Abstract
Head and neck paragangliomas (HNPGLs) are rare neuroendocrine tumors belonging to the family of pheochromocytoma/paraganglioma neoplasms. Despite advances in understanding the pathogenesis of these tumors, the growth potential and clinical outcome of individual cases remains largely unpredictable. Over several decades, surgical resection has long been the treatment of choice for HNPGLs. However, increasing experience in various forms of radiosurgery has been reported to result in curative-like outcomes, even for tumors localized in the most inaccessible anatomical areas. The emergence of such new therapies challenges the traditional paradigm for the management of HNPGLs. This review will assist and guide physicians who encounter patients with such tumors, either from a diagnostic or therapeutic standpoint. This review will also particularly emphasize current and emerging knowledge in genetics, imaging, and therapeutic options as well as the health-related quality of life for patients with HNPGLs.
Introduction
Current Views on Molecular Origins
-
Clinical Presentation, Diagnosis, and Staging
Clinical presentation
Pathology
Current view of malignancy
Current key steps in assessing a patient with HNPGL
Current diagnosis of HNPGLs
Emerging HNPGL-specific functional imaging
Current approaches for initial staging
Imaging follow-up of mutation carriers
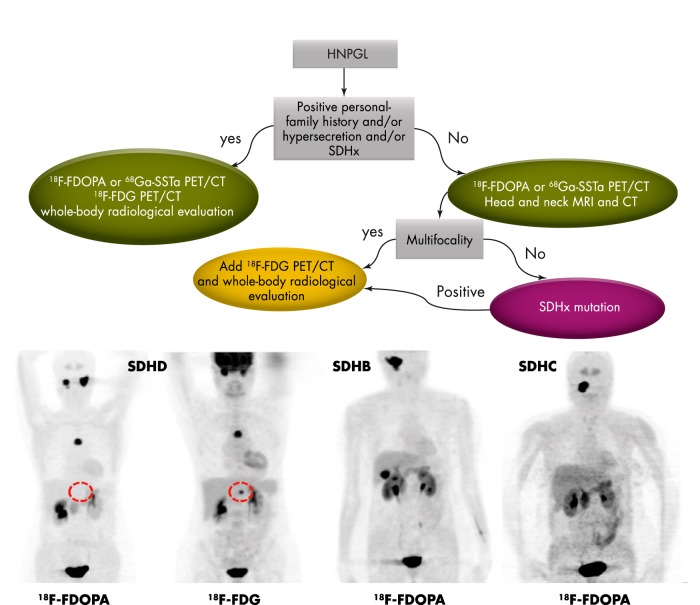
Current proposed imaging algorithm in the diagnosis and localization of HNPGLs
-
Treatment Goals and Options
Natural history of HNPGLs and treatment goals
Observation
Surgical indications and considerations
Therapeutic radiation
Multifocality and/or Hereditary HNPGLs
Metastatic HNPGLs
-
Near-Future Therapeutic Options
Therapeutic radiation
Targeted therapies
Radionuclide therapy
I. Introduction
Head and neck paragangliomas (HNPGLs, often referred to as glomus tumors) belong to the pheochromocytoma (PHEO)/paraganglioma (PGL) family. Based on the classification published in 2004 by the World Health Organization, the term PHEO should be reserved solely for adrenal PGL (1). HNPGLs account for approximately 20% of PGLs in unselected series (2), 0.6% of head and neck tumors, and 0.03% of all tumors (3).
Embryogenesis of paraganglial cells has received considerable attention over the last century. Evidence for the origin of sympathetic ganglia, adrenal medulla, and the carotid body was pursued by a number of labs, but it was largely the work of Le Douarin and Kalcheim (4) using quail/chick chimeras that led to the resolution of their neural crest (NC) origin. Later, immunohistochemical, in situ hybridization, and transgenic animal studies as well as single-cell electroporation methods using fluorescent NC progenitors added significantly to the knowledge of the mechanisms required for the correct specification, migration, and differentiation of multipotent NC progenitors during development (5). Beyond the carotid body, few data are available on the embryology of other paraganglionic cells associated with the parasympathetic system, probably because these cells are relatively late in differentiating. However, these peripheral cells produce a selection of neurotransmitter-like compounds that are common to other NC derivatives. They are embedded in several sensory ganglia whose neurons may be of NC or placodal origin but whose supporting cells are crest-derived. Therefore, it is widely assumed that these cells are also of NC origin. The number of NC-derived neuroendocrine cells decreases postnatally by modes that are still enigmatic (ie, apoptosis and autophagy) (5).
PGLs may arise from paraganglia and extra-adrenal cell clusters that persist postnatally. HNPGLs and a subset of thoracic PGLs are closely aligned with the distribution of the parasympathetic nervous system and act as chemoreceptors. The carotid body is a prime example of a chemoreceptor organ that mediates reflex hyperventilation during hypoxemia via activation of the respiratory center. Exceptional cases of PGLs of the cervical sympathetic chain have been reported. HNPGLs arise preferentially from the jugular bulb (glomus jugulare PGLs [JPs]), along the tympanic branch of the glossopharyngeal nerve or the auricular branch of the vagus nerve (glomus tympanicum PGLs [TPs]), along the vagus nerve (glomus vagale PGL [VPs]), and from the carotid body (glomus caroticum PGLs [CBPs]). The overall incidence of HNPGLs ranges from 1 in 30 000 to 1 in 100 000, with CBPs making up nearly 60% of cases, followed by JPs (23%), VPs (13%), and TPs (6%). (6). It has now been established that 35% or more of HNPGLs are associated with recognized genetic defects. In hereditary syndromes, JPs, VPs, and CBPs are observed in 26%, 31%, and 39%, respectively (7). TPs are extremely rare in patients who carry one of the well-studied PGL susceptibility genes. Unusual or exceptional locations have also been reported, such as PGLs of the larynx, thyroid, sinonasal region, nasopharynx, orbit, and tongue. The reported malignancy risks are variable and probably overestimated by cancer registries (due to unregistered unresected tumors) (8) or old literature (underdiagnosis). In more recent series, malignant cases were present in less than 3.5% (9).
This review will particularly emphasize current and emerging knowledge in genetics, imaging, and therapeutic options as well as the health-related quality of life for patients with HNPGLs.
II. Current Views on Molecular Origins
At of the beginning of 2014, one-third or more of PHEOs/PGLs were found to have a familial etiology, with germline mutations in 1 of more than 12 different PGL susceptibility genes. The molecular era of HNPGLs began in 2000, with the description of the first succinate dehydrogenase subunit D (SDHD) gene mutation in the PGL1 syndrome (10). Hereditary HNPGLs are most commonly associated with germline mutations in one of the SDH subunit genes (A through D, collectively referred to as SDHx) or its flavination factor SDHAF2. Mutations in other genes (ie, Von Hippel-Lindau/VHL, MYC-associated factor X/MAX, and transmembrane protein 127/TMEM127) are rarely involved in the pathogenesis of these tumors. PHEOs/PGLs of various genetic backgrounds can be segregated by their transcription profile into 2 main clusters (cluster 1 and cluster 2).
Cluster 1 comprises genes that are associated with the hypoxic response, and cluster 2 contains tumors that activate kinase signaling and protein translation. Cluster 1 contains tumors with mutations of VHL, components of the SDH complex, hypoxia-inducible factor (HIF)-2α (HIF2A, also known as EPSA1), the prolyl hydroxylase domain 2 gene (PHD2), and fumarate hydroxylase (FH). Cluster 2 contains tumors with RET, NF1, TMEM127, and MAX mutations (11).
Of all the known genetic mutations, mutations in SDHD are currently the leading cause of hereditary HNPGLs (>50%), followed by SDHB (20%–35%) and SDHC (15%) mutations (12–14). Some correlations between the genes involved and tumor location have been found. SDHD-linked patients have a 75% risk of developing HNPGLs throughout their life, with concomitant thoracoabdominal PGLs in about 10% of cases (7). SDHB-linked PGL syndrome is characterized by a high rate of retroperitoneal PGL or HNPGLs, but a combination of sympathetic and parasympathetic PGLs are less frequently found (<5%) (7). Furthermore, it should be noted that multiple PGLs at a very young age found in several family members may be related to SDHAF2 mutations (15). So far, only HNPGLs have been reported in these rare families. The inheritance pattern of the SDHB and SDHC genes is autosomal dominant, whereas for SDHD and SDHAF2 genes, the disease occurs only when the mutations are inherited from the father, which is consistent with maternal imprinting (16). Until present, mutations in SDHA have been described in several isolated cases. Major predictors of hereditary HNPGLs are a family history of PGL, especially those related to SDHD mutations (although it is not always present due to a low penetrance of some other mutations, low morbidity, and any symptoms related to HNPGLs); a previous history of adrenal or extra-adrenal PGL; and multifocality or a characteristic syndromic presentation (9, 14).
Currently, a syndromic presentation includes the existence of other tumor types associated with the presence of a PGL (eg, renal cell carcinoma, gastrointestinal stromal tumor [GIST], and pituitary adenomas can also be related to SDHx mutations) (17, 18). Another syndrome, originally described by J. Aidan Carney (19) in 1977, called Carney's triad associates tumors in at least 2 of the 5 following organs in the same patient: stomach (gastric GIST), lungs (pulmonary chondroma), paraganglionic system (extra-adrenal PGL), adrenal cortex (adenoma), and esophagus (leiomyoma). At present, a specific gene mutation has not been discovered with Carney triad; however, very few patients were found to have SDHx gene mutations. Recently, DNA methylation at the gene locus of the SDH subunit C has been found in Carney triad-related tumors, leading to loss of the SDHC protein (20).
Subjects with an inherited germline heterozygous SDH mutation have an increase risk of developing PHEOs/PGLs. SDH acts as a tumor supressor in the paraganglionic system. Tumorigenesis requires somatic loss of heterozygosity (second hit).
The 4 SDHx genes encode the 4 subunits of the SDH enzyme (also named mitochondrial complex II). This membrane complex catalyzes the oxidation of succinate to fumarate in the tricarboxylic acid cycle and the respiratory chain. Deleterious mutations in any of the SDH genes (biallelic inactivation) invariably result in decreased SDH activity and/or a significant reduction or complete absence of its protein (21–23).
This results in the accumulation of succinate and the generation of reactive oxygen species (ROS) due to disrupted electron transport. The accumulation of succinate is associated with depletion of fumarate, limited capacity to use oxidative phosphorylation for ATP production, accelerated cell proliferation and growth, and tumor development, most commonly PHEO/PGL. The accumulation of succinate and depletion of fumarate in these tumors results in a very high succinate to fumarate ratio, which was recently introduced as a promising new metabolic marker for the presence of these tumors (24–26, 181).
The accumulation of succinate and other oncometabolites has been illustrated in other tumor models with inherited and acquired alterations in enzymes of the tricarboxylic acid cycle, such as fumarate in cases of fumarate hydroxylase gene mutations (27) and 2-hydroxyglutarate in mutations in 1 of the 2 isocitrate dehydrogenase genes (IDH1/2) (28). The accumulation of some oncometabolites in SDHx-tumors or other metabolically related tumors may inhibit 2-oxoglutarate–dependent dioxygenases that include members of the EglN1–3 (also called prolyl hydroxylases [PHDs] 1–3) family, which have a key role in the regulation of the stability of HIF-α. The HIF-α signaling pathway promotes tumorigenesis, abnormal angiogenesis, decreased apoptosis, cell migration, and metastatic spread (29, 30). These events are occurring despite a normal oxygen supply, causing these tumors to have a pseudohypoxic phenotype (31) (Figure 1).
Figure 1.
Relationship between SDH complex dysfunction and tumorigenesis. Abbreviations: IMM, inner mitochondrial membrane; 2-OG, 2-oxoglutarate.
Although HIF-1α and HIF-2α share some redundant functions (ie, angiogenesis, glucose transport, lipid metabolism, and pH homeostasis), they also exhibit unique functions such as the regulation of the expression of the glycolytic enzymes and pyruvate dehydrogenase kinase 1 by HIF-1α and erythropoiesis and cellular iron metabolism by HIF-2α (30). They may even exihibit opposing activities in stem cell regulation and some cancers via differential regulation of c-Myc/Max complex stability and interactions with specific client proteins. HIF-1α seems to have a positive effect on cell differentiation, whereas HIF-2α maintains an undifferentiated state. These findings have also been recently demonstrated in chromaffin cell lines (32). HIF-1α and HIF-2α are both tumor-promoting and tumor-suppressing, depending on the context. The DNA binding and transcriptional activities of c-Myc require heterodimerization with Max. In cancer cells with normal levels of c-Myc, HIF-1α inhibits c-Myc transcriptional activity. However, when c-Myc is overexpressed, the high levels of c-Myc protein maintain c-Myc–Max heterodimers through mass action. On the other hand, HIF-2α appears to cooperate with c-Myc and hence positively affect normal c-Myc as well as deregulated c-Myc. These findings explain the antitumorigenic effects of HIF-1α and protumorigenic effects of HIF-2α in certain cancers, such as renal cell carcinoma (33).
Molecular profiling studies have provided evidence of pseudohypoxia and increased angiogenesis in SDHx and VHL PGLs due to predominant upregulation of HIF-2α (34–41). Aerobic glycolysis has been preferentially observed in VHL-related tumors. However, these findings have not been fully replicated in HNPGLs (42). Thus, different expression profiles of HNPGLs vs other PGLs are most likely influenced by their specific location rather than their genetic background (41).
Recently, epigenetic mechanisms have been implicated in the pathogenesis of PGLs due to the finding that inhibition of ten-eleven translocation DNA hydroxymethylases and jumonji C domain-containing histone demethylases by succinate impairs DNA and histone methylation and subsequently contributes to dedifferentiation, invasion, and migration properties of SDHx-related tumors (43–46). Overproduction of ROS also contributes to the inactivation of PHDs (Figure 1). Additionally, somatic mutations may play a role in tumorigenesis.
III. Clinical Presentation, Diagnosis, and Staging
A. Clinical presentation
HNPGLs range in spectrum from small lesions (usually detected in PGL mutation carriers) to large unresectable masses, often invading cranial nerves and sometimes the brainstem. HNPGLs are almost always nonsecreting tumors (up to 95%), and thus, they are often discovered on imaging studies or revealed by symptoms of cervical mass and/or compression or infiltration of adjacent structures (eg, hearing loss, tinnitus, cervical mass, dysphagia, and cranial nerve palsies). HNPGLs are distinguished from other tumors by a newly introduced biochemical marker, 3-methoxytyramine (a dopamine metabolite), that is elevated in almost one-third of patients with HNPGL, in contrast to plasma or urine metanephrines, which are rarely elevated (47–50). Therefore, accurate diagnosis of nonsecreting PGLs can be made using PGL-specific anatomical and functional imaging studies as discussed later in the review (see Section III.F).
B. Pathology
PGLs are encapsulated ovoid tumors that exhibit a typical endocrine pattern with chief cell nests (zellballen pattern). The stroma surrounding and separating the nests is mainly composed of fibrovascular tissue. Sustentacular cells may be seen at the periphery of the cell nests. On immunohistochemical studies, the chief cells express neuroendocrine markers (chromogranin, synaptophysin, and neuron-specific enolase), whereas the sustentacular cells are positive for the S100 protein. At the present time, there are no reliable pathological criteria for malignancy in PHEOs/PGLs (51). Pathology may help distinguish between nodal metastasis and multifocal PGLs. As previously mentioned, the absence of SDHB immunoexpression is indicative of a germline mutation in one of the SDH genes (22). Additionally, a subset of these tumors may also be immunohistochemically negative for SDHA, which is highly suggestive of the presence of SDHA mutation (52). These immunohistochemical studies can be used in addition to conventional staining as a screening method to guide genetic testing.
C. Current view of malignancy
At the present time, there are no reliable cytological, histological, immunohistochemical, or molecular criteria for malignancy (51). The diagnosis of malignancy remains strictly based on the finding of tumor loci where paraganglial cells are not usually present, such as the lymph nodes, lung, bone, or liver. In the absence of metastases at initial presentation, the diagnosis can only be made when metastases are discovered, which may often be too late if a patient did not undergo appropriate follow-up or if treatment options failed.
Nevertheless, recently, some new studies have attempted to predict metastatic potential through measurements such as elevated plasma methoxytyramine (47, 53), the presence of tumor necrosis, high Ki-67 index and/or mitotic count (54), overexpression of HIF-α and its target genes in tumors (55, 56), or extremely high mRNA copy numbers of a variant of carboxypeptidase E in tumors (57).
Based on our extensive clinical experience, metastatic disease is often seen with large tumors (usually over 5–6 cm) and tumors presenting with significant necrosis on histopathological examination. However, tumor size is not often applicable as a predictor for metastatic disease for HNPGLs as it is for PHEOs (58, 59). Abdominal PGLs with an underlying SDHB mutation are associated with a higher risk of aggressive behavior, development of metastatic disease, and death. Overall, the malignancy risk for SDHB mutation-associated tumors has been estimated to be 30% (range 20%–70%). It is therefore possible that this relationship may also be applied to HNPGLs, although properly designed studies have not been done (60–63) (Table 1). The increased malignancy risk associated with SDHB mutations is still largely unexplained. A more pronounced epigenetic state or activation of epithelial-to-mesenchymal transition compared with other SDH-mutated tumors were recently proposed to explain this specificity (44, 64). Tumors with gross tumor infiltration into surrounding structures and/or related to SDHB mutations should be considered at a higher risk for malignancy. In other situations, tumors should be characterized as having a low malignant potential. It should be noted that the uncertainty regarding the exact risk of malignancy can lead to different medical decisions between teams, based on their experience, with regards to appropriate patient evaluation (including genetic testing) and follow-up. In the near future, the use of the second-generation sequencing will rapidly allow us to screen for many cancer-related genes in tumors and to further predict their behavior including metastatic potential. Metabolomics may become another promising area to help predict behavior of these tumors.
Table 1.
Most Common Clinical Presentations of SDHx-Related HNPGLs and Malignancy Risksa
| Gene | Inheritance | Family History | Tumor Number in Head and Neck | Concomitant Sympathetic PGLs, % | Malignancy Risk, %b | Related Conditions |
|---|---|---|---|---|---|---|
| SDHA | AD | Low | Single | None reportedc | 10c | GIST |
| SDHB | AD | Low | Multiple | <5 | 20–30 | GISTs, RCCs |
| SDHC | AD | Low | Single | Rare | Rare | GISTs |
| SDHD | AD (paternal) | High | Multiple | 10–15 | <5 | GISTs, pituitary adenomas |
| SDHAF2 | AD (paternal) | High | Multiple | None reported | None reported | None reported |
Abbreviations: AD, autosomal dominant; RCC, renal cell carcinoma.
SDHD and SDHAF2 mutation carriers develop tumors only when the mutation is inherited from the father; the latter is found very rarely and only in young adults. Non-KIT/PDGFRA GIST may be caused by mutations in one of the SDH genes and can be associated with PGL in Carney-Stratakis syndrome. Renal cell carcinoma has been mainly described as a component of SDHB-associated syndromes but may also occur in the presence of other SDHx mutations. Pituitary adenomas have been mainly reported in PGL patients with SDHD germline mutations but may also occur in the presence of other SDHx mutations.
All primary sites pooled.
Further study needed.
D. Current key steps in assessing a patient with HNPGL
The following algorithm outlines our approach for assessing a patient with an HNPGL: 1) first, confirm the diagnosis of HNPGL using specific PGL biochemistry and imaging. If an HNPGL is confirmed, then 2) rule out the presence of multiple tumors by carefully reviewing imaging studies. Ruling out multiplicity is a crucial step, because the presence of multiplicity guides the final decision about whether an operation could be performed. If no multiplicity is present, evaluate the size of the primary tumor and the involvement of surrounding structures. If the anatomical location allows surgery to be performed, then again reassess biochemistry to decide whether adrenergic blockade should be used. 3) Most HNPGLs are nonsecreting tumors; thus, elevations of metanephrines (usually at least 3 times above the upper reference limit) may be indicative of concurrent PHEO or thoracoabdominal PGLs. This is also seen in hereditary PHEOs/PGLs, often in SDHx-related ones. If metanephrines are elevated, then 4) perform computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen/pelvis first, followed by a chest CT or MRI. If any tumor is found, surgical removal of that tumor has priority over resection of an HNPGL. It is strongly recommended that any surgery on an HNPGL or other PHEOs/PGLs be performed by a very experienced surgeon, preferably at tertiary medical centers. 5) Simultaneously with step 4, screen a patient for predisposing germline mutations. Evaluation and counseling for index cases and relatives are performed by clinical geneticists in most countries. The choice of the genes to be tested may be guided by clinical presentation and ex vivo biomarkers. This strategy will change in time with the clinical implementation of next-generation sequencing-based gene panels for the diagnosis of inherited PGL syndromes. 6) If a surgical procedure is not considered as the primary option, consider other therapeutic modalities, including therapeutic radiation or chemotherapy as discussed below (in Section IV.D).
E. Current diagnosis of HNPGLs
HNPGLs are often nonsecreting tumors (up to 70%–95% based on whether methoxytyramine or metanephrines are measured) and thus often discovered on imaging studies or revealed by the presence of cervical/skull base masses with or without compression or infiltration of adjacent structures (eg, hearing loss, tinnitus, cervical mass, dysphagia, and cranial nerve palsies). In nonsecreting cases, accurate diagnosis of an HNPGL is made by thorough clinical examination together with MRI and PGL-specific functional imaging studies (50) (Tables 2 and 3). HNPGLs usually demonstrate marked enhancement of intratumoral vessels after contrast administration on CT and low signal on T1-weighted images and an intermediate to high signal on T2-weighted MRI images; they also often enhance intensely after gadolinium injection on MRI. Flow signal voids in the tumor are typical of PGLs, with a salt-and-pepper appearance on spin-echo sequences. MR angiography also demonstrates intratumoral arterial vessels (65–69). The 3-dimensional (3D) time-of-flight and 3D gadolinium sequences have been shown to be highly informative, with sensitivities and specificities of 90% and 94% (66) and 100% and 94% (67), respectively. There is a recent trend of using time-resolved 4D gadolinium MR angiography (69). The time-resolved imaging of contrast kinetics technique (TRICKS) enables evaluation of both intratumoral vessels (early arterial phase) and tumor perfusion, including capillary permeability. Fusion images between 3D-volumetric interpolated breath-hold examination fat-saturated T1-weighted and 4D-MR angiography are particularly informative. Diffusion-weighted imaging, which is dependent on tissue cellularity, may be useful in preoperative characterization and prognosis assessment of PGL, but further evaluation is needed (70).
Table 2.
Localization and Classification of HNPGLs and Their Main Clinical and Radiological Differential Diagnoses
| Type | Localization | Classification | Differential Diagnoses |
|---|---|---|---|
| Carotid body PGL | Carotid bifurcation | Shamblin's | Carotid artery aneurysm |
| Vasculitis of the carotid artery | |||
| Lymphadenopathy | |||
| Schwannoma | |||
| Branchial cleft cyst | |||
| Accessory thyroid gland | |||
| Vagal PGL | Retrostyloid parapharyngeal (carotid space) | Netterville's | Schwannoma Neurofibroma Lymph node metastasis (nasopharynx/oropharynx, thyroid cancer) Lymphoma Ganglioneuroma, hemangiopericytoma Miscellaneous lesions (ie, abscess, internal carotid artery aneurysm, internal jugular vein thrombosis, vascular malposition, organizing hematoma) |
| Tympanic PGL | Middle ear and hypotympanum | Fisch and Mattox's | Middle ear adenoma (carcinoid) |
| Glasscock and Jackson's | Meningioma Aberrant vascular structure (jugular vein, internal carotid artery) |
||
| Jugular PGL | Jugular foramen | Fisch and Mattox's | Schwannoma |
| Meningioma | |||
| Plasmocytoma | |||
| Carcinoma (primary and metastatic) | |||
| Chondrosarcoma | |||
| Tumor extension from nasopharyngeal carcinoma |
Table 3.
Differential Diagnosis of Post-Styloid Retropharyngeal Masses: Imaging Features on MRI and Molecular Imaging
| PGL | Nodal Metastasis | Schwannoma | Neurofibroma | |
|---|---|---|---|---|
| MRI | Flow voids (salt-and-pepper appearance on T2-weighted images) | Variable appearance on spin-echo images | Moderate to high signal intensity on T2 | Moderate to high signal intensity on T2 Central low signal on T2 may be seen (target sign) |
| Rapid (numerous arterial feeders), intense and homogeneous enhancement | Heterogeneous enhancement | Heterogeneous enhancement in large tumors with degenerative changes (cystic, pseudocystic) | Moderate and delayed homogeneous enhancement | |
| [18F]FDG | Moderate to high uptake (highly elevated in SDHx-tumors) | Low to high uptake depending on the primary tumor | Low to high uptake (even in benign cases) | Low uptake (high uptake values in malignant forms) |
| [18F]FDOPA | High uptake | No significant uptake | No significant uptake | No significant uptake |
| SST-based imaginga | High uptake | No significant uptake (possible high uptake in nodal metastases from thyroid cancers of follicular origin or nasopharyngeal cancers) | No significant uptake | No significant uptake |
SST-based imaging is somatostatin receptor imaging with octreoscan or 68Ga-labeled somatostatin analogs.
Radionuclide imaging techniques now play a crucial role in the evaluation of HNPGLs. This is enabled by the increasing availability of radiopharmaceuticals that have been developed to target receptors, transporter systems, or metabolic pathways that are specific to HNPGLs, essentially, uniquely, and precisely performing so-called in vivo histology.
Several excellent studies have demonstrated the superiority of Octreoscan for HNPGLs compared with [123I/131I]MIBG (metaiodobenzylguanidine), with sensitivities of 89% to 100% and 18% to 50%, respectively (71–76). However, the sensitivity of this imaging modality needs to be revised downward because some lesions can be only a few millimeters in size and therefore are not detectable by even the best available cameras (sensitivity of 75% with single-photon emission computerized tomography [SPECT] or SPECT/CT) (7). The recently introduced hybrid SPECT/CT cameras have increased diagnostic confidence in image interpretation and enhanced sensitivity, but practical constraints such as long imaging times remain important limitations. Positron emission tomography (PET) imaging is a highly sensitive functional imaging technique for HNPGLs.
F. Emerging HNPGL-specific functional imaging
[18F]Fluorodopa ([18F]FDOPA) PET/CT, which was initially developed to investigate dopaminergic neurotransmission, was found to be a highly sensitive (>95% for HNPGLs) and specific (95%–100%) imaging modality for PGL detection, especially for HNPGLs (77–81). [18F]FDOPA is taken up via an amino acid transporter (L-type amino acid transporter 1 and 4F2 heavy chain) decarboxylated into [18F]fluorodopamine by aromatic l-amino acid decarboxylase, and concentrated in intracellular vesicles. [18F]FDOPA PET was also found to be superior to octreoscan (77, 79) in the detection of HNPGLs and to be excellent for both SDHx (79) and non-SDHx (78) HNPGLs. Currently, this imaging modality is the preferred technique in the detection of these tumors. However, [18F]FDOPA is not routinely available at most imaging centers worldwide. [18F]Fluorodopamine, which uses the cellular norepinephrine transporter system expressed on PGL cells, is not recommended for the evaluation of these tumors due to its suboptimal sensitivity. Nevertheless, the advantage of this radiopharmaceutical is that it has almost 100% specificity, unlike [18F]FDOPA, which can also detect additional sympathetic PGLs in addition to HNPGLs.
PET/CT imaging with 68Ga-labeled somatostatin analogs ([68Ga]SSTa) has excellent preliminary results in localizing HNPGLs (82–86). This imaging modality is a rapidly evolving one, awaiting comparison with [18F]FDOPA for its sensitivity. It is expected that this method will be as good as [18F]FDOPA PET, and because it does not require a cyclotron to make the radiotracer, it may surpass [18F]FDOPA PET in the near future. Moreover, another advantage of somatostatin analogs is that they can be used in the radioactive treatment of these tumors ([177Lu] DOTA-Tyr3-octreotate [DOTATATE]), unlike DOPA analogs.
[18F]FDG PET plays an interesting role in the evaluation of an HNPGL. Although this imaging modality is not specific, data have clearly shown that [18F]FDG PET has a superior sensitivity over CT/MRI or [123/131I]MIBG in the localization of SDHx-related PHEOs/PGLs as well as their metastatic counterparts (87).
Evaluation of parapharyngeal space tumors involves careful consideration of clinical and imaging information to distinguish vagal PGLs from peripheral nerve sheath tumors (schwannomas and neurofibroma), nodal metastases (nasopharynx/oropharynx and thyroid cancer), other rare primary tumors, and a variety of uncommon miscellaneous lesions (88) (Table 3).
In clinical oncology, it is [18F]FDG PET that has become the first-line imaging modality in the follow-up of patients with SDHB-related metastatic tumors, often used as the single imaging modality to monitor patient responses to chemotherapy or radiotherapy (RT) without simultaneous use of CT or MRI (88–92) (Figure 2, Table 3).
Figure 2.
Proposed imaging algorithm.
G. Current approaches for initial staging
The aim of pretherapeutic imaging is to provide the most complete staging of the disease. In this way, treatment can be tailored to each specific situation.
The initial staging of an HNPGL is currently based on the use of anatomical and functional imaging approaches to determine the tumor extension into the bone and surrounding soft tissue, to determine tumor multiplicity not only in the head and neck area but also elsewhere in the body, and finally, to exclude any metastases. Although rare, malignancy often requires dramatically different patient management. When metastases are present, the goal of imaging is to precisely assess the anatomy of these tumors to carefully select patients who could benefit from aggressive but complete surgical resection with the intent to cure as opposed to debulking strategies (93). Thus, anatomic imaging serves as the first-line modality in the locoregional staging of these tumors. CT offers several advantages over MRI (eg, better spatial resolution and fewer motion artifacts) and enables better evaluation of the temporal bone extension of JP and TP. MRI provides better soft-tissue contrast than CT and thus offers unique information for tumor delineation. MRI is also preferred if RT or stereotactic radiosurgery (SRS) is considered. Several classifications (ie, Fisch and Mattox's or Glasscock and Jackson's for TP and JP, Netterville's for VP, and Shamblin's for CBP) help predict surgical outcome and should be used in the evaluation of these patients (94–97) (Table 4).
Table 4.
Classifications of HNPGLs
| Classification | Localization | Primary Subclass | Secondary Subclass | Tertiary Subclass |
|---|---|---|---|---|
| Shamblin's | Carotid Body PGL | I: splaying of the carotid bifurcation with little attachment to the carotid vessels | II: partial involvement of the carotid vessels | III: complete involvement of the carotid vessels |
| Netterville's | Vagal PGL | A: confined to the neck | B: in contact with the jugular foramen | C: extended into or beyond the jugular foramen with or without intracranial extension |
| Fisch and Mattox's | Tympanic PGL | A: limited to the middle ear cleft | B: limited to the tympanomastoid compartment | |
| Jugular PGL | C1: located in the jugular foramen, erosion of the carotid foramen | C2: vertical segment of the carotid canal involved C3: horizontal segment of the carotid canal involved C4: foramen lacerum and cavernous sinus involved |
De: intracranial extradural extension (De1/2 depending on the displacement of the dura) Di: intracranial intradural extension (Di1/2/3 depending on the depth of invasion into the posterior cranial fossa) |
To determine whether additional HNPGLs are present, MRI is inferior to [18F]FDOPA PET/CT. Therefore, it is currently recommended that all patients with HNPGLs, especially those with larger tumors or tumors in critical locations (eg, JP), are assessed by [18F]FDOPA or [68Ga]DOTA-SSTa PET/CT. Although both functional imaging modalities are relatively new and more studies are needed, the published results are strongly supportive and convincing and have been validated in many centers that evaluate HNPGLs (82–86) (Tables 3 and 5).
Table 5.
Comparison of Different Imaging Techniques for the Localization and Staging of HNPGLs
| Sensitivity, % | Specificity, % | Locoregional Staging | Malignancy Prediction | Advantages | Mean Estimated Effective Dose Equivalent | |
|---|---|---|---|---|---|---|
| CT | 80–90 | 90 | High | Unreliablea | High spatial resolution Low motion artifacts Accurately delineates temporal bone extension |
3 mSv for unenhanced CT and 5 mSv for CT angiogram |
| MRI | 80–90 | 90 | High | Unreliablea | No iodinated media No radiation exposure Accurately delineates soft tissue extension |
None |
| PET/CT-[18F]FDOPA | >90 | >95 | Low | Unreliablea | High tumor/background uptake ratio (TBR) Highly sensitive regardless genotype Low uptake in normal adrenals |
0.025 mSv/MBq for [18F]FDOPA and 0.5–3 mSv for low-dose CT |
| PET/CT-[18F]FDG | 80 | 80–90 | Low | Unreliablea,b | Highly sensitive in SDHx-tumors Accurately detects concomitant abdominal PGLs that can be missed by [18F]FDOPA |
0.019 mSv/Mbq for [18F]FDG and 0.5–3 mSv for low-dose CT |
| PET/CT-[68Ga]DOTATATE | >90 | 90 | Low | Unreliablea | High TBR Extemporaneously prepared |
0.021 mSv/Mbq for [68Ga]DOTATATE and 0.5–3 mSv for low-dose CT |
In the absence of metastases, there is no reliable assessment tool for distinguishing benign from malignant PGLs.
Low tumor [18F]FDG uptake has an excellent negative predictive value for SDHx mutations.
The clinical impact of detecting additional tiny nonfunctional lesions by molecular imaging may be subject to debate. Four major arguments support the use of highly sensitive functional imaging in addition to cross-sectional imaging for detecting these tumors:
Complete surgical resection is curative for patients with HNPGLs. However, for many tumors, eg, large and locally advanced JPs, complete resection is inadvisable due to their specific anatomical location. It is expected that early detection of JP with functional imaging may minimize complications related to mass effect and facilitate curative treatment of these tumors (see Section IV).
For tumors that are good candidates for tumor resection, lower cranial nerve injury represents a major risk in the surgical resection of HNPGLs, up to 20% for CBP (especially for Shamblin class III) and seemingly 100% for VP. In patients with an apparently single tumor, cranial nerve palsy may significantly compromise subsequent surgery to the contralateral side. Therefore, identification of an additional tiny tumor may change the management strategy from surgery to radiosurgery (RS).
It is widely accepted that tumors with an underlying SDHB mutation are associated with a higher risk of aggressive behavior. It is therefore expected that the early detection and treatment of SDHB-related tumors may potentially reduce the occurrence of metastases.
Detection of these lesions by functional imaging facilitates their evaluation by anatomical imaging and subsequent follow-up.
Functional imaging also enables the localization of extracervical PGLs/PHEOs and the diagnosis of malignancy.
It is notable that multifocality mainly occurs in hereditary PGL syndromes. However, even nowadays, some patients may exihibit multiple tumors without any mutations found in susceptibility genes. Furthermore, the genetic status is often unknown at the time of the imaging work-up. For all these reasons, we recommend combining anatomic and functional imaging in the evaluation of all patients with extra-adrenal PGLs, including HNPGLs.
In the near future, the most promising radiopharmaceuticals may be [68Ga]DOTATATE and [68Ga] DOTA-Tyr3-octreotide (DOTANOC), due to the important fact that they do not require a cyclotron on site and because there is already extensive previous experience using octreoscan to target somatostatin receptor subtype 2, namely SST2 (7, 71, 75–77). Among different [68Ga]DOTA-SST receptor analogs, [68Ga]DOTATATE enables better visualization of bone metastases than [68Ga]DOTANOC, due to the lower background (presumed bone marrow) activity (99). This finding is of huge importance, because vertebral body metastases are common in malignant HNPGLs. All of these tracers should be evaluated on advanced PET/MRI integrated systems for better characterization of residual masses and target volume delineation for radiation therapy or RS. This might not be crucial for benign tumors, where SRS already provides excellent local control (100), but rather for malignant lesions for which results could hopefully be improved (8, 101, 102).
H. Imaging follow-up of mutation carriers
Mutation carriers, especially those with SDHD mutations, may develop asymptomatic HNPGLs during their lifespan (103). The penetrance of SDHD-related PGLs is modulated by genomic imprinting, resulting in an almost complete absence of disease after maternal transmission (16). Paternal transmission is associated with incomplete penetrance (43%–100%) (7, 103, 104). In contrast to SDHD, SDHB mutations are at lower risk of asymptomatic PGLs in mutation carriers, consistent with a lower penetrance of the disease (105).
The optimal follow-up algorithm has not yet been validated in hereditary HNPGLs but most likely requires a more frequent and complete imaging work-up than for their sporadic counterparts. MRI offers several physical advantages over CT and does not expose patients to ionizing radiation, which is critical in a patient population submitted to lifelong imaging surveillance. Follow-up should include annual biochemical screening, and MRI can be delayed to 3-year intervals. Indications for PET imaging studies should be discussed on an individual basis.
Published data regarding the occurrence of new primary tumors in SDHx mutation-related patients with a single tumor or with negative imaging is still missing. In our experience, most of the new tumors detected by PET imaging have been missed by previous evaluations using SPECT imaging. The occurrence of truly new primary tumors and their locations are extremely variable between patients, even within the same families, with time intervals between tumor detection ranging from several months to several years.
I. Current proposed imaging algorithm in the diagnosis and localization of HNPGLs
Based on the currently available imaging techniques for the diagnosis and staging of PGLs, we propose the following approach to investigate a patient with HNPGL:
For diagnosis, the specificity provided by functional imaging techniques using [18F]FDOPA PET/CT or [68Ga]SSTa is superior to anatomical imaging.
For detecting additional tumor sites (multifocality and metastases), functional imaging techniques are superior to anatomical imaging. Based on the most recent studies, both SDHx and non-SDHx HNPGLs are well detected by [18F]FDOPA PET/CT or [68Ga]DOTA-SSTa. Importantly, SDHx-related tumors outside of the head and neck are better visualized by [18F]FDG PET/CT.
For determining the locoregional extension of HNPGLs, anatomic imaging modalities remain the first-line procedures. Temporal bone extension of TPs and JPs is better evaluated by CT, whereas MRI enables better evaluation of soft-tissue extension.
This algorithm should be adapted to the practical situation within each institution and should evolve with time as new techniques become available. [18F]FDOPA and [68Ga]DOTA-SSTa are currently available in many clinical and research centers around the world.
IV. Treatment Goals and Options
A. Natural history of HNPGLs and treatment goals
A better understanding of the natural history of the disease could improve treatment decisions. In patients with HNPGLs, data suggest little to no growth over time in most cases. To our knowledge, only 2 studies have specifically examined the natural history of HNPGLs (for a total of 95 tumors) (106, 107). The authors did not find any patient with clinically meaningful tumor growth during the follow-up period (4 and 5 years). Among the tumors that grew, the growth rate ranged from 1 to 2 mm/y. However, a study showed that most patients with apparently sporadic HNPGL in the Netherlands suffer from one founder SDHD mutation, which was the location of one of the aforementioned natural history studies. This mutation seems to be associated with a rather benign clinical course (with no impact on overall survival compared with the general population) and, obviously, lower growth rates. Such low rates may also be related to the fact that most people in the Netherlands live at sea level.
Long-term exposure to high altitude induces a response of O2-sensitive glomus cells that release substances (eg, endothelin-1) that induce neural stem cell-dependent carotid body hypertrophy necessary for acclimatization to chronic hypoxemia (108). It has been shown that very high altitudes also increase the rate of sporadic PGL (109). Higher altitudes may also facilitate the likelihood of early and multifocal tumor development in SDHx mutation carriers (ie, facilitating the second hit) (110). Therefore, the 2 studies on the natural history of tumor growth should probably not be extrapolated to worldwide HNPGL patients. Other studies estimated a tumor doubling time between 4.2 (111) and 13.8 years (112) for HNPGLs.
Four major disease-specific factors may have a negative effect on survival: 1) cardiovascular death related to excessive catecholamine production by HNPGL and/or a concomitant sympathetic PHEO/PGL, or a fatal stroke due to embolization initiating from the carotid artery due to its manipulation during open surgery; 2) aspiration pneumonia secondary to the loss of lower cranial nerve function; 3) compression of the brainstem by a temporal bone tumor; and 4) metastases. Even if the survival of HNPGL patients has not extensively been studied, it appears that for benign HNPGLs not invading adjacent structures, the disease itself has no significant impact on survival (113). By contrast, the quality of life (QOL) of these patients is markedly reduced by the local extension and progression of HNPGL (114, 115). Thus, active treatment should be considered in symptomatic cases, in progressive disease, and in cases at higher risk of malignancy.
B. Observation
Observation may be considered in asymptomatic cases with a low risk of malignancy. However, provided the increasing life span, even slowly growing tumors may progress in the long term and cause delayed and irreversible complications. A wait-and-scan policy could be the primary option for defining the growth pattern. Patients undergoing such an approach should be informed that many tumors continue to grow, and that they may eventually require treatment. These patients should be monitored using MRI. The patient's psychological and social status, treatment preferences, and baseline QOL need to be evaluated. For instance, some patients can express a wish for complete removal of their HNPGLs. Such an approach needs to be thoroughly discussed with the patients.
C. Surgical indications and considerations
Complete surgical resection is curative for patients with HNPGLs; however, for many tumors, eg, large and locally advanced JPs, complete resection is inadvisable due to their specific anatomical location.
1. Surgery for carotid body PGL
These tumors require special attention due to a low but constant malignancy risk across series. Shamblin class I and II tumors (adherent or partially surrounding the carotid vessels) are good candidates for tumor resection with a low risk of cranial nerve palsy and vascular morbidity (116, 117). Shamblin class III tumors (completely surrounding the carotid vessels) are at a much higher surgical risk and are typically better treated by radiation therapy. Patients with a CBT and an SDHB mutation should undergo a complete surgical resection, even in Shamblin class III tumors whenever possible. In a very recent study, Ellis et al (61) demonstrated that patients with SDHB mutations benefited from more aggressive surgical interventions due to the high malignant potential of their HNPGLs.
Advances in vascular surgical techniques have greatly reduced the risks of perioperative complications such as carotid injury, stroke, and death (118, 119). The resection of CBPs is performed via a transcervical approach (118, 119). Due to the risk of intra-operative carotid artery injury, every surgeon performing CBP resection should be familiar with the use of intraluminal vascular shunts as well as vascular reconstructive techniques. After proximal and distal control of the carotid vessels and the internal jugular vein has been obtained, special care has to be taken to avoid injury of the lower cranial nerves closely related to the tumor (118). Lower cranial nerve injury represents the major risk in surgical resection of CBPs today. In a 1995 review, Anand et al (119) reported on the results of 1181 CBPs that underwent resection. Cranial nerve deficits were seen in 21.8% of cases. It must be mentioned, however, that most of those palsies occurred in patients with very large tumors (Shamblin class III). In patients with Shamblin class I and II tumors, the risk for iatrogenic cranial nerve damage was very low. To exclude lymph node metastases, a selective neck dissection in regions IIA, IIB, and III should be performed (120). Long-term results after complete surgical resection of CBPs are excellent, with cure rates reported to be as high as 89% to 100% (121, 122).
Besides a detailed personal medical history and family history, thorough preoperative examination and planning is crucial in all patients with CBTs. Although those tumors are rarely hormonally active, one has to keep in mind the risk of intraoperative hypersecretion of vasoactive substances. Another important risk is that plaques in the common and internal carotid arteries can break free during intraoperative manipulation and lead to serious neurologic consequences as severe as complete unilateral stroke. Therefore, patients with CBTs need to be seen by an endocrinologist and a cardiologist before surgery. Patients with Shamblin class III tumors that are scheduled for surgical resection should undergo preoperative angiography with embolization of their tumor. During that same angiography, a balloon occlusion test should be performed, because those patients often require temporary intraoperative interruption of the ipsilateral common and/or internal carotid artery. If a 20-minute balloon occlusion is accepted without problems, then one may proceed with the planning of the surgery.
2. Surgery for vagal PGL
Surgery of VPs should be performed in rapidly enlarging tumors and large tumors that are compressing vital structures (such as the trachea). In other cases, therapeutic radiation is recommended as a first-line approach.
VPs are also resected via a transcervical approach. The risk for major vascular injury is smaller than in patients with CBPs. The downside of VP resection is the fact that vagal nerve function can only be preserved in very rare cases (123). Long-term results after resection regarding the risk of tumor recurrence are excellent, with cure rates well over 90% (123).
3. Surgery for tympanic PGL
Most patients with TP present with conductive hearing loss and/or pulse synchronous tinnitus at diagnosis. Surgery is the treatment of choice for TPs, because complete tumor resection is possible in the vast majority of cases. Ossicular chain reconstruction can be achieved in most patients, which results in excellent hearing outcomes. Furthermore, facial palsy is a very infrequent side effect (124).
Surgical resection of TPs is performed via a retroauricular approach. In small tumors, a standard stapedectomy-type transcanal approach is entirely adequate. In larger tumors, a postauricular extended facial recess or an infratympanic extended facial recess approach with facial nerve monitoring is recommended. Surgical results are excellent, with very low morbidity. Forest et al (124) reported on their results with surgical resection of 80 TPs. The cure rate was 92.5%, with only 1 temporary facial nerve palsy.
4. Surgery for jugular PGL
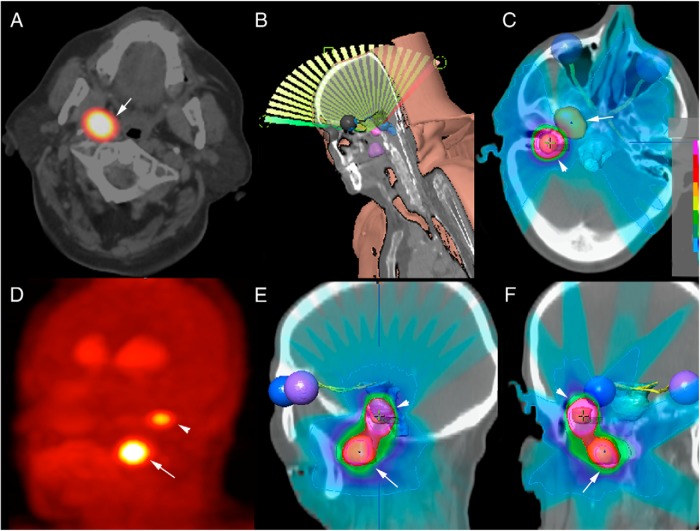
The optimal management of these tumors remains controversial. High rates of morbidity (cranial nerve injuries), incomplete resection, and the aggressive behavior of these tumors are the main arguments to advocate upfront radiation therapy. Surgery should be considered for patients with catecholamine excess, rapidly progressive disease, or large tumors with brainstem compression. In these cases, adjuvant radiation therapy should be discussed in cases with residual tumor (93). Patients with Fisch class C1 tumors may be very good candidates for complete resection, with very little morbidity in the hands of a very experienced surgeon (Figure 3).
Figure 3.
Radiosurgical planning in a patient with sporadic multifocal PGL (vagal and jugular locations). [18F]FDOPA PET/CT showing vagal (arrows) and jugular (arrowheads) locations in a sporadic case (axial view) centered over the vagal PGL (A) and maximum intensity projection (MIP) (D). 3D reconstruction of single-fraction dynamic conformal arc RS planning (B) based on 3D [18F]FDOPA PET/MR fusion image using the iPlanDose 4.5 (BrainLab) treatment planning software. Organs at risk (brainstem, eyes, optic nerves, optic chiasm, and optic tracts) as well as the 2 tumors are auto-segmented (on T1-weighted MRI) and manually delineated (on [18F]FDOPA PET/CT images) and are represented in 3D. Beams and isodoses are represented using color wash display on axial (C), sagittal (E), and coronal (F) views.
JPs are the most difficult HNPGLs to be surgically removed. Any surgical removal that is considered should be first discussed with experienced specialists for these tumors. JPs are resected via an infratemporal approach in most cases. In classes C1, C2, and Dei1/2, the juxtacondylar approach represents an alternative technique (126). Neuromonitoring of cranial nerves VII, IX, X, XI, and XII has been shown to be greatly important and beneficial. Suárez et al (123) reviewed the results of 1084 JP patients who underwent surgical resection. Long-term tumor control was obtained in 78.2% of patients. However, a total of 1183 preoperative cranial nerve palsies increased to 2148 definitive postoperative cranial nerve palsies. Thus, surgery resulted in 965 new cranial nerve deficits. Such clinical data suggest that there is only a very small fraction of patients who will benefit overall from an operative approach to these tumors, and all patients must be assessed extremely carefully and only by a very experienced surgeon.
5. Preoperative embolization
If surgery is planned, then preoperative embolization is mandatory in all JPs (127) and Shamblin class III CBTs (111). In large VPs, some Fisch class B TPs, and some Shamblin class II tumors, embolization is a useful tool (128). In all other HNPGLs, the potential risks of preoperative embolization clearly outweigh its potential benefits.
The primary goal of preoperative embolization of HNPGLs is to help obtain a dry surgical field, which facilitates the identification of adjacent lower cranial nerves and vascular structures. Thereby, it not only minimizes intraoperative blood loss but also the risk of surgical morbidity, namely postoperative lower cranial nerve palsies and vascular damage. Especially in JPs, it should also increase the chance of obtaining complete surgical resection, minimizing the risk of tumor recurrence. However, embolization is an invasive procedure with potentially life-threatening side effects. Therefore, the risk of potential complications has to be weighed against the advantages mentioned. Preoperative embolization of HNPGLs should be performed only by very experienced vascular neuroradiologists. Possible minor side effects of embolization include fever as well as local pain and hematomas in the groin. Severe side effects can include skin necrosis, amaurosis, lower cranial nerve palsies, various neurological deficits including unilateral palsy, and even deadly outcomes (111).
With regard to the complications of embolization, Persky et al (111) reported on 47 patients with 53 HNPGLs. All 53 tumors underwent preoperative embolization with complications seen in 6 subjects (12.8%). Those complications were 2 vagal palsies, 1 jugular foramen syndrome, 1 facial palsy, 1 temporary unilateral palsy, and 1 asymptomatic dissection of the vertebral artery.
The question of whether cervical HNPGLs need to undergo routine preoperative embolization is a matter of controversial discussion. Many authors are in favor of embolization of CBTs and vagal PGs. Miller et al (128) reported on a group of 19 patients with VPs; 16 VPs underwent resection, and 5 of those 16 were embolized preoperatively. In those 5 cases, the authors reported shorter operating times, a smaller incidence of cranial nerve deficits, and less intraoperative blood loss. Wang and colleagues (118) compared 17 CBPs that underwent embolization with a group of 12 CBTs that were not embolized. The intraoperative blood loss was significantly higher in patients that did not undergo embolization. On the other hand, at the University of Leiden in the Netherlands, even Shamblin class III tumors did not undergo preoperative embolization. In a December 2013 study, Paridaans et al (129) reported on 45 consecutively operated CBPs in 41 patients (Shamblin I, 7 tumors; Shamblin II, 22 tumors; Shamblin III, 16 tumors) at Leiden University Medical Center between 2006 and 2011. None of the tumors were embolized. Intraoperative mean blood loss was as low as 129 mL. In our opinion, Shamblin class I and II tumors as well as most VPs do not need to undergo routine preoperative embolization. For very large VPs as well as for patients with Shamblin class III tumors, embolization may still be a very valuable tool.
In contrast, preoperative angiographic embolization is regarded as the international gold standard in the treatment of class C and D JPs (127, 130). Without embolization, it would not be possible to completely resect these neoplasms in the vast majority of cases. Embolization is clearly not indicated in Fisch class A tumors. In large Fisch class B tumors, it may occasionally be a useful tool.
D. Therapeutic radiation
1. Radiobiological considerations
The radiobiological basis for conventionally fractionated RT is relatively well understood (131). In contrast, single-session and hypofractionated ablative RS are often regarded to be merely extensions of open surgical resection and appear to act through different molecular pathways.
Conventionally fractionated RT is best modeled by means of the linear quadratic formula, which quantitatively estimates the effect of radiation on cells using the concept of biological effective dose (BED).
Specifically, BED = D × (1 + d/α/β), where D is total dose, d is dose per fraction, and α/β is tissue radiosensitivity (132). RT is believed to typically work by damaging DNA and eventually lead to postmitotic cell death. Because HNPGLs arise from the highly specialized radiation-resistant cells of the neural crest, these tumors are believed to have a low α/β, perhaps even as low as 2. Neoplasms bearing such low α/β ratios are characterized by slow turnover rates and robust repair mechanisms (high β-component) and are generally highly resistant to radiation therapy (ie, renal cancer). However, such tumors prove to be sensitive to extreme-high-dose single-session radiation as embodied in RS techniques. If RT is used to treat HNPGLs, the usual way to overcome radioresistance is to increase the total radiation dose at the expense of surrounding normal tissues. For HNPGLs, this remains an important limitation given surrounding critical neuroanatomical structures. In practice, when treating HNPGLs, the RT total dose is typically limited to 46 Gy, which in the setting of 2-Gy fractions results in a BED of 90 Gy2 (d = 2, α/β = 2 symbolized by Gy2). Developed by neurosurgeons, RS (also called SRS) uses submillimetric targeting accuracy and steep dose gradients to enable high doses to be delivered in a single treatment session lasting from a few minutes to an hour. In contrast to standard RT doses, a single SRS session can administer a Dmax as high as 35 Gy, which has an associated BED of 640 Gy2 has been reported. With RS, cancer histology is not predictive (182) of tumor response, so as a result, the concept of radioresistance is far less applicable than with RT.
RS is believed to destroy tumors via newly described pathways that differ significantly from classical radiobiological mechanisms (133) including 1) the induction of early endothelial inflammation (134) and apoptosis via the sphingomyelin pathway (135) that results in microvascular damage and tumor death (136–138), 2) the enhancement of tumor immunogenicity inducing a sustained immunologic response akin to a tumor vaccine (139, 140), and 3) a delayed vascular injury causing a protracted ischemic tumoricidal effect.
2. Radiotherapy
Despite major technological breakthroughs in the radiotherapeutic treatment of HNPGLs, since 1957, reported local control rates have remained more or less constant and generally in excess of 80%. Among the 804 patients included in 34 different RT series between 1962 and 2009, the median local control rate is generally in excess of 90% (100, 123, 141, 142). Nevertheless, among many of these older retrospective series, local control may have been overestimated by relying on inadequate follow-up tools such as noncontrast CT imaging and in some cases only clinical evaluation.
Our experience demonstrates elevated tumor uptakes using specific tracers even years after RT (D. Taieb, unpublished observations) despite tumor stabilization or even shrinkage. This observation is consistent with reports that persistent neuroendocrine cells can be found years after RT within PGLs that have been seemingly successfully treated (143–145). Given a natural history typically characterized by very slow growth, many researchers question the evidence that RT can ever completely eradicate PGL (106).
Meanwhile among older radiation therapy series complication rates are not insignificant and can range from mild to transient, and even include severe and permanent events such as osteoradionecrosis (146, 147).
An ongoing concern using RT to manage any slow-growing benign tumor in a young patient remains the risk of secondary malignancy. Dose and volume ranges are similar (often smaller) to highly prevalent head and neck squamous cell carcinomas for which no increase in secondary malignancy is of concern after RT (148). Nevertheless, some authors estimated a theoretical risk of 0.28% (149, 150), which is hardly supported by the follow-up of these 804 patients (100). Nowadays, RT has gained precision with the integration of modern imaging (conformal 3D modalities, based on CT and more recently using MRI and PET-CT fusion), leading to a reduced and more accurate treated volume, thereby decreasing the likelihood of complications within surrounding normal tissues. The way photons are delivered within defined target volumes also radically evolved through other technological breakthroughs (intensity-modulated radiation therapy provided better dose conformality, image-guided radiation therapy enabled tumor tracking of moving targets, and volumetric modulated arc therapy further enhanced conformality and dramatically decreased treatment time) (151, 152). These improvements remain inferior to stereotactic precision but take part in the recent decrease in side effects after RT (100, 148). Patients achieved symptomatic improvement in more than 70% of cases across 34 published series (100), in agreement with reviews estimating partial/complete resolution of symptoms in more than 60% of cases (123, 141, 142). In the landscape of clinical oncology, local control is defined by growth arrest (stable disease) or tumor shrinkage (partial or complete response). The latter occurred in 61% of patients, using Response Evaluation Criteria In Solid Tumors criteria (100). Retrospectively, 10-year local control rates are roughly achieved in up to 90% after RT, based on various assessments, with CT and/or MRI being used systematically only since the 1990s (148) and with 10-year survival rates of 95%. Streamlined dedicated software integrating multimodal imaging (PET/MR) will enable delineations of biological target volumes (153, 154), possibly leading to better results even assessed by modern imaging. Nevertheless, it remains unlikely that massive dose escalation will be considered for RT and that these technological improvements will rather be restrained to achieving better therapeutic ratios through normal tissue sparing. As explained below, such dose rates seem insufficient for malignant and metastatic PGLs.
3. Radiosurgery/stereotactic radiosurgery
RS (or SRS) is the most spatially precise form of therapeutic radiation. This attribute, when combined with the steepest possible surrounding dose gradient, enables this procedure to be most typically administered in a single outpatient session lasting less than 1 hour. There are 3 major types of radiosurgical instruments. The oldest radiosurgical method, the GammaKnife, uses a frame affixed to the skull for target localization and patient immobilization (155). More recently, image-guided targeting technology enables a noninvasive frameless approach to the precision targeting of RS. The pioneer in image-guided RS, the CyberKnife, uses real-time dual-projection x-ray correlation to a pre-SRS CT (156, 157, 159, 160). Meanwhile, modified conventional medical linear accelerators have recently demonstrated the capacity to administer highly accurate therapeutic radiation by using a pretreatment cone beam CT (such as the Truebeam STx series) for patient positioning (161, 162) (Figure 3).
By combining targeting accuracy with the steepest possible dose gradients, the above technologies have together made ablative RS readily available throughout much of the world. By virtue of such, RS has made it possible to lower the rate of side effects, almost to the point of being negligible, without any apparent loss of local tumor control; in most recent clinical series, tumor control approaches 100%.
Because the first use of RS to ablate HNPGLs began in the early 1990s, the maximum duration of post-RS follow-up is now approximately 20 years. For slow-growing tumors like HNPGL, this length of follow-up would seem adequate to assess the efficacy of RS as a primary treatment, especially among a population of patients that was typically deemed to be poor candidates for open surgical resection. This length of follow-up also provides a relatively thorough appreciation for any and all long-term treatment-related complications, including the hypothetical risk of second malignancy, which has been estimated to be <1/1000 (163). Nevertheless, as for any treatment modality, patients need to be informed of that theoretical risk when being consented for such an option. However, we do not feel that an extended and specific follow-up is required for RS patients in regard to that theoretical risk compared with their surgical counterparts.
In a recent literature review extending from 1994 through 2010 and including 375 patients treated with radiosurgical ablation as either an initial or postsurgical salvage procedure, MRI-assessed local control was achieved in 98% after a mean follow-up of 31 months (range 1–193) (100). This measure of efficacy (in terms of tumor control) is mirrored in the findings of a recent meta-analysis (19 series) that revealed evidence of symptom resolution in 95% of cases (164). Finally, complication rates were only 3%, and no case seems to exceed grade 2 toxicity. Moreover, no case of secondary malignancy was reported. In summary, published studies, with continuously increasing length of follow-up, uniformly document radiosurgical ablation of PGLs to be highly effective in terms of tumor control and symptom relief, and achieve this objective with minimal toxicity.
The benefits of RS among patients with PGLs is consistent with the outcome observed among a variety of other benign brain and skull base lesions, and even most small malignant lesions, such as brain metastases (165). When combined with the fact that open surgical resection has an operative mortality of 1/100 (163), it would appear that there is now enough evidence to use ablative SRS as a frontline modality and from a practical standpoint, such treatment should be deemed curative. The published evidence to date does not justify the use of RS over surgical resection in cases of secretory HNPGLs in large part because of the delay in altering secretory function (Figure 3). Considerations of QOL further strengthen the argument, first made 2 decades ago by Springate and Weichselbaum (150), for using RS as a frontline procedure, given the frequent cranial nerve injuries associated with conventional operation.
V. Multifocality and/or Hereditary HNPGLs
Patients with multifocal HNPGLs represent a special therapeutic challenge. In these patients, an individual therapeutic approach is absolutely necessary. Important factors that need to be kept in mind include, but are not limited to, the number and localization of tumors, unilateral or bilateral tumors, the size and stage of tumors, hereditary or nonhereditary status, SDHB-associated tumors vs other hereditary tumors, patient age, general patient condition, and patient's preference. Whenever a surgeon and a patient agree to plan resection of multiple HNPGLs, certain general rules should be followed.
Subjects with bilateral HNPGLs should not undergo a 1-step surgical approach, because this bears the risk of bilateral cranial nerve palsies resulting in severe disabilities (166) (Figure 3). Surgery should be used with great caution in patients at risk for bilateral palsy of the vagal nerve. This is important in any patient with a 1-sided vocal cord palsy and a CBT or a JP on the other side. In these cases, patients need to be extremely well-informed about the potential risks of a surgical approach as well as alternative therapeutic options. In most patients with a unilateral vocal cord palsy and a vagal PGL on the contralateral side, surgery is not a good option, because resection of the vagal PGL leads to ipsilateral vocal cord palsy in the vast majority of cases. On the other hand, in patients with Fisch class A tumors, there is no perioperative risk for the ipsilateral vagal nerve. These patients are always excellent candidates for complete surgical resection regardless of their cranial nerve status.
When it comes to planning a 2-step surgical approach in patients with bilateral HNPGLs, there are no internationally accepted rules on which tumor to resect first. Velegrakis et al (167) recommended always starting with the larger tumor. Depending on the outcome of the first operation, the remaining HNPGL(s) can be observed or, if needed, treated by surgery or radiation therapy. Sobol and Dailey (166) suggested that a 1-step surgical approach may be an option for selected patients with multiple unilateral HNPGLs. They reported on a 27-year-old patient with a CBT and a VP on the right side. During the resection of the VP, the vagal nerve had to be killed. The ipsilateral class I CBP could therefore be resected without the risk of additional cranial nerve palsies during the same surgery. Whenever a tumor on the first side has successfully been resected without cranial nerve palsies, resection of the contralateral tumor may be planned. In cases of JPs, there should be at least 9 to 12 months between surgical interventions because resection of the jugular bulb is usually necessary and venous collaterals need time to develop. Patients with multiple HNPGLs and malignancy need a different therapeutic approach. Whenever there is a curative setting, one should discuss aggressive surgical resection that may not always be able to preserve all lower cranial nerves. In cases of widely metastatic disease, surgical intervention should usually not be performed.
All patients, with or without a family history, should be screened for germline mutations (including large gene rearrangements) in one of the SDH subunit genes, especially in the SDHB, -C, and -D genes. Clinical parameters can help establish the order in which these 3 genes should be tested. In cases of multiple HNPGLs, solitary HNPGL with a positive family history, or a combination of HNPGLs and abdominal PGLs, screening should be first directed at SDHD, followed by SDHB and SDHC. By contrast, patients with solitary HNPGL and a negative family history or malignant HNPGL should undergo genetic screening for SDHB, followed by SDHD and SDHC. This approach can be modified under certain circumstances. If the family history confirms a phenotype transmission from mother to child, showing the lack of the parent-of-origin effect seen in SDHD mutations, the SDHB gene should be tested first, followed by the SDHC gene if necessary (168). This testing strategy will also probably change in time with the clinical implementation of next-generation sequencing-based gene panels for the diagnosis of inherited PGL syndromes (169). The benefit of such panels is to get all known associated genes and decreases the chance of partial incomplete testing, often caused by loss of follow-up. It will also significantly increase our understanding of phenotype and penetrance.
Knowing a patient's genetic status is crucial, especially for those with a single HNPGL, because they are at an increased risk of developing additional ipsi- or contralateral tumors or even malignant disease.
Thus, severe and permanent surgery-induced injuries may compromise subsequent operations. An individual treatment regimen is absolutely necessary in patients with hereditary HNPGLs. Before any treatment is started, these patients have to be extensively discussed at an interdisciplinary tumor board that is familiar with hereditary HNPGLs, and all the treatment options have to be discussed with the patient as well.
The type of germline mutation plays an important role. SDHC mutation carriers do not have a high risk of developing more than 1 HNPGL over their lifetime, and thus, they should probably be treated similarly to sporadic cases. In contrast, SDHD and SDHAF2 mutations carriers have a very high risk of developing multiple HNPGLs. Surgery could be an option in these patients, but a step-by-step approach may be necessary to avoid bilateral neurologic complications if multiple tumors are present.
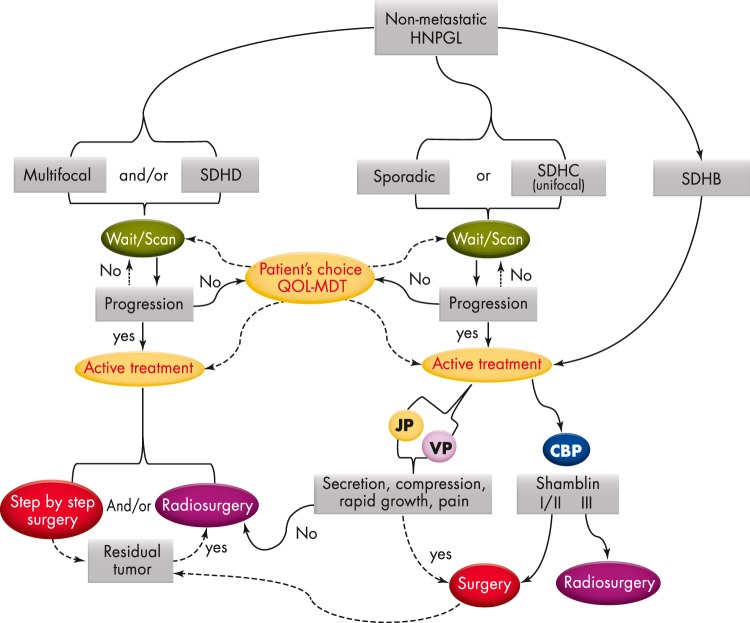
SDHB mutation carriers are a special problem because they have a higher risk for malignancy. Ellis et al (61) have recently demonstrated that even SDHB patients with benign CBPs present with worse disease-free survival after resection. The authors therefore recommended a more aggressive surgical approach to these patients. It is expected that the early detection and treatment of SDHB-related HNPGLs may minimize complications related to mass effect, facilitate curative treatment, and potentially reduce the occurrence of metastases. Refer to Figure 4 for a proposed clinical algorithm for management of HNPGLs.
Figure 4.
Clinical algorithm for management of HNPGLs.
VI. Metastatic HNPGLs
Metastatic HNPGLs can be diagnosed at initial presentation or upon the development of metastases after complete resection of the primary tumor (the mean time of recurrence is about 6 years). Rarely, very aggressive invasion of surrounding structures is considered to indicate the presence of malignancy. Metastatic PGLs often have an unpredictable natural history. Recently, it has been shown that the 1-year progression-free survival of therapy-naive malignant PGLs was 46% (170). Anatomical and functional imaging is necessary for localizing metastases as well as for selecting patients who may be candidates for RT.
Optimal treatment strategies have not been defined to date because the relevant literature mostly comprises case reports or small series, with a frequent mixing of both sympathetic PGL and HNPGL and with different management options (102). Nevertheless, several treatment options are possible, depending on the clinical situation. There are some important considerations that must be taken into account, including the size of the tumor and its feasibility to be removed surgically, the presence of any metastatic lesions, multiplicity, the patient's current status (age, severe cardiac history, atherosclerosis, etc), and previous operations with some residual defects, as well as some other important limiting factors (low blood count related to previous treatment, predisposition for thrombosis and embolization, unusual head and neck anatomical defects, etc). Nevertheless, whenever feasible, surgical removal of the primary tumor is recommended. This can be done together with removal of local metastatic disease, especially when present in lymphatic nodes. Performing a single operation on multiple primary tumors is questionable and is usually not recommended. The reason is that, eg, if both carotid bodies are removed, a severe neurological deficit may occur after the operation of the first tumor and operating on the second tumor may significantly contribute to the morbidity of the patient. It is strongly recommended that only 1 primary tumor be removed first, with the second removed after a few weeks when the patient fully recovers. Exceptions are primary tumors on the same side of the neck, but even in that situation a surgeon must be very careful, because removing more than 1 tumor on one side may have additive neurological damage to a patient. Furthermore, it is important to consider whether the patient really needs to have his/her HNPGL removed. Currently, in most neck PGLs, except those related to SDHB, surgery is not indicated for tumors less than 2 cm in size, unless a neurological deficit is present (61). Surgery is recommended for tumors 2 cm or larger. Besides SDHB tumors, another exception can be JPs. In large tumors, surgery leads to a significant increase in morbidity and a high risk of persistent/recurrent disease.
Chemotherapy is reserved for more advanced disease stages. Treatment experience with different drugs and regimens is limited and associated with high-grade toxicities. The most effective chemotherapy regimen appears to be the CVD scheme including cyclophosphamide, vincristine, and dacarbazine. According to the National Cancer Database on malignant HNPGLs, the 5-year relative survival rate is 59.5% (76.8% for regionally confined carcinoma and 11.8% for distant metastasis) (8). Younger patients and those who may be treated with aggressive therapy may have a longer median survival (102). Other cytotoxics, alone or combined with antiangiogenics, like temozolomide and thalidomide, temozolomide-capecitabine, or sunitinib monotherapy, have shown encouraging preliminary results (172). Enrollment in clinical trials is strongly encouraged.
Another approach is to target isolated tumor cells directly with [131I]MIBG (in MIBG-positive tumors). Radionuclide therapy should also be encouraged within clinical trials, especially using 177Lu-radiolabeled somatostatin analogs (also called peptide receptor radionuclide therapy), which have shown promising results in other SST-expressing tumors, such as metastatic gastroenteropancreatic neuroendocrine tumors (173). Besides radiological imaging, PET imaging should be used to monitor tumor response to systemic therapies and radionuclide therapy (174, 175).
VII. Near-Future Therapeutic Options
A. Therapeutic radiation
Besides HNPGLs, real-time image-guided RT and gating technologies enable radiosurgical stereotactic accuracy for treating extracranial moving targets (133). Until present, there were almost no published clinical data on the primary treatment of PGL in other sites. Because the α/β ratio of head and neck tumors is seemingly the same as their extracranial counterparts, one would anticipate similar radiosensitivity. Consequently, 3 to 4 synchronous sites can be handled curatively in 1 to 3 outpatient sessions. This approach would permit the treatment of patients with multifocal and/or oligometastatic disease. For these malignant forms, a combination of antiangiogenics and RS also seems very promising for amplifying the tumor response to RS via 3 different mechanisms. RS triggers microvascular dysfunction via activation of the acidic sphingomyelinase signaling pathway (176). Vascular endothelial growth factor inhibitors may potentialize this effect and increase cell death. To this end, vascular endothelial growth factor inhibition should be initiated before RS (177), a schedule that may also prevent endothelial progenitor recruitment for repairing vasculature damages (178). These 2 effects, which lead to the disruption of neovasculature and the tumor's supply of oxygen and nutrients, support the pioneering work of Folkman (179). Finally, antiangiogenics can also restore microvascular function in a different mechanism and time frame than the previously mentioned 2 effects via vascular normalization, increased tumor pO2, decreased interstitial fluid pressure, and subsequently enhanced production of tumoricidal free radicals by RS (180). Antiangiogenics are still providing modest results in clinical settings for most malignancies (181, 182). A major issue to integrate them in curative strategies remains for these several coexisting mechanisms of action. RS could possibly exploit their true potential through an adequate combination with each of the 3 distinct mechanisms and respective optimal temporal sequencing. RS is indeed conferring to such a combination an overwhelming simplification given the unique fractions delivered over a few minutes (weeks for RT/chemotherapy). The optimal time to initiate antiangiogenic therapy for radiosensitization in a given patient remains to be determined and could therefore benefit from real-time monitoring of HNGPL tumor perfusion/interstitial fluid pressure/pO2 and pave the way for using these agents more appropriately in other malignancies.
B. Targeted therapies
We envision that future approaches will be based on better knowledge of the disease gained from discoveries made through the use of proteomics and genomics. These tumors will need to be well-characterized by these techniques through precise comparison with other types of PHEOs and PGLs. Then, a comparison of specific genes and proteins related to the development and presence of these tumors will emerge, and those results will serve as pivotal markers or targets for diagnostic and therapeutic approaches to PGLs. Identification of subgroups of molecular-specific phenotypes may assist in implementing novel personalized treatments for metastatic patients. Several key factors involved in the pathogenesis of SDHx-related PHEOs/PGLs could be targeted by specific agents to restore SDH or PHD activities, prevent ROS damaging, increase HIF-1/2α proteosomal degradation, disrupt cellular pH regulation and inhibit glycolysis, disrupt DNA and histone methylation, and finally disrupt alternative signaling pathways. These approaches are tightly interconnected and can also be combined.
C. Radionuclide therapy
This will not only accelerate how new functional imaging (and localization) of these tumors will be used, but these specific targets will help to synthesize radiopharmaceuticals that will indeed be very specific for these tumors and can also be used for their treatment. This is very important, because most of these tumors are currently not positive on [123I]MIBG scintigraphy. MIBG is a compound for treating metastatic sympathetic tumors using β-emitting particles when [131I]MIBG is applied. Uncovering specific developmental and pathogenic characteristics of these malignancies will help to introduce new treatment molecules for such tumors. This also includes the manipulation of norepinephrine transporters using histone deacetylases inhibitors (183). Results from molecular profiling studies now provide important and promising information in the identification of new therapeutic and imaging targets (41, 184). The development of new radiopharmaceuticals that target angiogenesis, apoptosis, hypoxia, and glycolysis might be of particular interest in the characterization of these tumors. It is also expected that these radiopharmaceuticals will serve as predictive biomarker classifiers.
[177Lu]DOTATATE will also be rapidly implemented into the therapeutic arsenal for HNPGLs (173), especially metastatic PGLs. Somatostatin analogs labeled with high-linear-energy-transfer α-emitters should likewise selectively deliver higher doses to SST-expressing tumors. As for other cancers, nuclear medicine will rapidly shift toward tumor-specific characterization tightly linked to appropriate therapy selections and individualized medicine, resulting in more optimal treatment with cost-effective outcome.
Acknowledgments
This research was supported by the Intramural Research Program of the Eucine Kennedy Shriver National Institute of Child Health and Development and the National Institute of Neurological Disorders and Stroke at the National Intitutes of Health.
Disclosure Summary: The authors declare no competing interests.
Footnotes
- [18F]FDOPA
- [18F] dihydroxyphenylalanine
- 3D
- 3-dimensional
- BED
- biological effective dose
- CBP
- carotid body PGL
- CT
- computed tomography
- DOTANOC
- DOTA-Tyr3-octreotide
- DOTATATE
- DOTA-Tyr3-octreotate
- DOTATOC
- DOTA-Tyr3-octreotide
- GIST
- gastrointestinal stromal tumor
- HIF
- hypoxia-inducible factor
- HNPGL
- head and neck paraganglioma
- JP
- glomus jugulare PGL
- MIBG
- metaiodobenzylguanidine
- MRI
- magnetic resonance imaging
- NC
- neural crest
- PET
- positron emission tomography
- PGL
- paraganglioma
- PHD
- prolyl hydroxylase
- PHEO
- pheochromocytoma
- QOL
- quality of life
- ROS
- reactive oxygen species
- RS
- radiosurgery
- RT
- radiotherapy
- SDH
- succinate dehydrogenase
- SDHD
- SDH subunit D
- SPECT
- single-photon emission computerized tomography
- SRS
- stereotactic radiosurgery
- SST
- somatostatin receptor
- TP
- glomus tympanicum PGL
- VP
- glomus vagale PGL.
References
- 1. DeLellis RA, Lloyd RV, Heitz PU, Eng C. Pathology and Genetics of Tumours of Endocrine Organs. Vol 8 3rd ed Lyon, France: World Health Organization; 2004 [Google Scholar]
- 2. Mannelli M, Castellano M, Schiavi F, et al. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94(5):1541–1547 [DOI] [PubMed] [Google Scholar]
- 3. Sykes JM, Ossoff RH. Paragangliomas of the head and neck. Otolaryngol Clin North Am. 1986;19(4):755–767 [PubMed] [Google Scholar]
- 4. le Douarin N, Kalcheim C. The Neural Crest. 2nd ed Cambridge, UK: Cambridge University Press; 1999 [Google Scholar]
- 5. Unsicker K, Huber K, Schober A, Kalcheim C. Resolved and open issues in chromaffin cell development. Mech Dev. 2013;130(6–8):324–329 [DOI] [PubMed] [Google Scholar]
- 6. Erickson D, Kudva YC, Ebersold MJ, et al. Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab. 2001;86(11):5210–5216 [DOI] [PubMed] [Google Scholar]
- 7. Gimenez-Roqueplo AP, Caumont-Prim A, Houzard C, et al. Imaging work-up for screening of paraganglioma and pheochromocytoma in SDHx mutation carriers: a multicenter prospective study from the PGL.EVA Investigators. J Clin Endocrinol Metab. 2013;98(1):E162–E173 [DOI] [PubMed] [Google Scholar]
- 8. Lee JH, Barich F, Karnell LH, et al. National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer. 2002;94(3):730–737 [DOI] [PubMed] [Google Scholar]
- 9. Jafri M, Whitworth J, Rattenberry E, et al. Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol (Oxf). 2013;78(6):898–906 [DOI] [PubMed] [Google Scholar]
- 10. Baysal BE, Ferrell RE, Willett-Brozick JE, et al. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287(5454):848–851 [DOI] [PubMed] [Google Scholar]
- 11. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14(2):108–119 [DOI] [PubMed] [Google Scholar]
- 12. Baysal BE, Willett-Brozick JE, Lawrence EC, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39(3):178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Piccini V, Rapizzi E, Bacca A, et al. Head and neck paragangliomas: genetic spectrum and clinical variability in 79 consecutive patients. Endocr Relat Cancer. 2012;19(2):149–155 [DOI] [PubMed] [Google Scholar]
- 14. Neumann HP, Erlic Z, Boedeker CC, et al. Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res. 2009;69(8):3650–3656 [DOI] [PubMed] [Google Scholar]
- 15. Kunst HP, Rutten MH, de Mönnink JP, et al. SDHAF2 (PGL2-SDH5) and hereditary head and neck paraganglioma. Clin Cancer Res. 2011;17(2):247–254 [DOI] [PubMed] [Google Scholar]
- 16. Baysal BE. Mitochondrial complex II and genomic imprinting in inheritance of paraganglioma tumors. Biochim Biophys Acta. 2013;1827(5):573–577 [DOI] [PubMed] [Google Scholar]
- 17. Papathomas TG, Gaal J, Corssmit EP, et al. Non-pheochromocytoma (PCC)/paraganglioma (PGL) tumors in patients with succinate dehydrogenase-related PCC-PGL syndromes: a clinicopathological and molecular analysis. Eur J Endocrinol. 2014;170(1):1–12 [DOI] [PubMed] [Google Scholar]
- 18. Pasini B, McWhinney SR, Bei T, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16(1):79–88 [DOI] [PubMed] [Google Scholar]
- 19. Carney JA. Carney triad. Front Horm Res. 2013;41:92–110 [DOI] [PubMed] [Google Scholar]
- 20. Haller F, Moskalev EA, Faucz FR, et al. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr Relat Cancer. 2014;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10(5):289–291 [DOI] [PubMed] [Google Scholar]
- 22. van Nederveen FH, Gaal J, Favier J, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol. 2009;10(8):764–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang C, Matro JC, Huntoon KM, et al. Missense mutations in the human SDHB gene increase protein degradation without altering intrinsic enzymatic function. FASEB J. 2012;26(11):4506–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lendvai N, Pawlosky R, Bullova P, et al. Succinate-to-fumarate ratio as a new metabolic marker to detect the presence of SDHB/D-related paraganglioma: initial experimental and ex vivo findings. Endocrinology. 2014;155(1):27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Imperiale A, Elbayed K, Moussallieh FM, et al. Metabolomic profile of the adrenal gland: from physiology to pathological conditions. Endocr Relat Cancer. 2013;20(5):705–716 [DOI] [PubMed] [Google Scholar]
- 26. Rao JU, Engelke UF, Rodenburg RJ, et al. Genotype-specific abnormalities in mitochondrial function associate with distinct profiles of energy metabolism and catecholamine content in pheochromocytoma and paraganglioma. Clin Cancer Res. 2013;19(14):3787–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Isaacs JS, Jung YJ, Mole DR, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8(2):143–153 [DOI] [PubMed] [Google Scholar]
- 28. Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462(7274):739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jokilehto T, Jaakkola PM. The role of HIF prolyl hydroxylases in tumour growth. J Cell Mol Med. 2010;14(4):758–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qin N, de Cubas AA, Garcia-Martin R, et al. Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: Insights from MAX. Int J Cancer. 2014;135(9):2054–2064 [DOI] [PubMed] [Google Scholar]
- 33. Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012;37(9):364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burnichon N, Rohmer V, Amar L, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94(8):2817–2827 [DOI] [PubMed] [Google Scholar]
- 35. Burnichon N, Vescovo L, Amar L, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985 [DOI] [PubMed] [Google Scholar]
- 36. Dahia PL, Hao K, Rogus J, et al. Novel pheochromocytoma susceptibility loci identified by integrative genomics. Cancer Res. 2005;65(21):9651–9658 [DOI] [PubMed] [Google Scholar]
- 37. Eisenhofer G, Huynh TT, Pacak K, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897–911 [DOI] [PubMed] [Google Scholar]
- 38. Favier J, Briere JJ, Burnichon N, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jochmanová I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. López-Jiménez E, Gómez-López G, Leandro-García LJ, et al. Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24(12):2382–2391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shankavaram U, Fliedner SM, Elkahloun AG, et al. Genotype and tumor locus determine expression profile of pseudohypoxic pheochromocytomas and paragangliomas. Neoplasia. 2013;15(4):435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merlo A, de Quiros SB, Secades P, et al. Identification of a signaling axis HIF-1α/microRNA-210/ISCU independent of SDH mutation that defines a subgroup of head and neck paragangliomas. J Clin Endocrinol Metab. 2012;97(11):E2194–E2200 [DOI] [PubMed] [Google Scholar]
- 43. Kaelin WG, Jr, McKnight SL. Influence of metabolism on epigenetics and disease. Cell. 2013;153(1):56–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Letouzé E, Martinelli C, Loriot C, et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23(6):739–752 [DOI] [PubMed] [Google Scholar]
- 45. Pollard PJ, Brière JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–2239 [DOI] [PubMed] [Google Scholar]
- 46. Xiao M, Yang H, Xu W, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26(12):1326–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eisenhofer G, Lenders JW, Siegert G, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. van Duinen N, Steenvoorden D, Kema IP, et al. Increased urinary excretion of 3-methoxytyramine in patients with head and neck paragangliomas. J Clin Endocrinol Metab. 2010;95(1):209–214 [DOI] [PubMed] [Google Scholar]
- 49. van Duinen N, Corssmit EP, de Jong WH, Brookman D, Kema IP, Romijn JA. Plasma levels of free metanephrines and 3-methoxytyramine indicate a higher number of biochemically active HNPGL than 24-h urinary excretion rates of catecholamines and metabolites. Eur J Endocrinol. 2013;169(3):377–382 [DOI] [PubMed] [Google Scholar]
- 50. Lenders JW, Duh QY, Eisenhofer G, et al. Pheochromocytoma and paraganglioma: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(6):1915–1942 [DOI] [PubMed] [Google Scholar]
- 51. Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397(2):155–177 [DOI] [PubMed] [Google Scholar]
- 52. Korpershoek E, Favier J, Gaal J, et al. SDHA Immunohistochemistry Detects Germline SDHA Gene Mutations in Apparently Sporadic Paragangliomas and Pheochromocytomas. J Clin Endocrinol Metab. 2011;96(9):E1472–E1476 [DOI] [PubMed] [Google Scholar]
- 53. Peitzsch M, Prejbisz A, Kroiss M, et al. Analysis of plasma 3-methoxytyramine, normetanephrine and metanephrine by ultraperformance liquid chromatography-tandem mass spectrometry: utility for diagnosis of dopamine-producing metastatic phaeochromocytoma. Ann Clin Biochem. 2013;50(Pt 2):147–155 [DOI] [PubMed] [Google Scholar]
- 54. de Wailly P, Oragano L, Radé F, et al. Malignant pheochromocytoma: new malignancy criteria. Langenbecks Arch Surg. 2012;397(2):239–246 [DOI] [PubMed] [Google Scholar]
- 55. Pinato DJ, Ramachandran R, Toussi ST, et al. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. Br J Cancer. 2013;108(2):429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Span PN, Rao JU, Oude Ophuis B, et al. Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant phaeochromocytoma/paraganglioma. Endocr Relat Cancer. 2011;18(3):323–331 [DOI] [PubMed] [Google Scholar]
- 57. Murthy SR, Pacak K, Loh YP. Carboxypeptidase E: elevated expression correlated with tumor growth and metastasis in pheochromocytomas and other cancers. Cell Mol Neurobiol. 2010;30(8):1377–1381 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58. Eisenhofer G, Lenders JW, Goldstein DS, et al. Pheochromocytoma catecholamine phenotypes and prediction of tumor size and location by use of plasma free metanephrines. Clin Chem. 2005;51(4):735–744 [DOI] [PubMed] [Google Scholar]
- 59. Zelinka T, Musil Z, Dušková J, et al. Metastatic pheochromocytoma: does the size and age matter? Eur J Clin Invest. 2011;41(10):1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Boedeker CC, Neumann HP, Maier W, Bausch B, Schipper J, Ridder GJ. Malignant head and neck paragangliomas in SDHB mutation carriers. Otolaryngol Head Neck Surg. 2007;137(1):126–129 [DOI] [PubMed] [Google Scholar]
- 61. Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E. The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. 2014;260(1):158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Hulsteijn LT, Dekkers OM, Hes FJ, Smit JW, Corssmit EP. Risk of malignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: a systematic review and meta-analysis. J Med Genet. 2012;49(12):768–776 [DOI] [PubMed] [Google Scholar]
- 63. Zheng X, Wei S, Yu Y, et al. Genetic and clinical characteristics of head and neck paragangliomas in a Chinese population. Laryngoscope. 2012;122(8):1761–1766 [DOI] [PubMed] [Google Scholar]
- 64. Loriot C, Burnichon N, Gadessaud N, et al. Epithelial to mesenchymal transition is activated in metastatic pheochromocytomas and paragangliomas caused by SDHB gene mutations. J Clin Endocrinol Metab. 2012;97(6):E954–E962 [DOI] [PubMed] [Google Scholar]
- 65. Johnson MH. Head and neck vascular anatomy. Neuroimaging Clin North Am. 1998;8(1):119–141 [PubMed] [Google Scholar]
- 66. van den Berg R, Schepers A, de Bruïne FT, et al. The value of MR angiography techniques in the detection of head and neck paragangliomas. European journal of radiology. 2004;52(3):240–245 [DOI] [PubMed] [Google Scholar]
- 67. Neves F, Huwart L, Jourdan G, Reizine D, Herman P, Vicaut E, Guichard JP. Head and neck paragangliomas: value of contrast-enhanced 3D MR angiography. AJNR Am J Neuroradiol. 2008;29(5):883–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. van den Berg R. Imaging and management of head and neck paragangliomas. Eur Radiol. 2005;15(7):1310–1318 [DOI] [PubMed] [Google Scholar]
- 69. Arnold SM, Strecker R, Scheffler K, et al. Dynamic contrast enhancement of paragangliomas of the head and neck: evaluation with time-resolved 2D MR projection angiography. Eur Radiol. 2003;13(7):1608–1611 [DOI] [PubMed] [Google Scholar]
- 70. Dong Y, Liu Q. Differentiation of malignant from benign pheochromocytomas with diffusion-weighted and dynamic contrast-enhanced magnetic resonance at 3.0 T. J Comput Assist Tomogr. 2012;36(4):361–366 [DOI] [PubMed] [Google Scholar]
- 71. Bustillo A, Telischi F, Weed D, et al. Octreotide scintigraphy in the head and neck. Laryngoscope. 2004;114(3):434–440 [DOI] [PubMed] [Google Scholar]
- 72. Duet M, Sauvaget E, Pételle B, et al. Clinical impact of somatostatin receptor scintigraphy in the management of paragangliomas of the head and neck. J Nucl Med. 2003;44(11):1767–1774 [PubMed] [Google Scholar]
- 73. Koopmans KP, Jager PL, Kema IP, Kerstens MN, Albers F, Dullaart RP. 111In-Octreotide is superior to 123I-metaiodobenzylguanidine for scintigraphic detection of head and neck paragangliomas. J Nucl Med. 2008;49(8):1232–1237 [DOI] [PubMed] [Google Scholar]
- 74. Muros MA, Llamas-Elvira JM, Rodríguez A, et al. 111In-pentetreotide scintigraphy is superior to 123I-MIBG scintigraphy in the diagnosis and location of chemodectoma. Nucl Med Commun. 1998;19(8):735–742 [DOI] [PubMed] [Google Scholar]
- 75. Schmidt M, Fischer E, Dietlein M, et al. Clinical value of somatostatin receptor imaging in patients with suspected head and neck paragangliomas. Eur J Nucl Med Mol Imaging. 2002;29(12):1571–1580 [DOI] [PubMed] [Google Scholar]
- 76. Telischi FF, Bustillo A, Whiteman ML, et al. Octreotide scintigraphy for the detection of paragangliomas. Otolaryngol Head Neck Surg. 2000;122(3):358–362 [DOI] [PubMed] [Google Scholar]
- 77. Charrier N, Deveze A, Fakhry N, et al. Comparison of [111In]pentetreotide-SPECT and [18F]FDOPA-PET in the localization of extra-adrenal paragangliomas: the case for a patient-tailored use of nuclear imaging modalities. Clin Endocrinol (Oxf). 2011;74(1):21–29 [DOI] [PubMed] [Google Scholar]
- 78. Gabriel S, Blanchet EM, Sebag F, et al. Functional characterization of nonmetastatic paraganglioma and pheochromocytoma by 18F-FDOPA PET: focus on missed lesions. Clin Endocrinol (Oxf). 2013;79(2):170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. King KS, Chen CC, Alexopoulos DK, et al. Functional imaging of SDHx-related head and neck paragangliomas: comparison of 18F-fluorodihydroxyphenylalanine, 18F-fluorodopamine, 18F-fluoro-2-deoxy-d-glucose PET, 123I-metaiodobenzylguanidine scintigraphy, and 111In-pentetreotide scintigraphy. J Clin Endocrinol Metab. 2011;96(9):2779–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Miederer M, Fottner C, Rossmann H, et al. High incidence of extraadrenal paraganglioma in families with SDHx syndromes detected by functional imaging with [18F]fluorodihydroxyphenylalanine PET. Eur J Nucl Med Mol Imaging. 2013;40(6):889–896 [DOI] [PubMed] [Google Scholar]
- 81. Timmers HJ, Chen CC, Carrasquillo JA, et al. Comparison of 18F-fluoro-l-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2009;94(12):4757–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kroiss A, Putzer D, Frech A, et al. A retrospective comparison between 68Ga-DOTA-TOC PET/CT and 18F-DOPA PET/CT in patients with extra-adrenal paraganglioma. Eur J Nucl Med Mol Imaging. 2013;40(12):1800–1808 [DOI] [PubMed] [Google Scholar]
- 83. Maurice JB, Troke R, Win Z, Ramachandran R, Al-Nahhas A, Naji M, Dhillo W, Meeran K, Goldstone AP, Martin NM, Todd JF, Palazzo F, Tan T. A comparison of the performance of 68Ga-DOTATATE PET/CT and 123I-MIBG SPECT in the diagnosis and follow-up of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39(8):1266–1270 [DOI] [PubMed] [Google Scholar]
- 84. Naji M, Al-Nahhas A. 68Ga-labelled peptides in the management of neuroectodermal tumours. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):61–67 [DOI] [PubMed] [Google Scholar]
- 85. Naji M, Zhao C, Welsh SJ, et al. 68Ga-DOTA-TATE PET vs. 123I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13(4):769–775 [DOI] [PubMed] [Google Scholar]
- 86. Sharma P, Thakar A, Suman K C S, et al. 68Ga-DOTANOC PET/CT for Baseline Evaluation of Patients with Head and Neck Paraganglioma. J Nucl Med. 2013;54(6):841–847 [DOI] [PubMed] [Google Scholar]
- 87. Blanchet EM, Gabriel S, Martucci V, et al. 18F-FDG PET/CT as a predictor of hereditary head and neck paragangliomas. Eur J Clin Invest. 2014;44(3):325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Taïeb D, Varoquaux A, Chen CC, Pacak K. Current and future trends in the anatomical and functional imaging of head and neck paragangliomas. Semin Nucl Med. 2013;43(6):462–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Timmers HJ, Eisenhofer G, Carrasquillo JA, et al. Use of 6-[18F]-fluorodopamine positron emission tomography (PET) as first-line investigation for the diagnosis and localization of non-metastatic and metastatic phaeochromocytoma (PHEO). Clin Endocrinol (Oxf). 2009;71(1):11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Timmers HJ, Kozupa A, Chen CC, et al. Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol. 2007;25(16):2262–2269 [DOI] [PubMed] [Google Scholar]
- 91. Taïeb D, Sebag F, Barlier A, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med. 2009;50(5):711–717 [DOI] [PubMed] [Google Scholar]
- 92. Taïeb D, Timmers HJ, Hindié E, et al. EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging. 2012;39(12):1977–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cosetti M, Linstrom C, Alexiades G, Tessema B, Parisier S. Glomus tumors in patients of advanced age: a conservative approach. Laryngoscope. 2008;118(2):270–274 [DOI] [PubMed] [Google Scholar]
- 94. Fisch U, Mattox D. Microsurgery of the Skull Base. Stuttgart/New York: Georg Thieme Verlag; 1988:148–281 [Google Scholar]
- 95. Fisch U. Infratemporal fossa approach to tumours of the temporal bone and base of the skull. J Laryngol Otol. 1978;92(11):949–967 [DOI] [PubMed] [Google Scholar]
- 96. Netterville JL, Jackson CG, Miller FR, Wanamaker JR, Glasscock ME. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch Otolaryngol Head Neck Surg. 1998;124(10):1133–1140 [DOI] [PubMed] [Google Scholar]
- 97. Shamblin WR, ReMine WH, Sheps SG, Harrison EG., Jr Carotid body tumor (chemodectoma). Clinicopathologic analysis of ninety cases. Am J Surg. 1971;122(6):732–739 [DOI] [PubMed] [Google Scholar]
- 98. Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med. 2012;2(12):a006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wild D, Bomanji JB, Benkert P, et al. Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2013;54(3):364–372 [DOI] [PubMed] [Google Scholar]
- 100. Lieberson RE, Adler JR, Soltys SG, Choi C, Gibbs IC, Chang SD. Stereotactic radiosurgery as the primary treatment for new and recurrent paragangliomas: is open surgical resection still the treatment of choice? World Neurosurg. 2012;77(5–6):745–761 [DOI] [PubMed] [Google Scholar]
- 101. Adjallé R, Plouin PF, Pacak K, Lehnert H. Treatment of malignant pheochromocytoma. Horm Metab Res. 2009;41(9):687–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moskovic DJ, Smolarz JR, Stanley D, et al. Malignant head and neck paragangliomas: is there an optimal treatment strategy? Head Neck Oncol. 2010;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Heesterman BL, Bayley JP, Tops CM, et al. High prevalence of occult paragangliomas in asymptomatic carriers of SDHD and SDHB gene mutations. Eur J Hum Genet. 2013;21(4):469–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hensen EF, Jansen JC, Siemers MD, et al. The Dutch founder mutation SDHD.D92Y shows a reduced penetrance for the development of paragangliomas in a large multigenerational family. Eur J Hum Genet. 2010;18(1):62–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hes FJ, Weiss MM, Woortman SA, et al. Low penetrance of a SDHB mutation in a large Dutch paraganglioma family. BMC Med Genet. 2010;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jansen JC, van den Berg R, Kuiper A, van der Mey AG, Zwinderman AH, Cornelisse CJ. Estimation of growth rate in patients with head and neck paragangliomas influences the treatment proposal. Cancer. 2000;88(12):2811–2816 [PubMed] [Google Scholar]
- 107. Langerman A, Athavale SM, Rangarajan SV, Sinard RJ, Netterville JL. Natural history of cervical paragangliomas: outcomes of observation of 43 patients. Arch Otolaryngol Head Neck Surg. 2012;138(4):341–345 [DOI] [PubMed] [Google Scholar]
- 108. Platero-Luengo A, Gonzalez-Granero S, Duran R, et al. An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell. 2014;156(1–2):291–303 [DOI] [PubMed] [Google Scholar]
- 109. Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol. 1973;4(2):251–263 [DOI] [PubMed] [Google Scholar]
- 110. Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet. 2003;113(3):228–237 [DOI] [PubMed] [Google Scholar]
- 111. Persky MS, Setton A, Niimi Y, Hartman J, Frank D, Berenstein A. Combined endovascular and surgical treatment of head and neck paragangliomas–a team approach. Head Neck. 2002;24(5):423–431 [DOI] [PubMed] [Google Scholar]
- 112. Foote RL, Pollock BE, Gorman DA, et al. Glomus jugulare tumor: tumor control and complications after stereotactic radiosurgery. Head Neck. 2002;24(4):332–338; discussion 338–339 [DOI] [PubMed] [Google Scholar]
- 113. de Flines J, Jansen J, Elders R, et al. Normal life expectancy for paraganglioma patients: a 50-year-old cohort revisited. Skull Base. 2011;21(6):385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. van Hulsteijn LT, Louisse A, Havekes B, et al. Quality of life is decreased in patients with paragangliomas. Eur J Endocrinol. 2013;168(5):689–697 [DOI] [PubMed] [Google Scholar]
- 115. Havekes B, van der Klaauw AA, Hoftijzer HC, et al. Reduced quality of life in patients with head-and-neck paragangliomas. Eur J Endocrinol. 2008;158(2):247–253 [DOI] [PubMed] [Google Scholar]
- 116. Patetsios P, Gable DR, Garrett WV, et al. Management of carotid body paragangliomas and review of a 30-year experience. Ann Vasc Surg. 2002;16(3):331–338 [DOI] [PubMed] [Google Scholar]
- 117. Papaspyrou K, Mewes T, Rossmann H, et al. Head and neck paragangliomas: Report of 175 patients (1989–2010). Head Neck. 2012;34(5):632–637 [DOI] [PubMed] [Google Scholar]
- 118. Wang SJ, Wang MB, Barauskas TM, Calcaterra TC. Surgical management of carotid body tumors. Otolaryngol Head Neck Surg. 2000;123(3):202–206 [DOI] [PubMed] [Google Scholar]
- 119. Anand VK, Alemar GO, Sanders TS. Management of the internal carotid artery during carotid body tumor surgery. Laryngoscope. 1995;105(3 Pt 1):231–235 [DOI] [PubMed] [Google Scholar]
- 120. Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128(7):751–758 [DOI] [PubMed] [Google Scholar]
- 121. Kollert M, Minovi A, Mangold R, Hendus J, Draf W, Bockmühl U. [Paraganglioma of the head and neck–tumor control, functional results and quality of life]. Laryngorhinootologie. 2006;85(9):649–656 [DOI] [PubMed] [Google Scholar]
- 122. Hinerman RW, Mendenhall WM, Amdur RJ, Stringer SP, Antonelli PJ, Cassisi NJ. Definitive radiotherapy in the management of chemodectomas arising in the temporal bone, carotid body, and glomus vagale. Head Neck. 2001;23(5):363–371 [DOI] [PubMed] [Google Scholar]
- 123. Suárez C, Rodrigo JP, Bödeker CC, et al. Jugular and vagal paragangliomas: Systematic study of management with surgery and radiotherapy. Head Neck. 2013;35(8):1195–1204 [DOI] [PubMed] [Google Scholar]
- 124. Forest JA, 3rd, Jackson CG, McGrew BM. Long-term control of surgically treated glomus tympanicum tumors. Otol Neurotol. 2001;22(2):232–236 [DOI] [PubMed] [Google Scholar]
- 125. Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol. 2012;9(7):378–390 [DOI] [PubMed] [Google Scholar]
- 126. Schipper J, Spetzger U, Tatagiba M, et al. Juxtacondylar approach in temporal paraganglioma surgery: when and why? Skull Base. 2009;19(1):43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Gstoettner W, Matula C, Hamzavi J, Kornfehl J, Czerny C. Long-term results of different treatment modalities in 37 patients with glomus jugulare tumors. Eur Arch Otorhinolaryngol. 1999;256(7):351–355 [DOI] [PubMed] [Google Scholar]
- 128. Miller RB, Boon MS, Atkins JP, Lowry LD. Vagal paraganglioma: the Jefferson experience. Otolaryngol Head Neck Surg. 2000;122(4):482–487 [DOI] [PubMed] [Google Scholar]
- 129. Paridaans MP, van der Bogt KE, Jansen JC, et al. Results from craniocaudal carotid body tumor resection: should it be the standard surgical approach? Eur J Vasc Endovasc Surg. 2013;46(6):624–629 [DOI] [PubMed] [Google Scholar]
- 130. Tasar M, Yetiser S. Glomus tumors: therapeutic role of selective embolization. J Craniofac Surg. 2004;15(3):497–505 [DOI] [PubMed] [Google Scholar]
- 131. Brenner DJ, Sachs RK, Peters LJ, Withers HR, Hall EJ. We forget at our peril the lessons built into the α/β model. Int J Radiat Oncol Biol Phys. 2012;82(4):1312–1314 [DOI] [PubMed] [Google Scholar]
- 132. Brenner DJ. Exploring two two-edged swords. Radiat Res. 2012;178(1):7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol. 2010;7(1):44–54 [DOI] [PubMed] [Google Scholar]
- 134. Sharp CD, Jawahar A, Warren AC, Elrod JW, Nanda A, Alexander JS. Gamma knife irradiation increases cerebral endothelial expression of intercellular adhesion molecule 1 and E-selectin. Neurosurgery. 2003;53(1):154–160; discussion 160–151 [DOI] [PubMed] [Google Scholar]
- 135. Peña LA, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53(5):615–621 [DOI] [PubMed] [Google Scholar]
- 136. Ch'ang HJ, Maj JG, Paris F, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11(5):484–490 [DOI] [PubMed] [Google Scholar]
- 137. Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89–91 [DOI] [PubMed] [Google Scholar]
- 138. Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293(5528):293–297 [DOI] [PubMed] [Google Scholar]
- 139. Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 2009;15(17):5379–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174(12):7516–7523 [DOI] [PubMed] [Google Scholar]
- 141. Suárez C, Rodrigo JP, Mendenhall WM, et al. Carotid body paragangliomas: a systematic study on management with surgery and radiotherapy. Eur Arch Otorhinolaryngol. 2014;271(1):23–34 [DOI] [PubMed] [Google Scholar]
- 142. Capatina C, Ntali G, Karavitaki N, Grossman AB. The management of head-and-neck paragangliomas. Endocr Relat Cancer. 2013;20(5):R291–R305 [DOI] [PubMed] [Google Scholar]
- 143. Glasscock ME, 3rd, Jackson CG, Johnson GD, Poe DS. Radiation therapy in chemodectoma treatment. Laryngoscope. 1988;98(4):465–466 [DOI] [PubMed] [Google Scholar]
- 144. Spector GJ, Compagno J, Perez CA, Maisel RH, Ogura JH. Glomus jugulare tumors: effects of radiotherapy. Cancer. 1975;35(5):1316–1321 [DOI] [PubMed] [Google Scholar]
- 145. Suit HD, Gallager HS. Intact Tumor Cells in Irradiated Tissue. Arch Pathol. 1964;78:648–651 [PubMed] [Google Scholar]
- 146. de Jong AL, Coker NJ, Jenkins HA, Goepfert H, Alford BR. Radiation therapy in the management of paragangliomas of the temporal bone. Am J Otol. 1995;16(3):283–289 [PubMed] [Google Scholar]
- 147. Al-Mefty O, Teixeira A. Complex tumors of the glomus jugulare: criteria, treatment, and outcome. J Neurosurg. 2002;97(6):1356–1366 [DOI] [PubMed] [Google Scholar]
- 148. Hinerman RW, Amdur RJ, Morris CG, Kirwan J, Mendenhall WM. Definitive radiotherapy in the management of paragangliomas arising in the head and neck: a 35-year experience. Head Neck. 2008;30(11):1431–1438 [DOI] [PubMed] [Google Scholar]
- 149. Lalwani AK, Jackler RK, Gutin PH. Lethal fibrosarcoma complicating radiation therapy for benign glomus jugulare tumor. Am J Otol. 1993;14(4):398–402 [PubMed] [Google Scholar]
- 150. Springate SC, Weichselbaum RR. Radiation or surgery for chemodectoma of the temporal bone: a review of local control and complications. Head Neck. 1990;12(4):303–307 [DOI] [PubMed] [Google Scholar]
- 151. Holt A, Van Gestel D, Arends MP, et al. Multi-institutional comparison of volumetric modulated arc therapy vs. intensity-modulated radiation therapy for head-and-neck cancer: a planning study. Radiat Oncol. 2013;8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lim CM, Clump DA, Heron DE, Ferris RL. Stereotactic body radiotherapy (SBRT) for primary and recurrent head and neck tumors. Oral Oncol. 2013;49(5):401–406 [DOI] [PubMed] [Google Scholar]
- 153. Onal C, Yuksel O, Topkan E, Pehlivan B. Bilateral glomus tumor treated with PET-CT based conformal radiotherapy: a case report. Cases J. 2009;2:8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Wahl RL, Herman JM, Ford E. The promise and pitfalls of positron emission tomography and single-photon emission computed tomography molecular imaging-guided radiation therapy. Semin Radiat Oncol. 2011;21(2):88–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Liscák R, Simonová G, Vymazal J, Janousková L, Vladyka V. Gamma knife radiosurgery of meningiomas in the cavernous sinus region. Acta Neurochir (Wien). 1999;141(5):473–480 [DOI] [PubMed] [Google Scholar]
- 156. Bianchi LC, Marchetti M, Brait L, et al. Paragangliomas of head and neck: a treatment option with CyberKnife radiosurgery. Neurol Sci. 2009;30(6):479–485 [DOI] [PubMed] [Google Scholar]
- 157. Gerszten PC, Ozhasoglu C, Burton SA, et al. CyberKnife frameless single-fraction stereotactic radiosurgery for benign tumors of the spine. Neurosurg Focus. 2003;14(5):e16. [DOI] [PubMed] [Google Scholar]
- 158. Martiniova L, Perera SM, Brouwers FM, et al. Increased uptake of [123I]meta-iodobenzylguanidine, [1F]fluorodopamine, and [3H]norepinephrine in mouse pheochromocytoma cells and tumors after treatment with the histone deacetylase inhibitors. Endocr Relat Cancer. 2011;18(1):143–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lim M, Bower R, Nangiana JS, Adler JR, Chang SD. Radiosurgery for glomus jugulare tumors. Technol Cancer Res Treat. 2007;6(5):419–423 [DOI] [PubMed] [Google Scholar]
- 160. Wegner RE, Rodriguez KD, Heron DE, Hirsch BE, Ferris RL, Burton SA. Linac-based stereotactic body radiation therapy for treatment of glomus jugulare tumors. Radiother Oncol. 2010;97(3):395–398 [DOI] [PubMed] [Google Scholar]
- 161. Henzel M, Hamm K, Gross MW, et al. Fractionated stereotactic radiotherapy of glomus jugulare tumors. Local control, toxicity, symptomatology, and quality of life. Strahlenther Onkol. 2007;183(10):557–562 [DOI] [PubMed] [Google Scholar]
- 162. Poznanovic SA, Cass SP, Kavanagh BD. Short-term tumor control and acute toxicity after stereotactic radiosurgery for glomus jugulare tumors. Otolaryngol Head Neck Surg. 2006;134(3):437–442 [DOI] [PubMed] [Google Scholar]
- 163. Muracciole X, Régis J. Radiosurgery and carcinogenesis risk. Prog Neurol Surg. 2008;21:207–213 [DOI] [PubMed] [Google Scholar]
- 164. Guss ZD, Batra S, Limb CJ, et al. Radiosurgery of glomus jugulare tumors: a meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81(4):e497–e502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Hayashi M, Chernov MF, Lipski SM, et al. Do we really still need an open surgery for treatment of patients with vestibular schwannomas? Acta Neurochir Suppl. 2013;116:25–36 [DOI] [PubMed] [Google Scholar]
- 166. Sobol SM, Dailey JC. Familial multiple cervical paragangliomas: report of a kindred and review of the literature. Otolaryngol Head Neck Surg. 1990;102(4):382–390 [DOI] [PubMed] [Google Scholar]
- 167. Velegrakis G, Kalikakis G, Karampekios S, Stefanaki K, Helidonis E. Bilateral paraganglioma of the vagus nerve [in German]. HNO. 2001;49(6):471–475 [DOI] [PubMed] [Google Scholar]
- 168. Boedeker CC, Hensen EF, Neumann HP, et al. Genetics of hereditary head and neck paragangliomas. Head Neck. 2014;36(6):907–916 [DOI] [PubMed] [Google Scholar]
- 169. Rattenberry E, Vialard L, Yeung A, et al. A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98(7):E1248–E1256 [DOI] [PubMed] [Google Scholar]
- 170. Hescot S, Leboulleux S, Amar L, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98(10):4006–4012 [DOI] [PubMed] [Google Scholar]
- 171. Cascón A, Tennant DA. From transcriptional profiling to tumor biology in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23(1):15–20 [DOI] [PubMed] [Google Scholar]
- 172. Jimenez C, Rohren E, Habra MA, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep. 2013;15(4):356–371 [DOI] [PubMed] [Google Scholar]
- 173. Zovato S, Kumanova A, Demattè S, et al. Peptide receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE in individuals with neck or mediastinal paraganglioma (PGL). Horm Metab Res. 2012;44(5):411–414 [DOI] [PubMed] [Google Scholar]
- 174. Argiris A, Mellott A, Spies S. PET scan assessment of chemotherapy response in metastatic paraganglioma. Am J Clin Oncol. 2003;26(6):563–566 [DOI] [PubMed] [Google Scholar]
- 175. Nakazawa A, Higuchi T, Oriuchi N, Arisaka Y, Endo K. Clinical significance of 2-[18F]fluoro-2-deoxy-d-glucose positron emission tomography for the assessment of 131I-metaiodobenzylguanidine therapy in malignant phaeochromocytoma. Eur J Nucl Med Mol Imaging. 2011;38(10):1869–1875 [DOI] [PubMed] [Google Scholar]
- 176. Truman JP, Garcia-Barros M, Kaag M, et al. Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS One. 2010;5(8):e12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lee JI, Itasaka S, Kim JT, Nam DH. Antiangiogenic agent, thalidomide increases the antitumor effect of single high dose irradiation (gamma knife radiosurgery) in the rat orthotopic glioma model. Oncol Rep. 2006;15(5):1163–1168 [PubMed] [Google Scholar]
- 178. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186 [DOI] [PubMed] [Google Scholar]
- 180. Jain RK. A new target for tumor therapy. N Engl J Med. 2009;360(25):2669–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Richter S, Peitzsch M, Rapizzi E, et al. Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab. [July 11, 2014] doi.org/10.1210/jc.2014–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71(2):484–490 [DOI] [PubMed] [Google Scholar]