation and characterization of mouse-human chimeric anti-HIV-1 mAbs with differential FcγR binding profile.
Mouse-human anti-HIV-1 mAbs were generated by replacing the constant region of the heavy chain (CH1–3) of human IgG1 (parental IgG subclass) with that of mouse IgG1 or mouse IgG2a. A null FcγR binding variant of mouse IgG1 (mIgG1 D265A) was also generated. (A) Relative FcγR binding profile (as assessed by SPR analysis (Table S1)) for the various mouse IgG Fc subclass variants. The affinity of mouse-human chimeric Fc domain variants of anti-HIV-1 mAbs for recombinant gp140YU-2 was determined by SPR analysis and representative SPR sensograms for 1–74 mAb (B) are presented (gp140 concentration ranging from 31.25–1000 nM). Affinity kinetics measurements for all the other anti-HIV mAbs are presented in Table S2. (C) The capacity of Fc domain variants of mouse-human anti- HIV-1 mAbs to bind to HEK293T cells expressing gp160ΔctYU2 was assessed by flow cytometry. GFP-expressing HEK293T cells (GFP) were used to determine background mAb binding. Data represent the geometric mean fluorescence intensity (Geo MFI) from three independent experiments. See also related Figure S1 (D) In vitro neutralization activity of mouse-human anti- HIV-1 mAbs against HIV-1YU-2 was determined by a standardized TZM-bl assay (Montefiori, 2005) and IC50 values are presented. (see also Table S3).
A number of anti-HIV-1 bNAbs were selected targeting various epitopes of HIV-1 Env, exhibiting variable degree of in vitro neutralization breadth and potency (Table S3, Figure 1D). These included: the anti-CD4 binding site (CD4bs) monoclonal antibodies (mAbs) 3BNC117 (Scheid et al., 2011), 3BNC60 (Scheid et al., 2011), and 1–74 (Scheid et al., 2009); 3BC176, a mAb recognizing a conformational V3/CD4i epitope (Klein et al., 2012a), PG16, an anti-V1/2 mAb (Walker et al., 2009) and the anti-V3 mAbs 1–79 (Scheid et al., 2009) and PGT121 (Pejchal et al., 2011; Walker et al., 2011). A non-HIV-1 reactive mAb (mGO53 (Wardemann et al., 2003)) was included as control. Mouse-human chimeric mAbs were expressed in mammalian cells (HEK293T cells) and characterized in terms of antigen specificity by ELISA (Figure S1), surface plasmon resonance (Figure 1B, Table S2) using recombinant gp140, and by flow cytometry using gp160-expressing cells (gp160Δct (deleted cytoplasmic tail)-HEK293T cells) (Figure 1C) to ensure that changes in the Fc domain had no impact on their capacity for Fab-mediated antigen recognition; an effect that was previously described for other (not anti-HIV-1) mAbs (Casadevall and Janda, 2012). While these mouse Fc domain variants exhibited differential capacity to engage mouse FcγRs (Table S1; Figure 1A), no differences in their antigen affinity and specificity were observed. Furthermore, no differences in the in vitro neutralization activity were evident among the various mouse IgG subclass variants (Table S3; Figure 1D).
The in vivo protective activity of anti-HIV-1 bNAbs is dependent upon FcγR engagement
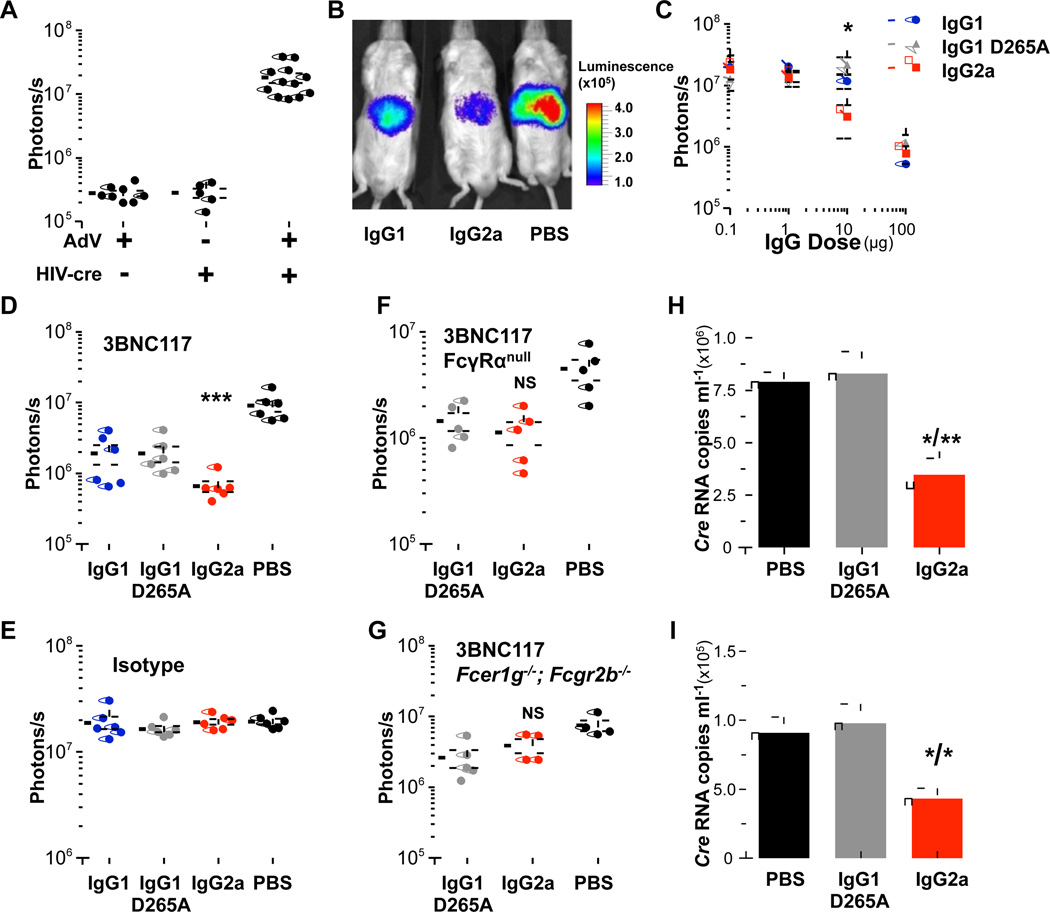
Given the differential capacity of the mouse-human chimeric IgG subclass variants of anti-HIV-1 bNAbs to engage particular classes of FcγRs, comparison of their in vivo activity would determine the specific contribution of particular FcγR classes required for optimal mAb in vivo function. Using a recently-described mouse model of HIV-1 entry (Pietzsch et al., 2012) that is based on a principle closely-related to the well-established in vitro TZM-bl neutralization assay (Montefiori, 2005), we assessed the in vivo activity of the various mouse subclass variants of anti-HIV-1 bNAbs. As determined previously (Pietzsch et al., 2012), in vivo infection of luciferase reporter mice (ROSA-Luc) with HIV-1 Cre pseudovirus was accomplished by administration of adenoviral particles (1011, i.v., 48 hours prior to HIV-1YU-2 Cre pseudovirus challenge) coding for the receptors required for HIV-1 entry (hCD4 and hCCR5)(Figure 2A). Following challenge with HIV-1 Cre pseudovirus, infection was quantified non-invasively by whole body imaging.
Figure 2. Mouse IgG2a subclass variants of 3BNC117 exhibit improved in vivo activity.
The in vivo activity of mouse-human chimeric Fc variants of 3BNC117 was assessed in a mouse model for HIV entry (Pietzsch et al., 2012) using luciferase reporter mice. (A) Infection with HIV-1YU-2 Cre pseudovirus was accomplished by adenoviral-mediated expression of human CD4 and CCR5. (B-D) Enhanced in vivo activity was observed for mouse IgG2a subclass variants of 3BNC117, compared to mouse IgG1 and mouse IgG1 D265A (B: representative in vivo luminescence image; C: 3BNC117 variants administered at different doses (0.1–100 µg/ml, i.p.), data are presented as the mean ± SEM, n=5/group, *p<0.05 mouse IgG2a vs. mouse IgG1 D265A; D: 3BNC117 administered at a single concentration (50 µg, i.p.) n=5–6/group, ***p<0.001 compared to PBS-treated group. (E) Isotype subclass variants of a non-HIV-1 reactive mAb (mGO53, 500 µg) displayed no protective activity, n=5–6/group. (F-G) No significant difference in the in vivo activity was observed between mouse IgG2a and mouse IgG1 D265A (FcγR null binding) variants of 3BNC117 (50 µg, i.p.) in two strains of FcγR deficient mice (FFcγRα null;GFcer1g−/−; Fcgr2b−/−) NS: not significant mouse IgG2a vs. PBS or mouse IgG1 D265A. (H–I) Enhanced in vivo activity of mouse IgG2a 3BNC117 was accompanied by improved virus clearance. Serum pseudovirus content (1 (H) and 3 (I) days following HIV-1YU-2 Cre pseudovirus infection) was determined using a qRT-PCR-based assay specific for Cre. Data are presented as the mean ± SEM. *p<0.05; **p<0.01 compared to PBS/mIgG1 D265A groups; n=4 mice/group.
Mouse IgG subclass variants of 3BNC117 (mouse IgG1, mouse IgG1 D265A, and mouse IgG2a) with differential capacity to engage mouse FcγRs were administered (i.p.) to luciferase reporter mice 24 h prior to challenge with HIV-1YU-2 Cre pseudovirus. Comparison of the in vivo activity of different mouse-human chimeric IgG subclass variants of 3BNC117 revealed that the mouse IgG2a variant exhibited significantly enhanced in vivo protective activity compared to its mouse IgG1 and mouse IgG1 D265A counterparts (Figure 2B–D). Titration of the mAb dose revealed a specific range at which mouse IgG2a exhibits improved in vivo activity; lower doses (<1 µg) were not sufficient to prevent infection, while at higher ones (100 µg) Fab-mediated neutralization activity dominates masking any Fc effector contribution by mouse IgG2a subclass variant (Figure 2C). Minimal activity was observed in isotype subclass variants of a non-HIV-1 reactive antibody (mGO53 (Wardemann et al., 2003))(Figure 2E).
To provide further evidence on the role of Fc-FcγR interactions in the augmented in vivo protective activity of mouse IgG2a subclass variant of 3BNC117, its activity was assessed in two mouse strains (crossed to ROSA-Luc) deficient for all classes of mouse FcγRs: (i) FcγRα null encompassing targeted deletion of the all mouse FcγR α chain-encoding genes (Fcgr1, Fcgr2b, Fcgr3, Fcgr4)(described and characterized in (Smith et al., 2012)) and (ii) Fcer1g−/−, Fcgr2b−/−double knockout mice for the FcR γ chain (required for the expression of all activating FcγRs) and FcγRIIb-encoding genes. In contrast to wild-type mice, comparison of the in vivo activity between mouse IgG2a and mouse IgG1 D265A (FcγR null binding variant, exhibiting only Fab-mediated activity) revealed no significant difference in FcγR-deficient mice (FcγRα null, Figure 2F; Fcer1g−/−, Fcgr2b−/−, Figure 2G), suggesting the involvement of FcγR-, but not complement-mediated pathways in the enhanced in vivo activity of 3BNC117 mouse IgG2a subclass variant.
Given the selective binding specificity of mouse IgG2a for activating FcγR, like FcγRIV and FcγRI, it is likely that viral opsonization by mouse IgG2a mAbs promotes their clearance by circulating or tissue-resident FcγR-expressing effector cells, contributing thereby to the observed enhanced in vivo activity of mouse IgG2a mAbs. To determine the consequences of mAb-mediated virus opsonization on clearance and subsequent prevention of viral entry, circulating viral content was measured in mice that have been administered either mouse IgG2a or mouse IgG1 D265A (FcγR null binding) variants of 3BNC117. Serum viral RNA was quantified by reverse-transcriptase quantitative PCR and as it is evident in Figure 2H–I, significantly lower viral titers were observed on day 1 and 3 post-infection in mice that have previously received mouse IgG2a 3BNC117 compared to untreated (PBS) or mouse IgG1 D265A–treated ones. These findings suggest that the observed enhancement in FcγR-mediated in vivo protective activity of mouse IgG2a 3BNC117 could be attributed to improved clearance of mAb-opsonized viruses prior to infection.
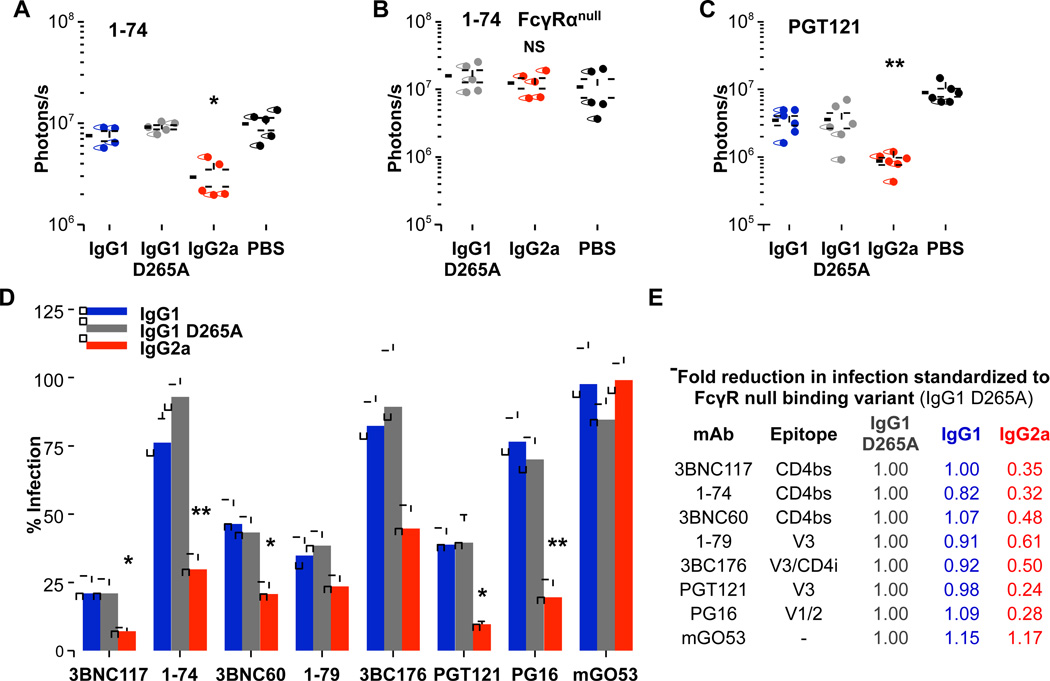
To determine whether the enhanced in vivo activity observed for the mouse IgG2a subclass variant of the broadly neutralizing anti-CD4bs mAb 3BNC117 also extends to mAbs with low neutralization potency, we assayed mouse subclass variants of 1–74, a poorly neutralizing, anti-CD4bs mAb (IC50 >50 µg/ml for HIV-1YU-2). Mouse IgG2a 1–74 mAb exhibited significantly augmented in vivo activity compared to mouse IgG1 and mouse IgG1 D265A variants (Figure 3A); an effect that was not evident in FcγR-deficient (FcγRα null) mice (Figure 3B). Similar to anti-CD4bs mAbs, increased in vivo effector activity of mouse IgG2a subclass variants was observed in mAbs with differential epitope specificities (CD4bs, V1/2, V3), indicating that augmented FcγR-mediated in vivo protective activity is independent of Fab-antigen interactions relating to epitope targeting (Figure 3C–E). Furthermore, no differences in mAb serum concentration at the time of HIV-1 Cre pseudovirus challenge were observed among the various mouse IgG subclass variants, confirming that the enhanced in vivo activity of mouse IgG2a mAbs could not be attributed to differential mAb biodistribution (Figure S2). These findings suggest a clear role for Fc effector activity of anti-HIV-1 mAbs in preventing viral entry and revealed that selective engagement of activating, but not inhibitory FcγR by mouse IgG2a subclass mAbs induces increased in vivo protective activity; an effect not dependent on antibody potency or epitope specificity.
Figure 3. Enhanced in vivo activity of mouse IgG2a Fc variants of anti-HIV-1 mAbs is not restricted by epitope specificity or neutralization potency.
Mouse Fc domain variants of anti-HIV-1 mAbs were administered to luciferase reporter mice (i.p. 3BNC60, 3BNC117: 50 µg; 1–74: 500 µg; 1–79: 350 µg; 3BNC176: 150 µg; PGT121, PG16: 200 µg; mGO53: 500 µg) 24 hrs prior to HIV-1YU-2 Cre pseudovirus infection and their in vivo activity was assessed by whole body imaging. (A–B) Significant enhancement in the in vivo activity of mouse IgG2a subclass variants of 1–74 was observed in (A) wild-type, but not in (B) FcγR-deficient mice, n=5–6/group, *p<0.05 compared to PBS-treated group; NS: not significant. (C–E) Increased in vivo potency of the mouse IgG2a variant was also evident for mAbs targeting different epitopes. (C) PGT121, n=6/group, **p<0.01 compared to PBS; (D) Overview of the in vivo activity of the different mouse IgG Fc subclass variants of the various anti-HIV-1 mAbs. Maximal infection obtained from PBS-injected mice was used to calculate % infection; data are presented as the mean ± SEM; n=5–7 mice/group, *p<0.05, **p<0.01 compared to the respective mouse IgG1 D265A mAb variant. (E) Quantitative comparison of infection (infection index: averaged % infection of mouse IgG2a- or mouse IgG1-treated over mouse IgG1 D265A) between the Fc domain variants standardized to the mouse IgG1 D265A variant of each respective mAb. See also Figure S2.
Development of human IgG1 anti-HIV-1 bNAbs with selectively enhanced FcγR binding profile through Fc domain engineering
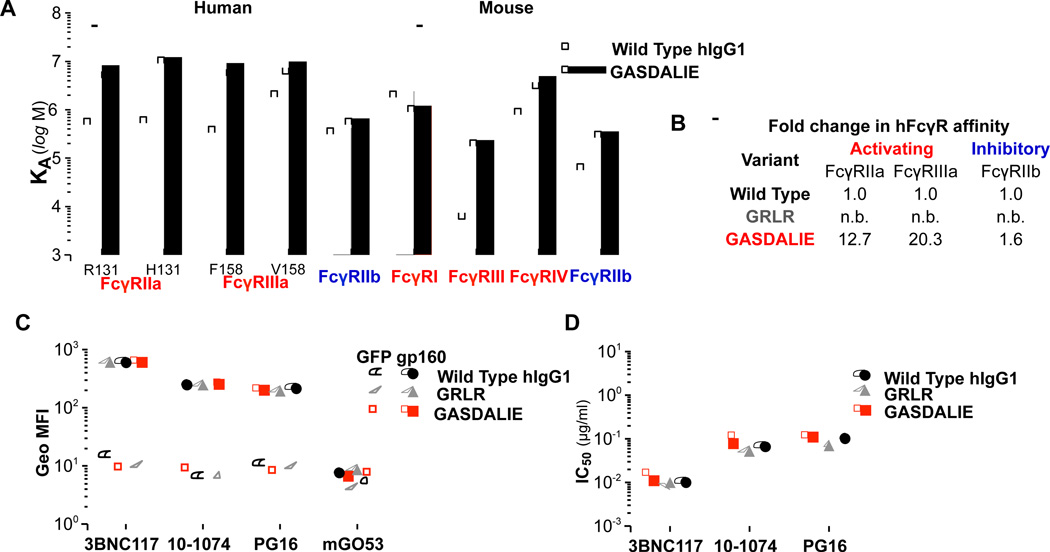
Given our finding using wild-type mice that preferential engagement of activating but not inhibitory FcγRs augmented the in vivo bNAb activity, human IgG1 Fc domain variants of anti-HIV-1 bNAbs (3BNC117, 10–1074 (Mouquet et al., 2012), and PG16) were generated to selectively engage with enhanced activity human activating FcγRs, like human FcγRIIIa and FcγRIIa, with minimal modulation of FcγRIIb binding capacity (Table S4; Figure 4A–B). Additionally, an Fc domain variant of hIgG1 that is deficient in binding to all classes of human and mouse FcγRs was generated. Wild-type hIgG1 and Fc domain variants (enhanced for activating FcγR binding; G236A/S239D/A330L/I332E (GASDALIE)(Smith et al., 2012); FcγR null binding: G236R/L328R (GRLR)(Horton et al., 2010)) of 3BNC117, 10–1074 and PG16 were expressed in mammalian cells and characterized in terms of their affinity and specificity for HIV-1 Env (Figure 4C and S3; Table S5) and in vitro neutralization (Figure 4D; Table S6). No differences in their antigen specificity and in vitro neutralization activity were detected among the various Fc domain variants, despite their differential capacity to engage the various human and mouse FcγRs (Figure 4A, Table S4).
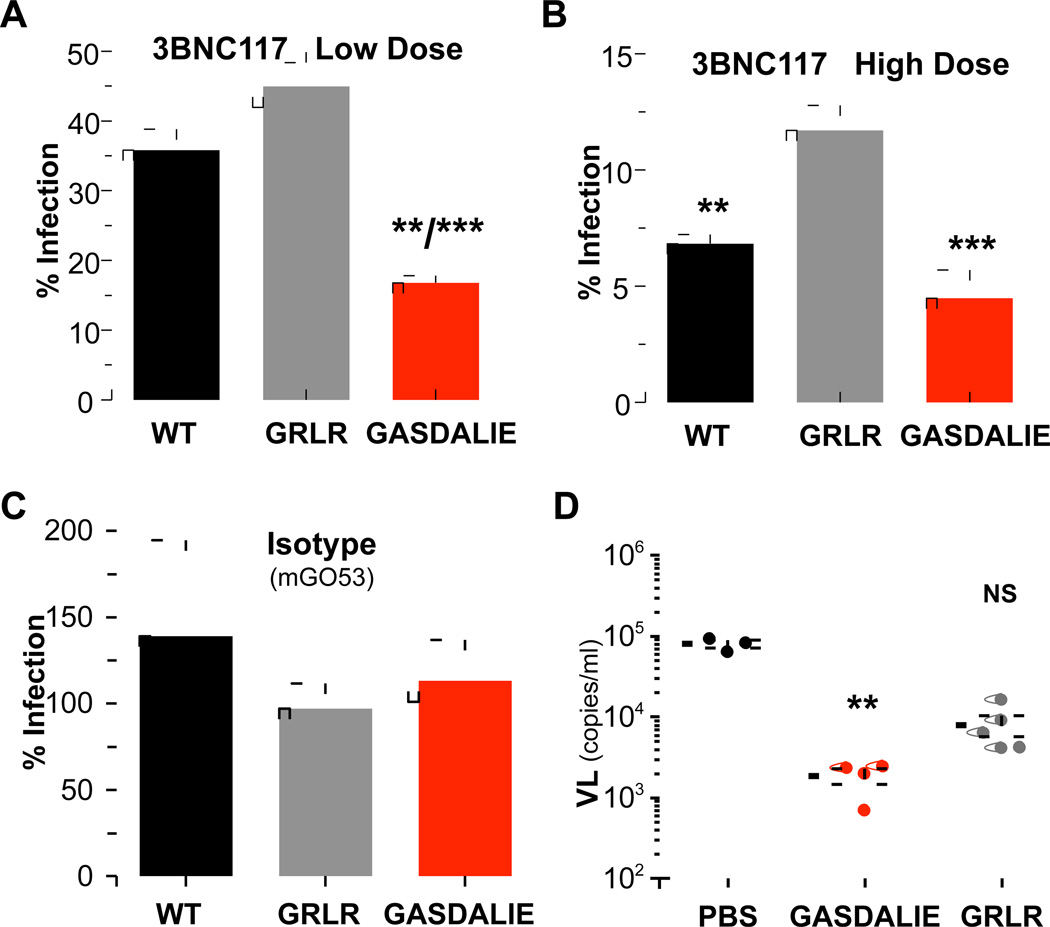
Figure 4. Generation and characterization of Fc domain variants of anti-HIV-1 mAbs with selective FcγR binding capacity.
Fc domain variants (GRLR and GASDALIE) of hIgG1 anti-HIV-1 mAbs with differential FcγR affinity (A–B; see also Table S4 (A: IgG Fc domain variant affinity against different classes and common allelic variants of human and mouse FcγRs; B: Fold enhancement in the affinity of Fc domain variants compared to wild-type human IgG1 for human FcγRs. n.b.: no binding)) were generated and characterized in terms of (C) antigen specificity (see also related Figure S3) and (D) in vitro neutralization activity. (C) Binding of anti-HIV-1 mAb hIgG1 Fc domain variants to gp160ΔctYU-2- or GFP-expressing HEK293T cells was assessed by flow cytometry. Data represent the geometric mean fluorescence intensity (Geo MFI) from three independent experiments. (D) In vitro neutralization activity (against HIV-1YU-2) of human Fc domain variants of anti-HIV-1 mAbs was determined by a standardized TZM-bl assay (Montefiori, 2005)(see also Tables S5 and S6).
Fc optimized variants of anti-HIV-1 human IgG1 bNAbs exhibit improved in vivo activity
Although our findings suggest that preferential engagement of activating FcγRs through mouse IgG2a subclass variants modulates the in vivo bNAb activity, the use of a murine model system does not allow direct comparison of mAb Fc effector activity in humans. To overcome this problem, we have recently developed a mouse model, in which the mouse FcγRs were deleted and all the human FcγRs were expressed as transgenes, faithfully recapitulating the human-specific FcγR expression pattern and diversity (Smith et al., 2012). These mice have been previously characterized and successfully used for the study of human IgG Fc effector activity in vivo (Bournazos et al., 2014; DiLillo et al., 2014; Smith et al., 2012). Luciferase reporter mice were crossed to FcγR humanized mice and the activity of anti-HIV-1 human IgG1 bNAbs and Fc domain variants was evaluated. Comparison of their in vivo activity revealed that the Fc domain variant of 3BNC117 with enhanced binding capacity to engage activating FcγRs (GASDALIE) also demonstrated increased protective activity compared to wild type human IgG1 3BNC117 (Figure 5A–B). Furthermore, 3BNC117 GRLR Fc variant, which exhibits minimal binding to all classes of human FcγRs displayed significantly reduced in vivo protective activity compared to wild type human IgG1 and to the GASDALIE Fc variant. These effects were not accompanied by any differences in the mAb biodistribution, with comparable serum IgG levels detected at the time of virus challenge irrespective of the Fc variant (Figure S4A). Furthermore, no in vivo protective activity was observed when a non-HIV-1 reactive mAb (mGO53) was used as control (Figure 5C). These findings clearly highlight the contribution of Fc-FcγR interactions in the in vivo mAb activity using a mouse system that closely resembles the unique pattern of human FcγR structural and functional diversity.
Figure 5. Enhancement of the in vivo activity of anti-HIV-1 mAbs through Fc domain engineering for selective FcγR engagement.
(A–C) The in vivo activity of 3BNC117 Fc variants with differential FcγRs binding capacity was assessed in luciferase reporter mice crossed to FcγR humanized mice. Wild-type (WT) IgG1, GRLR, or GASDALIE variants of 3BNC117 were administered (i.p.) to mice (A: low dose, 20 µg, **p<0.01 compared to WT; ***p<0.001 compared to GRLR; B: high dose, 100 µg, ***p<0.001/**p<0.01 compared to GRLR; C: Isotype control (mGO53), 100 µg) 24 h prior to infection with HIV-1 Cre pseudovirus. Maximal infection obtained from PBS-injected mice was used to calculate % infection and data are presented as the mean ± SEM. n=5–6.group (D) Fc domain variants (GRLR and GASDALIE) of 10–1074 mAb were administered (200 µg, i.v.) to NRG mice prior to transfer of in vitro HIV-1YU-2-infected human CD4+ T cells (2×107, i.v.). Measurement of plasma viral load (24 h post inoculation of infected cells) revealed a substantial reduction in viremia in mice that have previously received 10–1074 GASDALIE. n=3–5 mice/group, **p<0.01 compared to PBS-treated mice. NS: not significant. See also Figure S4.
In an attempt to provide additional evidence on the importance of Fc effector pathways for the in vivo activity of anti-HIV-1 mAbs, the activity of Fc domain variants of anti-HIV-1 mAbs was evaluated in two different mouse models of HIV-1 infection that recapitulate the complete virus cycle of HIV-1. Human IgG1 Fc domain variants of the 10–1074 bNAb exhibiting differential binding capacity for human and mouse FcγRs (GRLR: FcγR null binding; GASDALIE: improved FcγR binding) were administered (200 µg, i.v.) to NRG (non-obese diabetic (NOD), Rag1−/−, Il2rg−/−) mice prior to transfer of in vitro HIV-1YU-2-infected human CD4+ T cells (2×107, i.v.). Measurement of plasma viral load (24 h post inoculation of infected cells) revealed a substantial reduction in viremia in mice that have previously received 10–1074 GASDALIE, but not the GRLR Fc variant (Figure 5D).
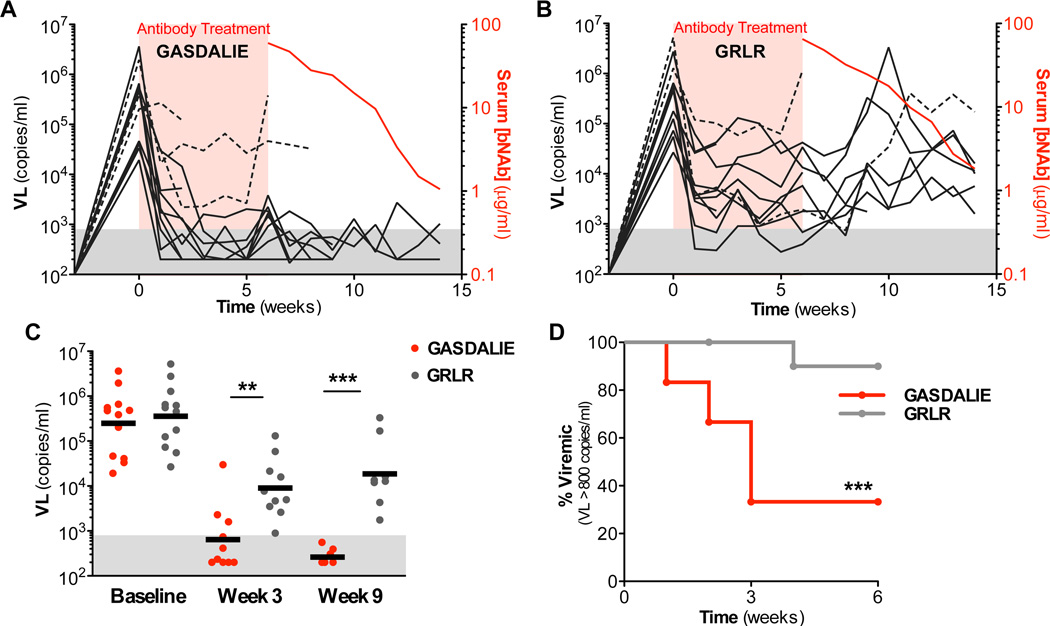
Concurrent administration of bNAbs targeting different epitopes of HIV-1 Env has been shown to confer sustained suppression of viremia in humanized mouse models of HIV-1 infection (Horwitz et al., 2013; Klein et al., 2012b). This model has been previously used to evaluate the therapeutic potential of several anti-HIV-1 bNAbs and involves the use of NRG mice reconstituted with human fetal liver-derived CD34+ haematopoietic stem cells (HSCs)(Klein et al., 2012b). To determine the contribution of the Fc effector function in the in vivo therapeutic activity of broadly neutralizing anti-HIV-1 mAbs, HIV-1YU-2-infected humanized mice were treated with a combination of 3BNC117, 10–1074, and PG16 (200 µg per mAb, every 5–7 days) either as GASDALIE or GRLR Fc variants, which exhibit enhanced or diminished FcγR binding capacity, respectively (Table S4). Both variants exhibited comparable binding capacity to human or mouse FcRn/β2 microglobulin and similar in vivo half-life profile (Figure S4B–F). In comparison to mice treated with the FcγR null binding (GRLR) variant of 3BNC117, 10–1074, and PG16, GASDALIE-treated mice exhibited faster and sustained reduction in viral load with a significantly higher proportion of them demonstrating durable (lasting >3 consecutive weeks) viremia suppression (viremia levels below detection limit (<800 copies/ml)) (Figure 6A–D). Sequencing of HIV-1 gp120 from GASDALIE-treated mice that failed to completely suppress viremia (3/12) revealed recurrent mutations in some of the bNAb-recognizing epitopes within gp120 (Figure S5). Furthermore, when we monitored viremia for several weeks (up to 8) following cessation of bNAb treatment in decaying serum anti-HIV-1 bNAb concentration, GASDALIE-treated mice exhibited suppressed viremia even when serum bNAb concentration dropped below 3 µg/ml; an average concentration previously determined when viral rebound occurred in mice treated with the same combination (3BNC117, 10–1074 and PG16) of wild-type hIgG1 bNAbs (Halper-Stromberg et al., 2014). In summary, these findings strongly support that the in vivo protective activity of anti-HIV-1 mAbs is highly dependent upon Fc-FcγR interactions and Fc optimized bNAb variants with enhanced FcγR binding capacity exhibited improved in vivo protective activity.
Figure 6. In vivo therapeutic activity of anti-HIV-1 bNAbs requires FcγR effector function in HIV-1-infected humanized mice.
Humanized NRG mice were infected with HIV-1YU-2 and 3 weeks post-infection (viral load >104 copies/ml) were treated for 6 weeks (red-shaded area) with a combination of 3BNC117, 10–1074, and PG16 mAb Fc domain variants exhibiting either enhanced binding capacity for activating FcγRs (GASDALIE; A) or diminished binding to all classes of FcγRs (GRLR; B). Plasma viral load was quantified at weekly intervals to assess the capacity of anti-HIV-1 bNAb Fc variants to suppress viremia. Each line represents an individual mouse and dotted lines represent mice with evidence of recurrent mutations in mAb-targeting epitopes of gp120 (Figure S5). Quantitation of serum bNAb concentration (right axis, red line) following cessation of antibody therapy revealed sustained viremia suppression in GASDALIE-treated mice at limiting anti-HIV-1 bNAb serum levels. (C–D) Comparison of their in vivo therapeutic activity revealed that GASDALIE mAb variants induced a significant and faster reduction in viral load (C) resulting in substantially lower proportion of mice with viremia levels above the detection limit (>800 copies/ml)(D); n=12/group **p<0.05, *** p<0.01 GASDALIE vs. GRLR-treated groups. See also Figure S5.
Discussion
Since the first clinical use of mAbs for the treatment of inflammatory and neoplastic disorders, it became apparent that Fc effector activity plays an essential role in the therapeutic potential of an antibody (Nimmerjahn and Ravetch, 2012). For example, the therapeutic outcome of a number of mAbs was associated with allelic variants of FcγR genes that affect the receptor capacity for IgG binding (Nimmerjahn and Ravetch, 2012). This ever-increasing body of evidence suggests a significant role of FcγR-mediated interactions in the in vivo activity of antibody-based therapeutics.
Despite previous evidence on the role of FcγR effector activity in clinical progression of AIDS (Forthal et al., 2007) and mAb-mediated pre-exposure prophylaxis (Hessell et al., 2007), the contribution of Fc effector function to the in vivo activity of anti-HIV-1 bNAbs has not been yet determined, mainly due to the lack of an in vivo model system for HIV-1 entry that recapitulates the unique pattern of human FcγR expression and diversity. Current strategies to measure the neutralization activity of anti-HIV-1 mAbs involve well-established in vitro assays (TZM-bl cell-based assays), which generally offer no information on the antibody effector activity, since they lack FcγR-expressing effector leukocytes. Likewise, in vitro conditions of assays commonly used for the study of antibody Fc effector activity fail to reproduce the in vivo complexity that governs FcγR-mediated pathways. Non-human primates have been widely used for the in vivo study of HIV-1 infection and a role for Fc effector function in the mAb-mediated anti-viral activity has been previously demonstrated (Hessell et al., 2007). However, given the relative limitations that non-human primate models present, the precise role of Fc-FcγR interactions in the neutralizing activity of anti-HIV-1 bNAbs cannot be systematically studied. For example, non-human primate models display substantial FcγR genomic variation and their FcγR functional and structural characteristics are not concordant with human FcγR physiology (Nguyen et al., 2011)(S.B. and J.V.R., unpublished observations).
It is therefore likely that any evidence on the role of Fc effector activity obtained through the use of in vitro assays or non-human primate in vivo models might not represent accurately the complexity and diversity of human FcγR physiology. Indeed, recent studies indicated that the relative contribution of FcγR-mediated pathways to the in vivo mAb activity diverges greatly between humans and non-human primates and many mechanistic insights obtained from one species were not replicated to the other. For example, although Fc-engineered glycovariants of b12 (non-fucosylated b12) with enhanced affinity for human and rhesus FcγRIIIa demonstrated substantially improved in vitro Fc effector activity in NK cell activation, ADCC and ADCVI assays using human FcγR-expressing effector leukocytes, there was no augmentation in the capacity of this mAb variant to protect macaques against mucosal SHIV challenge (Moldt et al., 2012). Similarly, in another study (Dugast et al., 2014), passive transfer of antibodies failed to protect macaques following SHIV challenge, despite their exceptional in vitro ADCC activity using human-derived effector leukocytes.
To precisely delineate the FcγR-mediated mechanisms that participate in the in vivo activity of anti-HIV-1 bNAbs, we utilized a recently developed mouse model for HIV-1 entry (Pietzsch et al., 2012) as well as human leukocyte-reconstituted mice, as models for bNAb-mediated therapy. In agreement with previous observations on the requirement of Fc effector activity of anti-HIV-1 mAbs to confer pre- and post-exposure prophylaxis in macaques and humanized mice, respectively (Halper-Stromberg et al., 2014; Hessell et al., 2007), our study demonstrated a key role for FcγR-mediated interactions in the in vivo activity of anti-HIV-1 bNAbs. Assessment of the in vivo activity of mouse-human chimeric anti-HIV-1 bNAbs in wild type mice revealed significantly augmented activity of mouse IgG2a subclass variants through enhanced opsonization and clearance of viral particles from circulation. These protective effects of mouse IgG2a reflect the capacity of this subclass to interact with activating FcγRs, like FcγRIV and FcγRI (Table S1, Figure 1A). Indeed, in several mouse models of antibody-mediated cellular cytotoxicity, such differences in the capacity of the various mouse IgG subclasses to engage activating or inhibitory FcγRs have been shown to be predictive of the in vivo mAb activity (Abboud et al., 2010; DiLillo et al., 2014; Li and Ravetch, 2011; Nimmerjahn et al., 2010; Nimmerjahn and Ravetch, 2005).
Since engagement of activating, but not inhibitory FcγRs conferred augmented in vivo protective activity, we developed Fc domain variants of human IgG1 to exhibit selectively enhanced binding capacity to activating human FcγRs. As the amino acid backbone of the Fc domain is the main determinant for Fc-FcγR interaction, introduction of certain mutations in the Fc region that participates in the FcγR binding interface modulates the capacity of an IgG molecule to interact with FcγRs (Lazar et al., 2006; Shields et al., 2001). This approach has been previously employed by several groups to successfully generate antibodies with improved Fc effector functions, including ADCC and opsonization through enhancement of Fc-FcγR interactions (Bournazos et al., 2014; DiLillo et al., 2014; Heider et al., 2011; Horton et al., 2010; Lazar et al., 2006; Smith et al., 2012). Similarly, in our study, Fc domain variants (GASDALIE) of anti-HIV-1 bNAbs that exhibited enhanced binding capacity for activating human FcγRs, such as FcγRIIa and FcγRIIIa (Figure 4A–B) also presented augmented in vivo protective and therapeutic activity compared to wild-type or FcγR null binding variants (GRLR). Despite the obvious limitations that animal models present to reflect precisely the complexity of human HIV-1 infection (duration of infection, host immune responses, alterations in effector cellular compartments), our findings strongly support the importance of activating FcγR engagement in the bNAb-mediated anti-viral protection.
In summary, our findings clearly indicate that the in vivo activity of anti-HIV-1 mAbs is largely determined by Fc domain interactions. Indeed, the in vivo protective activity of anti-HIV mAbs correlates precisely with their capacity to engage activating FcγRs, but not with their in vitro neutralization activity. For example, poorly neutralizing anti-HIV-1 mAbs, like 1–74, exhibit potent in vivo activity when expressed as Fc subclass variants capable for activating FcγR engagement (Figure 3A). Likewise, antibodies with exceptionally high neutralization potency readily lose their in vivo therapeutic activity, when their capacity for FcγR engagement is diminished (Figure 6). These findings highlight the substantial role of FcγR-mediated mechanisms in the in vivo protective activity of anti-HIV-1 bNAbs, completely revising our current understanding of the mechanisms of antibody-mediated viral neutralization. Contrary to the typical notion that viral neutralization by antibodies is simply the result of blocking viral entry via Fab-antigen interactions, our observations strongly suggest that Fc-mediated effector functions contribute substantially to the activity of anti-HIV-1 mAbs.
Understanding the significance of Fc-FcγR interactions in the protective activities of anti-HIV-1 antibodies would provide useful insights for the development of vaccination strategies to elicit antibodies with enhanced capacity to activate FcγR-mediated pathways. Additionally, in view of the recent successful therapeutic application of several anti-HIV-1 mAbs with exceptionally broad and potent neutralizing activity to effectively control viral replication in non-human primate (Barouch et al., 2013; Shingai et al., 2013) and humanized mouse models (Horwitz et al., 2013; Klein et al., 2012b), optimization of their in vivo therapeutic efficacy is necessary to accelerate their potential clinical use for the treatment of HIV-1 infection in humans. Although previously-employed strategies to successfully generate anti-HIV bNAbs with increased potency mainly focused on enhancing the specificity and breadth of Fab-antigen interactions (Diskin et al., 2011), in the present report, we suggest that modifications in the Fc domain to modulate its selective interaction with different FcγRs can greatly influence the protective activity of bNAbs. These findings suggest novel strategies for designing and developing therapeutic antibodies with improved in vivo efficacy for the prevention and treatment of HIV-1 infection in humans.
Experimental Procedures
A detailed description of the experimental procedures is provided in the Extended Experimental Procedures section.
In Vivo HIV-1 Pseudovirus Infection and Bioluminescence Imaging
Mice were injected with 1011 adenoviral particles i.v. (AdV-hCCR5–2A–hCD4) 48 h prior to infection with HIV-1 Cre pseudovirus. For in vivo infection, HIV-1YU-2 pseudovirus (3–5.5×1010) was injected through the lateral vein. On day 4 post-infection with pseudovirus, mice were anesthetized with 2% (v/v) isoflurane and injected i.p. with 4.5 mg D-Luciferin (Regis Technologies). For FcγR humanized mice, mice were anesthetized with 2% (v/v) isoflurane and ketamine/xylazine (75 mg and 15 mg/kg of body weight, respectively; i.p.) and the peritoneal cavity was surgically exposed prior to in vivo imaging. Bioluminescence was acquired using an IVIS Lumina II platform (Caliper Life Sciences) and data were analyzed using Living Image imaging software (Caliper Life Sciences).
Viral Load Quantification
For the quantification of serum HIV-1YU-2 Cre pseudovirus levels, viral RNA was purified from serum (100 µl) and Cre RNA content of the samples was quantitated by one step real time RT-PCR. Plasma HIV-1 viral load was quantified as previously described (Horwitz et al., 2013; Klein et al., 2012b). The lower limit of detection for this assay was previously determined to be 800 copies/ml (Klein et al., 2012b). Additional details are presented in the Extended Experimental Procedures section.
In Vitro HIV-1 infection of CD4+ T cells
Human PBMCs were purified from peripheral blood leukocyte units (New York Blood Center) using Ficoll density gradient medium (Ficoll-Paque Plus, GE Healthcare), following by isolation of CD4+ T cells by negative magnetic immunoselection (Human CD4+ T cell isolation kit (negative selection), Miltenyi Biotech). Purified CD4+ T cells (5×106 cells/ml in complete RPMI media) were stimulated with PHA (5 µg/ml; Sigma) and recombinant human IL-2 (50 U/ml; Peprotech) for 48 h prior to infection with HIV-1YU-2 (50 ng p24/107 cells for 4 h). Following infection, cells were incubated in fresh complete RPMI media, supplemented with IL-2 (50 U/ml) for 60 h. Prior to injection (2×107, i.v.) to NRG mice, cells were washed twice in PBS.
In Vivo HIV-1 Infection and mAb Treatment
Humanized NRG mice were generated by intrahepatic human CD34+ HSCs injection of sublethally irradiated neonatal NRG mice, as previously described (Horwitz et al., 2013; Klein et al., 2012b). Mice (>10 weeks) with a measurable human CD45+ graft were infected following i.p. injection of HIV-1YU-2 (57.5 ng p24). Viral load was quantified 3 weeks post-infection and mice with viral loads >104 copies/ml were included in treatment experiments. Antibodies (3BNC117, 10–1074, PG16, 200 µg of each mAb, 600 µg total in PBS containing 2 mg IVIG, Gammagard) were administered s.c. every 5 days for the first two weeks and weekly thereafter for 6 weeks.
This dosing schedule was sufficient to maintain serum levels of each mAb >10 µg/ml over the course of the treatment (Figure S4F). IVIG (5 mg) was administered s.c. 6 hours prior to the first antibody injection. No neutralization activity or gp140 reactivity was observed for the IVIG preparation, as assessed by TZMbl assay and anti-gp140 ELISA, respectively.
Statistical Analysis
Results from multiple experiments are presented as mean ± standard error of the mean (SEM). One-way ANOVA or Kruskal-Wallis test was used to test for differences in the mean values of quantitative variables, and where statistically significant effects were found, post-hoc analysis using Bonferroni t-test or Dunn’s multiple comparison test was performed, respectively. For infection curve comparison, viremia rates (sustained aviremia was defined as VL <800 copies/ml for >3 consecutive weeks) were analyzed with the log-rank (Mantel-Cox) test. Data were analyzed with Graphpad Prism software (Graphpad) and P values of <0.05 were considered to be statistically significant.
Supplementary Material
Highlights.
Anti-HIV-1 mAb-mediated protective activity depends on Fc effector function.
Engagement of activating FcγR is critical for optimal anti-HIV-1 mAb activity.
Fc domain variants of anti-HIV-1 mAbs exhibit improved protective activity.
Enhanced therapeutic activity of anti-HIV-1 mAbs through selective FcγR engagement.
Acknowledgements
We wish to thank all members of the Laboratory of Molecular Genetics and Immunology (Rockefeller University) for the helpful discussions and technical help. We also thank B. Donovan and A. Ploss (Laboratory of Virology and Infectious disease, Rockefeller University) for providing the hCD4/hCCR5 adenovirus, L. Nogueira, A. Halper-Stromberg, J. Horwitz and H. Gruell (Laboratory of Molecular Immunology, Rockefeller University) for technical help and advice, J. Scheid and H. Mouquet (Rockefeller University) for providing the antibody plasmids and L. Stamatatos (Seattle Biomedical Research Institute) for providing the gp140SF162. Research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers AI081677 and AI100148 (to J.V.R. and M.C.N.), by the CTSA grant #UL1TR000043 to Rockefeller University and Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery AI 100663 (to M.C.N.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research support was also provided by the Bill & Melinda Gates Foundation grant OPP1033115 to J.V.R. and M.C.N, and CA-VIMC#1032144 to M.S.S. S.B. was supported by an NBC Career Development Award (U54AI057158) and an American Heart Association (AHA) postdoctoral fellowship (13POST14100003).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
S.B. designed and performed experiments, analyzed data and wrote the manuscript, F.K. generated humanized mice and designed experiments, J.P. assisted in HIV entry experiments, M.S.S. performed the in vitro neutralization assays, M.C.N. designed experiments, J.V.R. designed and directed the study.
All authors declare no conflict of interest.
References
- Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, Scharff MD, Casadevall A. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman ME, Dugast AS, McAndrew EG, Tsoukas S, Licht AF, Irvine DJ, Alter G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J Virol. 2013;87:5468–5476. doi: 10.1128/JVI.03403-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi DE, Sheoran AS, Rich CM, Richard L, Chapman-Bonofiglio S, Tzipori S. Evaluation of Fab and F(ab')2 fragments and isotype variants of a recombinant human monoclonal antibody against Shiga toxin 2. Infect Immun. 2010;78:1376–1382. doi: 10.1128/IAI.00867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger S, Tussiwand R, Schlaepfer E, Mazzucchelli L, Heikenwalder M, Kurrer MO, Behnke S, Frey J, Oxenius A, Joller H, et al. Disseminated and sustained HIV infection in CD34+ cord blood cell-transplanted Rag2−/−gamma c−/− mice. Proc Natl Acad Sci U S A. 2006;103:15951–15956. doi: 10.1073/pnas.0604493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bournazos S, Chow SK, Abboud N, Casadevall A, Ravetch JV. Human IgG Fc domain engineering enhances antitoxin neutralizing antibody activity. J Clin Invest. 2014;124:725–729. doi: 10.1172/JCI72676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A, Janda A. Immunoglobulin isotype influences affinity and specificity. Proc Natl Acad Sci U S A. 2012;109:12272–12273. doi: 10.1073/pnas.1209750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, Vachieri SG, Pinna D, Minola A, Vanzetta F, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Tan GS, Palese P, Ravetch JV. Broadly neutralizing hemagglutinin stalk-specific antibodies require FcγR interactions for protection against influenza virus in vivo. Nat Med. 2014;20:143–151. doi: 10.1038/nm.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin R, Scheid JF, Marcovecchio PM, West AP, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, et al. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 2011;334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugast AS, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, et al. Lack of Protection following Passive Transfer of Polyclonal Highly Functional Low-Dose Non-Neutralizing Antibodies. PLoS One. 2014;9:e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal DN, Gach JS, Landucci G, Jez J, Strasser R, Kunert R, Steinkellner H. Fc-glycosylation influences Fcγ receptor binding and cell-mediated anti-HIV activity of monoclonal antibody 2G12. J Immunol. 2010;185:6876–6882. doi: 10.4049/jimmunol.1002600. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Bream J, Jacobson LP, Phan TB, Montoya B. FcgammaRIIa genotype predicts progression of HIV infection. J Immunol. 2007;179:7916–7923. doi: 10.4049/jimmunol.179.11.7916. [DOI] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Ding H, Kappes JC, Wang A, Thung I, Phan T. IgG2 inhibits HIV-1 internalization by monocytes, and IgG subclass binding is affected by gp120 glycosylation. AIDS. 2011;25:2099–2104. doi: 10.1097/QAD.0b013e32834b64bd. [DOI] [PubMed] [Google Scholar]
- Halper-Stromberg A, Lu C-L, Klein F, Horwitz, Joshua A, Bournazos S, Nogueira L, Eisenreich, Thomas R, Liu C, Gazumyan A, Schaefer U, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014 doi: 10.1016/j.cell.2014.07.043. In Press - http://dx.doi.org/10.1016/j.cell.2014.1007.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider KH, Kiefer K, Zenz T, Volden M, Stilgenbauer S, Ostermann E, Baum A, Lamche H, Kupcu Z, Jacobi A, et al. A novel Fc-engineered monoclonal antibody to CD37 with enhanced ADCC and high proapoptotic activity for treatment of B-cell malignancies. Blood. 2011;118:4159–4168. doi: 10.1182/blood-2011-04-351932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Horton HM, Bernett MJ, Peipp M, Pong E, Karki S, Chu SY, Richards JO, Chen H, Repp R, Desjarlais JR, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116:3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Gaebler C, Mouquet H, Sather DN, Lehmann C, Scheid JF, Kraft Z, Liu Y, Pietzsch J, Hurley A, et al. Broad neutralization by a combination of antibodies recognizing the CD4 binding site and a new conformational epitope on the HIV-1 envelope protein. J Exp Med. 2012a;209:1469–1479. doi: 10.1084/jem.20120423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012b;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. Antibodies in HIV-1 vaccine development and therapy. Science. 2013;341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JI, Licht AF, Dugast AS, Suscovich T, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. Divergent antibody subclass and specificity profiles but not protective HLA-B alleles are associated with variable antibody effector function among HIV-1 controllers. J Virol. 2014;88:2799–2809. doi: 10.1128/JVI.03130-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A. 2006;103:4005–4010. doi: 10.1073/pnas.0508123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, Finstad SL, Jin C, Landucci G, Alpert MD, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol. 2012;86:6189–6196. doi: 10.1128/JVI.00491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005;12 doi: 10.1002/0471142735.im1211s64. 12.11. [DOI] [PubMed] [Google Scholar]
- Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, et al. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A. 2012;109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DC, Scinicariello F, Attanasio R. Characterization and allelic polymorphisms of rhesus macaque (Macaca mulatta) IgG Fc receptor genes. Immunogenetics. 2011;63:351–362. doi: 10.1007/s00251-011-0514-z. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Lux A, Albert H, Woigk M, Lehmann C, Dudziak D, Smith P, Ravetch JV. FcgammaRIV deletion reveals its central role for IgG2a and IgG2b activity in vivo. Proc Natl Acad Sci U S A. 2010;107:19396–19401. doi: 10.1073/pnas.1014515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Divergent immunoglobulin g subclass activity through selective Fc receptor binding. Science. 2005;310:1510–1512. doi: 10.1126/science.1118948. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun. 2012;12:13. [PMC free article] [PubMed] [Google Scholar]
- Pejchal R, Doores KJ, Walker LM, Khayat R, Huang PS, Wang SK, Stanfield RL, Julien JP, Ramos A, Crispin M, et al. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez LG, Zolla-Pazner S, Montefiori DC. Antibody-DEPENDENT, FcγRI-mediated neutralization of HIV-1 in TZM-bl cells occurs independently of phagocytosis. J Virol. 2013;87:5287–5290. doi: 10.1128/JVI.00278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, Diskin R, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, et al. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, et al. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, et al. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P, DiLillo DJ, Bournazos S, Li F, Ravetch JV. Mouse model recapitulating human Fcgamma receptor structural and functional diversity. Proc Natl Acad Sci U S A. 2012;109:6181–6186. doi: 10.1073/pnas.1203954109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.