Abstract
PURPOSE
To investigate the frequency and types of systemic findings in patients with apparently isolated uveal coloboma.
DESIGN
Cross-sectional observational study.
METHODS
SETTING
Single-center ophthalmic genetics clinic.
STUDY POPULATION
Ninety-nine patients with uveal coloboma seen at the National Eye Institute.
OBSERVATIONAL PROCEDURE
Results of audiology testing, echocardiogram, brain magnetic resonance imaging, renal ultrasound, and total spine radiographs.
MAIN OUTCOME MEASURE
Prevalence of abnormal findings on systemic testing.
RESULTS
Uveal coloboma affected only the anterior segment in 8 patients, only the posterior segment in 23 patients, and both anterior and posterior segments in 68 patients. Best-corrected visual acuity (BCVA) of eyes with coloboma was ≥20/40 in 45% of eyes; 23% of eyes had BCVA of ≤20/400. The majority of patients (74%) had good vision (>20/60) in at least 1 eye. Ten of the 19 patients (53%) who underwent echocardiography had abnormalities, with ventral septal defects being the most prevalent. Abnormal findings were observed in 5 of 72 patients (7%) who had a renal ultrasound and in 5 of 29 patients (17%) who underwent a brain MRI. Audiology testing revealed abnormalities in 13 of 75 patients (17%), and spine radiographs showed anomalies in 10 of 77 patients (13%). Most findings required no acute intervention.
CONCLUSIONS
Although some patients with coloboma had evidence of extraocular abnormalities, the majority of findings on routine clinical examination did not require acute intervention, but some warranted follow-up. Results from the systemic evaluation of patients with coloboma should be interpreted with caution and in view of their clinical context.
Uveal coloboma is a rare eye malformation caused by failure of the optic fissure to close during the fifth to seventh weeks of fetal life.1 It has an estimated prevalence of 1 in 10 000 in the United States.2,3 A coloboma may appear as an isolated finding or as part of a broader systemic syndrome. The most common syndromic form of coloboma is the CHARGE syndrome, an acronym for coloboma, heart defects, atresia choanae, retarded growth and development, genitourinary anomalies, and ear anomalies/deafness. Important clinical features not reflected in the CHARGE acronym include orofacial clefts, facial palsies, and vestibular abnormalities.4 While many patients with syndromic forms of uveal coloboma will present clinically in infancy, the phenotypic spectrum is quite wide.
This broad phenotypic spectrum raises an important question for the practicing ophthalmologist and clinical geneticist, namely: in a new patient presenting with seemingly isolated uveal coloboma, are there additional systemic tests that should be completed to rule out a mild form of syndromic involvement?
In this study, we reviewed our 8 years of experience with comprehensive ocular and systemic phenotyping at the National Institutes of Health (NIH) Clinical Center of patients with uveal coloboma. The purpose of this study was to evaluate the yield of systemic testing, such as audiology, renal ultrasound, echocardiography, and neuroimaging, and the prevalence of non–clinically evident abnormalities among patients with uveal coloboma.
METHODS
This was an observational, prospective cross-sectional study of patients who were seen at the National Eye Institute (NEI) between April 2004 and June 2012. Registration information is available at the National Institutes of Health database at http://www.clinicaltrials.gov (identifiers: NCT00076271, NCT00368004, NCT01778543, and NCT000708955). The study was approved by the NEI Institutional Review Board or the Combined Neuroscience Institutional Review Board of the NIH, depending on the time frame. Informed consent/assent was obtained from the patients and/or their parents. The study conformed to all federal or state laws. All methods adhered to the tenets of the Declaration of Helsinki for research involving human subjects, and the study was conducted in accordance with Health Insurance Portability and Accountability Act regulations.
Because the purpose of this study was to assess the yield of systemic testing on patients presenting with coloboma to an ophthalmologist, subjects with CHARGE syndrome or another clearly identifiable syndrome were excluded from analysis (Supplemental Table 1, available at AJO.com). Patients who appeared otherwise generally healthy, but who had abnormalities detected on specialized testing (such as karyotype), were also excluded. Some patients were excluded because of developmental eye abnormalities described as “coloboma” by the referring physician, but who had defects not related to optic fissure closure (eg, macular “coloboma”).
If available, existing outside medical records were reviewed and included in this analysis. Tests that had been previously performed and documented as normal were not repeated. A detailed history was obtained, including questions about birth, development, and family history. Ophthalmologic evaluation included assessment of best-corrected visual acuity (BCVA), ocular motility and alignment, anterior segment examination, and dilated funduscopy. For infants and developmentally delayed children who could not participate in formal visual acuity assessment, the ability of the patient to fix and follow, the presence of any ocular fixation preference, and/or binocular Teller visual acuity was noted. Because of the age of most participants, microphthalmia was judged by clinical examination, as axial lengths could only rarely be obtained. All patients were examined by the same pediatric ophthalmologist (B.P.B.).
Depending on the patient’s age, developmental status, cooperation, and history of previous testing, further systemic investigations were obtained. Systemic testing included audiology evaluation, brain magnetic resonance imaging (MRI), skeletal x-rays, echocardiogram, kidney ultrasound, routine blood chemistries including kidney and liver function testing, and urinalysis. After a preliminary analysis of approximately 60 subjects, some systemic testing (eg, echocardiography in the absence of a detectable heart murmur) was eliminated because of its low clinical yield. In addition, systemic testing was performed only on those individuals able to cooperate without sedation. As such, not every patient received every test.
RESULTS
PATIENT CHARACTERISTICS
A total of 99 patients with an initial diagnosis of nonsyndromic uveal coloboma were investigated in the NEI Ophthalmic Genetics Clinic over an 84-month period. The mean age at the time of initial examination was 9.5 years (range 4 months – 43 years). There were 52 female and 47 male patients. The majority of the patients (80%) were white. The remaining patients were Latino (9%), black or African-American (5%), Asian (5%), and American Indian (1%).
OCULAR FINDINGS
Of all 99 patients with uveal coloboma, bilateral involvement was observed in 71 patients (71%). Among the 28 patients with unilateral involvement, the right eye was affected in 15 patients and the left eye was involved in 13 patients. Among the 71 patients with bilateral involvement, 4 had bilateral anterior segment involvement (lens and/or iris) only, 14 had bilateral posterior segment involvement (optic disc and/or retina) only, and 53 had involvement of both the anterior and posterior segments. Twenty-one of the 71 patients with bilateral involvement had discordance of the location of coloboma between the 2 eyes. In 19 patients, 1 eye had only anterior involvement with both anterior and posterior segments involved in the fellow eye. Interestingly, only 1 patient had only posterior involvement in 1 eye and anterior and posterior involvement in the fellow eye; similarly, 1 patient had only anterior involvement in 1 eye and only posterior involvement in the fellow eye. Systemic anomalies appeared in approximately equal frequency between patients with bilateral and unilateral colobomas (42% vs 39%; odds ratio 1.1; 95% confidence interval 0.5–2.8; P = .78).
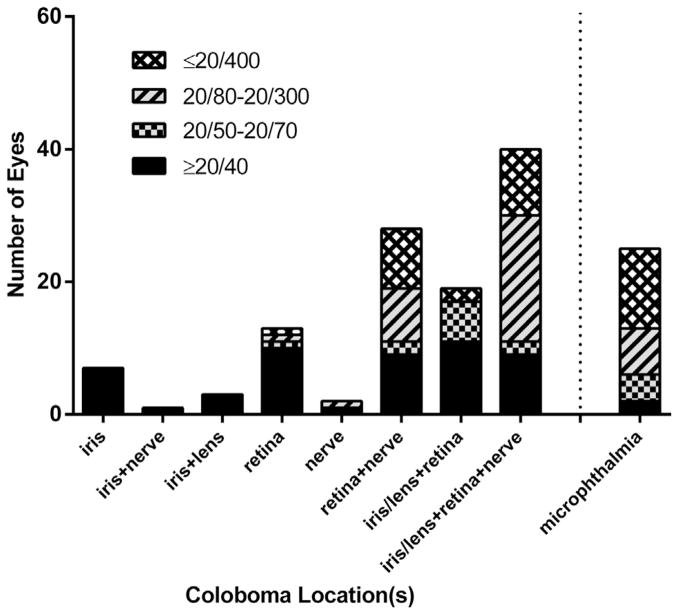
Visual acuity could not be quantitated in 33 patients because of either young age or developmental delay. Among the 66 patients in whom visual acuity could be assessed, 49 patients (74%) had good visual function (>20/60 in at least 1 eye), 8 patients (12%) were visually impaired (<20/60 but >20/200 in at least 1 eye), and 9 patients (14%) were legally blind (≤20/200 in the better eye). Visual acuity of the eye affected with coloboma ranged from 20/12.5 to no light perception (NLP). Most of the affected eyes had 20/40 or better vision (Table 1). However, 27 of the 117 eyes (23%) analyzed were 20/400 or worse. Figure 1 depicts the visual acuities based on coloboma location. All eyes with only anterior segment involvement had vision ≥20/40. Eyes with combined retinal and optic disc involvement, as well as eyes that were microphthalmic, had a higher frequency of visual impairment.
TABLE 1.
Visual Acuity of Eyes Affected With Uveal Coloboma
| Visual Acuity | n (%) |
|---|---|
| ≥20/40 | 53 (45) |
| 20/50–20/70 | 11 (9) |
| 20/80–20/160 | 16 (14) |
| 20/200–20/300 | 10 (9) |
| ≤20/400 | 27 (23) |
| HM | 14 |
| CF | 3 |
| LP | 1 |
| NLP | 1 |
CF = count fingers; HM = hand movement; LP = light perception; NLP = no light perception.
FIGURE 1.
Distribution of visual acuity based on uveal coloboma location and presence of microphthalmia (per eye).
Eight patients (8%) had involvement of the anterior segment only, 23 patients (23%) had involvement of the posterior segment only, and the remaining 68 subjects (69%) had both anterior and posterior segment findings (Table 2). Anterior segment-only findings included 7 patients with isolated iris coloboma and 1 patient with both iris and lenticular colobomas. Among the patients with isolated posterior segment involvement, chorioretinal coloboma was present in 3 patients and combined optic disc and chorioretinal colobomas were present in 20 patients. A total of 69 patients (153 eyes) had involvement of the optic nerve. Of these 153 eyes, the visual acuity could be measured in 106 eyes. Forty-one eyes (39%) had a visual acuity of 20/40 or better, 36 eyes (34%) had a vision of <20/40 but >20/400, and 29 eyes (27%) had a visual acuity of 20/400 or worse. Concurrent ocular abnormalities are summarized in Table 3. In order of decreasing frequency, the most common coexisting ocular anomalies were microphthalmia (29%), strabismus (19%), cataract (15%), microcornea (14%), and nystagmus (14%).
TABLE 2.
Location of Uveal Coloboma
| Location of Coloboma | Unilateral Involvement | Bilateral Involvement | Total |
|---|---|---|---|
| Anterior segment, n/N (%) | 8/99 (8) | ||
| Iris | 3 | 4 | 7 |
| Lens | 0 | 0 | 0 |
| Iris and lens | 1 | 0 | 1 |
| Posterior segment, n/N (%) | 23/99 (23) | ||
| Optic disc | 0 | 0 | 0 |
| Retina | 2 | 1 | 3 |
| Optic disc and retina | 6 | 14 | 20 |
| Anterior and posterior segments, n/N (%) | 68/99 (69) | ||
| Iris and optic disc | 1 | 0 | 1 |
| Iris and retina | 6 | 8 | 15 |
| Iris, optic disc, and retina | 3 | 33 | 36 |
| Iris, lens, retina | 0 | 5 | 5 |
| Iris, lens, optic disc, retina | 7 | 5 | 12 |
| Total | 28 | 71 | 99 |
TABLE 3.
Prevalence of Other Ocular Abnormalities Among Patients With Uveal Coloboma
| Other Ocular Disorders | Total, n/N (%) |
|---|---|
| Microphthalmia | 29/99 (29) |
| Microcornea | 14/99 (15) |
| Amblyopia | 10/99 (10) |
| Nystagmus | 14/99 (14) |
| Cataract | 15/99 (16) |
| Anisometropia | 1/99 (1) |
| Retinal detachment | 1/99 (1) |
| Fetal vascular remnant | 6/99 (6) |
| Strabismus | 18/99 (19) |
| Esotropia | 14 |
| Exotropia | 2 |
| Mixed | 2 |
PHYSICAL EXAMINATION AND DEVELOPMENTAL STATUS
A detailed physical examination was performed either by the pediatric or internal medicine service at the NIH or by the patient’s own physician. Thirty-three patients had their examination done at the NIH. Table 4 lists the details of the significant findings, which included growth retardation, failure to thrive, developmental delay, dysmorphic features, and skin findings. When identifiable syndromes were excluded, the dysmorphic features did not follow any specific pattern. High-resolution karyotype was initially performed on 36 patients; 3 had abnormal results, but these were excluded from this analysis, given that they were clearly syndromic patients. As such, we discontinued sending karyotype on apparently nonsyndromic patients.
TABLE 4.
Physical Examination and Developmental Findings in Patients With Uveal Coloboma
| Finding | n/N (%) |
|---|---|
| Growth abnormalities | 2/99 (2) |
| Failure to thrive | 1 |
| Retarded growth | 1 |
| Macrocephaly | 1/99 (1) |
| Dysmorphic features | 13/99 (13) |
| High-arched palate | 4 |
| Single palmar crease | 1 |
| Midfacial hypoplasia | 1 |
| Bifid thumb | 1 |
| Pectus excavatum | 1 |
| Incomplete Sydney crease | 1 |
| Craniosynostosis | 1 |
| Hypotonic face | 1 |
| Small jaw | 1 |
| Epicanthal folds | 1 |
| Skin findings | 7/99 (7) |
| Sacral dimple | 2 |
| Preauricular skin tags | 1 |
| Ear tags | 1 |
| Macular hyperpigmented lesion on occipital scalp | 1 |
| Capillary hemangioma on thigh | 1 |
| Forehead telangiectasia | 1 |
| Developmental delay | 16/99 (16) |
| Speech | 6 |
| Motor | 6 |
| Speech and motor | 4 |
Among the 16 patients with reported developmental delay, 3 patients (19%) had posterior colobomas and 13 patients (81%) had both anterior and posterior colobomas. Fourteen of the 16 patients (88%) had bilateral disease. There was not a statistically significant difference between coloboma laterality and developmental delay (Fisher exact test, P = .22). Among the 20 eyes affected with coloboma in the 16 patients with developmental delay, 8 eyes (40%) had visual acuities of >20/40, while 6 eyes (30%) had visual acuities of <20/200. Microphthalmia was observed in 5 patients (31%), and cardiac, urogenital, and central nervous system anomalies were present in 25% (4/16), 19% (3/16), and 38% (6/16) of the patients with developmental delay, respectively. There was no clear correlation between coloboma morphology and the presence of specific systemic findings.
ECHOCARDIOGRAPHY
Of the 19 patients who were examined by echocardiography, 10 patients (53%) had cardiac anomalies (Table 5). Seven individuals had a single finding: ventricular septal defect (VSD) (3 patients), atrial septal defect (ASD) (2 patients), mitral valve prolapse (1 patient), and patent foramen ovale (PFO) (1 patient). The other 3 patients each had 2 cardiac abnormalities: ASD and patent ductus arteriosus (PDA), pulmonary stenosis and PFO, and pulmonary stenosis and ASD. Three patients underwent cardiac surgical repair during infancy: 2 for ASD and 1 for VSD. In all cases, the abnormalities were ascertained in infancy and based on historical records.
TABLE 5.
Extraocular Abnormalities on Systemic Diagnostic Testing in Patients With Uveal Coloboma
| Abnormal Finding | n/N (%) |
|---|---|
| Cardiac abnormalities | 10/19 (53) |
| VSD | 3 |
| ASD | 2 |
| Mitral valve prolapse | 1 |
| PFO | 1 |
| ASD/PDA | 1 |
| Pulmonary stenosis/PFO | 1 |
| ASD/VSD | 1 |
| Urogenital abnormalities | 5/72 (7) |
| Bilateral hydronephrosis | 1 |
| Small left kidney with multiple small cysts | 1 |
| Slightly small left kidney | 1 |
| Cyst in left kidney | 1 |
| Missing left kidney | 1 |
| Skeletal abnormalities | 10/77 (13) |
| Osteopenia | 4 |
| Missing 2 thoracic vertebrae | 1 |
| Fused C2-C3 vertebrae | 1 |
| Spina bifida occulta T8 | 1 |
| Small left cervical rib | 1 |
| Missing T12 ribs | 1 |
| Incomplete blocked vertebrae at C6-C7 | 1 |
| Brain abnormalities | 5/29 (17) |
| Single focal subcortical white matter abnormality in right frontal lobe | 1 |
| Increased T1 signal in cerebellum | 1 |
| Prominent perivascular spaces | 1 |
| Small arachnoid cyst in left middle cranial fossa | 1 |
| 6-mm descent of cerebellar tonsils to C1 arch; prominent perivascular spaces; delayed myelination; periventricular white matter lesion of L temporal lobe; 2 L subependymal cysts | 1 |
| Audiology findings | 13/75 (17) |
| Bilateral conductive hearing loss | 5 |
| Unilateral conductive hearing loss | 5 |
| Bilateral mixed hearing loss | 1 |
| Bilateral sensorineural hearing loss | 2 |
ASD = atrial septal defect; PDA = patent ductus arteriosus; PFO = patent foramen ovale; VSD = ventricular septal defect.
RENAL ULTRASONOGRAPHY
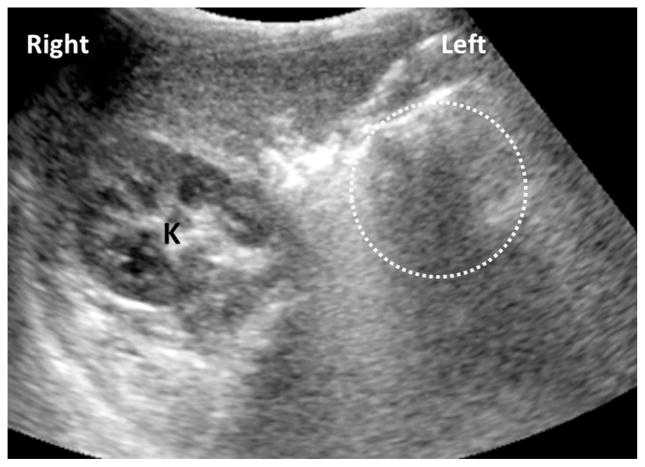
Renal ultrasonography was performed on 72 patients. Five patients (7%) had abnormal findings (Table 5). The abnormal findings were bilateral hydronephrosis, slightly small kidney, small kidney with multiple cysts, absent kidney (Figure 2), and a 6-mm cyst in the kidney. Blood pressure measurements taken during their NIH visit and laboratory analyses for blood urea nitrogen and creatinine were within normal limits for age and sex.
FIGURE 2.
Solitary right kidney in a patient with uveal coloboma. An axial ultrasound image demonstrates the right kidney in a normal position and orientation (K). However, the left renal fossa is empty (circle) and the left kidney was not found in an ectopic location.
SPINAL X-RAY
Skeletal or vertebral abnormalities were noted in 10 of the 77 patients (13%) who underwent imaging. Table 5 summarizes these findings, which included osteopenia and fused cervical vertebrae (Klippel-Feil anomaly).
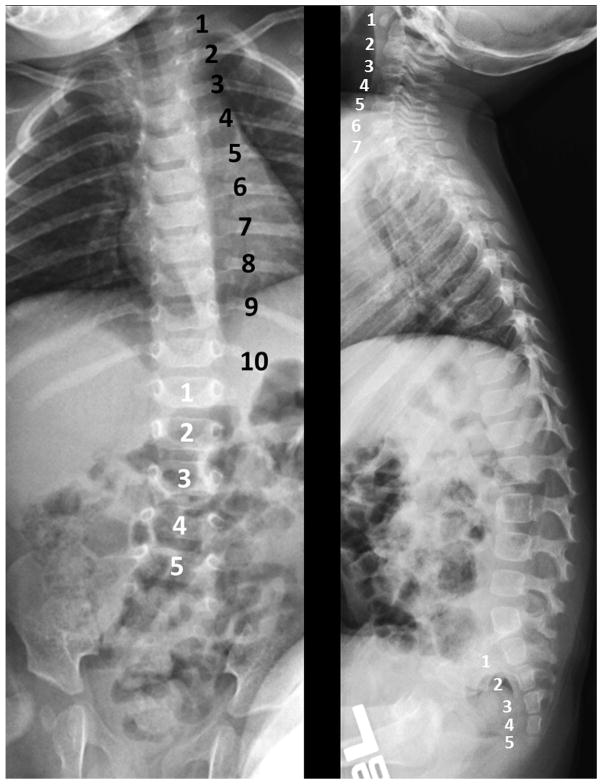
In 2 unrelated and nonconsanguineous families, vertebral anomalies appear to co-segregate with uveal coloboma (Supplemental Figures 1 and 2, available at AJO.com). In these families, vertebral findings include missing vertebral ribs (Figure 3), scoliosis, transitional vertebra, and spina bifida occulta. Coloboma appears to be incompletely penetrant and exhibits variable expressivity in individuals from Family 1, several of whom were found to have variants of the spinal bones, particularly in those subjects who linked the 2 affected lineages in this family. In both families, an autosomal-dominant pattern of inheritance seems most likely, although an X-linked inheritance pattern could not be excluded based on the pedigree in Family 2. The proband in Family 2 was missing a kidney as well. A similar finding was reported, but not verified, in 2 individuals from Family 1: one has coloboma, while the other is the son of a female subject with a missing vertebra. Linkage analysis was unsuccessful in determining a possible locus for this coloboma-spine association. Whole exome sequencing analysis is currently in progress.
FIGURE 3.
Segmentation error detected on spine x-ray in a patient with uveal coloboma. (Left) Anteroposterior and (Right) lateral radiographs of the spine demonstrating 10 rib-bearing vertebrae (black numbers). The number of cervical, lumbar, and sacral vertebrae (white numbers) is normal.
BRAIN MAGNETIC RESONANCE IMAGING
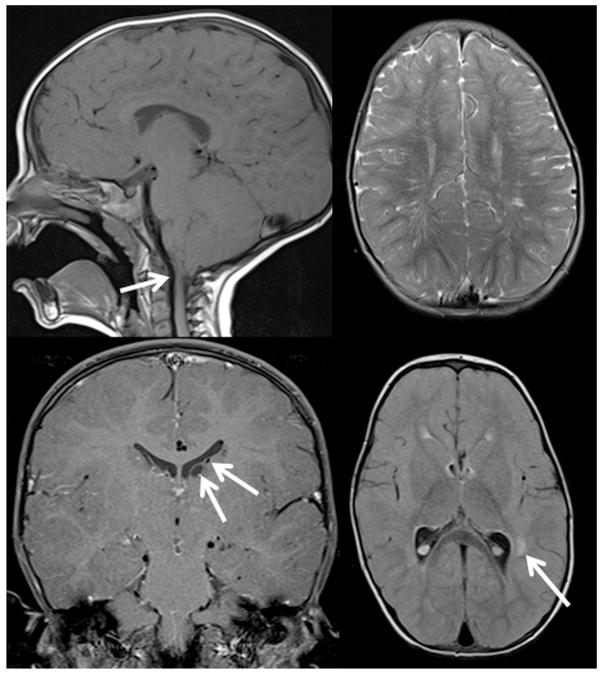
A brain MRI was performed in 29 patients. The mean age of these patients was 7.2 years (range 4 months – 27 years). Anomalies of the central nervous system were documented in 5 patients (17%). The mean age of the patients with anomalies was 10.9 years (range 3.1–34.7 years). The findings are documented in Table 5. One patient had multiple findings, including type 1 Chiari malformation, prominent perivascular spaces, periventricular white matter lesions, and subependymal cysts (Figure 4). This patient also had speech and motor developmental delay.
FIGURE 4.
Brain magnetic resonance imaging findings in a 26-month-old patient with bilateral uveal colobomas involving the iris and retina. (Top left) Type I Chiari malformation; the tips of the cerebellar tonsils lie 6 mm below the inferior margin of the foramen magnum (arrow), at the same level as the posterior arch of C1. (Top right) Prominent perivascular (Virchow-Robin) spaces are unusually numerous in the white matter of the cerebral hemispheres. (Bottom left) There are 2 subependymal cysts on the left side (arrow). (Bottom right) There are several nonenhancing lesions of indeterminate etiology (arrow) located in the perivascular white matter of the left temporal lobe.
AUDIOLOGY TESTING
Audiology evaluation was completed in 75 patients and 13 (17%) had positive findings (Table 5). Ten of these individuals had evidence of conductive hearing loss, likely secondary to middle ear pathology from a prior or present history of otitis media. One patient had mild sensorineural hearing loss, and 1 had profound hearing loss by history that was sufficient to warrant bilateral cochlear implantation. The patient with profound hearing loss was a 9-year-old girl with bilateral colobomas (iris OD and combined optic disc and chorioretinal colobomas OU) whose visual acuities were 20/400 OD and 20/200 OS. Her other systemic testing was normal. Hearing aids were used by 2 patients in 1 ear each.
DISCUSSION
Uveal coloboma can exist in isolation or in association with other ocular and systemic abnormalities. The identification of a coloboma often prompts clinicians to screen for associated systemic anomalies. To the best of our knowledge, this is the first study to systematically review the findings of diagnostic tests obtained in clinically nonsyndromic patients with coloboma at a single center. The findings in this study confirm that there is considerable ocular and systemic phenotypic variability among uveal coloboma patients.
In comparing the results of the ocular examination in our study with 2 recent population-based studies on coloboma (Supplemental Table 2, available at AJO.com), we found several differences. In a retrospective review of 33 pediatric patients, Nakamura and associates showed that 36% of patients had only anterior colobomas, 39% had only posterior involvement, and 29% had both anterior and posterior colobomas.5 Concurrent ocular anomalies included 33% with amblyopia and 30% with strabismus. In a prospective study of 135 children with newly diagnosed anophthalmia, microphthalmia, and typical coloboma (AMC), Shah and associates reported that the location of the colobomas were as follows: 14% involving only the anterior segment, 43% involving only the posterior segment, and 43% with both anterior and posterior colobomas.6,7 Compared to these 2 studies, we found a higher frequency of combined anterior and posterior colobomas (68%). Consistent with previous reports, patients with coloboma affecting the retina and optic nerve and those with microphthalmia have a more guarded visual prognosis.8,9 Compared to Nakamura’s study, we found a lower prevalence of amblyopia (10% vs 33%) and strabismus (19% vs 30%) among our cohort of coloboma patients. Nonetheless, our clinical experience supports a trial of glasses and/or patching in patients with uveal coloboma—even those with significant posterior segment involvement—as some children respond to these measures.
Differences in findings among studies may be explained by ascertainment methodology, examination techniques, and/or demographics. The Nakamura cohort retrospectively reviewed the records of patients in Olmsted County, Minnesota, ascertaining their subjects via a reported diagnosis code and with an upper age limit of 19 years.5 Shah’s cohort of patients was prospectively recruited across various clinical sites by multiple ophthalmologists in the United Kingdom through an established surveillance system.6 In contrast, our subjects were prospectively recruited as part of an NIH clinical research protocol and examined by a single ophthalmologist. As such, our study includes an ascertainment bias for patients willing to participate in research, and may be less likely to miss patients with asymptomatic posterior segment findings.
Other differences among studies include laterality of malformations and visual function. Among the 60 eyes with unilateral colobomas in the study by Shah and associates, the right eye (n = 40) was affected more than the left (n = 20) (P = .012).6 However, we did not identify significant laterality in our unilateral coloboma cases (15 OD vs 13 OS). Shah also reported that 44% of their subjects were bilaterally visually impaired (defined as BCVA “outside normal limits”) or severely visually impaired (defined as BCVA <3/60). We used a more specific classification for visual impairment, and found that 12% of our patients were visually impaired (<20/60 but >20/200 in at least 1 eye) and 14% were legally blind (<20/200 in the better eye). Using a definition that approximates that used by Shah, we found that 33% of our patients were bilaterally visually impaired or severely visually impaired. Furthermore, while Shah found that systemic abnormalities were more common in children with bilateral AMC (70% vs 47%; odds ratio 2.7; P = .006), we did not find a significant difference between our bilateral (42%) and unilateral (39%) cases (odds ratio 1.1; P = .78). This difference in correlation may be related to several factors, including recruitment ascertainment bias, multiple examiners, and inconsistent criteria for testing. The patients in the studies by Shah and associates were recruited through a rigorous nationwide monthly surveillance program that included both syndromic and nonsyndromic patients, as well as infants/neonates. Our recruitment, by definition, was biased toward families who were willing to participate in research, did not have a clearly identifiable syndrome, and were not seen neonatally, and (possibly) because patients with visual impairment are more likely to seek participation in research. The cohort in Shah and associates’ study likely captured a wider phenotypic spectrum, which, in addition to colobomas, included any patients with primary anophthalmia and microphthalmia. In contrast, our study recruited patients primarily based on the diagnosis of uveal coloboma, with concurrent microphthalmia/anophthalmia as a secondary feature.
Coloboma can be associated with systemic abnormalities of varying severity. In an analysis of 82 patients with uveal colobomas, Maumenee and Mitchell showed that a substantial portion of their patients (27%) had midline defects such as clefting, cardiovascular and urogenital anomalies, and intracranial defects.10 In another study of 48 pediatric patients with chorioretinal colobomas, 18 (38%) had other systemic abnormalities, including 6 patients with CHARGE association and 1 patient with transposition of the great vessels.11 In our study, we found that 50% of our patients had at least 1 abnormal finding on systemic evaluation and 12% of patients had 2 abnormal tests. Congenital developmental defects that result in coloboma may be associated with malformation of various other organ systems that are developing during similar phases of embryogenesis and/or use similar molecular mechanisms of development.
Echocardiograms were obtained in less than a quarter of our patients. Most of the abnormalities were noted during infancy in patients who had a cardiac murmur. Consistent with prior studies,5,12 a spectrum of congenital heart disease was present, including ASD, VSD, and PFO. Similar to our population, Nakamura and associates observed patients with combined cardiovascular anomalies5: 2 with ASD and PDA, 1 with ASD and pulmonic valvular stenosis, and 1 with PDA and PFO. We also had 1 patient with combined ASD and pulmonic valvular stenosis and another patient with ASD and PDA. Whether the combined cardiac malformations are related to the specific basis of the coloboma or to other factors such as modifying genes or intrauterine environment is speculative.
Urogenital abnormalities were found in 7% of our patients who were examined by renal ultrasonography. In an analysis of 24 patients with known CHARGE syndrome who were examined by renal ultrasound to screen for urinary abnormalities, Ragan and associates found that 5 patients had renal abnormalities of structure and number: solitary kidney in 2 patients, renal hypoplasia in 2 patients, and bilateral duplex kidneys in 1 patient.13 Three out of the 5 patients with renal anomalies had either unilateral renal agenesis or hypoplasia. Renal development is initiated at the fifth week of gestation,14 corresponding to the same time period when failure of embryonic fissure closure results in coloboma. Signs of renal impairment, as determined by the presence of hypertension, dipstick proteinuria, and blood urea nitrogen and creatinine levels, were not present in any of our subjects. There were no genital abnormalities by history, but a detailed examination was not performed on all patients at NIH. In a systematic meta-analysis of patients with solitary kidneys, Westland and associates showed that unilateral renal agenesis is not an entirely harmless disease, as hypertension, microalbuminuria, and end-stage renal disease can develop.15 As such, patients with coloboma, particularly those with possible syndromic coloboma, should be advised to be monitored regularly for renal disease. For instance, those with a single or dysplastic kidney should be monitored until adulthood, once at least every 1–2 years, for proteinuria and for possible urinary reflux. For those with 2 normal kidneys, particularly for those patients without a syndromic genetic diagnosis (eg, PAX2 mutation), there is currently no solid evidence that they are at an increased risk for kidney disease. In such cases, clinicians must use their clinical acumen to decide whether periodic screening is warranted.
Among the 13 patients with hearing loss, 10 had mild or moderate conductive hearing loss in 1 or both ears, which was associated with tympanometric evidence of reduced middle ear mobility. This hearing loss may be transient or intermittent, and should be monitored along with otologic management. One patient had a mild sensorineural hearing loss and 1 child had sufficient sensorineural hearing loss for bilateral cochlear implantation. Prevalence of mild or greater hearing loss among the pediatric and adolescent patients in our cohort was 18.5% (12/65 patients), which is substantially higher that the average prevalence of 3.1% in these age groups in the United States.16 Coloboma is considered a risk factor for hearing loss, and despite passing newborn hearing screening, an infant with coloboma should have at least 1 diagnostic audiologic evaluation by the age of 24–30 months.17 Early audiology assessment is especially important for coloboma patients, since vision may be impaired and early detection of even mild hearing loss can prompt timely interventions that can have significant beneficial impact on speech, language, learning, and academic outcomes.
Brain imaging in our population revealed findings that were mainly of minimal clinical significance. The MRI findings noted in our patients included prominent vascular spaces, subependymal cysts, and arachnoid cysts. Groeschel and associates reported that the prevalence of prominent vascular spaces (Virchow-Robin spaces) was 1.6% in adults, but this anomaly was not found among any children.18 However, there was no specific comment about the particular pattern of prominent vascular spaces (état criblé) noted in our study. One of our patients had a small arachnoid cyst in the left middle cranial fossa. The prevalence of arachnoid cysts in normal adults is 1%, most commonly located in the middle cranial fossa.19 Larcos and associates showed that the frequency of subependymal cysts detected by neonatal ultrasound is approximately 1%.20 Most of the patients in their study were premature infants. The authors felt that the cysts were of no particular neurologic consequence and that most cysts resolve with time.
The majority of findings on spinal radiograph required no acute intervention. We identified 2 families with autosomal-dominant pedigrees in which the proband and some affected family members had coloboma and missing vertebrae. We postulate that the mutated gene or genes in these families causes both the vertebral and ocular malformations. Based on the pedigrees, penetrance is incomplete. This observation suggests a developmental relationship between optic fissure closure and body segmentation in these patients. As such, identification of a segmentation abnormality on spine x-ray—although rare—can suggest a pattern of inheritance that affects genetic counseling. Sequencing of the coding sequence of GDF6 did not reveal any sequence variants in affected members of either family. Whole genome sequencing is currently in progress in our study. Klippel-Fiel anomaly and coloboma have been noted in patients with mutations in the GDF3 gene, a member of the bone morphogenetic protein family.21 It is interesting to note that 4 patients had apparent osteopenia of unclear etiology; this observation may be a clue to the developmental processes involved in these patients.
Coloboma is a multifaceted disorder that likely involves both genetic and environmental factors. The genetics of coloboma is complex.22 A search on the Online Mendelian Inheritance in Man (OMIM) database alone yields over 200 entries associated with coloboma, many of which are syndromic forms of disease. Most patients with apparently isolated coloboma present as sporadic cases; dominant, X-linked, and recessive pedigrees, however, have been reported. Although numerous genes have been associated with coloboma, they likely collectively explain <20% of cases. As such, there is no “high-yield” molecular test for such patients at this time and we have not performed systematic genetic screening of our patients. The advent of high-throughput sequencing technologies now allows us to begin to address this problem, improving our understanding of genetic vs environmental causes and our ability to provide meaningful genetic counseling.
Our study has several limitations. Subjects willing and able to participate in a clinical research study may not be representative of all patients with coloboma. Furthermore, our patients were ascertained in an outpatient setting in an ophthalmic genetics practice. We would anticipate, for example, that studies that include patients in a newborn nursery or in a clinical genetics practice would have a higher prevalence of medically serious systemic findings than those found in our study. We would also anticipate that the rate of finding subtle abnormalities would be affected by the practice patterns of the examining physician. While we have striven for uniformity in systemic testing, not every patient could cooperate with every test, and tests that required sedation (eg, brain MRI) were not obtained unless felt to be clinically indicated. We also altered our protocol after an interim analysis showed some tests to be of low yield. Nonetheless, we feel that our population approximates that of an outpatient ophthalmology setting for patients presenting for evaluation of seemingly isolated uveal colobomas.
What testing, then, ought to be pursued in such a setting? While no study can replace the clinical acumen of the physician treating an individual patient, the present study can provide guidelines. For example, a physical examination by a trained clinician can detect dysmorphic features and a heart murmur—findings that would likely influence additional evaluation. Of note, most of the cardiac anomalies detected in our series were diagnosed neonatally, making the likelihood of a positive echocardiogram in an otherwise seemingly healthy child low. The risks of a procedure should also be weighed against its benefits. In our study, brain MRI findings in the absence of symptoms had a low yield for clinically actionable findings. As such, we do not routinely perform this test on every patient, especially if anesthesia is required. Kidney ultrasound is noninvasive and does not require anesthesia or ionizing radiation. The prevalence of occult kidney malformations is low, and those found did not require acute intervention. Nonetheless, for those with significant positive findings, such as those with a missing kidney, clinical behavior is altered and regular follow-up with a nephrologist is recommended. Audiology findings were also generally mild, but in a child with visual impairment, early action on any condition affecting hearing may be important to maximize speech and language development. Lastly, skeletal imaging generally did not result in findings that would significantly alter clinical management in the short term. However, certain findings such as osteopenia should be monitored with appropriate follow-up diagnostic assessment. Taking these risks and benefits into account, our current practice is to obtain a physical examination (which may guide further evaluation), a baseline audiology assessment, a kidney ultrasound, and a spine x-ray on nearly all patients presenting with apparently isolated uveal coloboma.
Supplementary Material
Acknowledgments
The authors thank Dr Elias Traboulsi (Cole Eye Institute, Cleveland, Ohio, USA), Dr Adele Schneider (Einstein Healthcare System, Department of Genetics, Philadelphia, Pennsylvania, USA), and Dr Richard Weleber (Casey Eye Institute, Oregon Health & Science University, Portland, Oregon, USA) for referring patients and for reviewing the manuscript. We also thank Casey Hadsall, Jennifer Sarchet, and John Rowan (National Eye Institute, Bethesda, Maryland, USA) for their technical support in the clinic.
Biographies
 Nancy Huynh, MD, is currently an ophthalmic genetics fellow at the National Eye Institute, National Institutes of Health. She obtained her BA degree from Yale University and her MD degree from Harvard Medical School. Dr Huynh completed her ophthalmology residency at the Massachusetts Eye and Ear Infirmary in 2012. Her research interests are in ophthalmic genetics, clinical epidemiology, and healthcare policy.
Nancy Huynh, MD, is currently an ophthalmic genetics fellow at the National Eye Institute, National Institutes of Health. She obtained her BA degree from Yale University and her MD degree from Harvard Medical School. Dr Huynh completed her ophthalmology residency at the Massachusetts Eye and Ear Infirmary in 2012. Her research interests are in ophthalmic genetics, clinical epidemiology, and healthcare policy.
 Brian P. Brooks, MD, PhD, is the chief of the Unit of Genetic and Developmental Eye Disease at the National Eye Institute, National Institutes of Health. He studied biochemistry at the University of Maryland, and obtained his MD and PhD degrees from the University of Pennsylvania. He completed his ophthalmology residency and pediatric ophthalmology fellowship at the University of Michigan, and trained in medical genetics at the National Human Genome Research Institute.
Brian P. Brooks, MD, PhD, is the chief of the Unit of Genetic and Developmental Eye Disease at the National Eye Institute, National Institutes of Health. He studied biochemistry at the University of Maryland, and obtained his MD and PhD degrees from the University of Pennsylvania. He completed his ophthalmology residency and pediatric ophthalmology fellowship at the University of Michigan, and trained in medical genetics at the National Human Genome Research Institute.
Footnotes
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and none were reported. This work was supported by the intramural research programs of the National Eye Institute, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Deafness and Other Communication Disorders, and the Clinical Center of the National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, Maryland, USA. Contributions of authors: design of study (N.H., D.B., D.L.L., E.H.B., S.H., C.C.B., J.B.K., B.P.B.); acquisition of data (B.P.B., N.H., D.B., T.G., E.L.D., W.Z., D.L.L., E.H.B., S.H., C.C.B., J.B.K., T.M.B., I.H.M., B.J.B., B.P.B.); interpretation of data (N.H., D.B., T.G., B.P.B.); preparation of manuscript (N.H., B.P.B.); review and approval of manuscript (N.H., W.Z., D.L.L., E.H.B., S.H., C.C.B., J.B.K., T.M.B., I.H.M., B.J.B., B.P.B.).
References
- 1.Chang L, Blain D, Bertuzzi S, Brooks BP. Uveal coloboma: clinical and basic science update. Curr Opin Ophthalmol. 2006;17(5):447–470. doi: 10.1097/01.icu.0000243020.82380.f6. [DOI] [PubMed] [Google Scholar]
- 2.Stoll C, Alembik Y, Dott B, Roth MP. Congenital eye malformations in 212,479 consecutive births. Ann Genet. 1997;40(2):122–128. [PubMed] [Google Scholar]
- 3.Stoll C, Alembik Y, Dott B, Roth MP. Epidemiology of congenital eye malformations in 131,760 consecutive births. Ophthalmic Paediatr Genet. 1992;13(3):179–186. doi: 10.3109/13816819209046487. [DOI] [PubMed] [Google Scholar]
- 4.Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A. 2010;152A(3):674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura KM, Diehl NN, Mohney BG. Incidence, ocular findings, and systemic associations of ocular coloboma: a population-based study. Arch Ophthalmol. 2011;129(1):69–74. doi: 10.1001/archophthalmol.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SP, Taylor AE, Sowden JC, et al. Anophthalmos, microphthalmos, and Coloboma in the United kingdom: clinical features, results of investigations, and early management. Ophthalmology. 2012;119(2):362–368. doi: 10.1016/j.ophtha.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 7.Shah SP, Taylor AE, Sowden JC, et al. Anophthalmos, microphthalmos, and typical coloboma in the United Kingdom: a prospective study of incidence and risk. Invest Ophthalmol Vis Sci. 2011;52(1):558–564. doi: 10.1167/iovs.10-5263. [DOI] [PubMed] [Google Scholar]
- 8.Olsen TW, Summers CG, Knoloch WH. Predicting visual acuity in children with colobomas involving the optic nerve. J Pediatr Ophthalmol Strabismus. 1996;22(1):47–51. doi: 10.3928/0191-3913-19960101-12. [DOI] [PubMed] [Google Scholar]
- 9.Hornby SJ, Adolph S, Gilbert CE, Dandona L, Foster A. Visaul acuity in children with coloboma: clinical features and a new phenotypic classification system. Ophthalmology. 2000;107(3):511–520. doi: 10.1016/s0161-6420(99)00140-2. [DOI] [PubMed] [Google Scholar]
- 10.Maumenee IH, Mitchell TN. Colobomatous malformations of the eye. Trans Am Ophthalmol Soc. 1990;88:123–132. [PMC free article] [PubMed] [Google Scholar]
- 11.Daufenbach DR, Ruttum MS, Pulido JS, Keech RV. Chorioretinal colobomas in a pediatric population. Ophthalmology. 1998;105(8):1455–1458. doi: 10.1016/S0161-6420(98)98028-9. [DOI] [PubMed] [Google Scholar]
- 12.Wyse RK, al-Mahdawi S, Burn J, Blake K. Congenital heart disease in CHARGE association. Pediatr Cardiol. 1993;14(2):75–81. doi: 10.1007/BF00796983. [DOI] [PubMed] [Google Scholar]
- 13.Ragan DC, Casale AJ, Rink RC, Cain MP, Weaver DD. Genitourinary anomalies in the CHARGE association. J Urol. 1999;161(2):622–625. [PubMed] [Google Scholar]
- 14.Woolf AS, Hillman KA. Unilateral renal agenesis and the congenital solitary functioning kidney: developmental, genetic and clinical perspectives. BJU Int. 2007;99(1):17–21. doi: 10.1111/j.1464-410X.2006.06504.x. [DOI] [PubMed] [Google Scholar]
- 15.Westland R, Schreuder MF, Ket JCF, van Wijk JA. Unilateral renal agenesis: a systematic review on associated anomalies and renal injury. Nephrol Dial Transplant. 2013;28(7):1844–1855. doi: 10.1093/ndt/gft012. [DOI] [PubMed] [Google Scholar]
- 16.Mehra S, Eavey RD, Keamy DG. The epidemiology of hearing impairment in the United States: newborns, children, and adolescents. Otolaryngol Head Neck Surg. 2009;140(4):461–472. doi: 10.1016/j.otohns.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Pediatrics. Joint Committee on Infant Hearing Year 2007 position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120(4):898–921. doi: 10.1542/peds.2007-2333. [DOI] [PubMed] [Google Scholar]
- 18.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48(10):745–754. doi: 10.1007/s00234-006-0112-1. [DOI] [PubMed] [Google Scholar]
- 19.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 20.Larcos G, Gruenewald SM, Lui K. Neonatal subependymal cysts detected by sonography: prevalence, sonographic findings, and clinical significance. AJR Am J Roentgenol. 1994;162(4):953–956. doi: 10.2214/ajr.162.4.8141023. [DOI] [PubMed] [Google Scholar]
- 21.Ye M, Berry-Wynne KM, Asai-Coakwell M, et al. Mutation of the bone morphogenetic protein GDF3 causes ocular and skeletal anomalies. Hum Mol Genet. 2010;19(2):287–298. doi: 10.1093/hmg/ddp496. [DOI] [PubMed] [Google Scholar]
- 22.Moosajee M, Gregory-Evans CY. Advances in the molecular genetics of ocular coloboma. Expert Rev Ophthalmol. 2006;1(2):209–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.