Abstract
In organotypic hippocampal slice cultures, principal neurons form aberrant excitatory connections with other principal cells in response to slicing-induced deafferentation, similar to mechanisms underlying epileptogenesis in post-traumatic epilepsy. To investigate the consequences of this synaptogenesis, we recorded field potential activity from area CA3 during perfusion with the complete growth medium used during incubation. At 7 days in vitro (DIV), slice cultures only displayed multi-unit activity. At 14 DIV the majority displayed population bursts reminiscent of interictal-like spikes, but sustained synchronous activity was rare. Bandpass filtering of interictal discharges revealed fast ripple-like complexes, similar to in vivo recordings. Spontaneous ictal-like activity became progressively more prevalent with age: at 21 DIV 50% of organotypic hippocampal slice cultures displayed long lasting, ictal-like discharges that could be suppressed by phenytoin, whereas interictal-activity was not suppressed. The fraction of cultures displaying ictal events continually increased with incubation time. Quantification of population spike activity throughout epileptogenesis using automatic detection and clustering algorithms confirmed the appearance of interictal-like activity before ictal-like discharges, and also revealed high frequency pathological multi-unit activity in slice cultures at 14–17 DIV. These experiments indicate that interictal-like spikes precede the appearance of ictal-like activity in a reduced in vitro preparation. Epileptiform activity in cultures resembled in vivo epilepsy, including sensitivity to anticonvulsants and steadily increasing seizure incidence over time, although seizure frequency and rate of epileptogenesis were higher in vitro. Organotypic hippocampal slice cultures comprise a useful model system for investigating mechanisms of epileptogenesis as well as developing anti-epileptic and anti-epileptogenic drugs.
Keywords: Epileptogenesis, growth medium, interictal spikes, ictal discharges, organotypic hippocampal slices, phenytoin
Introduction
Although electrographic interictal spikes are so reliably associated with a propensity for spontaneous seizures that their presence is used diagnostically (Engel, 1989), the reasons for this robust association are entirely unknown (de Curtis and Avanzini, 2001). We recently hypothesized that interictal spikes might drive epileptic circuit formation (Staley et al., 2005). One characteristic of epileptogenesis is the appearance of axons returning to excite neurons in their network of origin, known as “sprouting”(Sutula and Dudek, 2007). Spikes are the synchronous discharge of neuron populations, an activity possibly guiding the synapse formation of newly sprouted axons within their circuit of origin following the developmental “fire together, wire together” axiom. Once formed, spike timing-dependent plasticity rules would predict that such synapses would be strengthened by synchronous activation of the population during spiking, a mechanism observed in a variety of in vitro preparations (Schneiderman et al., 1994; Bains et al., 1999; Abegg et al., 2004; Behrens et al., 2005; Hellier et al., 2007).
The hippocampal organotypic slice culture method (Gahwiler, 1981; Stoppini et al., 1991) is well established and has been used in a wide range of studies, particularly investigations of plasticity of mono-synaptically connected pairs of neurons and pharmacologically induced epileptiform discharges (Gahwiler et al., 1997). While most properties of organotypic hippocampal slice cultures resemble their in vivo counterparts (Gahwiler, 1984; De Simoni et al., 2003), neurons in this preparation exhibit robust sprouting as a consequence of massive deafferentation and de-efferentation occurring during tissue slicing (Zimmer and Gahwiler, 1984; Frotscher and Gahwiler, 1988). Despite this robust sprouting, however, spontaneous epileptiform activity has rarely been observed (McBain et al., 1989; Lahtinen et al., 2001; Abegg et al., 2004). If spiking drives some aspects of epileptogenesis, organotypic slices that developed spiking would be expected to progressively develop ictal-like epileptiform activity, while organotypic slices not developing spikes should not develop any epileptiform activity. We tested this idea using prolonged recordings of population activity from area CA3 under perfusion with complete growth medium at physiological temperature in slice cultures from 7–30 days in vitro in the absence of pharmacological manipulations. Our recordings demonstrate an unexpectedly high incidence of spontaneous epileptiform activity. Interictal-like spikes and short bursts of population activity developed before the appearance of ictal-like discharges of more than 3 minutes duration.
Materials and Methods
Organotypic hippocampal slice cultures – roller tube method
Roller-tube type organotypic slice cultures were prepared as described by Gähwiler (1981): Briefly, isolated hippocampi from P8 Sprague-Dawley (Charles River Laboratories, Wilmington, MA) rat pups were cut into 350 μm slices on a McIlwain tissue chopper (Mickle Lab Eng. Co., Surrey, UK). Slices were mounted in clots of chicken plasma (Cocalico Biologicals, Reamstown, PA) and thrombin (Sigma-Aldrich, St. Louis, MO) on poly-L-lysine (Sigma-Aldrich, St. Louis, MO) coated glass coverslips (Electron Microscopy Sciences, Hatfield, PA) and incubated in roller tubes (Nunc, Roskilde, Denmark) at 36 °C in 1 mL growth medium: 25% horse serum (Sigma-Aldrich, St. Louis, MO), 50% Eagles basal medium (Invitrogen, Carlsbad, CA), 25% Hanks balanced salt solution (Invitrogen, Carlsbad, CA) with 5.6 mM glucose, 0.8 mM GlutaMAX (Invitrogen, Carlsbad, CA) and 30 μg/mL gentamicin (Invitrogen, Carlsbad, CA). After 48h, 30 μL antimitotics (30 μg/mL cytosine-β-D-arabino-funanoside, 30 μg/mL uridine and 30 μg/mL 5-fluro-2′-deoxyuridine; Sigma-Aldrich, St. Louis, MO) were added to inhibit glial overgrowth, followed by a change of growth medium after 24h. Subsequently, growth medium was changed every 7 days.
Organotypic hippocampal slice cultures – interface method
Modified interface-type cultures were prepared as previously described earlier (Berdichevsky et al., 2009). Briefly, hippocampal slices of 350 μm thickness were dissected from P3-4 Sprague Dawley rat pups (Charles River Laboratories, Wilmington, MA) and placed into polydimethylsiloxane (Sylgard 184, Dow Corning, Midland, MI) wells on poly-D-lysine (Sigma-Aldrich, St. Louis, MO) coated 35 mm tissue-culture dishes (Becton Dickinson, Franklin Lakes, NJ). The dishes were filled with just enough serum-containing medium composed of 25% horse serum (Sigma-Aldrich, St. Louis, MO), 25% Hanks balanced salt solution and 50% Eagles basal medium with 1 mM GlutaMAX and 30 μg/ml gentamicin (all Invitrogen, Carlsbad, CA) to cover the bottom of the dish and incubated in a humidified 5% CO2 incubator at 37° C. Serum-free medium (Neurobasal A/B27, with 0.5 mM GlutaMAX and 30 μg/ml gentamicin, all Invitrogen, Carlsbad, CA) was substituted on the second DIV, and subsequently used in all medium changes (every 3 days).
Field potential recordings – roller tube cultures
Organotypic slice cultures were transferred from the roller tube to a submerged recording chamber while still attached to the glass coverslip, under continuous perfusion with 60 mL re-circulated, fresh growth medium bubbled with 95%O2/5%CO2 at 36 °C. Field potential recordings (60 min) were performed under IR-DIC visualization using a FN-1 upright microscope (Nikon, Tokyo, Japan) with a growth medium filled glass pipette positioned in CA3 and a MultiClamp 700B amplifier (Molecular Devices, Union City, CA, USA). Signals were low-pass filtered at 4 kHz using a 4-pole Bessel filter and digitized at 10 kHz with a Digidata 1440A analog–digital interface (Molecular Devices, Union City, CA, USA).
Field potential recordings – interface cultures
Petri dishes with organotypic cultures were removed from tissue culture incubator, and transferred to an interface recording chamber with a humidified 95% air / 5% CO2 atmosphere at 37° C. Cultures were recorded in their original serum-free medium. Field potential recordings were carried out with a tungsten recording electrode placed in area CA1, visualized with a stereo microscope. Signals were amplified and low-pass filtered at 30 kHz with a multiple channel amplifer (Dagan Corporation, Minneapolis, MN), digitized, and recorded with custom-designed software dClamp written in Microsoft Visual C++ (Microsoft, Redmond, WA).
Immunohistochemistry
After recording, organotypic slice cultures were rinsed with standard artificial cerebro-spinal fluid (ACSF) and fixed in 4% paraformaldehyde for 2h. Slices were removed from coverslips, permeabilized for 4h in 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO), and blocked overnight in 10% goat serum (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS). Subsequently, slices were incubated in a primary antibody cocktail (rabbit anti-NeuN monoclonal IgG1 conjugated with Alexa Fluor 488 (1:100; Millipore, Billerica, MA), mouse anti-GAD67 monoclonal IgG2a (1:500; Millipore, Billerica, MA) in 5% goat serum in PBS) at 4°C for 3d. Following 4 washes in 1% goat serum in PBS and secondary antibody incubation (Alexa Fluor 647- conjugated goat anti-mouse IgG2a (Invitrogen, Carlsbad, CA) in 5% goat serum in PBS) for 3d at 4°C, slices were mounted for microscopy. Anti-NeuN and anti-GAD67 staining was imaged on a LSM 5 Pascal (Carl Zeiss, Göttingen, Germany) laser confocal microscope (serial confocal images at 3 μm intervals using 20x or 10x objectives with appropriate filters) with subsequent processing in Photoshop (Adobe, San Jose, CA) and ImageJ (NIH, Bethesda, MD).
Analysis
Pathological activity in field potential recordings was detected with custom software (Fig. 1) written in Microsoft Visual C++ (Microsoft, Redmond, WA) using a previously published spike detection method (White et al., 2006). Detected pathological spikes were characterized individually and grouped based on interspike intervals. Visualization, fitting and statistics were done in MatLab (MathWorks, Natick, MA) and SigmaPlot (Systat, Chicago, IL).
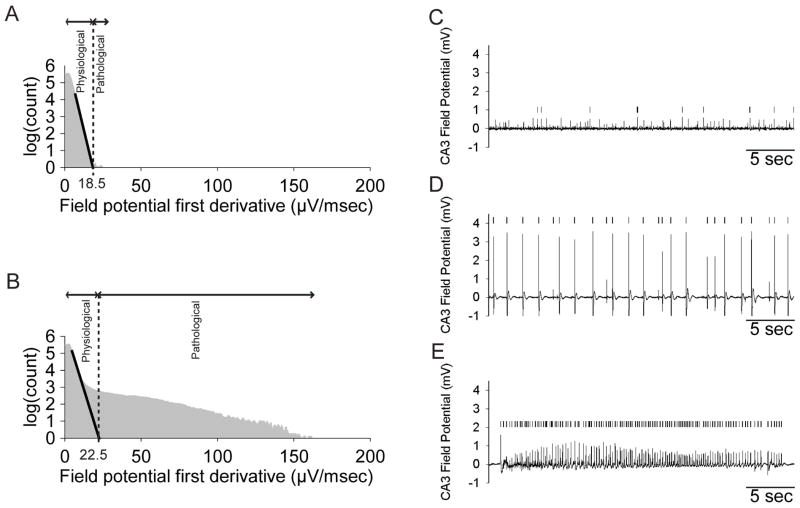
Figure 1. Automated detection of pathological spike activity.
A,B. Pathological spikes were detected in CA3 field potential traces using a previously published method (White et al., 2006). Briefly, a powerlaw (black line) is fitted to the steepest slope of the initial part of the first-derivative distribution of the signal (in grey, A. a 7 DIV slice culture with some pathological multi-unit activity, B. a 14 DIV slice culture with interictal-like discharges), yielding the cutoff between physiological and pathological activity as the intercept with the x-axis. This method reliably detected individual spikes (marked with vertical ticks | ) from C. pathological multi-unit activity, D. interictal-like activity and E. ictal-like discharges.
Results
Field potential recordings (60 min) were made from area CA3 of organotypic hippocampal slice cultures after 7–30 days in vitro (DIV) under constant perfusion with 60 mL re-circulated growth medium bubbled with 95%O2/5%CO2 at 36 °C. Each slice culture was visually inspected using IR-DIC (Fig. 2A,B), ensuring both slice integrity and maintained organotypic organization. Subsequently, a number of slice cultures were fixed and stained with antibodies targeting neuron specific nuclear protein NeuN (Mullen et al., 1992) and inhibitory interneuron specific protein GAD67 (Houser and Esclapez, 1994) (Fig. 2C,D). These stainings clearly demonstrate that organotypic organization, as well as both excitatory principal neurons (labeled for NeuN) and inhibitory interneurons (double labeled for NeuN and GAD67) were well-preserved in hippocampal slice cultures up to 30 DIV.
Figure 2. Organotypic hippocampal slice cultures spontaneously develop epileptiform discharges in the growth medium.
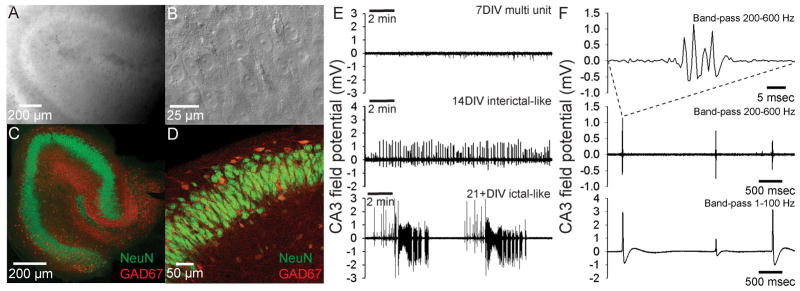
A. Low power IR-DIC image of hippocampal slice culture in the recording chamber shows preserved slice integrity and organotypic organization. B. High power IR-DIC image showing healthy pyramidal neurons in CA3. C. Immunohistochemical staining of a 30DIV slice with antibodies against NeuN (green) and GAD67 (red), respectively labeling neuronal nuclei and GABAergic cell bodies and processes. D. Higher power image showing CA3 pyramidal neurons labeled in green, GABAergic processes in red and double-labeled GABAergic interneuron cell-bodies in orange. E. Field potential recordings of spontaneous activity from CA3 of organotypic slice cultures. At 7 DIV only physiological multi-unit activity was recorded, but after 14 DIV the majority of slices displayed epileptiform, interictal-like spikes. This spontaneous epileptiform activity developed into a mix of interictal- and ictal-like discharges in slice cultures older than 21 DIV. F. Band-pass filtered (8-pole Bessel filter at 200 Hz, Gaussian filter at 600 Hz) field potential recording from area CA3 of a 14 DIV organotypic slice culture reveals fast ripple-like complexes (top trace). These fast ripple-like complexes occured at intervals larger than 1 sec (middle trace). Fast ripple-like complexes were associated with interictal-like spikes (bottom trace filtered w. 8 pole Bessel filter at 1 Hz, Gaussian filter at 100 Hz).
At 7 DIV, field potential recordings from CA3 of organotypic slice cultures revealed spontaneous multi-unit activity (Fig. 2E, top panel), but no population discharges. However, at 14–20 DIV the majority of recorded slice cultures exhibited spontaneous interictal-like discharges consisting of population spikes (Fig. 2E, middle panel). When band-pass filtered from 200–600 Hz, interictal-like spikes were associated with fast ripple-like complexes (Fig. 2F), similar to those recorded following kainate induced status epilepticus in rats (Bragin et al., 2004; Foffani et al., 2007). At 21-28+ DIV, virtually all slice cultures (11 of 12) displayed either interictal-like discharges, or mixed interictal-like and ictal-like activity (Fig. 2E, bottom panel). Periods of ictal-like discharges could last >3 minutes, including repeated after-discharges. Similar spontaneous epileptiform interictal- and ictal-like discharges were also recorded in interface-type cultures in neurobasal medium (Fig. 3). These results demonstrate that organotypic hippocampal slice cultures spontaneously develop epileptiform discharges under normal culturing conditions, - an activity evident when recording in the growth medium. Furthermore, interictal spikes precede the appearance of full-blown, ictal discharges during spontaneous epileptogenesis in vitro.
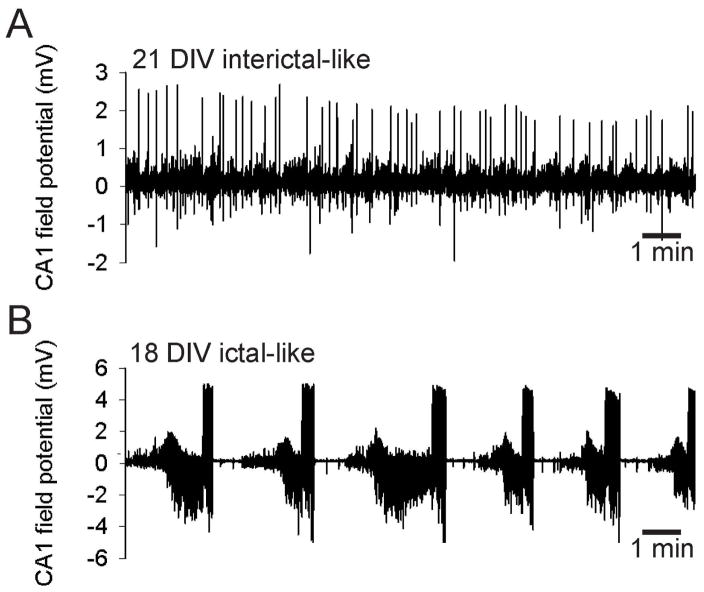
Figure 3. Spontaneous epileptiform activity in interface-type cultures.
A. Interictal-like acticity recorded from area CA1 in an interface-type culture after 21 DIV. B. Ictal-like activity recorded from area CA1 in an interface-type culture after 18 DIV.
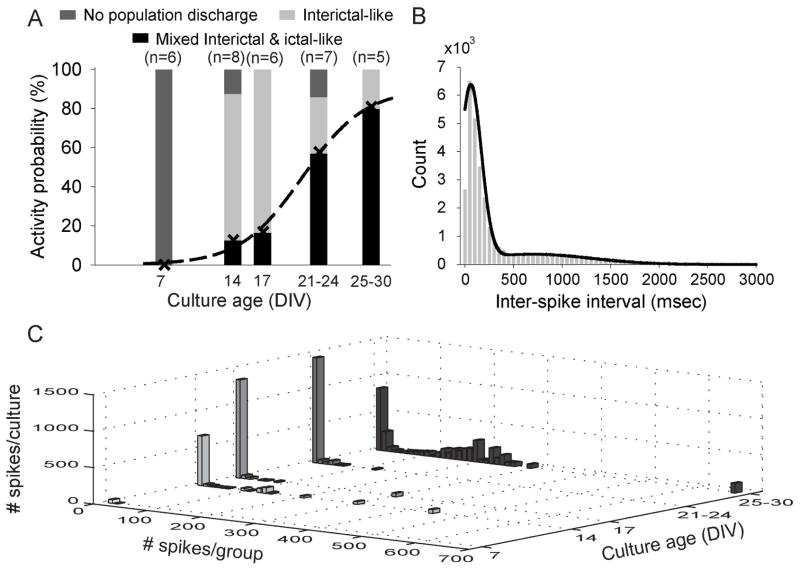
Grouping the recorded slice cultures according to age and classifying recorded spontaneous activity as either “No population discharge” (multi-unit activity only), “Interictal-like” or “Mixed Interictal- and ictal-like”, reveals a clear progression from multi-unit activity at 7 DIV over predominantly interictal-like population discharges at 14–17 DIV, to overwhelmingly ictal-like activity after 26–30 DIV (Fig. 4A). Interestingly, the probability P of recording spontaneous ictal-like activity (i.e. the ictogenesis) as a function of organotypic culture age could be fitted with a sigmoidal function (Fig. 4A, dashed line, r2 = 0.99) with P50 (50% of all cultures displaying ictal-like discharges) at 21 DIV. The probability P of ictal activity continued to rise with the number of days in vitro, reaching 80% for the oldest cultures recorded (26–30 DIV) while seemingly not reaching saturation. This developmental profile mirrors the progressive occurrence of spontaneous seizures recorded with chronic video-EEG monitoring in vivo following kainate-induced status epilepticus in rats (Williams et al., 2009), albeit on a compressed time-scale.
Figure 4. Spontaneous ictogenesis in organotypic hippocampal slice cultures mirrors in vivo developmental profile.
A. Spontaneous activity recorded in growth medium from CA3 of 32 organotypic slice cultures aged 7–30 DIV categorized as “No population discharge” (multi-unit activity only), “Interictal-like” (units and interictal-like spikes & bursts) and “Mixed interictal- and ictal-like” (units, interictal-like spikes & bursts and ictal-like discharges). The probability P of ictal-like discharges as function of age was fitted with a sigmoidal function (dashed line) with P50 = 21 DIV (r2 = 0.99). B. Pooled inter-spike-interval (ISI) distribution for all spikes detected (Fig. 1) in 10 cultures 14–30 DIV displaying mixed interictal- and ictal-like discharges. The distribution was fitted with the sum of two Gaussian distributions (black line), reflecting a population of low ISI (60±110 msec, high frequency, ictal-like activity) and a population of high ISI (700±600 msec, low frequency, interictal-like activity). C. The development of spike activity in groups of discharges with ISI ≤500 msec (frequency ≥2 Hz) as a function of organotypic slice culture age. The first bin of up to 10 consecutive spikes at ≥2 Hz discharge frequency includes single spikes with ISI ≥500 msec. Prolonged periods of pathological multi-unit activity with mean spike width <20 msec (Fig. 5) are excluded.
To further quantify the development of spontaneous epileptiform activity in hippocampal slice cultures, all pathological spikes in the recorded CA3 field potential were detected using a previously published algorithm (White et al., 2006), which discriminates between physiological and pathological activity based on a power-law fitted to the distribution of first derivatives of the recording (Fig. 1A,B). The method allowed for reliable detection of spikes in pathological multi-unit activity, interictal- and ictal-like discharges (Fig. 1C–E), making feasible the automatic characterization of individual spike parameters such as inter-spike interval (ISI) and spike width.
Pooling the inter-spike intervals for organotypic slice cultures displaying mixed interictal and ictal-like discharges (n = 10, 14–30 DIV) revealed a distribution consisting of two ISI populations (Fig. 4B, gray histogram). This distribution could be fitted with the sum of two Gaussians (Fig. 4B, black line), suggesting that a cutoff in the distribution of ISIs at 500 ms (2 Hz spike rate) could be used to distinguish between ictal-like and interictal-like activity. We used this cutoff to construct a histogram describing the distribution of spiking activity as a function of slice culture age (Fig. 4C), quantified as the average total number of spikes occurring per slice culture in different size groups of consecutive discharges during which the spike rate was at least 2 Hz.
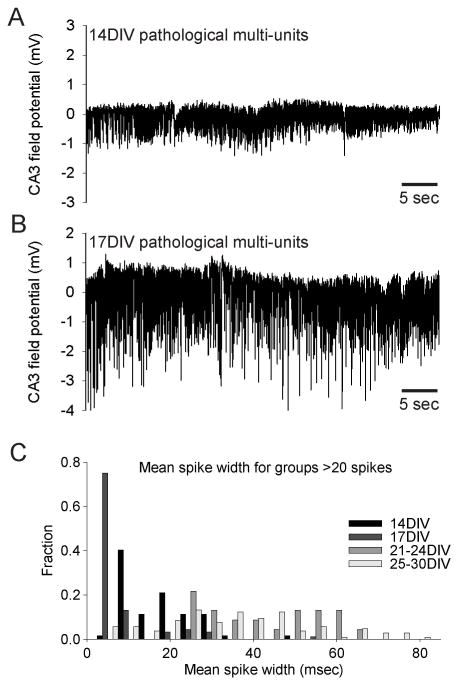
At 7 DIV (Fig. 4C) the number of spikes from pathological multi-unit activity detected per culture during 60 min is relatively low. In recordings from 14 and 17 DIV (Fig. 4C), the number of single spikes increases dramatically, along with small groups of consecutive spikes. At this age, long trains of high frequency, pathological multi-unit activity (up to ~700 consecutive spikes, ~70 sec duration and ~25 Hz discharge frequencies; Fig. 5A,B) easily discriminable based on mean spike width < 20 msec (Fig. 5C) also appears, - this activity was excluded from the summary histogram (Fig. 4C). Ictal-like activity at 14–17 DIV was relatively rare (see also Fig. 4A). At 21-24 DIV (Fig. 4C), the number of single spikes and small groups of spikes per slice culture again increases, with increased hyperexcitability and rising probability of ictal-like discharges reflected in the increased number of spikes at frequencies ≥2 Hz. This development continues at 25–30 DIV (Fig. 4C), where the number of single and few consecutive spikes decreases as the mean activity per slice culture dramatically progresses towards predominantly longer, high frequency discharges in the 100–300 consecutive spike-range. This quantification also emphasizes the progressive nature of in vitro epileptogenesis: While ictal-like discharges are present as early as 14 DIV and in 50% of all cultures at 21 DIV, they only become the dominant epileptiform activity type from 26–30 DIV.
Figure 5. Long lasting ≥2 Hz activity at 14.
–17 DIV.
A. High frequency pathological multi-unit activity at 14 DIV. B. High frequency pathological multi-unit activity at 14 DIV. C. Mean spike width distributions for groups of consecutive high frequency spikes at 14–30 DIV. Note that mean spike widths at 14–17 DIV predominantly fall below 20 msec, consistent with multi-unit discharges.
Spontaneous epileptiform discharges in organotypic slice cultures were only recorded during perfusion with growth medium. Notably, when cultures displaying robust epileptiform activity in growth medium were switched to standard ACSF, interictal- and ictal-like activity was completely suppressed within ~5 min (Fig. 6A). To investigate whether spontaneous breakdown of the glutamine supplement GlutaMAX to glutamate under the recording conditions could be inducing epileptiform activity, 100 min long recordings without and with GlutaMAX in the growth medium were performed at 7–8 DIV (Fig. 6B,C), where no population discharges were observed (Fig. 2E & 4A). If glutamine supplement breakdown was increasing ambient glutamate during the recordings, a gradual increase in uncorrelated neuronal activity would be expected with GlutaMAX in the medium, while medium without GlutaMAX should induce a gradual decrease in activity as leftover medium from the culture tube with GlutaMAX becomes diluted. Activity level throughout the 100 min recording was quantified in 5 min epochs, using the method previously described for discriminating physiological and pathological activity (Fig. 1). After the initial 5 min, recordings in medium with and without GlutaMAX settled to steady baseline activity level, indistinguishable between the two conditions (Fig. 6D), indicating that spontaneous degradation of glutamine supplement leading to excessive ambient glutamate was not occurring. Furthermore, when slice cultures were recorded without GlutaMAX at an age (30 DIV) where all cultures previously had been found to display either interictal- or ictal-like activity (Fig. 4A), epileptiform activity was not abolished but delayed beyond the initial 60 min of recording (Fig. 6E), demonstrating that decreased availability of glutamate (due to absence of glutamine supplement) merely lowers the frequency of epileptiform discharges. This suggests, in conjunction with the stable baseline activity of 7–8 DIV cultures in medium without or without GlutaMAX (Fig. 6D), that the mechanisms underlying epileptogenesis are intrinsic alterations in the cellular or network properties of organotypic slice cultures throughout their incubation period.
Figure 6. Spontaneous epileptiform discharges are the result of intrinsic alterations in organotypic slices but requires growth medium.
A. Robust ictal-like discharges in growth medium disappear within ~5 min of washing in standard ACSF. B. 100 min field potential recording from a 7 DIV hippocampal slice culture without GlutaMAX in the growth medium. C. 100 min field potential recording from a 7 DIV hippocampal slice culture with GlutaMAX in the growth medium. D. The threshold between pathological activity and physiological activity (Fig. 1) calculated in 5 min epochs of 100 min recordings with and without GlutaMAX for 7–8 DIV cultures. E. Without the glutamine supplement GlutaMAX in the recording medium, epileptiform activity in 21+ DIV cultures is delayed beyond the standard 60 min recording period.
To test the effect of standard anti-epileptic drugs (AEDs) on spontaneous epileptiform activity in hippocampal slice cultures, growth medium with a therapeutically relevant dose of 100 μM Phenytoin (Loscher et al., 1991) was perfused after 60 min of initial recordings in 22–27 DIV slice cultures (n = 3) displaying robust ictal-like discharges (Fig. 7A, top panel). During wash-in of Phenytoin (Pht) containing medium, ictal-like discharges were gradually shortened (Fig. 7A, middle panel) and after ~20 min, predominantly interictal-like spikes or short bursts remained (Fig. 7A, lower panel), comparable to the clinical effect observed in the EEG of patients with epilepsy (Duncan, 1987). This effect was quantified by detection of pathological activity with subsequent clustering of spikes into ≥2 Hz groups, as previously described (Fig. 1&4). The Pht-induced conversion of ictal-like to interictal-like discharges is clearly reflected in the strong reduction of groups of > 20 consecutive high frequency spikes and virtual abolishment of ≥2 Hz discharges in groups exceeding 100 consecutive spikes, accompanied by a moderate increase in the number of single spikes and bursts containing few consecutive spikes (Fig. 7B).
Figure 7. The anti-epileptic drug Phenytoin converts spontaneous ictal-like discharges to interictal-like activity.
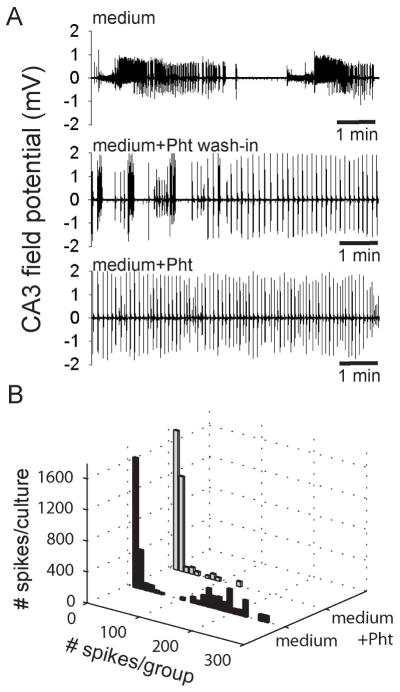
A. The duration of robust ictal-like discharges recorded in the growth medium of 3 hippocampal slice cultures (upper panel) was gradually shortened during wash-in of medium containing 100 μM phenytoin (middle panel). After 20 min wash-in, only interictal-like spikes and short burst persisted in the medium containing 100 μM phenytoin (lower panel). B. The transition from ictal-like activity in growth medium (black bars) to inter-ictal like activity following 20 min wash-in of medium containing 100 μM phenytoin (grey bars) is reflected in the decrease of ≥2 Hz spike activity spanning more than 20 consecutive spikes.
Discussion
Our findings demonstrate an unexpectedly high incidence of spontaneous epileptogenesis in organotypic hippocampal slice culture based on prolonged recordings from CA3 while bathed in growth medium (Fig. 2E). Epileptiform activity developed later than 7 DIV, with primarily interictal-like spikes and bursts at 14–17 DIV and preceding increasingly ictal-like discharges after 21 DIV (Fig. 4A). Spontaneous epileptiform activity is not limited to rollertube cultures or the growth medium used, as they were also present in interface-type cultures recorded in neurobasal medium (Fig. 3). These results support the possibility that interictal spikes may drive later stages of epileptogenesis, but because the incidence of spikes and subsequent seizures were both high, the planned correlation between spikes and subsequent seizures was not carried out.
Epileptiform activity has generally not been noted in prior studies using either roller culture or membrane-culture of organotypic hippocampal brain slices, perhaps as a consequence of the age at which the slices were utilized (Pavlidis and Madison, 1999; Lahtinen et al., 2001; Abegg et al., 2004). Spontaneous epileptiform discharges have been recorded intracellularly in CA1 pyramidal neurons (McBain et al., 1989), although without further classification or quantification of the discharges. These discrepancies cannot be explained by differences in the use of antibiotics, antimitotics or the glutamine supplement GlutaMAX in the present study, as none of the above studies applied these compounds. Furthermore, additional recordings with and without GlutaMAX in the recording medium neither increased nor abolished epileptiform activity (Fig. 6B–E). While the presently used Sprague-Dawley rat strain has been reported to have significantly lower seizure threshold than Wistar rats in the kindling model of epilepsy (Loscher et al., 1998), studies that do not mention epileptiform activity (Pavlidis and Madison, 1999) used Sprague-Dawley rats while the study that did observe epileptiform activity used Wistar rats. Further studies could potentially aid in explaining these discrepancies. The cause of spontaneous epileptogenesis in organotypic hippocampal slice cultures is likely the massive reorganization of the local network caused by reactive sprouting during the incubation period: In area CA3, the connection probability between pairs of pyramidal neurons increases from 1.5% in acute slices (Miles and Wong, 1986) to 56% in organotypic slice cultures after 14–28 DIV (Debanne et al., 1995). Such a dramatic increase in the clustering of local excitatory connections plays a large role in hyperexcitability and epileptogenesis (Dyhrfjeld-Johnsen et al., 2007), has been demonstrated as a seizure mechanism in hippocampal slice cultures treated with kainate (Bausch and McNamara, 2004) and is a hallmark of many in vivo epilepsy models including models of post-traumatic epilepsy (Pitkanen et al., 2009; Prince et al., 2009; Timofeev et al., 2009).
The spontaneous development of interictal-like discharges at 14–17 DIV preceded the development of predominantly ictal-like discharges after 21 DIV, as quantified using automated spike-detection and clustering algorithms (Fig. 4). This analysis confirmed a dramatic increase in the amount of consecutive spikes fired at frequencies ≥2 Hz in older slice cultures. A similar temporal relationship, with interictal spikes appearing prior to the first seizure, has been noted in prolonged radiotelemetric EEG recordings during kainate-induced epileptogenesis in vivo (White et al., 2006; Williams et al., 2009; White et al., 2009).
We also recorded fast ripple-like complexes associated with interictal spikes (Fig. 2F). These fast ripples are similar to high-frequency discharges recorded following in vivo kainate-induced epileptogenesis (Bragin et al., 2004; Foffani et al., 2007). Additional detailed investigations elucidating the mechanisms underlying these transitions from predominantly interictal-like to ictal-like discharges are necessary, possibly aided by intracellular recordings and optical imaging, and could greatly enhance our understanding of the mechanisms underlying epileptogenesis in general.
Epileptiform activity in this in vitro system bears many similarities to in vivo epilepsy, including the appearance of spikes before seizures (White et al., 2006), the progressive increase in seizure-incidence (Williams et al., 2009), the mix of interictal and ictal activity, and the suppression of ictal but not interictal activity by therapeutic concentrations of anticonvulsants. Differences from in vivo include a compressed time scale and a higher frequency of seizures; however these may prove to be great advantages if the organotypic preparation is used as a less time and cost-intensive model system for the study of epileptogenesis and the development of anti-epileptic and epileptogenic drugs. While eventual in vivo testing cannot be replaced, initial high density screening of candidate drugs could be done in organotypic hippocampal slice cultures. Additionally, investigating mechanisms of action of successful pharmacological candidates would be strongly facilitated by the relatively high frequency of interictal and ictal activity, permitting more rapid ascertainment of the effects of experimental manipulations on epilepsy. Furthermore, the accessibility of slice cultures makes multiple methods of acute and chronic population and single neuron recordings feasible, including both electrophysiological and optical imaging approaches.
Supplementary Material
Acknowledgments
The authors thank Michelle Mail for technical assistance. Supported by the NIH (NS34360-12 to KS)
Reference List
- Abegg MH, Savic N, Ehrengruber MU, McKinney RA, Gahwiler BH. Epileptiform activity in rat hippocampus strengthens excitatory synapses. J Physiol. 2004;554:439–448. doi: 10.1113/jphysiol.2003.052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Longacher JM, Staley KJ. Reciprocal interactions between CA3 network activity and strength of recurrent collateral synapses. Nat Neurosci. 1999;2:720–726. doi: 10.1038/11184. [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J Neurophysiol. 2004;92:3582–3595. doi: 10.1152/jn.01028.2003. [DOI] [PubMed] [Google Scholar]
- Behrens CJ, van den Boom LP, de HL, Friedman A, Heinemann U. Induction of sharp wave-ripple complexes in vitro and reorganization of hippocampal networks. Nat Neurosci. 2005;8:1560–1567. doi: 10.1038/nn1571. [DOI] [PubMed] [Google Scholar]
- Berdichevsky Y, Sabolek H, Levine JB, Staley KJ, Yarmush ML. Microfluidics and multielectrode array-compatible organotypic slice culture method. J Neurosci Methods. 2009;178:59–64. doi: 10.1016/j.jneumeth.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- De Simoni, Griesinger CB, Edwards FA. Development of rat CA1 neurones in acute versus organotypic slices: role of experience in synaptic morphology and activity. J Physiol. 2003;550:135–147. doi: 10.1113/jphysiol.2003.039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Physiology and pharmacology of unitary synaptic connections between pairs of cells in areas CA3 and CA1 of rat hippocampal slice cultures. J Neurophysiol. 1995;73:1282–1294. doi: 10.1152/jn.1995.73.3.1282. [DOI] [PubMed] [Google Scholar]
- Duncan JS. Antiepileptic drugs and the electroencephalogram. Epilepsia. 1987;28:259–266. doi: 10.1111/j.1528-1157.1987.tb04216.x. [DOI] [PubMed] [Google Scholar]
- Dyhrfjeld-Johnsen J, Santhakumar V, Morgan RJ, Huerta R, Tsimring L, Soltesz I. Topological determinants of epileptogenesis in large-scale structural and functional models of the dentate gyrus derived from experimental data. J Neurophysiol. 2007;97:1566–1587. doi: 10.1152/jn.00950.2006. [DOI] [PubMed] [Google Scholar]
- Engel J. Seizures and Epilepsy. Philadelphia, PA: F.A. Davis; 1989. [Google Scholar]
- Foffani G, Uzcategui YG, Gal B, Menendez de la PL. Reduced spike-timing reliability correlates with the emergence of fast ripples in the rat epileptic hippocampus. Neuron. 2007;55:930–941. doi: 10.1016/j.neuron.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Gahwiler BH. Synaptic organization of intracellularly stained CA3 pyramidal neurons in slice cultures of rat hippocampus. Neuroscience. 1988;24:541–551. doi: 10.1016/0306-4522(88)90348-x. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Development of the hippocampus in vitro: cell types, synapses and receptors. Neuroscience. 1984;11:751–760. doi: 10.1016/0306-4522(84)90192-1. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- Gahwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997;20:471–477. doi: 10.1016/s0166-2236(97)01122-3. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Grosshans DR, Coultrap SJ, Jones JP, Dobelis P, Browning MD, Staley KJ. NMDA receptor trafficking at recurrent synapses stabilizes the state of the CA3 network. J Neurophysiol. 2007;98:2818–2826. doi: 10.1152/jn.00346.2007. [DOI] [PubMed] [Google Scholar]
- Houser CR, Esclapez M. Localization of mRNAs encoding two forms of glutamic acid decarboxylase in the rat hippocampal formation. Hippocampus. 1994;4:530–545. doi: 10.1002/hipo.450040503. [DOI] [PubMed] [Google Scholar]
- Lahtinen H, Autere AM, Paalasmaa P, Lauri SE, Kaila K. Post-insult activity is a major cause of delayed neuronal death in organotypic hippocampal slices exposed to glutamate. Neuroscience. 2001;105:131–137. doi: 10.1016/s0306-4522(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Loscher W, Cramer S, Ebert U. Differences in kindling development in seven outbred and inbred rat strains. Exp Neurol. 1998;154:551–559. doi: 10.1006/exnr.1998.6948. [DOI] [PubMed] [Google Scholar]
- Loscher W, Fassbender CP, Nolting B. The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. II. Maximal electroshock seizure models. Epilepsy Res. 1991;8:79–94. doi: 10.1016/0920-1211(91)90075-q. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Boden P, Hill RG. Rat hippocampal slices ‘in vitro’ display spontaneous epileptiform activity following long-term organotypic culture. J Neurosci Methods. 1989;27:35–49. doi: 10.1016/0165-0270(89)90051-4. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. J Physiol. 1986;373:397–418. doi: 10.1113/jphysiol.1986.sp016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Pavlidis P, Madison DV. Synaptic transmission in pair recordings from CA3 pyramidal cells in organotypic culture. J Neurophysiol. 1999;81:2787–2797. doi: 10.1152/jn.1999.81.6.2787. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Immonen RJ, Grohn OH, Kharatishvili I. From traumatic brain injury to posttraumatic epilepsy: what animal models tell us about the process and treatment options. Epilepsia. 2009;50 (Suppl 2):21–29. doi: 10.1111/j.1528-1167.2008.02007.x. [DOI] [PubMed] [Google Scholar]
- Prince DA, Parada I, Scalise K, Graber K, Jin X, Shen F. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50 (Suppl 2):30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman JH, Sterling CA, Luo R. Hippocampal plasticity following epileptiform bursting produced by GABAA antagonists. Neuroscience. 1994;59:259–273. doi: 10.1016/0306-4522(94)90594-0. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sutula TP, Dudek FE. Unmasking recurrent excitation generated by mossy fiber sprouting in the epileptic dentate gyrus: an emergent property of a complex system. Prog Brain Res. 2007;163:541–563. doi: 10.1016/S0079-6123(07)63029-5. [DOI] [PubMed] [Google Scholar]
- Timofeev I, Bazhenov M, Avramescu S, Nita DA. Posttraumatic Epilepsy: The Roles of Synaptic Plasticity. Neuroscientist. 2009 doi: 10.1177/1073858409333545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Williams PA, Ferraro DJ, Clark S, Kadam SD, Dudek FE, Staley KJ. Efficient unsupervised algorithms for the detection of seizures in continuous EEG recordings from rats after brain injury. J Neurosci Methods. 2006;152:255–266. doi: 10.1016/j.jneumeth.2005.09.014. [DOI] [PubMed] [Google Scholar]
- White AM, Williams PA, Hellier JL, Clark S, Dudek FE, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02339.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2112. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Gahwiler BH. Cellular and connective organization of slice cultures of the rat hippocampus and fascia dentata. J Comp Neurol. 1984;228:432–446. doi: 10.1002/cne.902280310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.