Abstract
The placenta is a unique and highly complex organ that develops only during pregnancy and is essential for growth and survival of the developing fetus. The placenta provides the vital exchange of gases and wastes, the necessary nutrients for fetal development, acts as immune barrier that protects against maternal rejection, and produces numerous hormones and growth factors that promote fetal maturity to regulate pregnancy until parturition.
Abnormal placental development is a major underlying cause of Pregnancy-Associated Disorders that often result in preterm birth. Defects in placental stem cell propagation, growth, and differentiation are major factors that affect embryonic and fetal well being and dramatically increase the risk of pregnancy complications. Understanding the processes that regulate placentation is important in determining the underlying factors behind abnormal placental development. The ability to manipulate genes in a placenta-specific manner provides a unique tool to analyze development and eliminates potentially confounding results that can occur with traditional gene knockouts.
Trophoblast stem cells and mouse embryos are not overly amenable to traditional gene transfer techniques. Most viral vectors, however; have a low infection rate and often lead to mosaic transgenesis. While the traditional method of embryo transfer is intrauterine surgical implantation, the methodology reported here, combining lentiviral blastocyst infection and nonsurgical embryo transfer, leads to highly efficient and placental-specific gene transfer. Numerous advantages of our optimized procedures include increased investigator safety, a reduction in animal stress, rapid and noninvasive embryo transfer, and higher a rate of pregnancy and live birth.
Keywords: placenta, gene transfer, embryo transfer, preeclampsia, trophoblast, lentivirus
1. Introduction
Abnormal placental development has been identified as a major pathological finding associated with Pregnancy-Associated Disorders; including preeclampsia, intrauterine growth restriction, and placental insufficiency [1-4]. While the events associated with abnormal development remain under investigation, it is well established that Pregnancy-Associated Disorders can result in preterm birth, a leading cause of death in woman and children [1,2,5-8].
The placenta is the defining feature of all mammals and a unique organ that is formed transiently during pregnancy. It performs numerous functions for the developing fetus including attachment; nutrient, gas, and waste exchange; hormone secretion; ion transport; vascularization; and respiration. Gross defects in placental growth or differentiation can result in embryonic lethality. Understanding the processes that control placental organogenesis is important in determining the causes responsible for improper placental development [1-12].
Developing embryos are not overly amenable to traditional gene transfer techniques. Most viral vectors have a low rate of transduction, can lead to mosaicism, and are prone to gene silencing. Embryo transfer has traditionally been performed by surgical implantation. Our data indicate that by combining lentiviral blastocyst infection with nonsurgical intrauterine embryo transfer, we are able to achieve highly efficient and placental-specific gene transfer in mice. Lentivirally transduced genes have been shown to be expressed in vivo and are predominantly free of silencing [13-15].
Lentiviral gene transfer at the blastocyst stage of embryonic development has recently been shown to lead to placental-specific gene transfer [9,10,12,16,17]. Lentiviral transduction specifically targets gene transfer to the trophectoderm, the future placenta, of the developing blastocyst without altering the maternal or fetal genome and provides an important advantage over traditional gene knockouts. Placental transgenesis provides an important tool to evaluate organogenesis and assess new treatments for Pregnancy-Associated Disorders [9-11].
In this study, we have combined and optimized lentivirally-induced, placental-specific transgenesis with nonsurgical embryo transfer. This method provides a rapid, highly efficient, and cost effective method of embryo transfer to study the role of the placenta in development and disease states [18]. This method also eliminates the complications associated with the use of anesthetics and analgesics that occur during and after traditional surgical embryo transfer, while reducing maternal stress and producing a high rate of pregnancy and live birth [19].
2. Materials and Methods
2.1. Materials
M2 Media (#NC9460621), M2 Powder (#50-582-610), KSOM powder (#NC9505463), Polybrene (#NC9515805) were all obtained from Fisher Scientific. Non-surgical embryo transfer devices (#60010) were purchased from Paratechs Corporation. Mouse embryo culture Mineral Oil (#M8410), Acidic Tyrode's Solution (#T1788-100ml), Pregnant Mare Serum gonadotropin (PMSg) (G4877), and recombinant Human Chorionic Gonadotropin (hCG) (#C6322) were obtained from Sigma Aldrich Co. Metafectene (#T020-1.0) was obtained from Biontex. HIV type 1 p24 antigen ELISA 2.0 (#0801002) was purchased from ZeptoMetrix.
2.2. Induction of Superovulation and Pseudopregnancy
Upon arrival, animals were allowed to acclimate at least one week prior to the induction of superovulation and pseudopregnancy. To induce superovulation, C57BL/6 females (four-five weeks of age) were injected with 5IU of pregnant mare serum gonadotropin (PMSg) intraperitoneally (IP) at noon and subsequently injected IP 47 hours later with 5IU human chorionic gonadotropin (hCG). The primed females were then introduced to C57BL/6 adult males for overnight mating. C57BL/6 females were checked for a copulation plug the following morning and embryos were designated as embryonic day 0.5 (E0.5) [Table 1]. To produce pseudopregnant females, vasectomized ICR male bedding was added to the ICR female cages three days prior to mating to help induce estrus. All cages were kept undisturbed until the day of mating, when ICR female mice were introduced to the vasectomized male cage. Females were removed the following morning, checked for a copulation plug, and designated 0.5 days post coitum (0.5 dpc) [Table 1].
Table 1. Coordinated Timeline for Creating Transgenic Placentas via Lentiviral Infection and Nonsurgical Embryo Transfer.
The Upper Panel shows the timeline to produce large numbers of superovulated embryos and lentiviral infection. The Lower Panel shows the coordinated induction of pseudopregnancy and NSET to produce transgenic placentas.
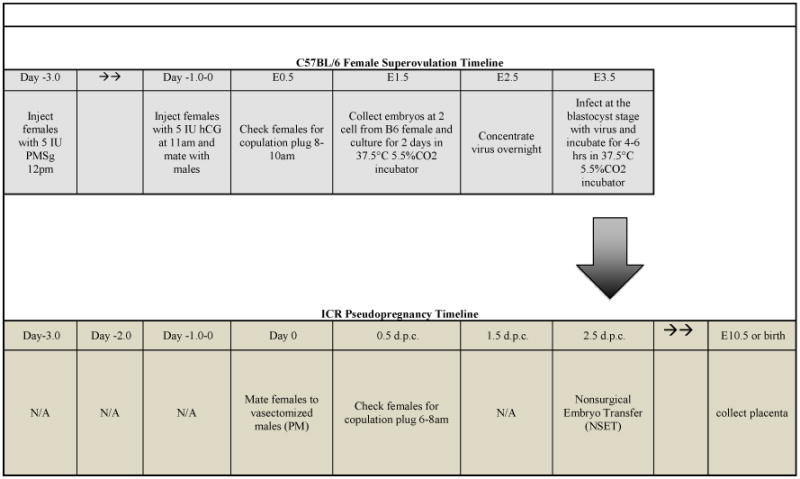
|
2.3. Two Cell Isolation and Microdrop Culture
Oviducts were obtained from superovulated C57BL/6 females at E1.5 for 2-cell isolation. The oviducts were teased apart and embryos were collected in M2 media. M2 media was used at room temperature within two hours, and has a shelf life of two weeks at 4°C. Isolated 2-cell embryos were placed in pre-equilibrated KSOM microdrops and cultured until E3.5. Microdrops were prepared by pipetting five, individual, 20μl drops of KSOM media in a p35 tissue culture plate and covered in 2ml of mineral oil. KSOM culture media was prepared according to the manufacturer's instructions and was pre-equilibrated overnight at 37.5°C and 5.5% CO2. KSOM culture media has a shelf life of two weeks at 4°C after preparation.
2.4. Lentiviral Production, Concentration, and Titer
293FT cells were cultured in DMEM/High glucose, 10% heat-inactivated fetal bovine serum (Biowest, S01520), 1% antibiotic-antimycotic (Thermo Scientific, SV30079.01), 1 mM sodium pyruvate (Mediatech, 25-000-CI), 2 mM glutaGRO Supplement (Mediatech, 25-105-CI), 0.1 mM NEAA Mixture (Lonza, 13-114E) and 500 μg/mL Geneticin sulfate (G418, InvivoGen, ant-gn) and transfected with pLv-[GFP]-V5 (lentiviral green fluorescent protein) and Virapower Lentiviral Packaging Mix (Invitrogen, K4975-00) using Metafectene as described by the manufacturer. Cell culture media was changed 24 hours post transfection and virus was collected at 48-65 hours. Virus containing media was centrifuged for 20 minutes at 3,000 rpm at room temperature and the supernatant was stored at −80°C. To concentrate lentivirus, viral supernatant (2ml) was mixed with 5× PEG-it viral precipitation solution (500μl) (System Biosciences, Lv810A-1) and incubated overnight at 4°C. The solution was centrifuged at 3,000 rpm for 30 minutes at room temperature and the lentiviral pellet was resuspended in 1XPBS and polybrene (8μg/ml) in a volume of 20μl. Dilutions of 1/80,000 and 1/200,000 of the concentrated virus were then used to determine the viral titer via use of HIV-1 p24 Antigen ELISA 2.0 according to the manufacturer's instructions. Briefly, samples were added to the microplate and incubated for 24 hours at 37 °C. The samples were subsequently washed and incubated with HIV-1 Detector Antibody for 1 hour at 37°C. The microplate was washed, substrate was added and then incubated at room temperature for 30 min. The reaction was stopped by adding Stop Solution and the plate was read within 15 min at 450 nm on an ELISA plate reader.
2.5. Blastocyst Infection and Nonsurgical Embryo Transfer
20μl of titered virus (1,500 ng/ml) was placed as a microdrop covered in mineral oil and allowed to incubate for 30 minutes at 37.5°C and 5.5% CO2. While the virus is equilibrating, blastocysts were removed from KSOM culture then washed and held in one 40μl drop of M2 media. To remove the zona pellucida, blastocysts were transferred and kept suspended in a 20μl drop of Acidic Tyrode's Solution until the zona pellucida was visibly removed. It is extremely important to keep blastocysts suspended and moving while in Acidic Tyrode's Solution to keep them from adhering. Upon zona pellucida dissolution, blastocysts were immediately and repeatedly washed in M2 media and then placed into a 40μl drop. 5μl of the above blastocyst-containing M2 media was added to 20μl of the titered virus (1,500 ng/ml) for a final viral infection rate of 1,200 ng/ml (25μl) and incubated 4-6 hours at 37.5°C, 5.5% CO2. Post-infection, blastocysts were washed extensively and held in M2 media. For nonsurgical embryo transfer, 10-20 blastocysts in M2 media (1.8μl) were expelled into the uterine horn of an unanesthetized 2.5 dpc pseudopregnant female according to the manufacturer's instructions. No post-operative care was required and surrogate (pseudopregnant) mothers were routinely monitored to ensure continual weight gain, indicative of pregnancy. A recent study has shown that when using viral vectors, animal bedding is safe to handle under normal BSL1 conditions 72 hours post infection [20].
2.6. Placental and Embryonic Dissection and Analysis
Following infection of blastocysts with a lentivirally-packaged, GFP-reporter construct and subsequent transfer to pseudopregnant females; placentas and developing embryos were dissected as previously described [21]. Resulting placentas and corresponding embryos were assessed for GFP expression by epifluorescence. Tissue sections (6μM) were processed and analyzed by epifluorescence or in situ hybridization with a cRNA probe to detect and confirm GFP mRNA expression [21-23].
2.7. Institutional Approvals
The Wright State University Institutional Biosafety Committee and the Laboratory Animal Care and Use Committee approved all experiments and procedures performed.
3. Results
The contribution the placenta provides to the developing embryo is essential to life. The ability to create placenta-specific transgenic mice provides a novel tool to analyze placental development and eliminates confounding results that occur in traditional knockouts that genetically alter both the embryo and placenta. Table 1 illustrates the coordinated methods used to produce high levels of superovulated and pseudopregnant females in preparation for lentiviral infection and embryo transfer.
Lentiviral GFP infection at the blastocyst stage of development resulted in highly-specific, uniform, and restricted expression to the external, single-celled trophectoderm, which gives rise to the placenta (Figure 1A). It is important to note that the cells of the inner cell mass, which give rise to the baby, are not infected. This level of specificity was confirmed by analyzing the expression in the entire fetal-maternal unit at E10.5, which exhibits localized and appropriate developmental restriction during early placenta formation (Figure 1B). Placental-specific expression in the trophoblast giant cells and the labyrinth were further verified in sectioned placentas at E10.5. Strong expression of GFP was observed in parietal trophoblast giant cells (*) that separate the maternal uterine decidua from the fetal-derived placenta (Figure 1C). GFP expression is also clearly evident in trophoblast cells of the labyrinth associated with both maternal (m) and fetal (f) blood spaces (Figure 1D). Examination at E12.5 further demonstrated that GFP expression was solely restricted to, but throughout, the entire placenta and was not expressed in embryonic or maternal decidual tissue (Figure 1E).
Figure 1. Trophoblast-specific epifluorescent GFP expression.
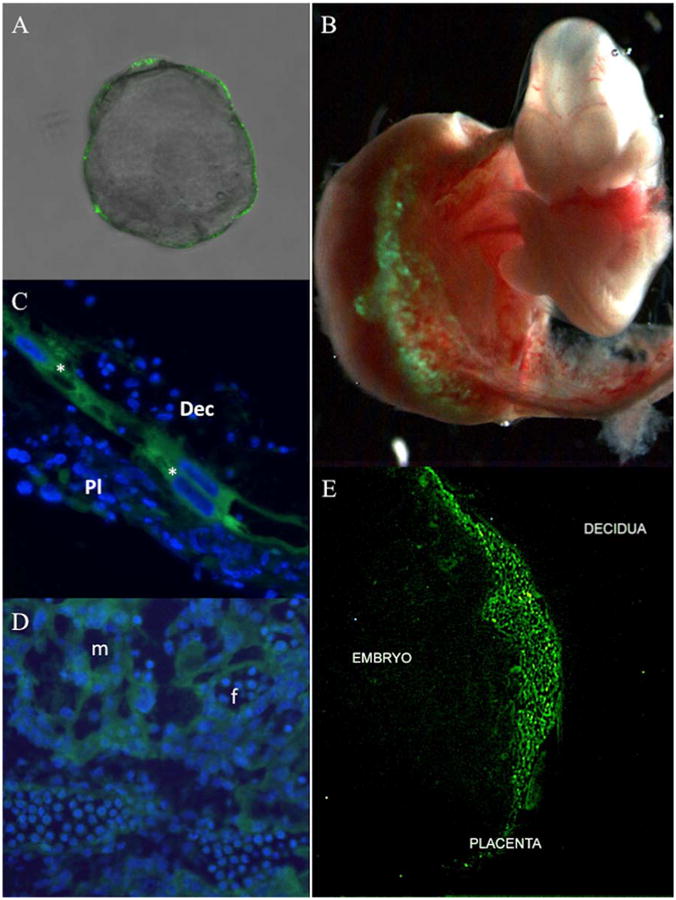
Mouse blastocysts (E3.5) devoid of zona pellucida were infected with Lv-CMV-GFP-V5 lentivirus for 4.5 hours and subsequently assessed for GFP expression via epifluorescent microscopy (A). Mouse blastocysts (E3.5) devoid of zona pellucida were infected with Lv-CMV-GFP-V5 and transferred into a pseudo-pregnant female. The feto-placental unit was collected at E10.5 and examined for GFP expression via epifluorescent microscopy (B). E10.5 placentas were sectioned to analyze GFP expression by epifluorescent microscopy in the region that distinguishes the maternal decidua from the developing placenta; (*) parietal trophoblast giant cells, (Dec) maternal uterine decidua, and (Pl) fetal-derived placenta (C); as well as trophoblasts in the placental labyrinth (D); (m) maternal and (f) fetal blood spaces are indicated. An E12.5 feto-placental unit was evaluated for GFP expression by epifluorescent microscopy that demarcates maternal (decidua), placental, and embryonic (embryo) tissue (E). Nuclei (blue) were stained with DAPI.
Placental GFP expression was maintained throughout pregnancy and was also present at birth (E20). mRNA in situ hybridization conducted on sections from fixed and paraffin-embedded placentas at E14.5 and E20 showed broad expression of GFP mRNA (Figure 2). Compared to uninfected control, which showed no expression (Figure 2A), GFP expression at E14.5 was detected in trophoblast giant cells (Figure 2B) as well as in spongiotrophoblast and to a lesser extent, glycogen trophoblast. In the labyrinth, expression was consistently observed in trophoblast cells associated with both maternal and fetal blood spaces (Figure 2C) in a pattern similar to and consistent with that of Syncytin-a expression, a lineage marker of syncytiotrophoblasts [22]. At E20, expression of GFP remained strong and was observed in trophoblast cells of both the labyrinth layer and junctional zone (Figure 2D). Our results also indicate that blastocyst infection and nonsurgical embryo transfer produces viable live births, as indicated by ICR pseudopregnant females (albino) that underwent embryo transfer after implantation with C57Bl/6 (black) blastocysts (Figure 3).
Figure 2. In situ hybridization of GFP expression at E14.5 and E20.
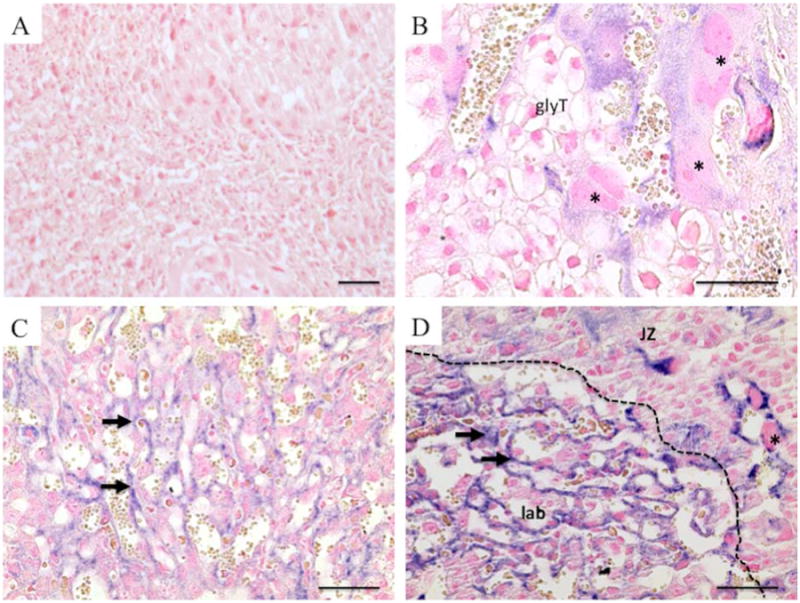
In situ hybridization was conducted on paraffin-embedded, 6 uM sections of control uninfected placentas (A, E14.5) and placentas derived from lentivirally-GFP transduced blastocysts collected at E14.5 (B, C) as well as E20 (D). Positive staining for GFP mRNA expression is stained purple. Arrows denote syncytiotrophoblasts; trophoblast giant cells are indicated by asterisks (*) and glycogen trophoblasts (glyT). (JZ) denotes the placental junctional zone and (lab) denotes the placental labyrinth. Scale bar = 100 μm.
Figure 3. Intrauterine Nonsurgical Embryo Transfer.

C57BL/6 two cell embryos were collected from superovulated females and cultured to the blastocyst stage. Blastocysts were transferred via noninvasive embryo transfer into albino ICR pseudopregnant females (foster moms) and allowed to develop to term. Shown is a foster mom (albino) and embryo transferred C57BL/6 (black) blastocysts that developed into offspring.
A previous report indicated a comparable rate of live births between traditional surgical and nonsurgical embryo transfer of approximately 24% [18]. Our data however, demonstrate that optimizing several factors, substantially elevates the rate of live births to 38%. In particular, a dedicated incubator maintained at 37.5°C and 5.5% CO2 increased the number of viable blastocysts. The use of M2 media, in comparison to KSOM or M16 media, during embryo transfer consistently produced a higher rate of pregnancy. Furthermore, the ability to perform embryo transfer without the need for surgery or anesthetics and the elimination of postoperative surgical recovery produced superior results in our studies compared to a currently published method [18]. Our results are consistent with a recent report that indicates that the nonsurgical embryo transfer reduces maternal stress levels compared to traditional surgical implantation [19].
4. Discussion and Conclusion
In this study, we have optimized numerous factors that contribute to higher rates of successful embryo transfer and placental transgenesis. Numerous advantages include the following: the use of a viral precipitate reagent, which increases investigator safety by substantially reducing the increased risk of aerosolization that is present in traditional viral concentration methods that can occur with ultracentrifugation as well as eliminating mouth pipetting of virally infected embryos. The addition of male bedding to female cages 3 days prior to mating, although not quantitatively determined, resulted in increased numbers of pseudopregnant females. This increase may be a result of female estrus induction in response to male scent factors. The use of nonsurgical embryo transfer provides a rapid and noninvasive method that reduces animal stress due to the elimination of surgery and post-surgical anesthetics. The increase in temperature and carbon dioxide likely maintained pH stability in KSOM culture media leading to higher rates of blastocyst viability [24]. Furthermore, the use of M2 as the optimal nonsurgical embryo transfer media is consistent with previous surgical uterine transfer techniques [24].
The advantages of nonsurgical embryo transfer with lentivirally-infected blastocysts to achieve placental-specific gene transfer opens exciting new avenues of research on placental development and the origins of Pregnancy-Associated Disorders, such as preeclampsia, intrauterine growth restriction, and placental insufficiency. Using a strong global promoter to drive GFP or luciferase markers can provide whole placental analysis at numerous levels, including bioimaging [25]. In addition, studies with a tet-inducible promoter and placental-specific gene transfer could be used to assess specific time windows critical to placental and embryonic development [26]. Promoter-specific constructs, such as PLII, Tpbpa, or hCYP, provide the ability to analyze cell-specific placental functions [27-29]. Lineage-specific and inducible promoters will allow for transgenic overexpression or gene knockdown and will provide a powerful new cross-disciplinary approach to assess numerous aspects of reproductive, placental, and developmental function. Furthermore, using this optimized methodology will accelerate our ability to assess and identify Placental-Associated Disorders and design treatments that originate specifically due to placental defects.
Acknowledgments
The pLv-GFP-V5 construct was kindly provided by Dr. Steven Berberich (Wright State University). Special thanks to Dr. David Ladle, Jackie Sisco, and the Microscopy core for their help. This work was supported in part by NIH grant HD059969 (TLB) and The Wright State University Endowment for Research on Pregnancy-Associated Disorders (TLB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circ. 2011;123:2856–2869. doi: 10.1161/CIRCULATIONAHA.109.853127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Preg. 2011 doi: 10.1155/2011/214365. Article ID 214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts JM, Escudero C. The placenta in preeclampsia. Preg Hypertension. 2001;2:72–83. doi: 10.1016/j.preghy.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross JC. Placental function in development and disease. Reprod Fertil Dev. 2006;18:71–76. doi: 10.1071/rd05121. [DOI] [PubMed] [Google Scholar]
- 5.CDC – The Centers for Disease Control and Prevention. 2014 Jan 2; www.http://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm.
- 6.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118:1566–73. doi: 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 7.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–59. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Ratajczak CK, Fay JC, Muglia LJ. Preventing preterm birth: the past limitations and new potential of animal models. Dis Model Mech. 2010;3:407–14. doi: 10.1242/dmm.001701. [DOI] [PubMed] [Google Scholar]
- 9.Malashicheva A, Kanzler B, Tolkunova E, Trono D, Tomilin A. Lentivirus as a tool for lineage specific gene manipulations. Genesis. 2007;45:456–9. doi: 10.1002/dvg.20313. [DOI] [PubMed] [Google Scholar]
- 10.Cross JC. Lentiviruses to the placental rescue. Nat Biotechnol. 2007;25:190–1. doi: 10.1038/nbt0207-190. [DOI] [PubMed] [Google Scholar]
- 11.Gultice AD, Kulkarni-Datar K, Brown TL. Hypoxia-inducible factor 1alpha mediates distinct steps of rat trophoblast differentiation in gradient oxygen. Biol Reprod. 2009;80:184–9. doi: 10.1095/biolreprod.107.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada Y, Ueshin Y, Isotani A, Saito-Fujita T, Nakashima H, Kimura K, et al. Complementation of placental defects and embryonic lethality by trophoblast-specific lentiviral gene transfer. Nat Biotechnol. 2007;25:233–237. doi: 10.1038/nbt1280. [DOI] [PubMed] [Google Scholar]
- 13.Ikawa M, Tanaka N, Kao WW, Verma IM. Generation of transgenic mice using lentiviral vectors: a novel preclinical assessment of lentiviral vectors for gene therapy. Mol Ther. 2003 Oct;8(4):666–73. doi: 10.1016/s1525-0016(03)00240-5. [DOI] [PubMed] [Google Scholar]
- 14.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002 Feb 1;295(5556):868–72. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 15.Jähner D, Stuhlmann H, Stewart CL, Harbers K, Löhler J, Simon I, Jaenisch R. De novo methylation and expression of retroviral genomes during mouse embryogenesis. Nature. 1982 Aug 12;298(5875):623–8. doi: 10.1038/298623a0. [DOI] [PubMed] [Google Scholar]
- 16.Georgiades P, Cox B, Gertsenstein M, Chawengsaksophak K, Rossant J. Trophoblast specific gene manipulation using lentivirus-based vectors. Biotechniques. 2007;42:322–5. doi: 10.2144/000112341. [DOI] [PubMed] [Google Scholar]
- 17.Kumasawa K, Ikawa M, Kidoya H, Hasuwa H, Saito-Fujita T, Morioka Y, et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc Natl Acad Sci USA. 2011;108:1451–1455. doi: 10.1073/pnas.1011293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green MA, Bass S, Spear BT. A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications. Biotechniques. 2009;47:919–924. doi: 10.2144/000113257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steele KH, Hester JM, Stone BJ, Carrico KM, Spear BT, Fath-Goodin A. Nonsurgical embryo transfer device compared with surgery for embryo transfer in mice. J Am Assoc Lab Anim Sci. 2013;52:17–21. [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter JD, Fang X, Ly CS, Suter KK, Gibbs D. Assessment of Hazard Risk Associated with the Intravenous Use of Viral Vectors in Rodents. Comparative Medicine. 2012;62:361–370. [PMC free article] [PubMed] [Google Scholar]
- 21.Natale DR, Starovic M, Cross JC. Phenotypic analysis of the mouse placenta. Methods Mol Med. 2006;121:275–93. doi: 10.1385/1-59259-983-4:273. [DOI] [PubMed] [Google Scholar]
- 22.Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135:2083–91. doi: 10.1242/dev.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes M, Natale BV, Simmons DG, Natale DR. Ly6e expression is restricted to syncytiotrophoblast cells of the mouse placenta. Placenta. 2013;34:831–5. doi: 10.1016/j.placenta.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. 3rd. New York: Cold Spring Harbor Laboratory Press; 2003. [Google Scholar]
- 25.Fan X, Ren P, Dhal S, Bejerano G, Goodman SB, Druzin ML, et al. Noninvasive monitoring of placenta-specific transgene expression by bioluminescence imaging. PLoS One. 2011;6:e16348. doi: 10.1371/journal.pone.0016348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan X, Petitt M, Gamboa M, Huang M, Dhal S, Druzin ML, et al. Transient, inducible, placenta-specific gene expression in mice. Endocrinology. 2012;153:5637–44. doi: 10.1210/en.2012-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shida MM, Jackson-Grusby LL, Ross SR, Linzer DI. Placental-specific expression from the mouse placental lactogen II gene promoter. Proc Natl Acad Sci USA. 1992;89:3864–8. doi: 10.1073/pnas.89.9.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calzonetti T, Stevenson L, Rossant J. A novel regulatory region is required for trophoblast specific transcription in transgenic mice. Dev Biol. 1995;171:615–26. doi: 10.1006/dbio.1995.1309. [DOI] [PubMed] [Google Scholar]
- 29.Kamat A, Graves KH, Smith ME, Richardson JA, Mendelson CR. A 500-bp region, approximately 40 kb upstream of the human CYP19 (aromatase) gene, mediates placenta-specific expression in transgenic mice. Proc Natl Acad Sci USA. 1999;96:4575–80. doi: 10.1073/pnas.96.8.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]


