Abstract
The mechanisms of diabetic painful neuropathy are complicated and comprise of peripheral and central pathophysiological phenomena. A number of proinflammatory cytokines are involved in this process. Tumor necrosis factor α (TNF-α) is considered to be one of the major contributors of neuropathic pain. In order to explore the potential role of inflammation in the peripheral nervous system of Type 1 diabetic animals with painful neuropathy, we investigated whether TNF-α is a key inflammatory mediator to the diabetic neuropathic pain and whether continuous delivery of TNFα soluble receptor from damaged axons achieved by HSV vector mediated transduction of DRG would block or alter the pain perception in animals with diabetic neuropathy. Diabetic animals exhibited changes in threshold of mechanical and thermal pain perception compared to control rats and also demonstrated increases in TNFα in the DRG, spinal cord dorsal horn, sciatic nerve and in the foot skin, 6 weeks after the onset of diabetes. Therapeutic approaches by HSV mediated expression of p55 TNF soluble receptor significantly attenuated the diabetes-induced hyperalgesia and decreased the expression of TNFα with reduction in the phosphorylation of p38MAPK in the spinal cord dorsal horn and DRG. The overall outcome of this study suggests that neuroinflammatory activation in the peripheral nervous system may be involved in the pathogenesis of painful neuropathy in Type 1 diabetes which can be alleviated by local expression of HSV vector expressing p55 TNF soluble receptor.
Keywords: Diabetic neuropathy, pain behavior, TNF-α, DRG, gene therapy, inflammation
1. Introduction
Painful neuropathy is a common, difficult to treat complication of diabetes. The pathogenesis of diabetic neuropathy is complex and involves multiple pathways. Lack of success in preventing neuropathy even with successful treatment of hyperglycemia suggests the presence of early mediators between hyperglycemia-induced metabolic and enzymatic changes in functional and structural properties of the nervous system. Metabolic changes induced by hyperglycemia lead to dysregulation of cytokines which can cause pain-related symptoms (Skundric and Lisak, 2003). Approximately 20–24% of diabetes patients experience neuropathic pain (Schmader, 2002). Increasing evidence shows that inflammatory cytokines may participate in the pathogenesis of insulin resistance and its complications (Fernandez-Real and Ricart, 2003). Subjects with Type 1 diabetic (T1D) neuropathy have shown an increase in plasma concentrations of inflammatory markers that are involved in insulin resistance (Goldberg, 2009; Gonzalez-Clemente et al., 2007). Increased level of tumor necrosis factor alpha (TNFα) is often demonstrated in patients with diabetic neuropathy, regardless of their glycemic control and cardiovascular risk factors that are associated with insulin resistance (Averill and Bornfeldt, 2009; King, 2008). Patients with painful neuropathy have shown increased IL-2 and TNFα mRNA and protein levels in blood (King, 2008). Studies have shown that patients with painful diabetic neuropathy (PDN) exhibit a different serum immune profile compared to patients with painless diabetic neuropathy which suggests that immune markers in blood are associated with diabetic neuropathic pain (Doupis et al., 2009; Uceyler et al., 2007). Results from these studies suggest that TNFα may play a pathogenic role in the development of diabetic neuropathy (Gonzalez-Clemente et al., 2005). Majority of these studies have been done in Type 2 diabetic subjects using serum immune profiles. It is unclear whether similar mechanisms exist in Type 1 diabetes. The relationship of these serum inflammatory markers to nociceptive pathways in the nervous system in T1D animals has not been explored. We undertook these studies to understand whether TNFα, a major contributor in other forms of neuropathic pain, plays a role in this process and that whether increases in TNFα in the peripheral nervous system of T1D animals are responsible for painful neuropathy. TNFα is a pleiotropic cytokine which mediates the inflammatory response and activation of the immune system (Marchand et al., 2005). The effects of TNFα are mediated by 2 specific cell surface receptors, 55-kD TNF receptor I and 75-kD TNF receptor II. The extracellular domains of these receptors are released by proteolytic cleavage to form soluble TNF (sTNF) receptor I and II (Horiuchi et al., 2010). Soluble TNF receptors act as TNF antagonists and inhibit TNFα-mediated proinflammatory effects.
A broad range of preclinical animal studies have shown that systemic delivery of neurotransmitters or anti-inflammatory agents could effectively prevent painful neuropathy. Unfortunately, administration of these agents in patients has resulted in a number of systemic side effects. Currently available treatments to effectively treat or prevent the progression of painful neuropathy are very limited. Earlier studies have shown that pretreatment with intraperitoneal administration of etanercept, a commercially available soluble human 75-kD TNF receptor, reduces mechanical allodynia in rats with the spinal nerve ligation or spinal cord hemi section model of peripheral neuropathic pain (Marchand et al., 2009; Schafers et al., 2003). In diabetic mice, both systemic or intrathecal administration of etanercept produced only a dose dependent reversal of tactile allodynia but not thermal hyperalgesia (Dogrul et al., 2011). This suggests that a better therapeutic approach is needed. To avoid systemic side effects, local expression of anti-inflammatory agent may overcome this consequence. Our study demonstrates that subcutaneous injection of replication defective HSV based vectors in the footpad to deliver and express the transgenes in primary sensory neurons of the DRG may prevent the progression of this condition. Previous studies have shown that footpad inoculation of HSV vector expressing p55TNF soluble receptor one week before nerve injury reduced mechanical allodynia and thermal hyperalgesia, and also resulted in a reduction of TNFα and concomitant reductions in interleukin (IL)-1α and phosphorylated p38 MAP kinase as well (Hao et al., 2007). Another spinal cord injury model of peripheral neuropathic pain involving footpad inoculation of HSV vector expressing p55TNF soluble receptor one week after injury showed alleviation of mechanical allodynia (Peng et al., 2006). In this study we investigated that continuous delivery of TNFα soluble receptor from damaged axons achieved by HSV vector mediated transduction of DRG in animals with Type 1 diabetes, blocks the nociceptive and stress responses in the DRG and spinal dorsal horn neurons by reducing the TNFα expression and also decreasing the downstream signaling molecule p38MAPK activation with improved pain perception. Additionally we found that the increases in TNFα in sciatic nerve and in the foot skin are also reduced in animals treated with the therapeutic vector in conjunction with the reduction of pain. Taken together these studies suggest that neuroinflammatory activation in the peripheral nervous system may be involved in the pathogenesis of painful neuropathy in Type 1 diabetes.
2. Materials and Methods
2.1. Animal studies: induction of diabetes and vector inoculation
Experiments were performed on male Sprague Dawley rats weighing 220–250 gms (Charles River, Portage, MI, USA) in compliance with approved institutional animal care and use protocols (IACUC, VAAAHS). Animals were rendered diabetic by IP injection of streptozotocin (STZ; 50 mg/kg). Three days after STZ injections, blood glucose levels were measured using OneTouch Ultra glucose meter (LifeScan, Inc; USA). Rats with greater than 300 mg/dl blood glucose level were included in the study (Table 1). Two weeks after the onset of diabetes, the T1D animals were inoculated with either HSV vector expressing p55 TNFα soluble receptor (vTNFsR) or control vector expressing lacZ (vZ) subcutaneously into the plantar surface of both hind paw (30 µl of 1 × 109 pfu/ml). Animals were grouped into four categories: control, diabetic alone, diabetic inoculated with vTNFsR (dia+vTNFsR) and diabetic inoculated with vZ (dia+vZ). All behavioral analyses were carried out by an observer blinded to the treatment group 4 weeks after vector inoculation (at 6 weeks of diabetes). A group of diabetic animals were evaluated at 2 weeks after hyperglycemia to assess the pain related behaviors at the time of vector treatment. A separate group of rats were injected in a similar fashion with vTNFsR and euthanized 7 days later for the studies of transgene expression in vivo.
Table 1.
Changes in metabolic parameters before and after the onset of diabetes.
| Pre-diabetic | 6 weeks post-diabetic | |||
|---|---|---|---|---|
| Weight (gm) | Glucose (mg/dL) |
Weight (gm) | Glucose (mg/dL) | |
| Control | 247.5 ± 9.1 | 114.3 ± 6.3 | 304 ± 10.2 *** | 121.5 ± 11.4 |
| STZ-Diabetic | 251.1 ± 6.5 | 118 ± 5.4 | 299.3 ± 9.2 *** | 401.8 ± 50.8 # |
Control and diabetic animals gained weight significantly (***P < 0.001) 6 weeks after the onset of diabetes compared to their pre-diabetic states. Animals treated with STZ have increased blood glucose level compared to the control animals (# P < 0.005) as measured 6 weeks post-diabetes.
2.2. Behavioral studies
Thermal hyperalgesia
The latency to hind paw withdrawal from a thermal stimulus was determined by exposing the plantar surface of the hind paw to radiant heat using a modified Hargreaves (Hargreaves et al., 1988) thermal testing device. Rats were placed in individual enclosures on a glass plate maintained at 30°C, and after a 30 min habituation period the plantar surface of the paw was exposed to a beam of radiant heat applied through the glass floor. Activation of the bulb simultaneously activated a timer, and both were immediately turned off by paw withdrawal or at the 20 sec cut-off time. Testing was performed by a blinded observer in triplicate at 5 min intervals.
Mechanical hyperalgesia
Mechanical nociceptive threshold was assessed using an analgesimeter (Ugo Basile, Comerio, VA, Italy) as described by Randall and Sellito (Randall and Selitto, 1957). A linearly increasing pressure was applied through a cone-shaped plastic tip with a diameter of 1 mm onto the dorsal surface of the hindpaw. The tip was positioned between the third and fourth metatarsus, and force applied until the rat attempted to withdraw its paw (paw withdrawal threshold to pressure). The pain threshold determined as the mean of three consecutive stable values expressed in grams was determined by a blinded observer.
2.3. Cell Culture experiment
DRG from adult rats were dissociated with 0.25% trypsin, 1mM EDTA for 30 minutes at 37°C with constant shaking and then plated on poly-D-lysine-coated coverslips (105 cells per well in a 24-well plate) in 500 µl of defined Neurobasal media containing B27, Glutamax I, Albumax II, and penicillin/streptomycin (Gibco-BRL, Carlsbad, CA), supplemented with 100 ng/ml of 7.0S NGF per ml (Sigma, St Louis, MO). 5-Fluoro-2'-deoxyuridine (5 µM; Sigma) and uridine (5 µM; Sigma) were added on days 1 and 3 to inhibit the growth of dividing cells. After 3 days in vitro, cells were exposed to hyperglycemic condition overnight; the medium was changed to 20 mM glucose (in addition to the basal 25 mM glucose) with Glutamax I, Albumax II, penicillin/streptomycin, but without B27 and NGF (cells are viable without NGF at this point). Control cells were exposed to an identical medium without B27 and NGF but with basal 25 mM glucose. For the TNFα experiment, cells were exposed to 15 ng/ml of TNFα overnight. All in vitro experiments were terminated after 16 hrs of exposure; cells were collected by cell lysis buffer and Western blot was performed.
2.4. Western blot
Cells from pooled DRG neurons in culture (3 wells per sample) or pooled samples of L4-6 DRG from each rat were homogenized with lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol and 1:100 dilution of Protease Inhibitor Cocktail, Phosphatase Inhibitor Cocktail (Sigma, St. Louis, MO). The homogenized tissues were centrifuged at 10,000 × g for 10 min at 4° C and the supernatant was stored at −80° C. An aliquot of supernatant was taken for protein estimation using a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Protein from DRG (20 µg protein per lane) was separated by PAGE, transferred to an Immobilon-P membrane (0.45 µm, Millipore, Bedford, MA), blocked with 5% nonfat milk, then incubated with the primary antibody (TNFα 1:400, Millipore, Bedford, MA; pp38 1:400, Santa Cruz Biotechnology, Santa Cruz, CA), followed by horseradish peroxidase-conjugated anti-rabbit IgG or anti-mouse IgG (1:5,000 Amersham, Piscataway, NJ) and visualized with ECL (Pierce, Rockford, IL). The membranes were reprobed with either β-actin (1:2000; Sigma) or p38 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA) as a loading control. The intensity of each band was determined by quantitative chemiluminescence using a PC-based image analysis system (ChemiDoc XRS System, Bio-Rad Laboratories) and normalized to the respective level of β-actin. Data are expressed as mean SEM (n = 5 animals per group).
2.5. qRT-PCR
Total RNA was isolated from L4–L6 DRG, spinal cord dorsal horn and sciatic nerve using a commercial kit (Qiagen, Valencia, CA, USA). cDNA was transcribed from the mRNA at 37°C using a Sensiscript Reverse Transcriptase kit (Qiagen, Valencia, CA, USA), and qRT-PCR was conducted using specific primers for Real time qPCR designed with NCBI primer-BLAST. The following PCR primers were used: for TNF (F: CGCCATCAAGAGCCCTTGCCC; R: GCAGGTCCCCCTTCTCCAGCT), for β-actin (F: AGATGGCCACTGCCGCATCC; R: AGAGCCTCGGGGCATCGGAA); Real time qRT-PCR for each sample was run in quadruplicate in a 20 µl reaction with SYBR Green Mastermix (Bio-Rad) using Mastercycler system (Eppendorf). The amplification protocol is as follows: 95°C for 3 min, followed by 40 cycles of 95°C for 10s for denaturation, 58°C for 45s for annealing and extension. Each Ct (cycle threshold) value was represented as relative mRNA level. All data was normalized to β-actin.
2.6. Immunohistochemistry
Rats were perfused transcardially with 0.9% NaCl followed by Zamboni’s fixative (Verdu et al., 1999). The DRG were removed, post-fixed with Zamboni’s fixative for 2 h, and then cryoprotected with 30% sucrose in phosphate buffered saline (PBS) overnight, cryostat sectioned at 10 µm and collected on gelatin-coated slides. Tissue sections were washed with PBS, incubated with blocking solution (PBS with 1% normal goat serum and 0.3% Triton X-100) for 1 h, then washed once before being incubated with the primary antibody anti-TNFα (1:400; Chemicon, Temecula, CA, USA), s100β (1:500); pp38 (1:500) and GFAP (1:1000; Santa Cruz Biotechnogy, Santa Cruz, CA) for 2 h at room temperature and washed three times. After incubation in the secondary fluorescent antibody, Alexa Fluor 594 goat anti-rabbit IgG (1:1000, Molecular Probes, Eugene, OR), for 1 h at room temperature, the specimens were washed 3 times and mounted in water-based Fluoromount G (Electron Microscopy Sciences, Fort Washington, PA).
2.7. Morphometric analysis
Digitized images of immunostained sections were captured with a Nikon E1000 microscope, and analysed using the MetaMorph computer-based image analysis system (Universal Imaging Corporation, Downingtown, PA, USA). The intensity of the immunostained cell bodies in DRG and spinal cord as well as peripheral nerve terminals in the subcutaneous tissue of the foot skin was determined using the image analysis software. Three cross-sections of the tissues from each animal were evaluated by an investigator blinded to the treatment group (data not shown).
2.8. Statistical analysis
The statistical significance of the difference between groups was determined by ANOVA (Systat 11) using Bonferroni’s correction for the multiple post hoc analyses. All results are expressed as mean ± SEM. The animal behavior experiments, with 8–10 animals per group, were repeated twice.
3. Results
3.1. vTNFsR expresses p55 TNF soluble receptor in vivo and reduces TNFα in DRG of diabetic animals
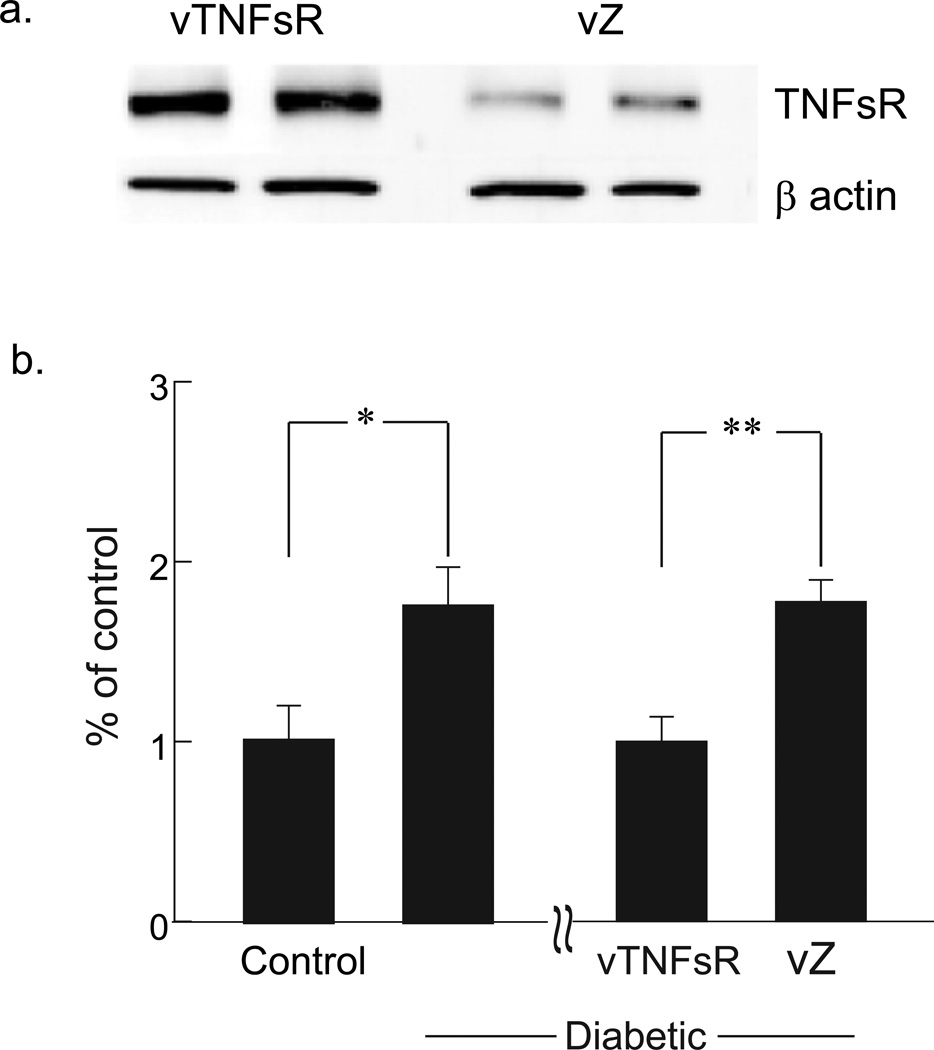
Rats inoculated with 3 × 107 pfu of vTNFsR subcutaneously in the footpad were euthanized after 7 days to examine the expression of p55 TNF soluble receptor in DRG. Western blot analysis of DRG showed increased expression of the receptor in vTNFsR vector inoculated animals compared to control vector vZ treated animals 7 days (Fig. 1a) and 4 weeks after the treatment (data not shown) confirming in vivo transgene expression of p55 TNF soluble receptor by the vector. Diabetic animals with painful neuropathy displayed a significant increase in TNFα mRNA in DRG 6 weeks after onset of diabetes compared to control animals as measured by real time PCR (Fig. 1b). Two weeks post-diabetic animals inoculated with vTNFsR showed a substantial and significant reduction in the amount of TNFα mRNA in DRG compared to animals inoculated with control vector vZ as measured 4 weeks after vector inoculation (Fig. 1b) by qRT-PCR.
Fig. 1. HSV-mediated increased expression of p55 TNF soluble receptor in DRG prevents the increase in TNFα mRNA expression in diabetic animals.
(a) Footpad inoculation of vTNFsR demonstrates an increased expression of p55 TNF soluble receptor in DRG after 7 days of administration compared to control vector treated DRG. (b) Significant increase in TNFα mRNA expression in DRG of diabetic animals with PDN (*P < 0.05; compared to control animals) is prevented in animals treated with vTNFsR vector (**P < 0.01; compared to vZ) 4 weeks after vector administration.
3.2. Subcutaneous inoculation of vTNFsR reduces thermal and mechanical hyperalgesia
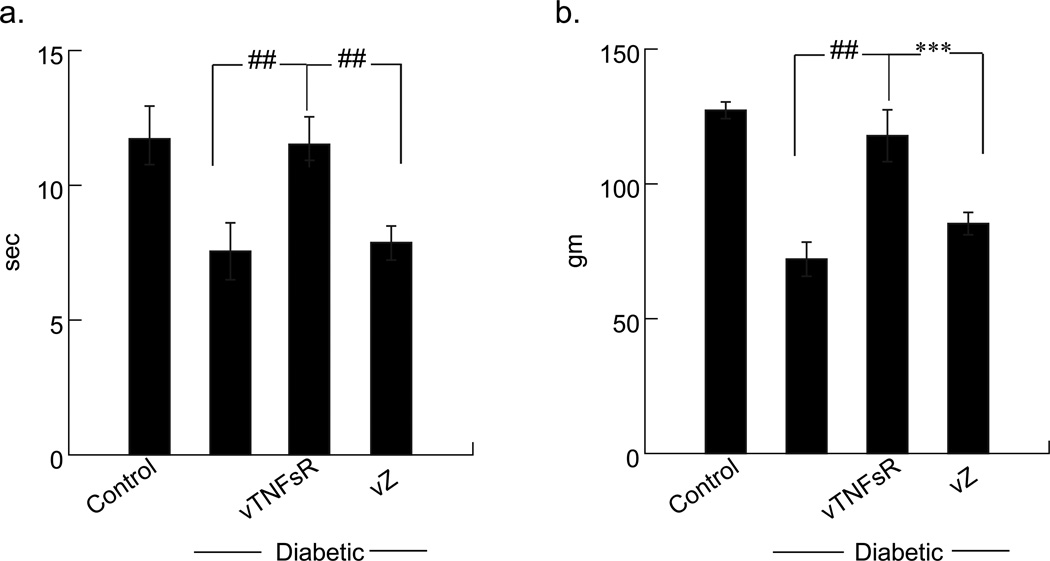
Diabetic animals were more sensitive to noxious thermal stimuli as manifested by a decrease in withdrawal latency (control 13.5 ± 0.3 sec; diabetic 8.82 ± 0.4 sec) compared to the control animals (##P < 0.0001) six weeks after the onset of diabetes. This deficit in thermal response latency was significantly restored in animals inoculated with vTNFsR. The thermal pain thresholds of animals inoculated with vTNFsR group was substantially improved (vTNFsR - 12.9 ± 1.2 sec; ##P < 0.0001) compared to diabetic alone or diabetic animals with vZ vectors (Fig. 2a). Diabetic animals tested for thermal hyperalgesia at 2 weeks after the onset of diabetes did not show any changes in thermal pain threshold, but exhibited a significant change in the mechanical pain threshold (data not shown). A significant reduction in paw withdrawal threshold by Randall Selitto test of mechanical hyperalgesia was detected in diabetic animals 6 weeks after the onset of diabetes compared to normal control animals (control 143 ± 8.1 gm; diabetic 62.7 ± 3.2 gm; ##P < 0.0001). Inoculation of vTNFsR significantly improved hind-paw withdrawal threshold (vTNFsR 141.8 ± 3.1 gm) compared to vZ-diabetic animals (75.5 ± 3.6 gm; ***P < 0.001) measured 6 weeks of diabetes and 4 weeks after vector inoculation (Fig. 2b). There were no difference between the diabetic alone and vZ vector treated group in both the behavioral tests.
Fig. 2. HSV-mediated expression of p55 TNF soluble receptor prevents the pain-related behaviors in diabetic animals 4 weeks after treatment.
(a) Diabetic animals demonstrate thermal hyperalgesia as manifested by a decrease in withdrawal latency to noxious thermal stimuli compared to the control animals (##P < 0.0001) six weeks after the onset of diabetes. Animals treated with vTNFsR show attenuation of thermal hyperalgesia compared to control vector treated animals. (b) Diabetic animals exhibit reduction in paw withdrawal threshold from mechanical pain stimuli 6 weeks after the onset of diabetes compared to normal control animals (##P < 0.0001). Animals treated with vTNFsR exhibit significantly improved hind-paw withdrawal threshold from mechanical pain compared to control vector vZ treated animals (***P < 0.001) All data are presented as mean ± SEM, n = 8–10 per group.
3.3. Vector mediated expression of p55TNF soluble receptor reduces TNFα and phospho-p38 MAPK in DRG in vivo
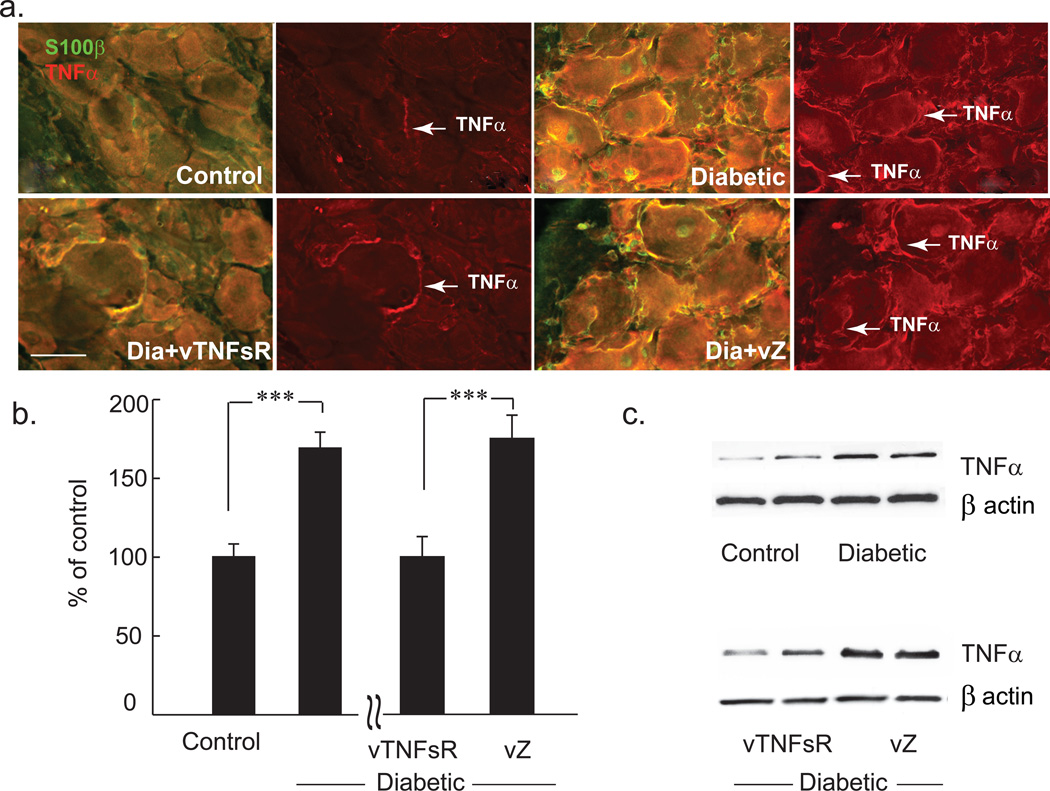
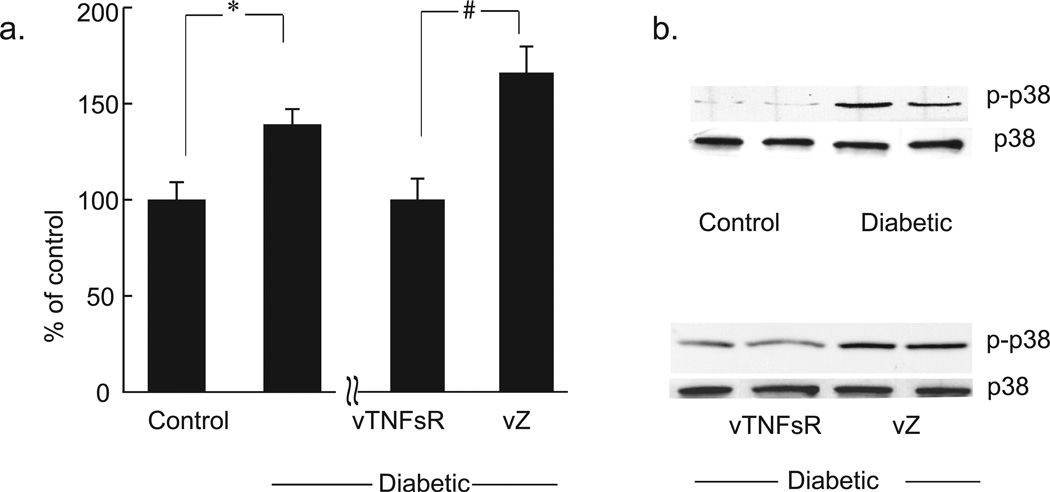
Diabetic animals with painful neuropathy displayed a significant increase in TNFα protein in DRG 6 weeks after onset of diabetes compared to control animals as measured by immunohistochemistry (Fig. 3a) and Western blot analysis (Fig. 3b–c). Animals inoculated with vTNFsR 2 weeks post-diabetes showed a substantial and significant reduction in the amount of TNFα in DRG at 4 weeks after vector inoculation compared to animals inoculated with vZ (Fig. 3b–c) by Western blot analysis. Immunohistochemistry of DRG of diabetic animals exhibited a significant increase in TNFα surrounding the DRG neurons and in the satellite cells. Treatment with vector vTNFsR showed a substantial decrease in TNFα in DRG (Fig 3a). Diabetic animals with painful neuropathy exhibited a significant increase in phospho-p38 MAPK protein in the DRG at 6 weeks after onset of diabetes compared to control animals, and showed a substantial reduction in the amount of pp38 in DRG at 4 weeks after vector inoculation compared to animals inoculated with vZ (Fig. 4a, b) as measured by Western blot.
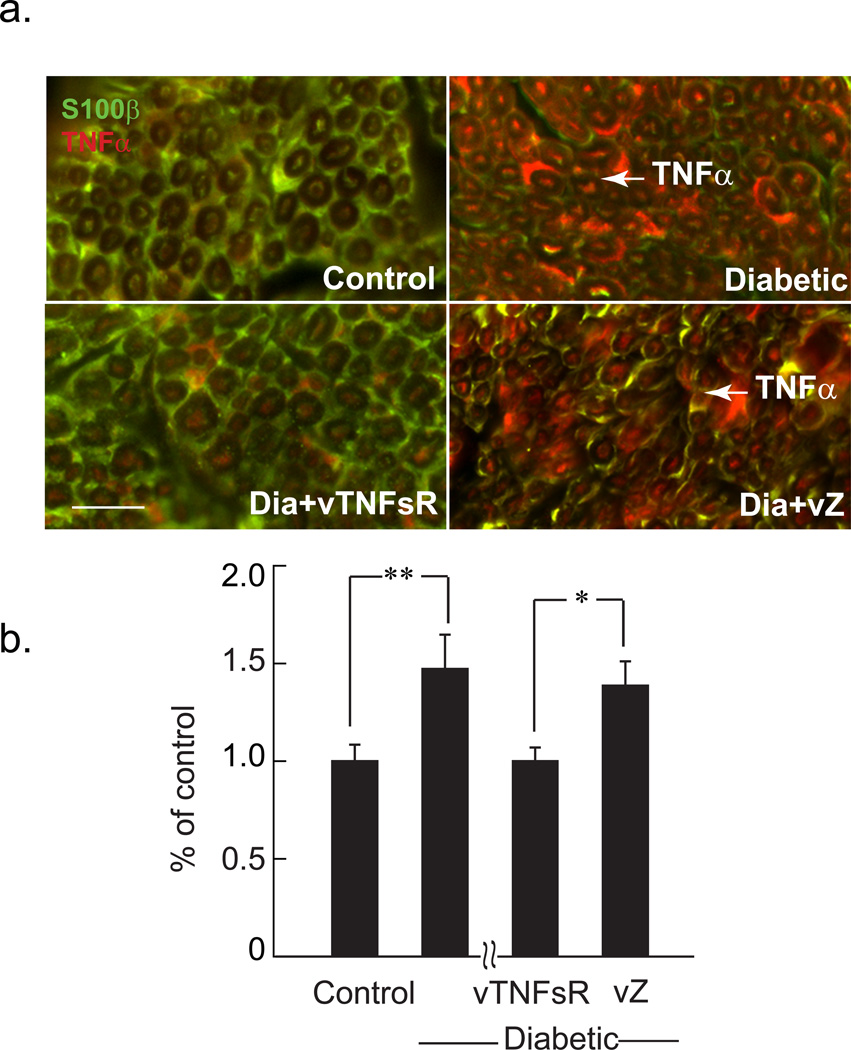
Fig. 3. Decreases in expression of TNFα in DRG of diabetic animals treated with vTNFsR.
Double-label immunostaining of TNFα (red) and s100β (green) reveals increased expression of TNFα in DRG of diabetic animals. Transduction of DRG with HSV vector expressing p55 TNF soluble receptor reduces TNFα expression in DRG of diabetic animals 4 weeks after inoculation as shown by immunohistochemistry (Bar = 50 µm) (a). Graph demonstrates the Western blot analysis of TNFα in the DRG of animals; *** P < 0.001; n = 5 animals per group. (b). Representative Western blots are showing two independent samples randomly chosen from the blot from each group (c).
Fig. 4. Treatment of vTNFsR reduces phosphorylation of p38MAPK protein in DRG of diabetic animals.
(a). Western blot analysis of phospho-p38 of DRG reveals that diabetic animals have increased phosphorylation of p38 compared to control animals (* P < 0.05; n=5). HSV-mediated expression of p55 TNF soluble receptor inhibits the phosphorylation of p-38 MAPK in DRG of the vTNFSR treated diabetic rats compared to those treated with control vector (# P < 0.005; n=5) by Western blot analysis. (b). Representative Western blots of DRG are showing random selection of two independent samples from each group.
3.4. Increased expression of TNFα in Schwann cells of the sciatic nerve in rats with PDN
We found that diabetic animals with painful neuropathy exhibited an increase in TNFα in the Schwann cells surrounding the sciatic nerve as shown in the cross section of the nerve in the immunohistochemical illustration (Fig. 5a), 6 weeks after onset of diabetes compared to control animals. Animals inoculated with vTNFsR 2 weeks after induction of diabetes by STZ showed a substantial and significant reduction in the amount of TNFα in Schwann cells of the sciatic nerve 4 weeks after vector inoculation compared to animals inoculated with vZ (Fig. 5a). Diabetic animals also exhibited a substantial increase in TNFα mRNA in the sciatic nerve at 6 weeks after onset of diabetes. Animals treated therapeutic vector showed a substantial reduction in TNFα mRNA compared to animals inoculated with vZ (Fig. 5b) as measured by real time PCR.
Fig. 5. vTNFsR treated animals demonstrate reduction in expression of TNFα in sciatic nerve 4 weeks after inoculation.
(a) Immunohistochemistry of nerve cross sections of the diabetic animals show a significant increase in TNFα (in red; shown in arrow) surrounding the schwann cells (s100β in green) 6 weeks after diabetes. Treatment with vector vTNFsR shows a substantial decrease in TNFα in schwann cells surrounding the axons of the treated animals (Bar = 50 µm). (b) Quantitative real time PCR analysis of the sciatic nerve of diabetic animals show increased TNFα mRNA expression compared to control animals (** P < 0.01, n = 5 per group) whereas 4 weeks of vector mediated expression of p55 TNF soluble receptor decreases the TNFα mRNA expression in sciatic nerve of vector treated animals compared to control vector treated group (* P < 0.05, n = 5 per group).
3.5. Amelioration of the increases in TNFα, GFAP and phospho-p38MAPK expression in diabetic spinal cord by vTNFsR vector treatment
Diabetic animals with painful neuropathy exhibited a significant increase in TNFα mRNA in the spinal cord dorsal horn at 6 weeks after onset of diabetes compared to control animals as measured by real time PCR (Fig. 6a). Immunohistochemical studies of the dorsal horn of the spinal cord of diabetic animals revealed significant increase of phosphorylated p38 MAPK expression. In other models, neuropathic pain is accompanied by astrogliosis in the dorsal horn of spinal cord. The immunohistochemistry of dorsal horn of spinal cord of diabetic animals showed substantial activation of astrocytes with increased GFAP (glial fibrillary acidic protein) immunoreactivity in animals with painful diabetic neuropathy. Animals treated with vTNFsR showed significant reduction in spinal GFAP immunoreactivity compared to vZ diabetic group (Fig. 6b). Similarly, diabetic rats inoculated with vTNFsR showed a reduction in phosphorylated p38 MAPK expression in the dorsal horn of the spinal cord compared to the animals inoculated with vZ (Fig. 6b).
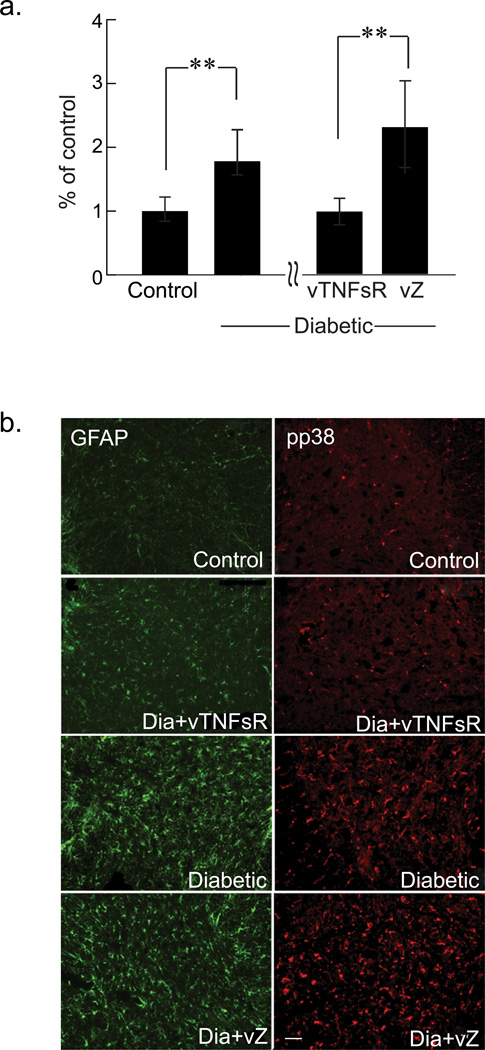
Fig. 6. Vector treatment ameliorates the levels of the markers of inflammation in the spinal cord of diabetic animals.
(a) Quantitative real time PCR analysis shows an increase in the amount of TNFα mRNA (corrected for β-actin) in the dorsal horn of the spinal cord of the diabetic animals compared to control animals (** P < 0.01, n = 5 per group). vTNFsR vector treated animals show decreased the TNFα mRNA expression in the spinal cord dorsal horn compared to vZ control vector treated animals (** P < 0.01, n = 5 per group). (b) GFAP and phospho-p38 immunohistochemistry of spinal cord dorsal horn reveals that diabetic animals have increased activation of astrocytes as well as increased phosphorylation of p38 compared to control animals. HSV-mediated expression of p55 TNF soluble receptor inhibits astrocytic activation and prevents the phosphorylation of p38 MAPK in dorsal horn of spinal cord of the diabetic rats (Bar = 20 µm).
3.6. Hyperglycemia increases TNFα in cells which may activate p38 MAPK signaling
In order to confirm whether hyperglycemia causes increases in inflammatory mediators, adult DRG neurons in culture were exposed to high glucose (20mM; overnight). The continuous exposure to high glucose caused a small increase of TNFα compared to control cells (Supplementary Fig. 1a, b) as quantified by Western blot analysis. To demonstrate the downstream signaling of TNFα and to determine whether TNFα can stimulate p38 MAPK signaling, the adult DRG neurons in culture were pre-incubated with 15 ng/ml of recombinant TNFα (rTNFα) for overnight. The cells exposed to rTNFα showed an increase in phosphorylation of p38 compared to control cells (Supplementary Fig. 1c, d).
3.7. Expression of p55TNF soluble receptor lowers the TNFα expression in the foot skin of vTNFsR vector inoculated group
From our studies, we found that there was a significant local increase of TNFα in foot skin of the diabetic animals. In contrast to generalized pain syndromes, where systemic cytokine levels were shown to differ from healthy controls, we only found moderate elevation of mRNA expression of TNFα in diabetic animals compared to control animals as measured by real time PCR (Fig. 7a). Immunohistochemical analysis of foot skin showed a substantial increase in TNFα in diabetic animals, whereas animals inoculated with vTNFsR 2 weeks after induction of diabetes by STZ showed a significant reduction in the amount of TNFα in the nerve fibers surrounding the sweat gland secretory cells, 4 weeks after vector inoculation compared to animals inoculated with vZ (Fig. 7b).
Fig. 7. HSV mediated expression of p55 TNF soluble receptor in DRG attenuates the increase in TNFα in the hind paw skin.
(a) STZ-diabetic animals show increased expression of TNFα mRNA compared to control animals (* P < 0.05, n = 5 per group). Vector mediated expression of p55TNF soluble receptor results in a decrease in the amount of TNFα mRNA in the hind paw skin of the diabetic animals compared to the control vector treated diabetic animals (* P < 0.05, n = 5 per group). (b) Double-label immunostaining of TNFα (red) and PGP 9.5 (green) of the paw skin demonstrates increased expression of TNFα in the nerve fibers as well as in the sweat gland of the diabetic animals compared to controls whereas vector treatment decreased the expression (Bar = 50 µm).
4. Discussion
Inflammation plays an important role in the development of Type 2 diabetes (Hotamisligil, 2006; Kaul et al., 2010; Roubicek et al., 2009). In this study we suggest that the activation of inflammatory cascade in the DRG, spinal dorsal horn, sciatic nerve and foot skin in combination or taken together contribute in the development of painful neuropathy in Type 1 model of diabetes. Several lines of evidence indicate that TNFα performs a key role in the development of chronic pain (Schafers et al., 2003; Zhang and Dougherty, 2013). Previous studies have shown that diabetic patients with painful neuropathy have increased inflammatory cytokines in serum (Uceyler et al., 2007) which may appear to play a pathogenic role in the development of diabetic neuropathy (Gonzalez-Clemente et al., 2005). This study determines the role of locally produced inflammatory mediato rTNFα in the DRG, spinal dorsal horn, sciatic nerve and foot skin of the rats with the development of painful diabetic neuropathy. This report also explores the possibility that potential pathogenic alterations of inflammatory mediators by local expression of p55TNF soluble receptor by gene transfer in the DRG can decrease the pain-related behaviors. In humans, TNFα has been shown to act as a pain mediator in neuropathic pain conditions, and nerve biopsies from patients with painful neuropathy show elevated levels of TNF-α expression, especially in Schwann cells (Calvo et al., 2012; Tanaka et al., 2002). Previous studies suggest that insulin therapy alone cannot suppress the production and/or activity of serum TNFα and does not inhibit the development of chronic diabetic complications in mice (Sharma et al., 2007). Metabolic changes induced by hyperglycemia leading to dysregulation of cytokine production causes pain-related symptoms (Skundric and Lisak, 2003). In addition, some inflammatory plasma markers (mainly related to TNFα) have been associated with the presence of diabetic retinopathy and nephropathy (Schram et al., 2005; Zoppini et al., 2001). Subjects with T1D neuropathy have increased level of TNFα in serum, despite their glycemic control and reduced cardiovascular risk factors (Averill and Bornfeldt, 2009; King, 2008). This suggests that TNFα may play a pathogenic role in the development of diabetic neuropathy (Gonzalez-Clemente et al., 2005). Studies on patients with type 2 diabetes with or without diabetic polyneuropathy exhibit a different immune profile. High levels of C-reactive protein (CRP) and IL-6, most consistently associated with diabetic polyneuropathy (Choudhary and Ahlawat, 2008; Hills and Brunskill, 2009) and other neuropathic deficits suggest that inflammation is associated with diabetic polyneuropathy and neuropathic impairments. Studies have shown that pharmacological inhibition of TNFα by etanercept, a TNFα antagonist, either by intravenous or intrathecal routes, produced dose dependent reversal of tactile allodynia but not thermal hyperalgesia in diabetic mice. Intraplantar routes were also ineffective (Dogrul et al., 2011). This study defines that early interruption of the neuroinflammatory cascade in the peripheral nervous system by intraplantar injection of HSV vector expressing anti-inflammatory mediator can prevent the development of painful neuropathy. Skin keratinocytes are major producers of pro-inflammatory cytokines including interleukin (IL)-1, -6, -8; TNF, and express a variety of cytokine receptors. The bidirectional communication between skin cells and the nervous system acts as a homeostatic unit to guarantee regulation during physiological and pathophysiological states (Hendrix and Peters, 2007). In human studies, it has been shown that diabetes induced prolonged expression of TNFα contributes to impaired healing (Xu et al., 2012). TNFα neutralization using systemically applied antibodies has been shown to have beneficial effects on impaired wound healing (Goren et al., 2007). This finding of a localized increase of proinflammatory cytokine in foot skin in conjunction with mild increases in the DRG along with pain related behaviors depicts that increases in the levels of TNFα in peripheral nervous system plays an important role in the development of pain. In diabetic animals, TNFα mRNA expression is increased in DRG, spinal cord dorsal horn, foot skin as well as in the sciatic nerve compared to control animals; whereas TNFα protein is substantially increased in DRG and foot skin, which may suggest that hyperglycemia causes an increase in inflammatory mediators in the peripheral nervous system, which may partly be responsible for the development of pain. We have also confirmed the above phenomenon from our cell culture experiment that hyperglycemia can cause upregulation of TNFα. In other injury models, astrogliosis in the dorsal horn of spinal cord is associated with neuropathic pain (McMahon et al., 2005). We found an increase in GFAP immunoreactivity in animals with painful diabetic neuropathy. Diabetic animals treated with vTNFsR showed significant reduction in activation of spinal astrocytes compared to diabetic animals treated with control vector vZ. A strong association has also been established between inflammation and p38 MAPK pathway. The activation of the p38 pathway plays an important role in the production of proinflammatory cytokines, such as IL1β, TNFα and IL-6, (Westermann et al., 2006); whereas interleukins and TNF also stimulate p38 signaling (Lokuta and Huttenlocher, 2005; Saklatvala et al., 1996). In recent studies it has been reported that NGF activated-p38 phosphorylation mediates mechanical allodynia in the db/db mouse by upregulation of a number of inflammatory mediators in the lumbar DRG. Intrathecal delivery of SB203580, a p38 inhibitor, significantly inhibited the development of mechanical allodynia as well as prevented the upregulation of COX2, iNOS and TNFα (Cheng et al., 2010). Previous studies with HSV mediated local expression of p55sTNFR to DRG have shown a reduction of TNFα with concomitant reductions in IL1β and pp38 MAPK in the spinal nerve ligation as well as in partial spinal cord injury models (Hao et al., 2007; Peng et al., 2006). The current study shows that HSV-mediated expression of p55sTNFR in DRG prevents pain-related behaviors in T1D animals with diabetic neuropathy, reduces GFAP expression and decreases the phosphorylation of p38 MAPK in DRG and dorsal horn of spinal cord of the diabetic rats. Our cell culture study demonstrates that addition of TNFα in the medium can cause activation of p38 MAPK pathway, suggesting that TNF is acting through the p38 MAPK pathway, which in turn acts as an inducer of TNF expression, contributing to the development of chronic inflammatory conditions, and may partly be responsible for painful neuropathy in the T1D model. In these studies we have also found an increase in TNFα in sciatic nerve and in the foot skin which is greatly reduced in animals treated with vTNFsR. The overall studies of the DRG, nerve, spinal cord and foot skin taken together suggest that blocking TNF expression can alter the peripheral neuroimmune activation, which is important in the pathogenesis of pain in diabetic neuropathy. The results from the vector mediated expression of p55sTNFR in the DRG confirm that decrease in neuroinflammation in the peripheral nervous system may provide a better therapeutic approach by avoiding systemic side effects in the pathogenesis of pain in diabetes.
Supplementary Material
(a) Representative Western blots show a substantial increase of TNFα in cultured DRG neurons exposed to high glucose (20mM; overnight) compared to control cells; samples are chosen randomly from the blot. (b) Graph demonstrates the increase in TNFα as determined by Western blot, normalized to control (* P < 0.05, n = 3 samples per group). (c) Representative Western blots show pre-incubation of cultured DRG neurons with 15 ng/ml of rTNFα overnight, increases the phosphorylation of p38 MAPK compared to control cells (blots showing two randomly chosen independent samples from each group). (d) Western blot analysis of pp38 MAPK demonstrates the increases in phosphorylation of p38 MAPK in the rTNFα treated samples, normalized to control (* P < 0.05, n = 3 samples per group).
Acknowledgements
This work has funded by National Institute of Health grant NS38850 to Dr. David J. Fink and by American Diabetes Association (Grant #7-12-BS-021) to MC. We acknowledge Drs. David Fink and Marina Mata for the resources and Vikram Thakur for propagation of HSV vector.
Abbreviations
- PDN
painful diabetic neuropathy
- T1D
Type 1 Diabetes
- DRG
dorsal root ganglia
- IL-2
interleukin-2
- TNF
tumor necrosis factor
- TNFsR
Soluble TNF receptors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
KM carried out the behavior tests and biochemical assays. MC contributed to the design and analysis of the study, behavior test and wrote the manuscript.
Conflict of interests
The authors declare that they have no conflict of interests.
References
- Averill MM, Bornfeldt KE. Lipids versus glucose in inflammation and the pathogenesis of macrovascular disease in diabetes. Curr Diab Rep. 2009;9:18–25. doi: 10.1007/s11892-009-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo M, Dawes JM, Bennett DL. The role of the immune system in the generation of neuropathic pain. Lancet Neurol. 2012;11:629–642. doi: 10.1016/S1474-4422(12)70134-5. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Dauch JR, Oh SS, Hayes JM, Hong Y, Feldman EL. p38 mediates mechanical allodynia in a mouse model of type 2 diabetes. Mol Pain. 2010;6:28. doi: 10.1186/1744-8069-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary N, Ahlawat RS. Interleukin-6 and C-reactive protein in pathogenesis of diabetic nephropathy: new evidence linking inflammation, glycemic control, and microalbuminuria. Iran J Kidney Dis. 2008;2:72–79. [PubMed] [Google Scholar]
- Dogrul A, Gul H, Yesilyurt O, Ulas UH, Yildiz O. Systemic and spinal administration of etanercept, a tumor necrosis factor alpha inhibitor, blocks tactile allodynia in diabetic mice. Acta Diabetol. 2011;48:135–142. doi: 10.1007/s00592-010-0237-x. [DOI] [PubMed] [Google Scholar]
- Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24:278–301. doi: 10.1210/er.2002-0010. [DOI] [PubMed] [Google Scholar]
- Goldberg RB. Cytokine and Cytokine-like Inflammation Markers, Endothelial Dysfunction and Imbalanced Coagulation in Development of Diabetes and Its Complications. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2008-2534. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Clemente JM, Mauricio D, Richart C, Broch M, Caixas A, Megia A, Gimenez-Palop O, Simon I, Martinez-Riquelme A, Gimenez-Perez G, Vendrell J. Diabetic neuropathy is associated with activation of the TNF-alpha system in subjects with type 1 diabetes mellitus. Clin Endocrinol (Oxf) 2005;63:525–529. doi: 10.1111/j.1365-2265.2005.02376.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Clemente JM, Vilardell C, Broch M, Megia A, Caixas A, Gimenez-Palop O, Richart C, Simon I, Martinez-Riquelme A, Arroyo J, Mauricio D, Vendrell J. Lower heart rate variability is associated with higher plasma concentrations of IL-6 in type 1 diabetes. Eur J Endocrinol. 2007;157:31–38. doi: 10.1530/EJE-07-0090. [DOI] [PubMed] [Google Scholar]
- Goren I, Muller E, Schiefelbein D, Christen U, Pfeilschifter J, Muhl H, Frank S. Systemic anti-TNF-alpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol. 2007;127:2259–2267. doi: 10.1038/sj.jid.5700842. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hendrix S, Peters EM. Neuronal plasticity and neuroregeneration in the skin -- the role of inflammation. J Neuroimmunol. 2007;184:113–126. doi: 10.1016/j.jneuroim.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Hills CE, Brunskill NJ. Cellular and physiological effects of C-peptide. Clin Sci (Lond) 2009;116:565–574. doi: 10.1042/CS20080441. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Kaul K, Hodgkinson A, Tarr JM, Kohner EM, Chibber R. Is inflammation a common retinalrenal-nerve pathogenic link in diabetes? Curr Diabetes Rev. 2010;6:294–303. doi: 10.2174/157339910793360851. [DOI] [PubMed] [Google Scholar]
- King GL. The role of inflammatory cytokines in diabetes and its complications. J Periodontol. 2008;79:1527–1534. doi: 10.1902/jop.2008.080246. [DOI] [PubMed] [Google Scholar]
- Lokuta MA, Huttenlocher A. TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J Leukoc Biol. 2005;78:210–219. doi: 10.1189/jlb.0205067. [DOI] [PubMed] [Google Scholar]
- Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Roubicek T, Bartlova M, Krajickova J, Haluzikova D, Mraz M, Lacinova Z, Kudla M, Teplan V, Haluzik M. Increased production of proinflammatory cytokines in adipose tissue of patients with end-stage renal disease. Nutrition. 2009;25:762–768. doi: 10.1016/j.nut.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Saklatvala J, Davis W, Guesdon F. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond B Biol Sci. 1996;351:151–157. doi: 10.1098/rstb.1996.0011. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Schram MT, Chaturvedi N, Schalkwijk CG, Fuller JH, Stehouwer CD. Markers of inflammation are cross-sectionally associated with microvascular complications and cardiovascular disease in type 1 diabetes--the EURODIAB Prospective Complications Study. Diabetologia. 2005;48:370–378. doi: 10.1007/s00125-004-1628-8. [DOI] [PubMed] [Google Scholar]
- Sharma S, Chopra K, Kulkarni SK. Effect of insulin and its combination with resveratrol or curcumin in attenuation of diabetic neuropathic pain: participation of nitric oxide and TNF-alpha. Phytother Res. 2007;21:278–283. doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- Skundric DS, Lisak RP. Role of neuropoietic cytokines in development and progression of diabetic polyneuropathy: from glucose metabolism to neurodegeneration. Exp Diabesity Res. 2003;4:303–312. doi: 10.1155/EDR.2003.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka H, Narisawa T, Mori N, Masuda M, Kishi D, Suzuki T, Takaba T. The left internal thoracic artery and radial artery composite graft in off-pump coronary artery bypass grafting. Ann Thorac Cardiovasc Surg. 2002;8:204–208. [PubMed] [Google Scholar]
- Uceyler N, Rogausch JP, Toyka KV, Sommer C. Differential expression of cytokines in painful and painless neuropathies. Neurology. 2007;69:42–49. doi: 10.1212/01.wnl.0000265062.92340.a5. [DOI] [PubMed] [Google Scholar]
- Verdu E, Vilches JJ, Rodriguez FJ, Ceballos D, Valero A, Navarro X. Physiological and immunohistochemical characterization of cisplatin-induced neuropathy in mice. Muscle Nerve. 1999;22:329–340. doi: 10.1002/(sici)1097-4598(199903)22:3<329::aid-mus5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Westermann D, Rutschow S, Van Linthout S, Linderer A, Bucker-Gartner C, Sobirey M, Riad A, Pauschinger M, Schultheiss HP, Tschope C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu W, Zhang L, Dorset-Martin W, Morris MW, Mitchell ME, Liechty KW. The role of microRNA-146a in the pathogenesis of the diabetic wound-healing impairment: correction with mesenchymal stem cell treatment. Diabetes. 61:2906–2912. doi: 10.2337/db12-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Dougherty PM. Dynamic effects of TNF-alpha on synaptic transmission in mice over time following sciatic nerve chronic constriction injury. J Neurophysiol. 2013;110:1663–1671. doi: 10.1152/jn.01088.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppini G, Faccini G, Muggeo M, Zenari L, Falezza G, Targher G. Elevated plasma levels of soluble receptors of TNF-alpha and their association with smoking and microvascular complications in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2001;86:3805–3808. doi: 10.1210/jcem.86.8.7786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Representative Western blots show a substantial increase of TNFα in cultured DRG neurons exposed to high glucose (20mM; overnight) compared to control cells; samples are chosen randomly from the blot. (b) Graph demonstrates the increase in TNFα as determined by Western blot, normalized to control (* P < 0.05, n = 3 samples per group). (c) Representative Western blots show pre-incubation of cultured DRG neurons with 15 ng/ml of rTNFα overnight, increases the phosphorylation of p38 MAPK compared to control cells (blots showing two randomly chosen independent samples from each group). (d) Western blot analysis of pp38 MAPK demonstrates the increases in phosphorylation of p38 MAPK in the rTNFα treated samples, normalized to control (* P < 0.05, n = 3 samples per group).