Summary
Canine immune thrombocytopenia (ITP) is analogous to human ITP, with similar platelet counts and heterogeneity in bleeding phenotype among affected individuals. With a goal of ultimately investigating this bleeding heterogeneity, a canine model of antibody-mediated ITP was developed. Infusion of healthy dogs with 2F9, a murine IgG2a monoclonal antibody to the canine platelet glycoprotein GPIIb (a common target of autoantibodies in ITP) resulted in profound, dose-dependent thrombocytopenia. Model dogs developed variable bleeding phenotypes, e.g. petechiae and haematuria, despite similar degrees of thrombocytopenia. 2F9 infusion was not associated with systemic inflammation, consumptive coagulopathy, or impairment of platelet function. Unexpectedly however, evaluation of cytokine profiles led to the identification of platelets as a potential source of serum interleukin-8 (IL8) in dogs. This finding was confirmed in humans with ITP, suggesting that platelet IL8 may be a previously unrecognized modulator of platelet-neutrophil crosstalk. The utility of this model will allow future study of bleeding phenotypic heterogeneity including the role of neutrophils and endothelial cells in ITP.
Keywords: ITP, canine, interleukin-8, animal model
Introduction
Immune thrombocytopenia (ITP) is a bleeding disorder in which platelet autoantibodies lead to thrombocytopenia and, sometimes, severe bleeding (Nieswandt et al, 2000). A major unanswered question in ITP is the variable bleeding tendency; specifically, why do some patients have more bleeding manifestations while others with equally low platelet counts have less? This clinical heterogeneity impairs the clinicians' ability to decide which patients need more aggressive management (Eldor et al, 1982; Kenet et al, 1998; Psaila et al, 2011). Further investigations are necessary to better understand, predict and correct the bleeding phenotype in ITP.
Although much has been learned from murine models of ITP, striking differences exist between mice and humans, including platelet counts (1 million platelets/μl in mice versus 150-400 ×109/l in humans), platelet volume (murine platelets are about half the size of human platelets), and platelet signalling pathways (Table I) (Levin & Ebbe, 1994; Semple, 2010; Ware, 2004).
Table I. Comparison of murine, canine and human platelets and canine and murine ITP model systems.
| Humans | Mice | Dogs | |
|---|---|---|---|
| Spontaneous ITP? | Yes | No (Semple, 2010) | Yes (Lewis & Meyers, 1996) |
| Platelet counts | 150-400 × 109/l | 1 million/μl (Ware, 2004) | 190-468 × 109/l |
| Platelet volumes | 7.5-10 fl | 4.7 ± 0.3 fl(Ware, 2004) | 7.9-13.8 fl |
| Platelet protease activated-receptors (PAR) | PAR 1 and 4 (Ware, 2004) | PAR 3 and 4 (Ware, 2004) | PAR 1 and 4 (Boudreaux et al, 2007) |
| Blood volume that can be safely sampled | N/A | 0.2 ml (Diehl et al, 2001) (25 g mouse) | 85 ml (Diehl et al, 2001) (10 kg dog) |
| Animal survival studies possible? | N/A | No | Yes |
| Result of ITP models utilizing antibodies to the fibrinogen receptor (GPIIbIIIa) | Variable bleeding, no systemic symptoms other than fatigue (with spontaneous disease) | Acute systemic reactions, hypothermia, incoordination (Nieswandt et al, 2000) | Variable bleeding, no systemic signs other than those relating to haemorrhage (Lewis & Meyers, 1996; O'Marra et al, 2011) |
ITP, immune thrombocytopenia; GPIIbIIIa, glycoprotein IIbIIIa complex; N/A, not applicable.
While there are currently no large animal models of ITP, dogs may be ideal for this purpose. Dogs develop spontaneous ITP with a similar disease course, heterogeneous bleeding phenotype, and response to treatment as adult humans with ITP (Lewis & Meyers, 1996). Dogs are much closer in size to humans, resulting in more similar rheology (Son et al, 2010; Suo et al, 2007) and metabolic rates (Schmidt-Nielsen & Pennycuik, 1961), and dogs and humans have similar platelet counts and platelet volumes. Hundreds of times more blood volume can be safely sampled from dogs than mice (Diehl et al, 2001), and non-terminal studies are possible in dogs. Finally, platelet signalling pathways are more analogous between dogs and humans, with canine and human platelets signalling through protease-activated receptors (PAR)1 and PAR4, while murine platelets utilize PAR3 and 4 (James Catalfamo, Cornell University, personal communication, November 8, 2012) (Boudreaux et al, 2007; Kahn et al, 1999; Nakanishi-Matsui et al, 2000; Ware, 2004).
We sought to develop a canine ITP model that would be highly translational because of the similarities between canine and human platelets and because dogs develop spontaneous ITP as do humans. An antibody-mediated model is desirable as an initial approach because it recapitulates ITP without the confounding co-morbidities or treatment effects present in clinical cases. We employed 2F9, a murine anti-platelet antibody targeting canine platelet glycoprotein IIb (GPIIb), to model the autoantibody-induced clearance of platelets in ITP. We selected an anti-GPIIb antibody because autoantibodies in human patients with ITP are most commonly directed against epitopes on GPIIbIIIa, the fibrinogen receptor, and GPIb-IX-V, the von Willebrand factor receptor (Beardsley & Ertem, 1998). Infusing antibodies against GPIIbIIIa in murine models leads to incompletely understood acute systemic reactions and hypothermia (Nieswandt et al, 2000; Nieswandt et al, 2003). Thus, these murine models fail to recapitulate the naturally-occurring disease in which the predominant clinical manifestations, if any, are mucocutaneous bleeding and fatigue (Newton et al, 2011).
The generation of platelet autoantibodies in ITP is the tip of the immunological iceberg. Autoantibodies form in ITP as the result of a complex loss of self-tolerance, including formation of autoreactive B and T cells, autoreactive cytotoxic T cells and decreased regulatory T cells (Gernsheimer 2008; Olsson et al, 2003). The immunopathogenesis of ITP has been extensively reviewed elsewhere (Semple & Provan 2012). Furthermore, in addition to accelerated platelet destruction, ineffective platelet production is now understood to be central in disease pathogenesis (Gernsheimer 2008; Chang et al, 1999; McMillan et al, 2004). We are beginning to recognize that ITP is probably a heterogeneous syndrome with a final common pathway of platelet autoantibodies and platelet destruction (Arnold 2012). Thus, our passive ITP model only recapitulates one segment of a complex disease.
Despite these limitations, ultimately, this novel large animal model should be useful to investigate the pathophysiology of autoantibody-mediated thrombocytopenia, as well as reasons for the differential bleeding tendency in affected humans and dogs.
Materials and Methods
Complete and detailed methods are provided in the Online Supplement. In brief, we developed a canine ITP model by infusion of purpose-bred research dogs with a murine monoclonal antibody, 2F9, recognizing canine GPIIb (Burstein et al, 1991; Suter et al, 2007; Fang et al, 2011). 2F9 is a platelet-specific IgG2a antibody developed by Burstein and colleagues by immunizing mice with canine platelets (Burstein et al, 1991). The 2F9 hybridoma was gifted to us by David Wilcox (Medical College of Wisconsin, Milwaukee, WI), and 2F9 antibody was produced from this hybridoma by the Tissue Culture Facility, University of North Carolina, Chapel Hill. Western blot analysis was performed to confirm the specific reactivity of 2F9 with GPIIb by using control dog platelets and platelets from dogs with Glanzmann thrombasthenia (GT) (Boudreaux et al, 2007).
To model the clinical disease with variable bleeding tendencies at platelet counts below 30 × 109/l, we performed a dose-titration study in two dogs, aiming for a target platelet nadir of 5–30 × 109/l. 2F9 was administered at a starting dose of 15 ng/kg i.v. The dose was increased by a factor of ten every 1–2 h until the platelet count fell in the target range. Time zero was when the platelet count first fell into the target nadir range. Subsequently, three dogs were employed in a dose-repeatability study, receiving starting 2F9 doses of 50 μg/kg, based on the dose-titration study results; additional doses were given as needed and were based on their decrease in platelet count in response to the first 2F9 dose. Three dogs served as controls. They received anti-yellow fever IgG2a, the isotype control antibody, at the highest cumulative effective dose of 2F9 administered (167 μg/kg).
Blood was drawn at the following time points: baseline, time zero (when platelet count first fell into the target range or 1 h after control antibody administration), 2, 4, 6, 8, 12 and 24 h, and then every 24 hours until platelet count recovered and new bleeding stopped, whichever came later (168-240 h; termed recovery). Blood sampling and processing details are described in the supplementary methods.
To compare our experimental model with spontaneous canine ITP, blood samples were also obtained from dogs with naturally-occurring ITP (n=6) or thrombocytopenia of other causes (n=4). Signalment information for these dogs with naturally-occurring thrombocytopenia is listed in Supplementary Table 1.
Bleeding quantification
Bleeding was assessed by one author at all time points using a bleeding scale adapted from the human ITP bleeding score (Supplementary Table 2) (Page et al, 2007); buccal mucosal bleeding times were performed at baseline and time zero as previously described (Brooks & Catalfamo, 1993).
Cytokine and Chemokine Analysis
Cytokine/chemokine profiles in dogs with spontaneous and modelled ITP were analysed in sera by a 13-plex Milliplex Map canine cytokine magnetic bead panel (Milllipore, Billerica, MA) that was read using a Bioplex 200 (BioRad, Hercules, CA).
Canine IL8 results by multiplex assay were confirmed using a Quantikine canine IL8 enzyme-linked immunosorbent assay (ELISA) (R&D, Minneapolis, MN). Serum IL8 was also measured in human patients with ITP. Adult ITP patients were recruited at the Platelet Disorder Center, Weill Cornell Medical College/New York Presbyterian Hospital, NY. Patients consented and were enrolled in this Institutional Review Board–approved study. Serum IL8 was measured using a high sensitivity human IL8 immunoassay (R&D).
Fibrinogen and D-dimer Analysis
Coagulation analytes were measured in citrated plasma in the Comparative Coagulation Laboratory, Animal Health Diagnostic Laboratory, Cornell University as previously described (Stokol et al, 2000; Delgado et al, 2009).
Statistical Analysis
To test for differences in outcomes across all time points by treatment group (2F9 or control antibody), repeated-measures generalized linear modeling (analysis of variance [ANOVA]) was performed, where time point and variable of interest were entered into the model. Similarly, to screen for differences in variables in dogs with spontaneous ITP, healthy dogs and experimental dogs, a repeated-measures ANOVA was performed. The sole dog with secondary ITP was not considered in these analyses. To control for multiple testing, a Bonferroni correction was used (for each set of hypotheses) to determine the alpha level for statistical significance that ensured a family-wise error rate of 0.05; a comparison was considered to be statistically significant here if P<0.002. When differences between groups were identified, the Wilcoxon rank sum test was used to compare pairs of different groups of dogs (dogs with spontaneous ITP, experimental ITP and control dogs, healthy dogs). The Wilcoxon rank sum test was also used to compare nadir platelet counts for control and 2F9-treated dogs. Results were considered significant if P<0.05.
To identify candidate predictors of bleeding, the association between bleeding scores and different mediators was determined by univariate association between variables using a t-test for correlations with appropriate degrees of freedom. Bleeding scores were treated as ordinate variables as described by Mantel-Haenszel. Again, a Bonferroni correction was applied, with P<0.002 considered significant. A t-test for correlations was also used to evaluate the relationship between platelet count and IL8 levels.
All calculations were performed using Stata version 10 (Statacorp LP). Graphs were drawn with GraphPad PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Results
Ex Vivo Characterization of 2F9
By Western blot analysis, we determined that 2F9 does not recognize any protein bands in canine GT platelets, indicating that 2F9 must bind to either GPIIb or GPIIIa (Figure 1) (Boudreaux & Catalfamo, 2001). In normal canine platelets, 2F9 reacts with a band in the non-reduced state that is most prominent at 124 kDa, with weaker areas extending to 148 kDa and 110 kDa. This band is close to the predicted size of canine GPIIb (Abuelo et al, 2012).
Figure 1. Antigenic specificity of 2F9.
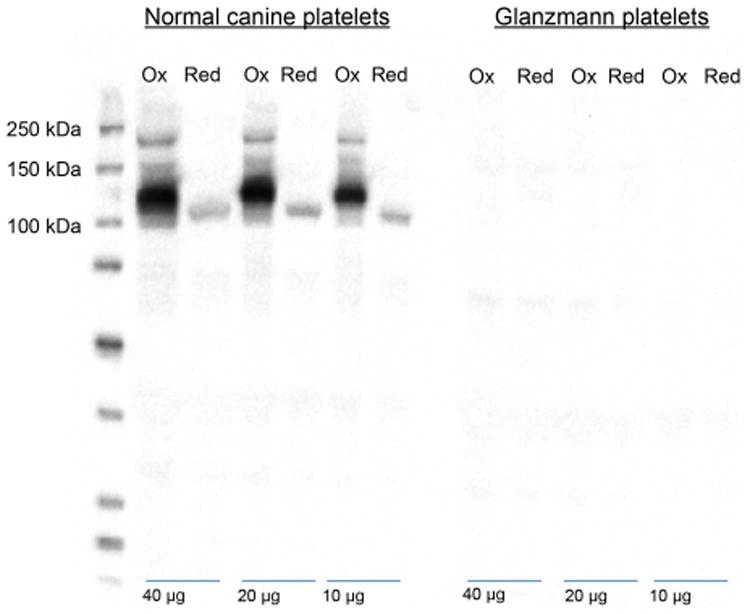
Resting platelets from normal and Glanzmann thrombasthenia (GT) dogs were lysed and 40, 20 and 10 μg were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis under non-reducing (Ox) and reducing (Red) conditions and then transferred onto a polyvinylidene difluoride membrane which was probed with 2F9 and detected with a goat anti-murine horseradish peroxidease antibody and enhanced chemiluminescence substrate. Note that no bands are observed in the GT platelet lanes indicating that 2F9 binds either GPIIb or GPIIIa. The prominent band at 124 kDa under non-reducing conditions (and broad band ranging from 110 to 145 kDa is most consistent with 2F9 binding GPIIb. The faint 108-kDa band under reducing conditions probably represents a small amount of GPIIb adhered to GPIIIa despite the presence of EDTA in the reaction conditions. If 2F9 recognized GPIIIa, a band should be present at 100 kDa under both non-reducing and reducing conditions (Boudreaux & Catalfamo, 2001).
Based on flow-cytometric studies, 2F9 did not lead to platelet activation (surface expression of P-selectin or phosphatidylserine) or inhibit platelet responsiveness to thrombin stimulation (data not shown).
In Vivo ITP Model Development
2F9 Induces a Severe, Dose-Dependent Thrombocytopenia
Within 2 h of a median cumulative 2F9 dose of 63 μg/kg (range 50.0-166.6 μg/kg), all dogs developed profound thrombocytopenia (11-28× 109/l) (Figure 2). Compared to the control group, platelet nadir was significantly lower (median (range): 6 (4-11) × 109/l vs. 200 (179-209) × 109/l; P=0.036) and change in platelet count from baseline to nadir was significantly greater in the 2F9-treated group (median (range): 238 (179-325) × 109/l vs. 4 (0-10) × 109/l; P=0.036). Platelet nadir was in our target range (5-30 × 109/l) and platelet count remained less than 40 × 109/l in all 2F9-treated dogs for 24 h. Nadir platelet counts averaged 3% of baseline. Dosing was predictable: in the dose-repeatability dogs, after an initial dose of 50 μg/kg 2F9, the second dose needed to reach the target nadir could be accurately calculated from the initial platelet decrease according to the following formula, where Δ platelet count is change in platelet count:
Figure 2. 2F9 induces a profound thrombocytopenia in vivo.
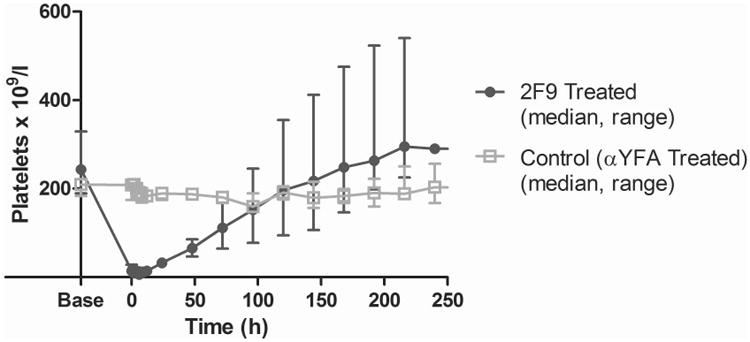
Dogs received a median cumulative dose of 63 μg/kg 2F9 intravenously. Platelet counts were determined at the indicated times. Time zero occurred when platelet count first fell within the target platelet nadir (5-30 × 109/l), a maximum of 2 h after the last 2F9 dose, or was defined as 1 h after control antibody administration. Injection of the isotype control antibody (αYFA) had no significant effect on the platelet count. Results are shown as median and range for 5 2F9-treated dogs and 3 control dogs.
Due to the dose-titration component of the study, dogs received up to 6 doses of 2F9 within a period of 3 days. Dose response details are tabulated in Supplementary Table 3. Platelet counts recovered in 5 to 10 days (median 6 days).
Dogs remained bright, alert, and responsive throughout the study. Temperature, pulse, and respiration were unchanged during antibody administration and remained within normal limits at all time points (not shown). Transient neutropenia was observed in two dogs (Supplementary Figure 1).
ITP Model Dogs Demonstrate Variable Bleeding
Thrombocytopenic dogs demonstrated a range of mild bleeding, from few cutaneous petechiae and/or ecchymoses to transient microscopic haematuria or transient melena. Bleeding scores ranged from 0 to 4 out of a possible 16 (Supplementary Figure 2). Overall, bleeding was clinically mild and required no supportive care. Interestingly, two dogs developed large delayed ecchymoses (up to 8.5 × 13 cm) when their platelet counts were already recovering (at 48 - 120 h in one dog and 120 - 192 h in the second animal). Figure 3 demonstrates one such ecchymosis and the platelet counts at which these bruises developed. Histological examination of these bruises following cutaneous biopsies revealed only perivascular haemorrhage with no evidence of inflammatory infiltrate, cellular necrosis or thrombosis (data not shown).
Figure 3. Delayed ecchymosis formation during platelet count recovery.
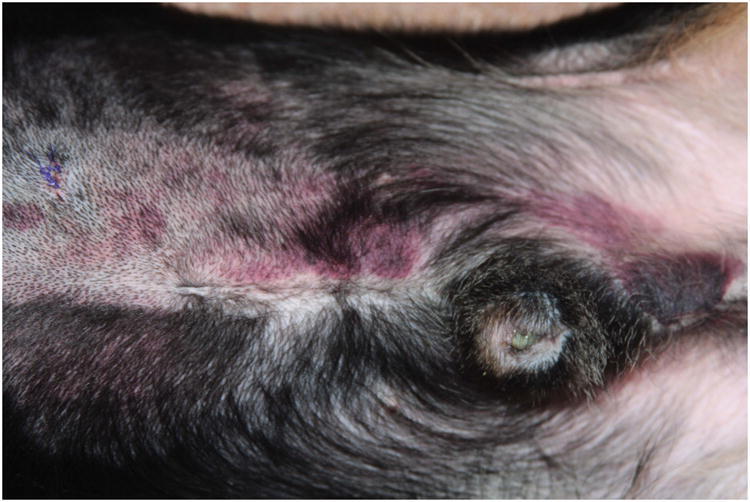
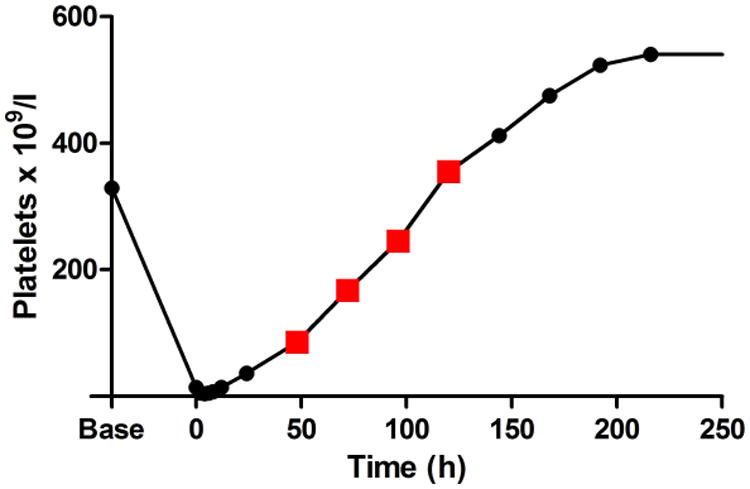
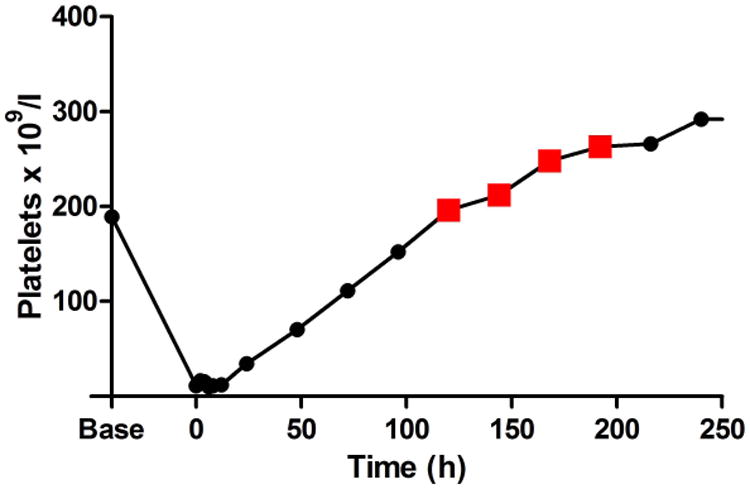
A. The photograph demonstrates a large abdominal and preputial ecchymosis (8.5 cm × 13 cm at its largest) in one 2F9-treated dog that began to form when platelet count had started to recover and continued to expand when the platelet count had exceeded baseline count. B. Red squares on the graph of platelet count over time demonstrate the period of active bleeding in this dog and in the second dog that developed a delayed ecchymosis (C).
Buccal mucosa bleeding times (BMBTs), performed as another way to quantify bleeding tendency, were variably prolonged at time zero in 2F9-treated thrombocytopenic dogs from 4.80±2.09 times baseline compared to 2.15±0.32 times baseline in control animals. Interestingly, the longest time-zero BMBTs were in the two dogs that later developed the delayed ecchymoses (>15 min and 13.13 min). Thrombocytopenic dogs all had prolonged BMBTs. When only the thrombocytopenic BMBTs were considered (platelet nadir in 2F9-treated dogs), there was no correlation between platelet count and BMBT (correlation coefficient -0.2108 (p>0.7336); Spearman rank correlation). However, negative conclusions must be interpreted in light of the small sample size.
Induced ITP Model Is Not Prothrombotic
Both fibrinogen and D-dimers increased slightly over time in both treated (n=5) and control (n=3) groups, but no difference (P=0.268 fibrinogen; P=0.457 D-dimers) was noted between experimental groups, indicating that, as expected, thrombocytopenia was not explained by disseminated intravascular coagulation (DIC) (Supplementary Figure 3).
Experimental ITP Cytokine/Chemokine Profile Models the Spontaneous Canine Disease and Identifies Platelets as an Important Source of Serum IL8
2F9 infusion generated negligible systemic inflammation, as assessed by white blood cell (WBC) count and serum cytokine measurement. Changes over time in TNF-α, MCP1, IL6, IL10, granulocyte-macrophage colony-stimulating factor (GM-CSF), IL2, CXCL10, IFN-γ, IL7, IL15, CXCL1 orthologue, and IL18 were not significantly different between 2F9 and αYFA treated dogs (Supplementary Figure 4). Unexpectedly, however, serum IL8 levels tracked faithfully with platelet count, suggesting that platelets are a major source of serum IL8 (Figure 4A-B). IL8 was significantly different between control and 2F9-treated dogs (P=0.000); WBC counts were not different between control and treated dogs over time (data not shown). These results were confirmed with an ELISA specific for canine IL8. Although absolute values of serum IL8 were higher in the multiplex assay than the ELISA, the relative IL8 decrease with platelet depletion was consistent. Although α-granules are known to contain IL8 (Gear & Camerini, 2003), platelets have not been previously described as a significant source of IL8 in serum. As IL8 is an important neutrophil chemokine, our finding may illuminate a novel mechanism of platelet-neutrophil crosstalk.
Figure 4. Serum IL8 tracks with platelet count.
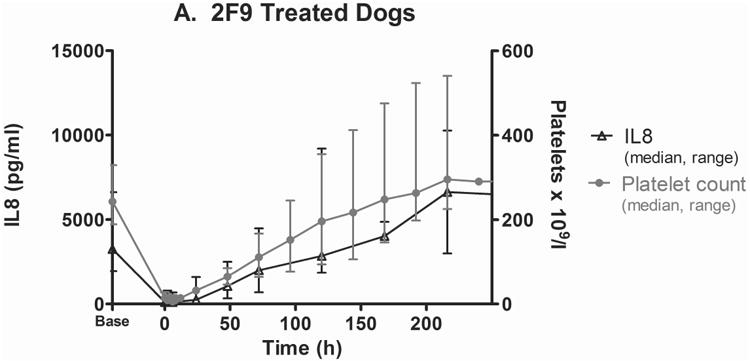
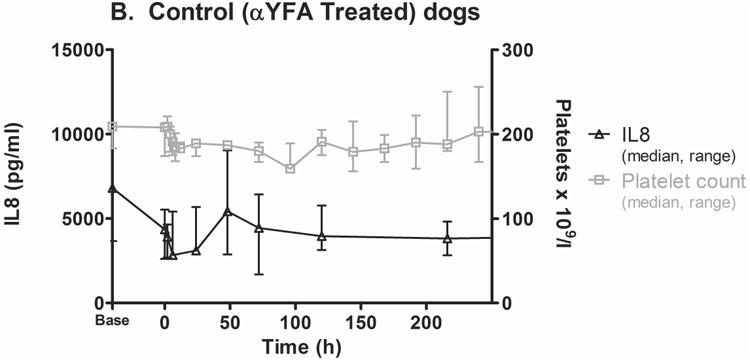
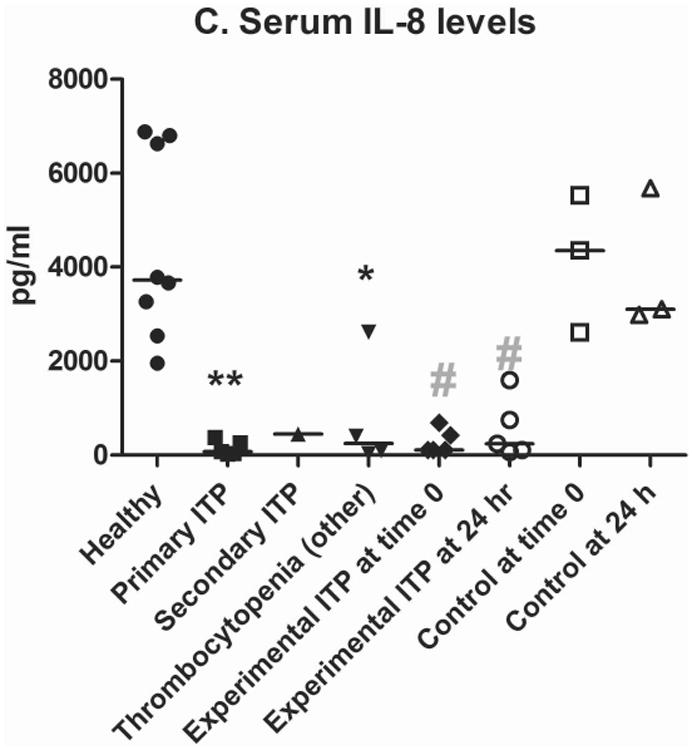
Serum IL8 (median, range) as measured by the Milliplex assay in 2F9-treated (A; n=5) and isotype control (αYFA)-treated dogs (B; n=3) compared to platelet count. C. Serum IL8 levels in dogs with naturally-occurring thrombocytopenia are decreased compared to healthy control dogs. **P=0.0016 healthy dogs (n=8) compared to primary ITP (n=5); *P=0.0162 healthy compared to thrombocytopenia other (n=4); #P=0.0357 experimental ITP at time 0 (n=5) compared to control at time 0 (n=3) and experimental at 24 h (n=5) compared to control at 24 h (n=3). Time zero is when platelet count first fell to target nadir of 5-30 × 109 platelets/l or 1 h after isotype control administration. Horizontal lines denote median values. Secondary ITP (n=1) was not considered in statistical analysis. Healthy dogs were not compared with control or experimental dogs because healthy dogs were these same dogs at baseline; instead control and experimental were compared to each other at matching time points.
In light of our unexpected IL8 findings in the dog antibody model, we investigated whether the same pattern of decreased IL8 was present in dogs with spontaneous ITP. Ten dogs with naturally-occurring thrombocytopenia were evaluated, including 5 with primary ITP, 1 with secondary thrombocytopenia due to Ehrlichiosis and 4 with thrombocytopenia of other causes (Supplementary Tables 1&4). Significantly decreased levels of serum IL8 were found in all dogs with naturally-occurring thrombocytopenia (primary ITP, P=0.0016; other causes of thrombocytopenia, P=0.0162) compared to healthy control dogs as shown in Figure 4C.
No cytokine/chemokine differences were detected between dogs with spontaneous ITP and the experimental ITP animals (repeated-measures ANOVA, Supplementary Table 4). The cytokine and chemokine profile of dogs with spontaneous primary ITP was similar to that of our experimental dogs at platelet nadir and at 24 h (Supplementary Table 4), suggesting that the ITP model recapitulates the naturally-occurring disease in dogs.
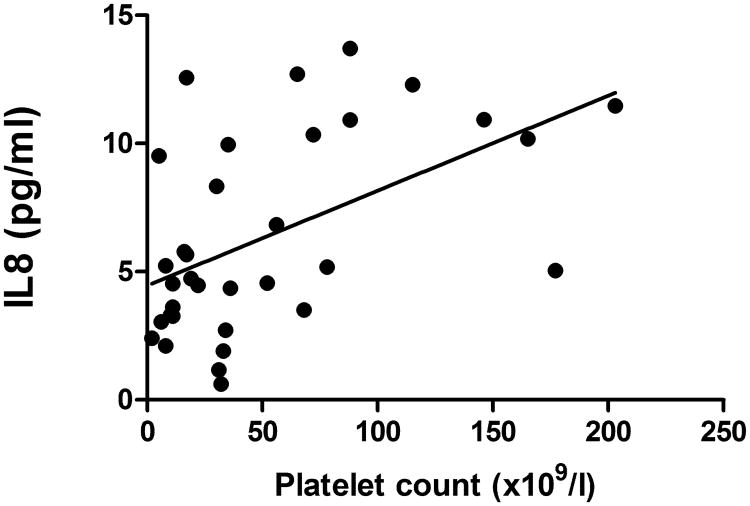
Finally, to determine whether serum IL8 levels track with platelet count in humans, serum IL8 levels were evaluated in human ITP patients with variable platelet counts (n=16). Serum IL8 levels were significantly correlated with platelet count (Spearman rank correlation=0.52; P=0.0410); (Figure 5). The nearly 100 times lower serum IL8 levels in humans compared to canines was not unexpected given previous reports and probably relates to differing IL8 levels in human versus canine platelets (Su et al, 1996; Gelaleti et al, 2012; Elewa et al, 2010).
Figure 5. Serum IL8 is directly related to platelet count in human ITP patients.
Serum IL8 in human ITP patients (n = 16) measured over up to three serial visits is correlated with platelet count (r =0.52, p =0.0410).
Identification of Candidate Bleeding Predictors
As a preliminary survey to identify candidate predictors of bleeding, we examined whether any correlation existed between the measured cytokine levels and bleeding scores. No correlations were found in experimental dogs, which is not surprising given their relatively low bleeding scores. In dogs with spontaneous primary ITP, IL10 levels were directly correlated with bleeding score (R2=0.6053, P<0.022) in univariate analysis, but this was not significant with multiple testing corrections. This relationship warrants further investigation in humans and dogs with ITP.
Discussion
We have developed a canine model of ITP that is highly analogous to the naturally occurring, spontaneous platelet autoantibody-mediated disease in dogs and humans. We characterized 2F9 as an antibody that targets the GPIIb/IIIa complex, with an immunoblotting pattern that is most consistent with GPIIb reactivity (Figure 1). These data confirm the suggestion by Burstein et al. (1991) that 2F9 targets GPIIb. The GPIIb specificity of 2F9 enhances its value for development of an ITP model, as the majority of platelet autoantibodies in ITP target epitopes on the fibrinogen receptor (GPIIbIIIa) and von Willebrand factor receptor (GPIb-IX-V) (Beardsley & Ertem, 1998). Furthermore, GPIIb is an ideal target when examining the role of endothelial integrity in ITP bleeding phenotype as GPIIb is located exclusively on platelets, while GPIIIa is also expressed by endothelial cells. However, it should be noted that in ITP patients with autoantibodies against the GPIIbIIIa complex, GPIIIa is targeted in the majority of cases (Mehta et al, 2000). We also demonstrated that 2F9, as assessed by flow cytometry, is not an activating or inhibitory antibody. This makes 2F9 an excellent choice to study platelet function in ITP.
Our in vivo studies demonstrated that the effects of 2F9 dosing were repeatable and the dose could be tailored to reach a desired platelet nadir. With a starting dose of 50 μg/kg, the initial platelet decrement could be used to calculate the additional dose necessary to reach a clinically relevant target nadir of 5-30 × 109 platelets/l, the range at which ITP patients are at risk for bleeding. Analogous to spontaneous ITP, experimental dogs demonstrated variable mucocutaneous bleeding, ranging from few cutaneous petechiae and/or ecchymoses to transient microscopic haematuria and melena (Cines et al, 2009; O'Marra, et al, 2011; Provan et al, 2010; Psaila et al, 2011; Psaila et al, 2009; Rozanski et al, 2002). Experimental dogs also had prolonged BMBTs, analogous to dogs with spontaneous thrombocytopenia (Jergens et al, 1987). Bleeding in these dogs was mild, probably reflecting the short duration of thrombocytopenia. Two dogs developed delayed ecchymoses during platelet recovery. As suggested by transmission electron micrographs of these skin lesions (not shown), these ecchymoses may be the result of altered endothelial integrity due to previous severe thrombocytopenia and loss of platelet-vascular stabilizing effects (Gimbrone, et al, 1969; Ho-Tin-Noe et al, 2011; Kitchens & Pendergast, 1986). Consistent thrombocytopathia due to persistent 2F9-platelet binding was excluded by daily flow cytometry evaluations (data not shown). Platelet function was not extensively pursued in this study but is a primary goal of future studies. Lastly, plasma Factor VIII level of activity was also normal at the time of bruising (data not shown).
Significantly, our model does not cause clinically significant signs of inflammation, such as fever, and the cytokine profile in experimental ITP dogs matched that of dogs with spontaneous primary ITP (Supplementary Table 4). Similarly, adult patients with ITP do not manifest clinical signs of systemic inflammation. Adult ITP is, however, marked by a type-1 immune response with elevated T-helper cell type 1 (Th1) cytokines (IL2, IFN-γ) and decreased Th2 cytokines (IL4 and IL5) (Panitsas et al, 2004; Semple et al, 1996). We did not observe a Th1 cytokine profile in our experimental or clinical dogs, but our sample size may have been too small to detect this pattern and we did not expect a passive ITP model to demonstrate a lymphocytic autoimmune response.
Our dog model sharply contrasts with murine models of ITP induced by antibodies against GPIIbIIIa. In these models, mice develop acute systemic reactions, uncoordinated movements and hypothermia through incompletely understood mechanisms involving platelet-activating factor (Nieswandt et al, 2000; Nieswandt et al, 2003). Thus, in terms of clinical manifestations, our canine model more accurately represents human ITP than do these murine models. Furthermore, murine models employ a species that does not naturally develop primary ITP, and that has inherent platelet differences from humans and dogs (Table 1). The fact that dogs develop naturally occurring ITP and have similar platelet physiology to humans is a major advantage of our model. What is learned from the canine passive ITP model can be directly extrapolated to the spontaneous dog model as illustrated by this study, and from there to the human disease.
Although canine ITP models have been previously described, they have not been well characterized and the platelet-depleting antibodies or heterologous serum employed in these models are no longer available (Makoto Hosono, Kinki University, personal communication, May 12, 2009) (Hosono et al, 1995; Joshi & Jain, 1977; Tocantins & Stewart, 1939). Thus, to our knowledge, our model represents the only available large animal ITP model.
There are several murine models that address the initial pathogenesis of ITP including the role of regulatory T cells and autoreactive T and B cells in the development of ITP (Nishimoto et al, 2012; Chow et al, 2010). The approaches used in these models, e.g. radiation and transplantation of sensitized cells, could also be pursued in dogs in the future. These models, though revolutionary in their ability to capture the early immunopathogenesis of ITP, have inherent limitations. Nishimoto and colleagues' model of regulatory T cell depletion is complicated by its potential to lead to multiple other concurrent autoimmune diseases in conjunction with ITP (Nishimoto et al, 2012). Moreover, in this model, mice were considered thrombocytopenic at a platelet count equivalent to a normal human platelet count (Nishimoto et al, 2012).
We recognize that our model has limitations similar to any passive antibody-mediated model of ITP in that it does not fully recapitulate the immunopathogenesis that leads to endogenous autoantibody production, nor does it address the role of autoreactive T cells in direct platelet destruction. Thus, it only models the humoral component of platelet destruction in ITP. However, it does clearly capture the events that occur downstream of autoantibody generation. As such, the model has many potential applications. It can be used to determine the efficacy and safety of selected ITP therapeutics targeting autoantibody-mediated platelet destruction. If the duration of thrombocytopenia is inadequate to assess treatment effects, repeated 2F9 administration or constant rate infusions of 2F9 could be administered, as repeated exposure to 2F9 over a 3-day period was well tolerated. An alternate strategy would be to employ a higher initial 2F9 dose, similar to that administered to our proof-of-concept dog (300 μg/kg), which induced absolute thrombocytopenia for 3 days (data not shown). The model could be further developed by generating caninized 2F9, which would allow unlimited 2F9 infusions.
As dogs in the model demonstrated variable bleeding, the dog model can also be used to test hypotheses about differential bleeding tendency. Inflammation destabilizing the vasculature is one suggested mechanism of thrombocytopenic bleeding. In a murine model of thrombocytopenia, only mice that had concurrent inflammation and thrombocytopenia developed haemorrhage (Goerge et al, 2008). Our model could be used to assess how bleeding phenotype differs when inflammation is induced locally or systemically in thrombocytopenic dogs. A major strength of our model for this application is that we have confirmed that the model itself is non-inflammatory. As previously discussed, the mild bleeding demonstrated by the model dogs was probably due to the short duration of thrombocytopenia. Prolonging the duration of severe thrombocytopenia with repeated 2F9 administration might be necessary to better explore thrombocytopenic bleeding manifestations. Given that variable 2F9 doses were required to reach our target platelet nadir, the influence of 2F9 dose on bleeding outcome could be explored in future studies. Individual variation in response to anti-platelet antibody might explain some of the bleeding variability in human and canine ITP patients. However, our study is not well powered to address this.
The relationship our model demonstrated between IL8 and platelet counts is intriguing (Figures 4-5). IL8 is a CXC chemokine that serves as a potent chemoattractant and neutrophil activator (Emadi et al, 2005; Van Damme et al, 1990). Many cells produce IL8, including endothelial cells, neutrophils, fibroblasts, mast cells, monocytes, macrophages and T-lymphocytes (Emadi et al, 2005; Gesser et al, 1995; Gibbs et al, 2001; Kaplanski et al, 1994; Larsen et al, 1989; Matsushima & Oppenheim, 1989; Rodenburg et al, 1999; Strieter et al, 1990a; Strieter et al, 1990b). Although platelets and megakaryocytes are known to contain IL8 in their α-granules, they have not been considered a major source of soluble IL8 (Gear &Camerini, 2003; Iannacone et al, 2008; Su et al, 1996; von Hundelshuasen & Weber, 2007). Studies have previously reported a direct correlation between serum IL8 and platelet count in patients with hepatocellular carcinoma (Ren et al, 2003; Elewa et al, 2010). However, the significance of this correlation was not explored, nor were these studies able to show, as ours did, a recovery of serum IL8 with platelet count recovery. In our study, serum IL8 was not correlated with other blood cell counts, including neutrophils (data not shown). Thus, our data suggest that platelets are a major source of serum IL8, although the possibility remains that other cells that require the presence of platelets to release IL8 are the actual source of IL8. We also found that a marked decrease in serum IL8 is present in naturally occurring canine (Figure 4C) and human (Figure 5) ITP. It is important to note that IL8 was similarly reduced in dogs with non-destructive causes of thrombocytopenia such as consumption (DIC) and myelodysplastic syndrome (Figure 4C, Supplementary Table 1), suggesting that the mechanism of thrombocytopenia was not related to serum IL8 depletion.
Although the role, if any, of IL8 in ITP pathogenesis is not known and not addressed in this study, we speculate a novel mechanism of platelet-neutrophil interaction. By recruiting leucocytes into inflamed tissues, platelets are now seen as important players in inflammatory disorders (Devi et al, 2010; Ho-Tin-Noe et al, 2011). Multiple mechanisms for platelet-leucocyte recruitment have been described, however, platelet secretion of IL8 has not, to our knowledge, been described as an important mechanism of leucocyte recruitment (Devi et al, 2010; Kameyoshi et al, 1992; Dole et al, 2005). As IL8 is a major neutrophil chemokine, this putative mechanism of platelet-neutrophil crosstalk warrants further investigation and this model offers an excellent opportunity to do so (Van Damme et al, 1990).
Two model dogs became neutropenic (Supplementary Figure 1), one only transiently. We confirmed that the dog with prolonged neutropenia was not septic. We were unable to detect 2F9 or canine IgG, IgM or complement binding of the dog's neutrophils (data not shown). The neutropenia resolved following administration of GM-CSF (Neupogen, Amgen, Thousand Oaks, CA). 2F9 does weakly bind neutrophils in the presence, but not in the absence, of platelets (data not shown). This is believed to be due to a nonspecific interaction of the Fc portion of platelet-bound 2F9 with neutrophil Fc receptors. The neutropenia may have been the result of this non-specific 2F9 neutrophil binding leading to neutrophil destruction via off-target complement fixation or phagocytosis of immune-complex opsonized neutrophils (Shashidharamurthy et al, 2008; Hart et al, 2004). The very nature of the mechanism of platelet depletion (platelet-antibody complexes) means neutrophils will be activated via their Fc receptor to some degree, probably enough to marginate and, with complement fixation, perhaps enough to deplete them. The neutropenia may have also been the result of generalized stimulation of the reticuloendothelial system secondary to 2F9 administration. Notably however, no relationship was identified between serum IL8 levels and neutrophil counts.
In dogs with primary ITP, the bleeding score directly correlated with serum IL10 levels. In some studies, but not others, decreased serum IL10 levels have been observed in human ITP with increasing levels during platelet recovery (Andersson et al, 2000; Lazarus et al, 2000; Panitsas et al, 2004). Our results warrant further investigation of the relationship between IL10 and bleeding tendency in both canine and human ITP.
An acknowledged limitation of our study is the small sample size. Differences between control and 2F9-treated dogs could have been missed due to limited power, some candidate bleeding predictors in clinical patients might have been overlooked and effect size estimates could be inaccurate. The bleeding score we used has not been validated in dogs, which may be a study limitation, but the scoring in model dogs was performed entirely by one author so as to avoid inter-rater variability and the bleeding score well-described the bleeding seen in the dogs.
In summary, we have developed a unique large animal model of ITP. This model is highly translational and representative of the naturally occurring disease in canines and humans, especially in terms of bleeding heterogeneity. Importantly, in this pilot study we have discovered that IL8 may play a role in mediating platelet-neutrophil interactions in canine and human ITP patients.
Supplementary Material
Acknowledgments
This work was supported in part by research funding from the North Carolina State University Center for Comparative Medicine and Translational Research (DNL, SKN, THF, MBB, NSK). DNL was supported by NIH grant T32 HL007149. We would like to acknowledge support from the NIH grant U34 HL115015, Determining Optimum Medical Therapy for ITP. The authors would like to thank David Wilcox for providing the 2F9 producing hybridoma that enabled this study, Gregg Dean for provision of the isotype control antibody hybridoma and colleagues and dogs at the Francis Owen Blood Research laboratory for providing a constant supply of normal canine blood. We profusely thank Dr. James Catalfamo for his tireless help with the Western blots. We thank Denise Esserman for her help with statistical analysis.
Footnotes
Presented in abstract form at the 54th annual meeting of the American Society of Hematology, Atlanta, GA, December 10, 2012 and at the American College of Veterinary Medicine 31st annual Forum, Seattle, WA, June 13, 2013.
Authorship: DNL conceived and supervised the studies, interpreted the results and wrote the manuscript. NSK, AJB, SKN, DAB, SLJ, MBB, JBB, SG and THF contributed to study design, data interpretation and writing of the manuscript. SEO was involved in essential reagent production. SG, MBB, KF, NSB, APP, and HSM performed research and collected data. AMR performed statistical analysis.
Conflict-of-interest disclosure: The authors have no conflicts of interest.
References
- Abuelo A, Catalfamo JL, Brooks MB. Platelets International. Endicott College; Beverly, MA: 2012. Platelet TMEM16F expression in canine Scott syndrome. [Google Scholar]
- Andersson PO, Stockelberg D, Jacobsson S, Wadenvik H. A transforming growth factor-beta1-mediated bystander immune suppression could be associated with remission of chronic idiopathic thrombocytopenic purpura. Annals of Hematology. 2000;79:507–513. doi: 10.1007/s002770000177. [DOI] [PubMed] [Google Scholar]
- Arnold DM. Immune thrombocytopenia: getting back to basics. American Journal of Hematology. 2012;87:841–842. doi: 10.1002/ajh.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley DS, Ertem M. Platelet autoantibodies in immune thrombocytopenic purpura. Transfusion Science. 1998;19:237–244. doi: 10.1016/s0955-3886(98)00037-x. [DOI] [PubMed] [Google Scholar]
- Boudreaux MK, Catalfamo JL. Molecular and genetic basis for thrombasthenic thrombopathia in otterhounds. American Journal of Veterinary Research. 2001;62:1797–1804. doi: 10.2460/ajvr.2001.62.1797. [DOI] [PubMed] [Google Scholar]
- Boudreaux MK, Catalfamo JL, Klok M. Calcium-diacylglycerol guanine nucleotide exchange factor I gene mutations associated with loss of function in canine platelets. Translational Research. 2007;150:81–92. doi: 10.1016/j.trsl.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Brooks M, Catalfamo J. Buccal mucosa bleeding time is prolonged in canine models of primary hemostatic disorders. Thrombosis and Haemostasis. 1993;70:777–780. [PubMed] [Google Scholar]
- Burstein SA, Friese P, Downs T, Epstein RE. Canine megakaryocytopoiesis: analysis utilizing a monoclonal antibody to a 140-kd dog platelet protein. Experimental Hematology. 1991;19:47–52. [PubMed] [Google Scholar]
- Chang M, Qian JX, Lee SM, Joubran J, Fernandez G, Nichols J, Knoppel A, Buzby JS. Tissue uptake of circulating thrombopoietin is increased in immune-mediated compared with irradiated thrombocytopenic mice. Blood. 1999;93:2515–2524. [PubMed] [Google Scholar]
- Chow L, Aslam R, Speck ER, Kim M, Cridland N, Webster ML, Chen P, Sahib K, Ni H, Lazarus AH, Garvey MB, Freedman J, Semple JW. A murine model of severe immune thrombocytopenia is induced by antibody- and CD8+ T cell-mediated responses that are differentially sensitive to therapy. Blood. 2010;115:1247–1253. doi: 10.1182/blood-2009-09-244772. [DOI] [PubMed] [Google Scholar]
- Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113:6511–6521. doi: 10.1182/blood-2009-01-129155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MA, Monreal L, Armengou L, Rios J, Segura D. Peritoneal D-dimer concentration for assessing peritoneal fibrinolytic activity in horses with colic. Journal of Veterinary Internal Medicine. 2009;23:882–889. doi: 10.1111/j.1939-1676.2009.0344.x. [DOI] [PubMed] [Google Scholar]
- Devi S, Kuligowski MP, Kwan RY, Westein E, Jackson SP, Kitching AR, Hickey MJ. Platelet recruitment to the inflamed glomerulus occurs via an alphaIIbbeta3/GPVI-dependent pathway. American Journal of Pathology. 2010;177:1131–1142. doi: 10.2353/ajpath.2010.091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- Dole VS, Bergmeier W, Mitchell HA, Eichenberger SC, Wagner DD. Activated platelets induce Weibel-Palade-body secretion and leukocyte rolling in vivo: role of P-selectin. Blood. 2005;106:2334–2339. doi: 10.1182/blood-2005-04-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldor A, Avitzour M, Or R, Hanna R, Penchas S. Prediction of haemorrhagic diathesis in thrombocytopenia by mean platelet volume. British Medical Journal. 1982;285:397–400. doi: 10.1136/bmj.285.6339.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elewa H, Abd-Elmeneem M, Hashem A, Alshehaby A. Study of interleukin 8 (IL8) serum level in patients with chronic liver diease due to hepatitis C virus (HCV) with and without hepatocellular carcinoma (HCC) International Journal of Hepatology. 2010;1:9–17. [Google Scholar]
- Emadi S, Clay D, Desterke C, Guerton B, Maquarre E, Charpentier A, Jasmin C, Le Bousse-Kerdiles MC for the French INSERM Reseach Network on MMM. IL-8 and its CXCR1 and CXCR2 receptors participate in the control of megakaryocytic proliferation, differentiation, and ploidy in myeloid metaplasia with myelofibrosis. Blood. 2005;105:464–473. doi: 10.1182/blood-2003-12-4415. [DOI] [PubMed] [Google Scholar]
- Fang J, Jensen ES, Boudreaux MK, Du LM, Hawkins TB, Koukouritaki SB, Cornetta K, Wilcox DA. Platelet gene therapy improves hemostatic function for integrin alphaIIbbeta3-deficient dogs. Proceedings of the National Academy of Sciences U S A. 2011;108:9583–9588. doi: 10.1073/pnas.1016394108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gear AR, Camerini D. Platelet chemokines and chemokine receptors: linking hemostasis, inflammation, and host defense. Microcirculation. 2003;10:335–350. doi: 10.1038/sj.mn.7800198. [DOI] [PubMed] [Google Scholar]
- Gelaleti GB, Jardim BV, Leonel C, Moschetta MG, Zuccari DA. Interleukin-8 as a prognostic serum marker in canine mammary gland neoplasias. Veterinary Immunology and Immunopathology. 2012;146:106–112. doi: 10.1016/j.vetimm.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Gernsheimer T. Epidemiology and pathophysiology of immune thrombocytopenic purpura. European Journal of Haematology. 2008;80:3–8. doi: 10.1111/j.1600-0609.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- Gesser B, Deleuran B, Lund M, Vestergard C, Lohse N, Deleuran M, Jensen SL, Pedersen SS, Thestrup-Pedersen K, Larsen CG. Interleukin-8 induces its own production in CD4+ T lymphocytes: a process regulated by interleukin 10. Biochemical and Biophysical Research Communications. 1995;210:660–669. doi: 10.1006/bbrc.1995.1711. [DOI] [PubMed] [Google Scholar]
- Gibbs BF, Wierecky J, Welker P, Henz BM, Wolff HH, Grabbe J. Human skin mast cells rapidly release preformed and newly generated TNF-alpha and IL-8 following stimulation with anti-IgE and other secretagogues. Experimentla Dermatology. 2001;10:312–320. doi: 10.1034/j.1600-0625.2001.100503.x. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Aster RH, Cotran RS, Corkery J, Jandl JH, Folkman J. Preservation of vascular integrity in organs perfused in vitro with a platelet-rich medium. Nature. 1969;222:33–36. doi: 10.1038/222033a0. [DOI] [PubMed] [Google Scholar]
- Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O'Donnell E, Zhao BQ, Cifuni SM, Wagner DD. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SP, Alexander KM, Dransfield I. Immune complexes bind preferentially to Fc gamma RIIA (CD32) on apoptotic neutrophils, leading to augmented phagocytosis by macrophages and release of proinflammatory cytokines. The Journal of Immunology. 2004;172:1882–1887. doi: 10.4049/jimmunol.172.3.1882. [DOI] [PubMed] [Google Scholar]
- Ho-Tin-Noe B, Demers M, Wagner DD. How platelets safeguard vascular integrity. Journal of Thrombosis and Haemostasis. 2011;9 Suppl 1:56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono M, Sone N, Endo K, Saga T, Kobayashi H, Hosono MN, Sakahara H, Yasunaga K, Konishi J. Kinetics of platelets in dogs with thrombocytopenia induced by antiglycoprotein IIb/IIIa receptor monoclonal antibody. Nuclear Medicine and Biology. 1995;22:71–76. doi: 10.1016/0969-8051(94)00072-r. [DOI] [PubMed] [Google Scholar]
- Iannacone M, Sitia G, Isogawa M, Whitmire JK, Marchese P, Chisari FV, Ruggeri ZM, Guidotti LG. Platelets prevent IFN-alpha/beta-induced lethal hemorrhage promoting CTL-dependent clearance of lymphocytic choriomeningitis virus. Proceedings of the National Academy of Sciences U S A. 2008;105:629–634. doi: 10.1073/pnas.0711200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jergens AE, Turrentine MA, Kraus KH, Johnson GS. Buccal mucosa bleeding times of healthy dogs and of dogs in various pathologic states, including thrombocytopenia, uremia, and von Willebrand's disease. American Journal of Veterinary Research. 1987;48:1337–1342. [PubMed] [Google Scholar]
- Joshi BC, Jain NC. Experimental immunologic thrombocytopenia in dogs: a study of thrombocytopenia and megakaryocytopoiesis. Research in Veterinary Science. 1977;22:11–17. [PubMed] [Google Scholar]
- Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. Journal of Clinical Investigation. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. Journal of Experimental Medicine. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, Dinarello CA. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84:4242–4248. [PubMed] [Google Scholar]
- Kenet G, Lubetsky A, Shenkman B, Tamarin I, Dardik R, Rechavi G, Barzilai A, Martinowitz U, Savion N, Varon D. Cone and platelet analyser (CPA): a new test for the prediction of bleeding among thrombocytopenic patients. British Journal of Haematology. 1998;101:255–259. doi: 10.1046/j.1365-2141.1998.00690.x. [DOI] [PubMed] [Google Scholar]
- Kitchens CS, Pendergast JF. Human thrombocytopenia is associated with structural abnormalities of the endothelium that are ameliorated by glucocorticosteroid administration. Blood. 1986;67:203–206. [PubMed] [Google Scholar]
- Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68:31–36. [PMC free article] [PubMed] [Google Scholar]
- Lazarus AH, Ellis J, Semple JW, Mody M, Crow AR, Freedman J. Comparison of platelet immunity in patients with SLE and with ITP. Transfusion Science. 2000;22:19–27. doi: 10.1016/s0955-3886(00)00004-7. [DOI] [PubMed] [Google Scholar]
- Levin J, Ebbe S. Why are recently published platelet counts in normal mice so low? Blood. 1994;83:3829–3831. [PubMed] [Google Scholar]
- Lewis DC, Meyers KM. Canine idiopathic thrombocytopenic purpura. Journal of Veterinary Internal Medicine. 1996;10:207–218. doi: 10.1111/j.1939-1676.1996.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Oppenheim JJ. Interleukin 8 and MCAF: novel inflammatory cytokines inducible by IL 1 and TNF. Cytokine. 1989;1:2–13. doi: 10.1016/1043-4666(89)91043-0. [DOI] [PubMed] [Google Scholar]
- McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103:1364–1369. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- Mehta YS, Pathare AV, Badakere SS, Ghosh K, Mohanty D. Influence of auto-antibody specificities on the clinical course in patients with chronic and acute ITP. Platelets. 2000;11:94–98. doi: 10.1080/09537100075706. [DOI] [PubMed] [Google Scholar]
- Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, Coughlin SR. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- Newton JL, Reese JA, Watson SI, Vesely SK, Bolton-Maggs PH, George JN, Terrell DR. Fatigue in adult patients with primary immune thrombocytopenia. European Journal of Haematology. 2011;86:420–429. doi: 10.1111/j.1600-0609.2011.01587.x. [DOI] [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Rackebrandt K, Gessner JE, Zirngibl H. Identification of critical antigen-specific mechanisms in the development of immune thrombocytopenic purpura in mice. Blood. 2000;96:2520–2527. [PubMed] [Google Scholar]
- Nieswandt B, Bergmeier W, Schulte V, Takai T, Baumann U, Schmidt RE, Zirngibl H, Bloch W, Gessner JE. Targeting of platelet integrin alphaIIbbeta3 determines systemic reaction and bleeding in murine thrombocytopenia regulated by activating and inhibitory FcgammaR. International Immunology. 2003;15:341–349. doi: 10.1093/intimm/dxg033. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Satoh T, Takeuchi T, Ikeda Y, Kuwana M. Critical role of CD4(+)CD25(+) regulatory T cells in preventing murine autoantibody-mediated thrombocytopenia. Experimental Hematology. 2012;40:279–289. doi: 10.1016/j.exphem.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Olsson B, Andersson PO, Jernas M, Jacobsson S, Carlsson B, Carlsson LM, Wadenvik H. T-cell-mediated cytotoxicity toward platelets in chronic idiopathic thrombocytopenic purpura. Nature Medicine. 2003;9:1123–1124. doi: 10.1038/nm921. [DOI] [PubMed] [Google Scholar]
- O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune-mediated thrombocytopenia. Journal of the American Veterinary Medical Association. 2011;238:346–352. doi: 10.2460/javma.238.3.346. [DOI] [PubMed] [Google Scholar]
- Page LK, Psaila B, Provan D, Michael Hamilton J, Jenkins JM, Elish AS, Lesser ML, Bussel JB. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. British Journal of Haematology. 2007;138:245–248. doi: 10.1111/j.1365-2141.2007.06635.x. [DOI] [PubMed] [Google Scholar]
- Panitsas FP, Theodoropoulou M, Kouraklis A, Karakantza M, Theodorou GL, Zoumbos NC, Maniatis A, Mouzaki A. Adult chronic idiopathic thrombocytopenic purpura (ITP) is the manifestation of a type-1 polarized immune response. Blood. 2004;103:2645–2647. doi: 10.1182/blood-2003-07-2268. [DOI] [PubMed] [Google Scholar]
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, Grainger J, Greer I, Hunt BJ, Imbach PA, Lyons G, McMillan R, Rodeghiero F, Sanz MA, Tarantino M, Watson S, Young J, Kuter DJ. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115:168–186. doi: 10.1182/blood-2009-06-225565. [DOI] [PubMed] [Google Scholar]
- Psaila B, Petrovic A, Page LK, Menell J, Schonholz M, Bussel JB. Intracranial hemorrhage (ICH) in children with immune thrombocytopenia (ITP): study of 40 cases. Blood. 2009;114:4777–4783. doi: 10.1182/blood-2009-04-215525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila B, Bussel JB, Frelinger AL, Babula B, Linden MD, Li Y, Barnard MR, Tate C, Feldman EJ, Michelson AD. Differences in platelet function in patients with acute myeloid leukemia and myelodysplasia compared to equally thrombocytopenic patients with immune thrombocytopenia. Journal of Thrombosis and Haemostasis. 2011;9:2302–2310. doi: 10.1111/j.1538-7836.2011.04506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clinical Cancer Research. 2003;9:5996–6001. [PubMed] [Google Scholar]
- Rodenburg RJ, van Den Hoogen FH, Barrera P, van Venrooij WJ, van De Putte LB. Superinduction of interleukin 8 mRNA in activated monocyte derived macrophages from rheumatoid arthritis patients. Annals of the Rheumatic Diseases. 1999;58:648–652. doi: 10.1136/ard.58.10.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozanski EA, Callan MB, Hughes D, Sanders N, Giger U. Comparison of platelet count recovery with use of vincristine and prednisone or prednisone alone for treatment for severe immune-mediated thrombocytopenia in dogs. Journal of the American Veterinary Medical Association. 2002;220:477–481. doi: 10.2460/javma.2002.220.477. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Pennycuik P. Capillary density in mammals in relation to body size and oxygen consumption. American Journal of Physiology. 1961;200:746–750. doi: 10.1152/ajplegacy.1961.200.4.746. [DOI] [PubMed] [Google Scholar]
- Semple JW. Animal models of immune thrombocytopenia. Annals of Hematology. 2010;89:S34–S44. doi: 10.1007/s00277-009-0882-8. [DOI] [PubMed] [Google Scholar]
- Semple JW, Provan D. The immunopathogenesis of immune thrombocytopenia: T cells still take center-stage. Current Opinion in Hematology. 2012;19:357–362. doi: 10.1097/MOH.0b013e3283567541. [DOI] [PubMed] [Google Scholar]
- Semple JW, Milev Y, Cosgrave D, Mody M, Hornstein A, Blanchette V, Freedman J. Differences in serum cytokine levels in acute and chronic autoimmune thrombocytopenic purpura: relationship to platelet phenotype and antiplatelet T-cell reactivity. Blood. 1996;87:4245–4254. [PubMed] [Google Scholar]
- Shashidharamurthy R, Hennigar RA, Fuchs S, Palaniswami P, Sherman M, Selvaraj P. Extravasations and emigration of neutrophils to the inflammatory site depend on the interaction of immune-complex with Fcgamma receptors and can be effectively blocked by decoy Fcgamma receptors. Blood. 2008;111:894–904. doi: 10.1182/blood-2007-04-085944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son KH, Lim CH, Song EJ, Sun K, Son HS, Lee SH. Inter-species hemorheologic differences in arterial and venous blood. Clinical Hemorheology and Microcirculation. 2010;44:27–33. doi: 10.3233/CH-2010-1248. [DOI] [PubMed] [Google Scholar]
- Stokol T, Brooks MB, Erb HN. Effect of citrate concentration on coagulation test results in dogs. Journal of the American Veterinary Medical Association. 2000;217:1672–1677. doi: 10.2460/javma.2000.217.1672. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Chensue SW, Standiford TJ, Basha MA, Showell HJ, Kunkel SL. Disparate gene expression of chemotactic cytokines by human mononuclear phagocytes. Biochemical and Biophysical Research Communications. 1990a;166:886–891. doi: 10.1016/0006-291x(90)90893-r. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Kasahara K, Allen R, Showell HJ, Standiford TJ, Kunkel SL. Human neutrophils exhibit disparate chemotactic factor gene expression. Biochemical and Biophysical Research Communications. 1990b;173:725–730. doi: 10.1016/s0006-291x(05)80095-6. [DOI] [PubMed] [Google Scholar]
- Su SB, Mukaida N, Matsushima K. Rapid secretion of intracellularly pre-stored interleukin-8 from rabbit platelets upon activation. Journal of Leukocyte Biology. 1996;59:420–426. doi: 10.1002/jlb.59.3.420. [DOI] [PubMed] [Google Scholar]
- Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- Suter SE, Vernau W, Fry MM, London CA. CD34+, CD41+ acute megakaryoblastic leukemia in a dog. Veterinary Clinical Pathology. 2007;36:288–292. doi: 10.1111/j.1939-165x.2007.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Tocantins LM, Stewart HL. Pathological anatomy of experimental thrombopenic purpura in the dog. American Journal of Pathology. 1939;15:1–24 29. [PMC free article] [PubMed] [Google Scholar]
- Van Damme J, Rampart M, Conings R, Decock B, Van Osselaer N, Willems J, Billiau A. The neutrophil-activating proteins interleukin 8 and beta-thromboglobulin: in vitro and in vivo comparison of NH2-terminally processed forms. European Journal of Immunology. 1990;20:2113–2118. doi: 10.1002/eji.1830200933. [DOI] [PubMed] [Google Scholar]
- von Hundelshuasen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circulation Research. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- Ware J. Dysfunctional platelet membrane receptors: from humans to mice. Thrombosis and Haemostasis. 2004;92:478–485. doi: 10.1267/THRO04090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.