Abstract
We have developed a method for cobalt-catalyzed, aminoquinoline- and picolinamide-directed sp2 C-H bond alkenylation by alkynes. Method shows excellent functional group tolerance and both internal and terminal alkynes are competent substrates for the coupling. The reaction employs Co(OAc)2*4H2O catalyst, Mn(OAc)2 cocatalyst, and oxygen from air as a terminal oxidant.
Keywords: Alkenes, C-H Activation, Alkynes, Cobalt
During the last decade, transition-metal catalyzed C-H bond functionalization methodology has emerged as an important chemistry tool that allows simplification and shortening of synthetic schemes.[1] Within last years, applications of C-H bond functionalization to synthesis of natural products and compounds of medicinal interest have emerged, showing the maturity of the methodology.[2] However, certain problems are still unsolved. For example, a general, functional group tolerant method for directed coupling of non-acidic sp2 C-H bonds with alkynes has yet to be described. Furthermore, most examples of sp2 C-H bond coupling with alkynes feature second-row transition metal catalysis.[3a–n] Directed alkenylation by employing alkenes is possible.[3o,p]
Following the pioneering work of Murai,[3a] a number of groups have reported directed or non-directed reactions of sp2 C-H bonds with alkynes catalyzed by second- or third row transition metals.[3] Use of more available first-row transition metals has been rare.[4] Only few examples describe nickel- or cobalt-catalyzed alkyne/sp2 C-H bond coupling. Notably, following earlier reports that low-valent cobalt species can activate and functionalize sp2 C-H bonds,[5] Yoshikai has developed a versatile system for cobalt-catalyzed, imine- and pyridine-directed alkenylation of sp2 C-H bonds with internal alkynes.[4f–h,l] Nakao and Hiyama have shown that Ni(cod)2 catalyzes coupling of sp2 C-H bonds with disubstituted acetylenes.[4d] Chatani has described nickel-catalyzed reaction of benzoic acid 2-pyridinylmethylamides with internal alkynes.[4e] However, directed coupling of both internal and terminal alkynes with sp2 C-H bonds is exceedingly rare.[4i,m] We report here a method for cobalt-catalyzed, aminoquinoline- and picolinamide-directed sp2 C-H bond coupling with alkynes. The reaction succeeds with terminal and internal alkynes, tolerates a wide range of functional groups on alkyne and arene, and allows for a removal of directing groups. Furthermore, first use of cobalt catalysis by employing bidentate, monoanionic auxiliaries is demonstrated.
In 2005, we introduced 2-aminoquinoline, picolinamide, and 2-pyridinylmethylamine auxiliaries for palladium-catalyzed sp2 and sp3 C-H bond functionalization.[6a,b] Subsequently, copper-catalyzed sp2 C-H bond sulfenylation, amination, fluorination, and etherification was described.[6c–f] Other groups have extensively used aminoquinoline, picolinamide, and other bidentate, monoanionic directing groups for palladium, ruthenium, iron, and copper-catalyzed C-H bond functionalization.[7] The near-universal efficiency of these directing groups for transition-metal catalyzed C-H bond functionalization presumably arises from the substrate acting as a tridentate, dianionic pincer that stabilizes high-valent transition metal intermediates (Figure 1).[6b,8]
Figure 1.
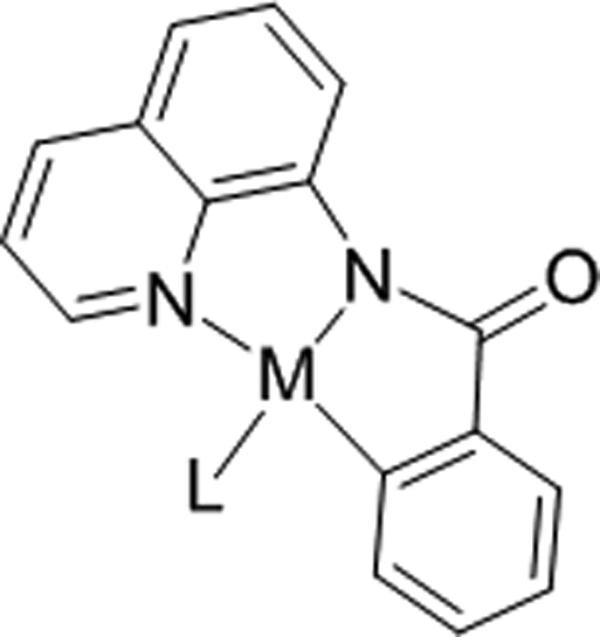
Aminoquinoline Directing Group.
We speculated that 8-aminoquinoline and picolinic acid auxiliaries would promote cobalt-catalyzed ortho-alkenylation of sp2 C-H bonds since Co(III) is known to activate sp2 C-H bonds[9] and carbon-carbon multiple bond insertion into Co(III)-C bonds has been demonstrated.[10]
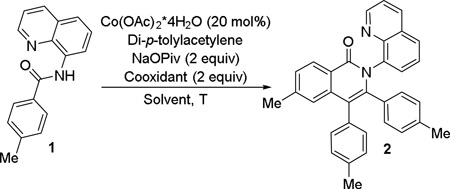
We decided to use readily available cobalt(II) acetate catalyst in combination with pivalate base. The reaction optimization was carried out with respect to solvent, reaction temperature, and cooxidant (Table 1). Entries 1–3 show that reaction is most efficient in trifluoroethanol solvent, presumably due to higher solubility of Co catalyst. Reaction is efficient at temperatures as low as 60 °C (entry 4). Potassium persulfate cannot be used as an oxidant (entry 6), while silver pivalate (entries 1–5) and Mn(OAc)2 (entries 8–10, 12) work well. Manganese(II) acetate was chosen as a cooxidant due to cost considerations. At least 1 equiv of Mn(OAc)2 is required (entry 9 vs. 12). Interestingly, reaction in degassed solvent affords only traces of product showing that presence of oxygen is essential (entries 9 vs. 10). Low conversion can be can be achieved without Mn(OAc)2 cocatalyst under an atmosphere of oxygen (entry 11). Cobalt(II) acetate tetrahydrate can be used instead of anhydrous salt with no decrease of reaction yields. No reaction was observed if Co(OAc)2 was omitted.
Table 1.
Optimization of Reaction Conditions.[a]
 | |||||
|---|---|---|---|---|---|
| Entry | T, °C | Time, h | Solvent | Cooxidant | Ratio 2/1 |
| 1[b,c] | 150 | 12 | DMF | AgOPiv | <1/99 |
| 2[b,c] | 150 | 12 | o-Cl2C6H4 | AgOPiv | 1/2 (33%)[h] |
| 3[b,c] | 150 | 12 | CF3CH2OH | AgOPiv | >99/1 (63%)[h] |
| 4 | 60 | 12 | CF3CH2OH | AgOPiv | >99/1 (65%)[h] |
| 5[b] | 25 | 12 | CF3CH2OH | AgOPiv | 1/4 (16%)[h] |
| 6 | 60 | 12 | CF3CH2OH | K2S2O8 | <1/99 |
| 7[d] | 60 | 2 | CF3CH2OH | PhI(OAc)2 | 1/1 (11%)[h] |
| 8 | 60 | 16 | CF3CH2OH | Mn(OAc)2 | 5/1 (79%)[h] |
| 9[e] | 80 | 2 | CF3CH2OH | Mn(OAc)2 | 1/2 (63%)[h] |
| 10[e,f] | 80 | 12 | CF3CH2OH | Mn(OAc)2 | <1/99 |
| 11[g] | 80 | 18 | CF3CH2OH | O2 | 1/1 (50%)[h] |
| 12[d] | 80 | 2 | CF3CH2OH | Mn(OAc)2 | 10/1 (87%)[h] |
Amide 0.1 mmol, solvent 0.7 mL. Conversions were determined by 1H NMR analysis.
Co(OAc)2 catalyst.
Cooxidant: 0.8 equiv.
Cooxidant: 1 equiv.
Cooxidant: 0.5 equiv.
Deoxygenated solvent.
Reaction vessel pressurized with O2.
NMR yield of 2 using 1,1,2-trichloroethane as internal standard in parentheses.
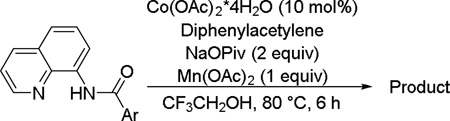
The reaction scope with respect to aminoquinoline amides is presented in Table 2. The reactions are successful for both electron-rich (entries 5, 7) and electron-poor (entries 2–4, 6) amides. Various functionalities, such as bromide (entry 3), nitro (entry 4), and iodide (entry 6) are tolerated. Furanecarboxylic and thiophenecarboxylic acids are reactive (entries 8 and 9) showing compatibility of reaction conditions with heterocycles. Reaction headspace volume is important as 1 equiv of O2 is consumed in the reaction.
Table 2.
Reaction Scope with Respect to Arylamides [a]
 | |||
|---|---|---|---|
| Entry | Ar | Product | Yield, % |
| 1 | C6H5 | 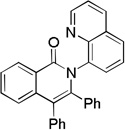 |
78 |
| 2 | 4-CF3C6H4 | 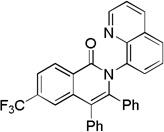 |
70 |
| 3 | 4-BrCC6H4 | 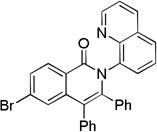 |
73 |
| 4 | 4-NO2C6H4 | 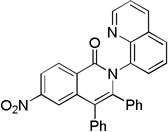 |
78 |
| 5 | 2-MeC6H4 | 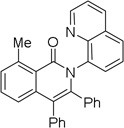 |
86 |
| 6 | 3-IC6H4 | 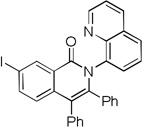 |
84 |
| 7[b] | 2-MeOC6H4 | 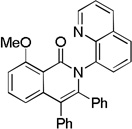 |
74 |
| 8[c] | 2-furyl | 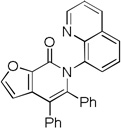 |
81 |
| 9[d] | 2-thiophenyl | 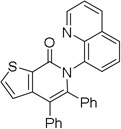 |
86 |
Amide 0.5 mmol, CF3CH2OH 5 mL, air. Yields are isolated yields. Please see Supporting information for details.
Time: 18 h.
Time: 16 h.
Time: 20 h.
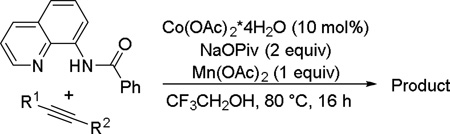
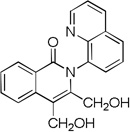
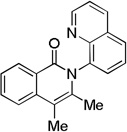
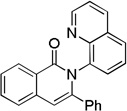
The reaction scope with respect to alkynes is presented in Table 3. The reaction is remarkably functional group tolerant, with free alcohol moiety (entry 1), ester (entry 6), silyl (entry 7), cyclopropyl (entry 8), and protected amine (entry 9) compatible with reaction conditions. Terminal alkynes with either aromatic (entry 3) or large substituents (entries 4, 7) afford products as a single regioisomers. Terminal alkynes with smaller substituents (entries 6, 8 and 9) as well as unsymmetric internal alkynes (entry 5) form regioisomer mixtures. However, selectivities are reasonably good, ranging from about 6/1 for ethyl propiolate (entry 6) to 14/1 for phenylmethylacetylene (entry 5).
Table 3.
Reaction Scope with Respect to Alkynes [a]
 | |||
|---|---|---|---|
| Entry | R1, R2 | Product | Yield, % |
| 1 | CH2OH CH2OH |
 |
95 |
| 2 | Me Me |
 |
96 |
| 3 | H Ph |
 |
95 |
| 4 | H tBu |
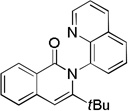 |
73 |
| 5[b] | Ph Me |
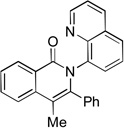 |
95 |
| 6[c] | CO2Et H |
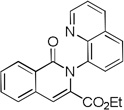 |
82 |
| 7 | TIPS H |
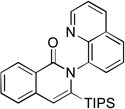 |
64 |
| 8[d] | cyclopropyl H |
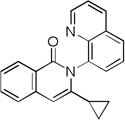 |
84 |
| 9[e] | CH2 NPhth H |
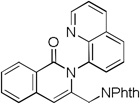 |
93 |
Amide 0.5 mmol, CF3CH2OH 5 mL, alkyne 1.2 equiv, air. Yields are isolated yields. Please see Supporting information for details.
Isolated as 14/1 isomer mixture.
Minor isomer (13%) also isolated.
Isolated as 13/1 isomer mixture.
Isolated as 7/1 isomer mixture, reaction time: 18 h.
Furthermore, aminoquinoline vinylamides are reactive (Scheme 1). Thus, cinnamic acid aminoquinoline amide was treated with 2-butyne and the cyclization product was isolated in 75% yield.
Scheme 1.
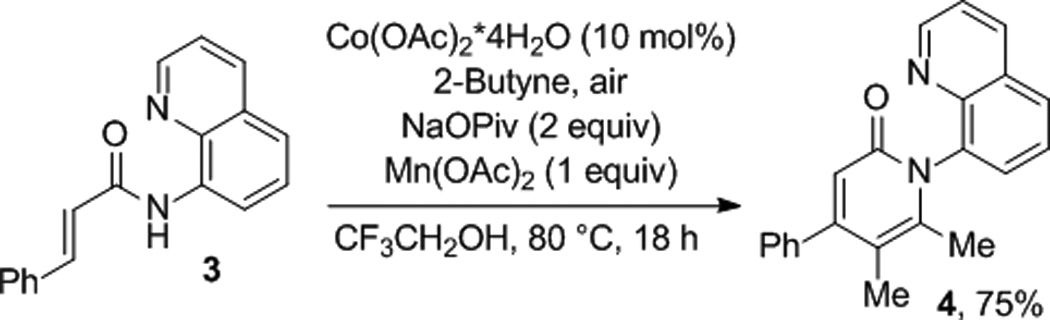
Cyclization with Vinyl Amide.
Picolinamide directing group can be used (Scheme 2), allowing functionalization of benzyl- and naphthylamine derivatives. Picolinamide of 1-naphthylamine was reacted with 2-butyne in the presence of catalytic Co(OAc)2*4H2O to afford the non-cyclized alkenylation product 6 in moderate yield. Similarly, 1-methylbenzylamine picolinamide reacted with 2-butyne to give cyclized product 8 in 44% yield. As shown before[6] picolinamidedirected reactions are less efficient affording lower yields of products, requiring higher temperatures, and longer reaction times.
Scheme 2.
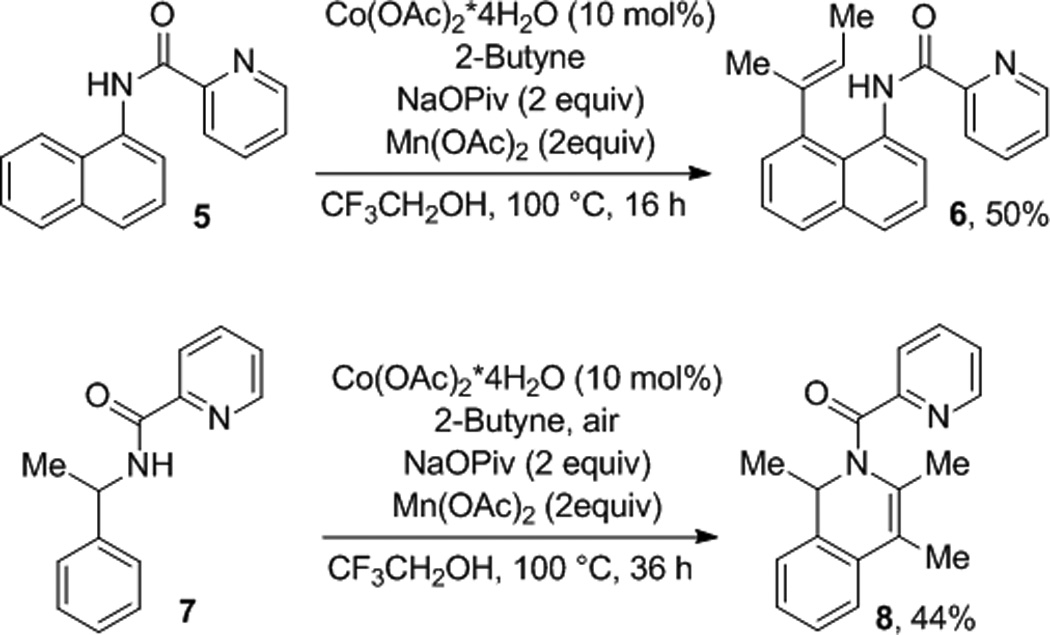
Picolinamide Directing Group.
The advantage of aminoquinoline and picolinamide directing groups lies in the possibility of their removal, affording useful functionalized products (Scheme 3). Base hydrolysis of 8 removes picolinamide, giving trimethylisoquinoline 9. Aminoquinoline directing group can be removed by treatment with CAN at room temperature. Cleavage of directing group is accompanied by oxidation of the double bond, affording ketolactone 10 in moderate yield.
Scheme 3.
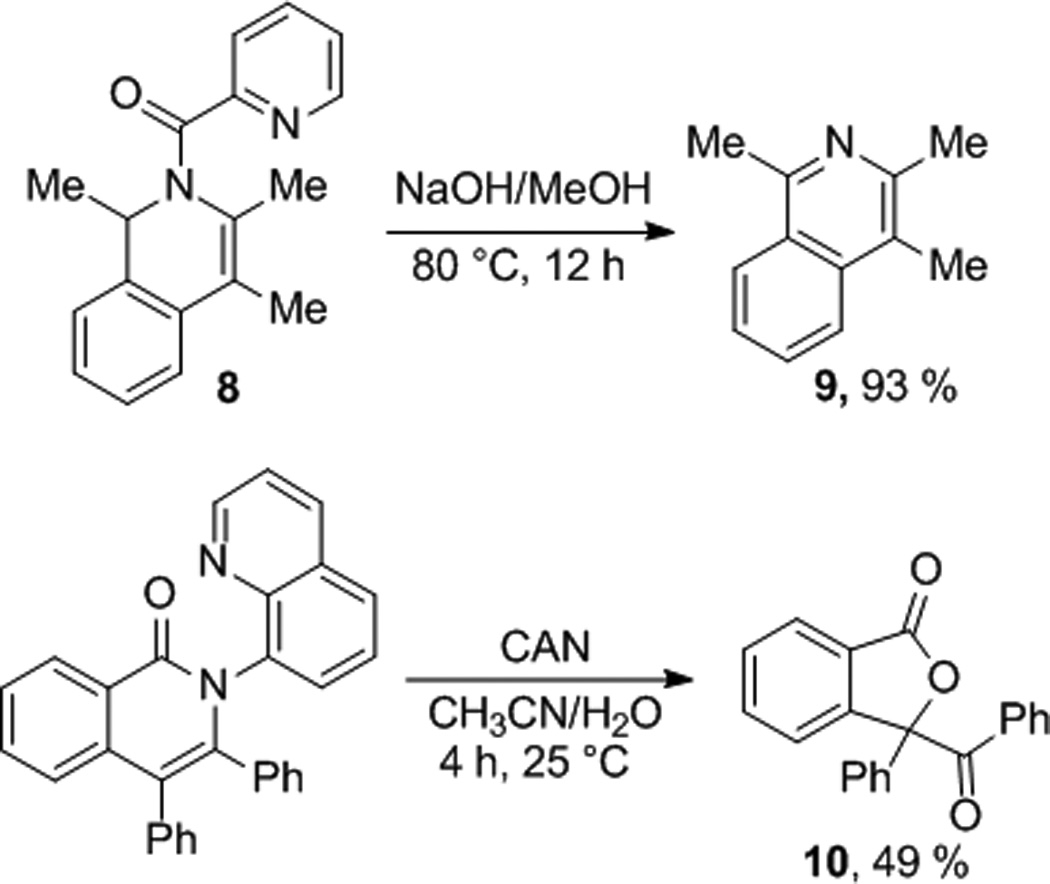
Directing Group Removal.
Based on the fact that aminoquinoline ligand stabilizes transition metals in high oxidation states, it is likely that the reaction proceeds via Co(III) intermediate 11 formed by oxidation of Co(OAc)2 in the presence of aminoquinoline amide ligand (Scheme 4). Insertion of alkyne into cobalt-aryl bond would provide 12. Alternative mechanism with cobalt acetylide intermediate is unlikely since (1) internal alkynes are reactive, and (2) terminal alkynes form product as a mixture of two isomers. Compound 12 could directly reductively eliminate 13, or Co(III) could be protonated to give 14 which could oxidatively cyclize to give 13. Formation of intermediate 14 is plausible since picolinamide of 1-naphthylamine reacts to form non-cyclic 6 (Scheme 2). To distinguish between those pathways, 14 was heated with Co(OAc)2 and Mn(OAc)2 at 60 °C. Complex reaction mixture was obtained and formation 13 was not observed, thus excluding intermediacy of 14.
Scheme 4.
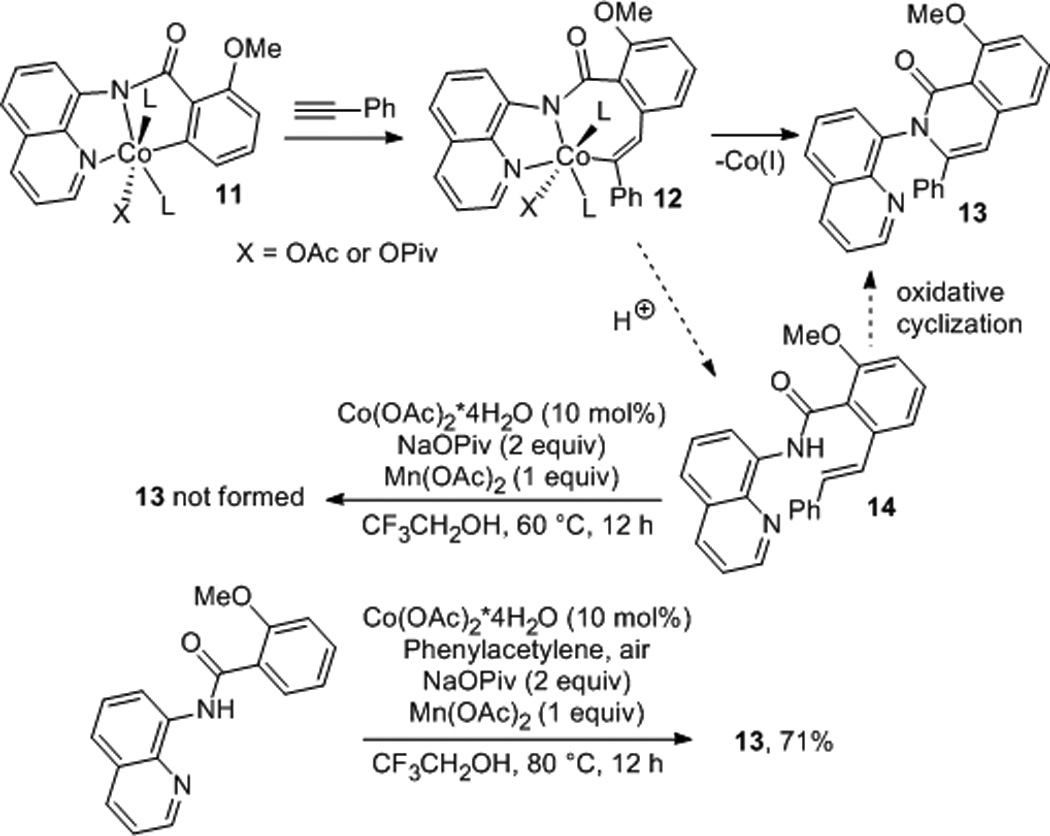
Mechanistic Considerations.
In conclusion, we have developed a highly general, functional-group tolerant method for cobalt-catalyzed, aminoquinoline- and picolinamide-directed coupling of alkynes with sp2 C-H bonds. The reaction employs Co(OAc)2*4H2O catalyst, Mn(OAc)2 cocatalyst, and oxygen from air as a terminal oxidant. Future directions of the work involve mechanistic studies of the transformation and attempts to isolate reaction intermediates.
Supplementary Material
Footnotes
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), and Camille and Henry Dreyfus Foundation for supporting this research.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.Reviews: Colby DA, Bergman RG, Ellman JA. Chem. Rev. 2010;110:624. doi: 10.1021/cr900005n. Ackermann L. Chem. Rev. 2011;111:1315. doi: 10.1021/cr100412j. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. 2009;121:5196. doi: 10.1002/anie.200806273. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem., Int. Ed. 2009;48:5094. doi: 10.1002/anie.200806273. Kitamura T. Eur. J. Org. Chem. 2009;8:1111. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. doi: 10.1021/cr100280d. Baudoin O. Chem. Soc. Rev. 2011;40:4902. doi: 10.1039/c1cs15058h. Kulkarni AA, Daugulis O. Synthesis. 2009:4087. Wendlandt AE, Suess AM, Stahl SS. Angew. Chem. 2011;123:11256. doi: 10.1002/anie.201103945. Wendlandt AE, Suess AM, Stahl SS. Angew. Chem., Int. Ed. 2011;50:11062. doi: 10.1002/anie.201103945. Hickman AJ, Sanford MS. Nature. 2012;484:177. doi: 10.1038/nature11008. Corbet M, De Campo F. Angew. Chem. 2013;125:10080. doi: 10.1002/anie.201303556. Corbet M, De Campo F. Angew. Chem., Int. Ed. 2013;52:9896. doi: 10.1002/anie.201303556.
- 2. Feng Y, Chen G. Angew. Chem. 2010;122:970. doi: 10.1002/anie.200905134. Feng Y, Chen G. Angew. Chem., Int. Ed. 2010;49:958. doi: 10.1002/anie.200905134. Gutekunst WR, Baran PS. J. Am. Chem. Soc. 2011;133:19076. doi: 10.1021/ja209205x. Gutekunst WR, Gianatassio R, Baran PS. Angew. Chem. 2012;124:7625. doi: 10.1002/anie.201203897. Gutekunst WR, Gianatassio R, Baran PS. Angew. Chem., Int. Ed. 2012;51:7507. doi: 10.1002/anie.201203897. Gutekunst WR, Baran PS. J. Org. Chem. 2014;79:2430. doi: 10.1021/jo4027148. Reviews: Gutekunst WR, Baran PS. Chem. Soc. Rev. 2011;40:1976. doi: 10.1039/c0cs00182a. Yamaguchi J, Yamaguchi AD, Itami K. Angew. Chem. Int. Ed. 2012;51:8960. doi: 10.1002/anie.201201666.
- 3.a) Kakiuchi F, Yamamoto Y, Chatani N, Murai S. Chem. Lett. 1995:681. [Google Scholar]; b) Dong C-G, Yeung P, Hu Q-S. Org. Lett. 2007;9:363. doi: 10.1021/ol062885a. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tunge JA, Foresee LN. Organometallics. 2005;24:6440. [Google Scholar]; d) Jia C, Piao D, Oyamada J, Lu W, Kitamura T, Fujiwara Y. Science. 2000;287:1992. doi: 10.1126/science.287.5460.1992. [DOI] [PubMed] [Google Scholar]; e) Chernyak N, Tilly D, Li Z, Gevorgyan V. Chem. Commun. 2010;46:150. doi: 10.1039/b919991h. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Ackermann L, Lygin AV, Hofmann N. Angew. Chem. 2011;123:6503. doi: 10.1002/anie.201101943. [DOI] [PubMed] [Google Scholar]; Ackermann L, Lygin AV, Hofmann N. Angew. Chem., Int. Ed. 2011;50:6379. doi: 10.1002/anie.201101943. [DOI] [PubMed] [Google Scholar]; g) Yi CS, Yun SY. J. Am. Chem. Soc. 2005;127:17000. doi: 10.1021/ja055608s. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Guimond N, Gouliaras C, Fagnou K. J. Am. Chem. Soc. 2010;132:6908. doi: 10.1021/ja102571b. [DOI] [PubMed] [Google Scholar]; i) Ueura K, Satoh T, Miura M. J. Org. Chem. 2007;72:5362. doi: 10.1021/jo070735n. [DOI] [PubMed] [Google Scholar]; j) Zhang YJ, Skucas E, Krische MJ. Org. Lett. 2009;11:4248. doi: 10.1021/ol901759t. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Colby DA, Bergman RG, Ellman JA. J. Am. Chem. Soc. 2008;130:3645. doi: 10.1021/ja7104784. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Guimond N, Fagnou K. J. Am. Chem. Soc. 2009;131:12050. doi: 10.1021/ja904380q. [DOI] [PubMed] [Google Scholar]; m) Hong P, Cho B-R, Yamazaki H. Chem. Lett. 1979:339. [Google Scholar]; n) Wang H, Grohmann C, Nimphius C, Glorius F. J. Am. Chem. Soc. 2012;134:19592. doi: 10.1021/ja310153v. [DOI] [PubMed] [Google Scholar]; o) Lu Y, Wang D-H, Engle KM, Yu J-Q. J. Am. Chem. Soc. 2010;132:5916. doi: 10.1021/ja101909t. [DOI] [PMC free article] [PubMed] [Google Scholar]; p) Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. doi: 10.1021/cr0104330. [DOI] [PubMed] [Google Scholar]
- 4.a) Matsuyama N, Hirano K, Satoh T, Miura M. Org. Lett. 2009;11:4156. doi: 10.1021/ol901684h. [DOI] [PubMed] [Google Scholar]; b) Wei Y, Zhao H, Kan J, Su W, Hong M. J. Am. Chem. Soc. 2010;132:2522. doi: 10.1021/ja910461e. [DOI] [PubMed] [Google Scholar]; c) Besseliévre F, Piguel S. Angew. Chem. 2009;121:9717. doi: 10.1002/anie.200904776. [DOI] [PubMed] [Google Scholar]; Besseliévre F, Piguel S. Angew. Chem., Int. Ed. 2009;48:9553. doi: 10.1002/anie.200904776. [DOI] [PubMed] [Google Scholar]; d) Nakao Y, Kashihara N, Kanyiva KS, Hiyama T. J. Am. Chem. Soc. 2008;130:16170. doi: 10.1021/ja807258m. [DOI] [PubMed] [Google Scholar]; e) Shiota H, Ano Y, Aihara Y, Fukumoto Y, Chatani N. J. Am. Chem. Soc. 2011;133:14952. doi: 10.1021/ja206850s. [DOI] [PubMed] [Google Scholar]; f) Lee P-S, Fujita T, Yoshikai N. J. Am. Chem. Soc. 2011;133:17283. doi: 10.1021/ja2047073. [DOI] [PubMed] [Google Scholar]; g) Ding Z, Yoshikai N. Angew. Chem. 2012;124:4776. doi: 10.1002/anie.201200019. [DOI] [PubMed] [Google Scholar]; Ding Z, Yoshikai N. Angew. Chem., Int. Ed. 2012;51:4698. doi: 10.1002/anie.201200019. [DOI] [PubMed] [Google Scholar]; h) Gao K, Lee P-S, Fujita T, Yoshikai N. J. Am. Chem. Soc. 2010;132:12249. doi: 10.1021/ja106814p. [DOI] [PubMed] [Google Scholar]; i) Zhou B, Chen H, Wang C. J. Am. Chem. Soc. 2013;135:1264. doi: 10.1021/ja311689k. [DOI] [PubMed] [Google Scholar]; j) Ikemoto H, Yoshino T, Sakata K, Matsunaga S, Kanai M. J. Am. Chem. Soc. 2014;136:5424. doi: 10.1021/ja5008432. [DOI] [PubMed] [Google Scholar]; k) Song W, Ackermann L. Chem. Commun. 2013;49:6638. doi: 10.1039/c3cc43915a. [DOI] [PubMed] [Google Scholar]; l) Yamakawa T, Yoshikai N. Org. Lett. 2013;15:196. doi: 10.1021/ol303259m. [DOI] [PubMed] [Google Scholar]; m) He R, Huang Z-T, Zheng Q-Y, Wang C. Angew. Chem. 2014;126:5050. doi: 10.1002/anie.201402575. [DOI] [PubMed] [Google Scholar]; He R, Huang Z-T, Zheng Q-Y, Wang C. Angew. Chem., Int. Ed. 2014;53:4950. doi: 10.1002/anie.201402575. [DOI] [PubMed] [Google Scholar]
- 5.a) Lenges CP, Grant BE, Brookhart M. J. Organomet. Chem. 1997;528:199. [Google Scholar]; b) Lenges CP, White PS, Brookhart M. J. Am. Chem. Soc. 1998;120:6965. [Google Scholar]; c) Wang A, Sun H, Li X. Organometallics. 2008;27:5434. [Google Scholar]
- 6.a) Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f. [DOI] [PubMed] [Google Scholar]; b) Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tran LD, Popov I, Daugulis O. J. Am. Chem. Soc. 2012;134:18237. doi: 10.1021/ja3092278. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Tran LD, Roane J, Daugulis O. Angew. Chem. 2013;125:6159. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tran LD, Roane J, Daugulis O. Angew. Chem., Int. Ed. 2013;52:6043. doi: 10.1002/anie.201300135. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Truong T, Klimovica K, Daugulis O. J. Am. Chem. Soc. 2013;135:9342. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Roane J, Daugulis O. Org. Lett. 2013;15:5842. doi: 10.1021/ol402904d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selected examples: Ni: Aihara Y, Chatani N. J. Am. Chem. Soc. 2014;136:898. doi: 10.1021/ja411715v. Song W, Lackner S, Ackermann L. Angew. Chem. 2014;126:2510. doi: 10.1002/anie.201309584. Song W, Lackner S, Ackermann L. Angew. Chem., Int. Ed. 2014;53:2477. doi: 10.1002/anie.201309584. Fe: Asako S, Ilies L, Nakamura E. J. Am. Chem. Soc. 2013;135:17755. doi: 10.1021/ja4106368. Ru: Rouquet G, Chatani N. Chem. Sci. 2013;4:2201. doi: 10.1002/anie.201301451. Pd: Zhang S-Y, He G, Nack WA, Zhao Y, Li Q, Chen G. J. Am. Chem. Soc. 2013;135:2124. doi: 10.1021/ja312277g. He G, Chen G. Angew. Chem. 2011;123:5298. He G, Chen G. Angew. Chem., Int. Ed. 2011;50:5192. doi: 10.1002/anie.201100984. Cu: Nishino M, Hirano K, Satoh T, Miura M. Angew. Chem. 2013;125:4553. doi: 10.1002/anie.201300587. Nishino M, Hirano K, Satoh T, Miura M. Angew. Chem., Int. Ed. 2013;52:4457. doi: 10.1002/anie.201300587.
- 8.Suess AM, Ertem MZ, Cramer CJ, Stahl SS. J. Am. Chem. Soc. 2013;135:9797. doi: 10.1021/ja4026424. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino T, Ikemoto H, Matsunaga S, Kanai M. Chem. Eur. J. 2013;19:9142. doi: 10.1002/chem.201301505. [DOI] [PubMed] [Google Scholar]
- 10.Brookhart M, Volpe AF, Lincoln DM, Horvath IT, Millar JM. J. Am. Chem. Soc. 1990;112:5634. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


