Abstract
Adams-Oliver syndrome (AOS) is a rare malformation syndrome characterized by the presence of two anomalies: aplasia cutis congenita of the scalp and transverse terminal limb defects. Many affected individuals also have additional malformations, including a variety of intracranial anomalies such as periventricular calcification in keeping with cerebrovascular microbleeds, impaired neuronal migration, epilepsy, and microcephaly. Cardiac malformations can be present, as can vascular dysfunction in the forms of cutis marmorata telangiectasia congenita, pulmonary vein stenoses, and abnormal hepatic microvasculature. Elucidated genetic causes include four genes in different pathways, leading to a model of AOS as a multi-pathway disorder. We identified an infant with mild aplasia cutis congenita and terminal transverse limb defects, developmental delay and a severe, diffuse angiopathy with incomplete microvascularization. Whole-genome sequencing documented two rare truncating variants in DOCK6, a gene associated with a type of autosomal recessive AOS that recurrently features periventricular calcification and impaired neurodevelopment. We highlight an unexpectedly high frequency of likely deleterious mutations in this gene in the general population, relative to the rarity of the disease, and discuss possible explanations for this discrepancy.
Keywords: Adams-Oliver syndrome, cutis aplasia, transverse terminal limb defects, brain malformation, DOCK6, RAC1, vasculogenesis, angiogenesis, pericyte
INTRODUCTION
Adams-Oliver syndrome (AOS) is a developmental disorder featuring the combination of aplasia cutis congenita of the scalp and transverse terminal limb defects, with or without other additional characteristic features, such as cutis marmorata telangiectatica congenita (20%) or other vascular abnormalities, cardiac malformations (20%), or neurological impairments [Snape et al., 2009]. It has an estimated incidence of 0.44/100,000 live births [Martínez-Frías et al., 1996], and it appears to be a highly heterogeneous disorder, with autosomal dominant and autosomal recessive forms. To date, two genes causing dominant forms, ARHGAP31 and RBPJ, and two genes causing recessive forms, DOCK6 and EOGT, have been discovered [Hassed et al., 2012; Southgate et al., 2011; Shaheen et al., 2011b; 2013; Cohen et al., 2013]. Many children with AOS are the only affected members of their family, and for them the mode of inheritance is unclear.
We report on the eighth patient with AOS caused by DOCK6 mutations and delineate the extensive angiopathy that accompanies this form of AOS. Individuals with this type of AOS have shown (in addition to transverse limb and scalp defects) microcephaly, optic nerve hypoplasia, variable developmental delay, seizures, dilated ventricles, periventricular calcifications, gastroschisis in one patient, pachygyria in one patient, and aortic valve dysplasia in one patient [Shaheen et al., 2011b; 2013]. Dedicator of cytokinesis 6 (DOCK6) is a guanine nucleotide exchange factor regulator of Cdc42 that activates Rho family guanosine triphosphatases Rac1 and Cdc42, and is required for normal function of the actin cytoskeletal structure. It is highly expressed in the growing edge of embryonic limb buds and in the heart in mice [Shaheen et al., 2011b].
MATERIALS AND METHODS
Whole-genome resequencing
Informed consent to participate in this study was provided by the family, and the protocol was approved by our institutional review board (H08-02077). DNA was extracted from peripheral blood from the proband and from saliva from the mother (DNA Genotek kit; Orasure Technologies, Bethlehem, USA) and quantified with picogreen (Qubit; Invitrogen, Carlsbad, USA). Whole-genome resequencing and variant calling was performed by Complete Genomics, Inc. (CGI; Mountain View, USA). Further variant annotation and analysis was performed with Ingenuity Systems Variant Analysis (Redwood City, USA) and the Family Genomics Toolkit (familygenomics.systemsbiology.net/software) developed at the Institute for Systems Biology (ISB) [Glusman et al., 2011]. Candidate variants were verified with Sanger sequencing.
Genomic microarray analysis
Genomic microarray analysis was performed on DNA from peripheral blood lymphocytes using the Genome Wide Human SNP Array 6.0 (Affymetrix, Santa Clara, USA). The data were analyzed with Genotyping Console v4.0 and Chromosome Analysis Suite v1.1 (Affymetrix). Copy number variants were called according to a 400 Kb threshold for a copy number gain and a 200 Kb threshold for a copy number loss.
Determination and comparison of DOCK6-AOS carrier frequency
Population frequencies of likely damaging DOCK6 alleles were compared to other recessive genes in order to estimate relative carrier frequency. Occurrences of stop-gain and canonical splice site variants were counted in DOCK6 and 19 other genes causative of similarly rare autosomal recessive disorders. Indels were not included because of the associated high false positive rate, and missense variants were not included because of suboptimal performance of in silico predictors for physiological effect of the altered protein. For estimating variant frequencies, we used Kaviar [Glusman et al., 2011]. In addition to 1000 Genomes (1000G) and the Exome Sequencing Project (ESP), Kaviar takes into account publically available personal genomes and exomes and also ISB’s in-house genomes, and combines all available data to compute integrated variant frequencies.
RESULTS
Clinical report
The proband was born to a healthy 36-year-old mother with two children and three spontaneous miscarriages from a prior relationship. Both parents are of northern European descent, are unrelated to one another and have unremarkable family histories. The father was unavailable for examination or testing. The mother had no evidence of distal limb reduction or cutis aplasia. There was a small amount of cigarette smoke exposure throughout the pregnancy. Mild symmetric intrauterine growth restriction was first noted at 19 weeks gestation and remained stable. Short digits and toes were queried on prenatal sonography. A female was delivered at 39 weeks gestation by emergency Cesarean for a non-reassuring fetal heart rate and resuscitation was required. Meconium was present in the amniotic fluid. Apgar scores were 2 at 1 minute, 4 at 5 minutes and 6 at 10 minutes. The umbilical cord pH was 7.15. Birth weight was 1990 g (− 3.2 SD), length was 42 cm (− 2.7 SD), and head circumference was 28.5 cm (− 1.6 SD). Placental pathology showed findings that suggested a diffuse thrombotic vasculopathy, in addition to smaller, overly tortuous vessels with poorly formed smooth muscle layers (Fig. 1). Some sections showed increased thickness of the smooth muscle layer with possible secondary stenosis while other areas showed very poor or asymmetric smooth muscle coverage of the placental vessels. Hypoglycemia occurred shortly after birth and was promptly treated. Thrombocytopenia, requiring platelet transfusion, was present until 12 days of age. Terminal transverse limb defects of the feet and hands were present with syndactyly of the second, third and fourth toes bilaterally (Fig. 2). There were multiple hairless patches of atrophic skin on the anterior scalp (Fig. 2).
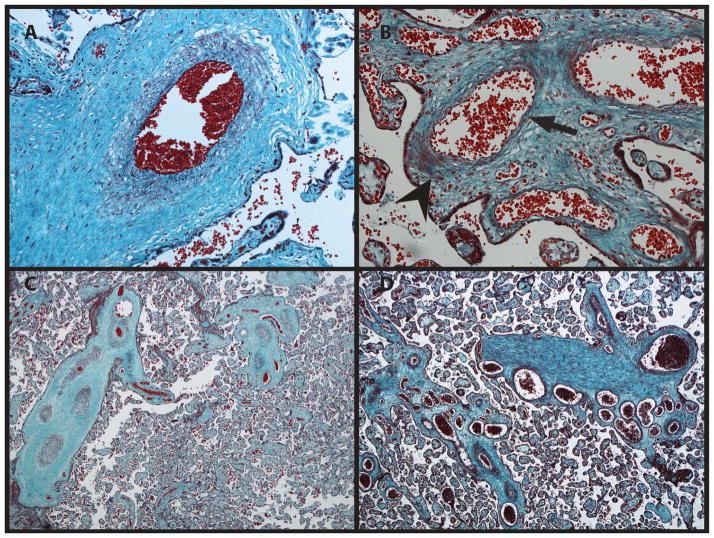
FIG. 1.
A. Microscopic image of a normal placenta at 200X stained with Masson trichrome. B. The patient’s placenta at 200X. The smooth muscular layer is markedly abnormal with an asymmetric layer of myocytes (arrowhead: clumped myoctyes; arrow: poor myocyte coverage), in keeping with failed pericyte migration. C. Control placenta at 40X. D. The patient’s placenta at 40X. The vessels are ectatic. A cluster of numerous veins is in keeping with tortuosity.
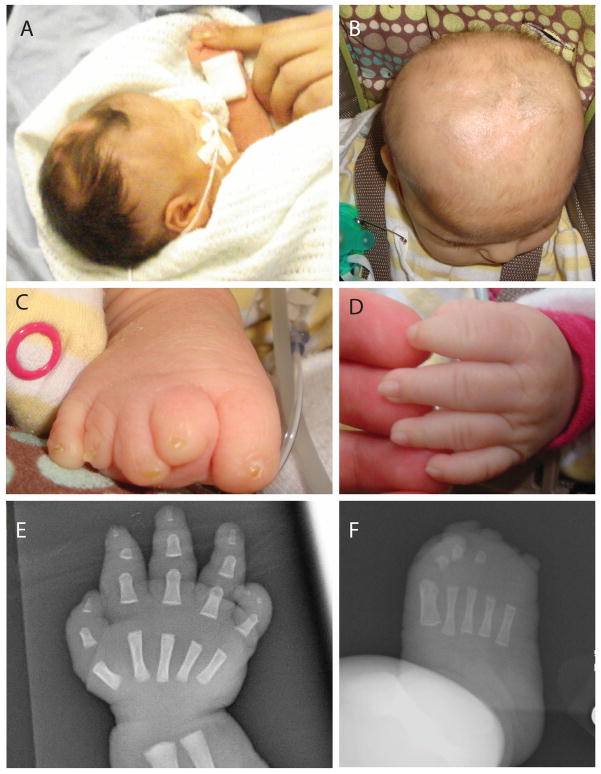
FIG. 2.
Photographs demonstrate hairless patches of the scalp at birth (A) and 4 months of age (B). The right foot shows distal hypoplasia of the toes and nails with partial soft tissue syndactyly of the second and third toes (C). Distal digital tapering, but an otherwise relatively normal left hand at 4 months of age (D). A radiograph of the right hand at 2 days shows appropriate bone formation with soft tissue tapering (E). A radiograph of the right foot shows hypoplastic bony structures of the 1st, 2nd, and 3rd toes, with no definite ossification beyond this.
An echocardiogram revealed tetralogy of Fallot plus bilateral superior vena cavae with the left draining into the coronary sinus. A renal ultrasound was normal. An ultrasound of her brain showed multiple small densities in the white matter as well as a hemorrhagic lesion in the posterior superior region of the right temporal lobe. A focal outpouching of the left lateral ventricle was in keeping with porencephaly. PCR and culture of a urine sample showed no evidence of cytomegalovirus infection. A brain MRI confirmed white matter insults of various ages. MR spectroscopy was normal.
At 15 days of age, she required resection of ischemic small bowel from the mid-jejunum to the ileocecal valve, and thereafter required total parental nutrition. Histopathology of the bowel wall documented numerous calcifications. Poor visual fixation was noted and an ophthalmology assessment found incomplete retinal vascularization, involving the maculae, and several focal hemorrhages. Vitrectomy was required for a total right-sided retinal detachment at 13 months. In her second year she began to experience generalized tonic-clonic seizures. Development has been markedly impaired with no independent sitting or speech by two years of age. Growth has been restricted despite optimization of her gastrostomy feeds and TPN.
Genetic findings
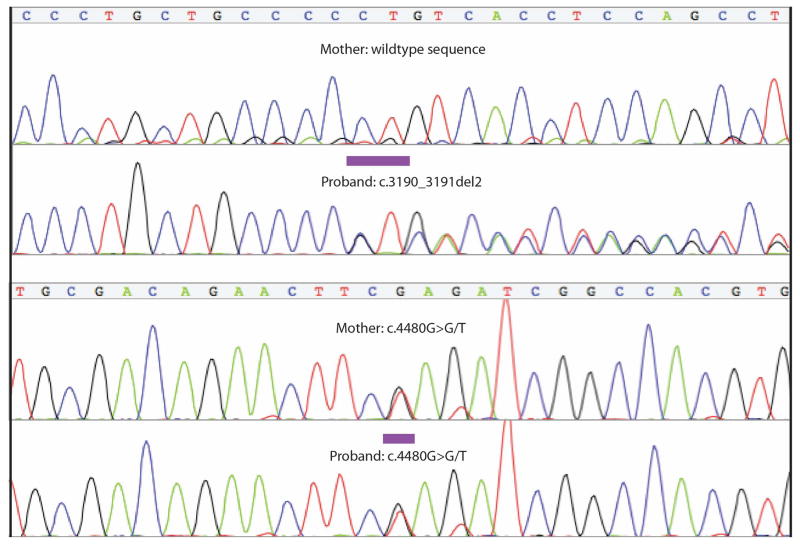
Genomic microarray analysis showed a normal 46, XX chromosomal complement. A normal G-banded karyotype at 550 band level resolution was also obtained, which ruled out a balanced translocation. Whole-genome resequencing with 47-fold average coverage identified 207,406 variants with <1% allele frequency (“rare”) as represented in 1000 Genomes, public access genomes from CGI, and the NHLBI Exome Sequencing Project (ESP). No rare, non-synonymous variants were evident in ARHGAP31, RBPJ, or EOGT. We observed two rare, putatively detrimental variants in DOCK6: a frameshift mutation at c.3190_3191del2 (p.L1064Vfs*60) with an allele frequency of 0.02% (computed using Kaviar) [Glusman et al., 2011], and a stop gain, c.4480G>T (p.E1494*), which had not been previously reported. Sanger sequencing confirmed the presence of both variants in the proband and showed the mother carries the novel stop gain mutation but not the previously observed deletion. These observations suggest compound heterozygosity in the proband (Fig. 3).
FIG. 3.
Chromatograms of c.3190_3191del2 in the proband and c.4480G>T in the mother and proband.
The carrier frequency for heterozygous stop-gain and splice-site single nucleotide variants (SNVs) in DOCK6 in the general population is 0.18% (Table I), which corresponds to a theoretical incidence under a simple recessive model of 0.34 per 100,000 individuals and would imply that 77% of the observed AOS cases are due to these variants. This frequency is greater than expected considering 1) the rarity of AOS, 2) the likelihood that DOCK6 accounts for a minority of the disease (other independent causal genes have been reported), and 3) the exclusion from the analysis of indels and missense variants, which could also cause disease. Comparisons of carrier frequency for stop-gain and splice variants with other similarly rare recessive disorders predicted incidence ranges of 0.3 – 1 per 100,000. For the majority of these genes, variant frequencies are low and consistent with the observed disease incidences. Several other disease genes, however, show higher than expected frequencies of likely damaging variants: in other words, the theoretical incidence of disease due to this subset of possible mutations approaches or exceeds the corresponding observed incidence.
TABLE I.
Frequency of stop-gain and splice site variants in DOCK6 and other autosomal recessive disease genes, observed in 1000 Genomes, the Exome Variant Server, and other databases, as mined by Kaviar (http://db.systemsbiology.net/kaviar/). A theoretical incidence for each disorder, based on the frequency of these variants, has been calculated and then compared to the clinically observed incidence. Disorders with greater than expected ratios of theoretical incidence to the observed incidences have been highlighted in the final column.
| Disease | Observed incidence per 100,000 | Gene | % of disease caused by gene | Frequency of truncating mutations (%) | Theoretical disease incidence per 100,000 | Ratio of theoretical to observed incidence |
|---|---|---|---|---|---|---|
| Alstrom syndrome | 0.14 | ALMS1 | 50% | 0.1575 | 0.248 | 1.772 |
| GM1 gangliosidosis | 0.5 (1) | GLB1 | 100% | 0.1344 | 0.181 | 0.361 |
| Alkaptonuria | 0.5 | HGD | 100% | 0.0068 | 0.0005 | 0.001 |
| Hurler syndrome | 0.57 | IDUA | 100% | 0.171 | 0.292 | 0.513 |
| Wolfram syndrome | 0.57 | WFS1 | >90% | 0.0962 | 0.093 | 0.162 |
| Netherton syndrome | 0.5 | SPINK5 | 100% | 0.0805 | 0.065 | 0.130 |
| Argininosuccinic aciduria | 0.45 | ASL | 100% | 0.0623 | 0.039 | 0.086 |
| Shwachman- Diamond syndrome | 0.55 | SBDS | >90% | 0.2924 | 0.855 | 1.555 |
| Werner syndrome | 0.45 | WRN | 90% | 0.2116 | 0.448 | 0.995 |
| Jervell and Lange-Nielson syndrome | 0.3 | KCNQ1 | 90% | 0.0136 | 0.002 | 0.006 |
| Niemann Pick A/B disease | 0.4 | SMPD1 | 100% | 0.034 | 0.012 | 0.029 |
| Bardet-Biedl syndrome | 0.7 | BBS1 | 23% | 0.0407 | 0.017 | 0.024 |
| AR malignant osteopetrosis | 0.75 | TCIRG1 | 50% | 0.1077 | 0.116 | 0.155 |
| Ataxia telangiectasia | 1 | ATM | 100% | 0.1558 | 0.243 | 0.243 |
| Congenital factor VII deficiency | 0.33 | F7 | 100% | 0.014 | 0.002 | 0.006 |
| Krabbe disease | 1 (2) | GALC | 100% | 0.3572 | 1.276 | 1.276 |
| Tyrosinemia | 0.8 (3) | FAH | 100% | 0.2227 | 0.496 | 0.620 |
| PMM2-CDG | 0.64 | PMM2 | 100% | 0.0676 | 0.046 | 0.071 |
| Cystinosis | 0.5 | CTNS | 100% | 0.0476 | 0.023 | 0.045 |
| Blackfan-Diamond anemia | 0.67 | RPS19 | 25% | 0.0772 | 0.060 | 0.089 |
| Adams-Oliver syndrome | 0.44 (4) | DOCK6 | <10%* | 0.1836 | 0.337 | 0.766 |
Incidences as reported in Orphanet, June 2013 Report, unless otherwise specifically cited. 1) Suzuki et al., 2008 2) Wenger, 2011 3) Mitchell et al., 2001 4) Martínez-Frías et al., 1996. AR - autosomal recessive.
From the authors’ experience in a genome-sequenced cohort of 13 probands (data not shown).
DISCUSSION
We identify diffuse angiopathy as the principal mechanism of morbidity in a child with AOS, in association with two truncating DOCK6 mutations. All DOCK6 disease alleles thus far reported are truncating, but it has yet to be resolved if are truly null or whether they can be hypomorphic. Shaheen et al. [2011b] observed production of a mutant transcript from RT-PCR in fibroblasts from an individual homozygous for p.T455Sfs*24, rather than nonsense-mediated decay.
Vascular abnormalities in our patient involved altered association of pericytes (vascular smooth muscle cells) with small vessels, and these findings were associated with poor placental function, bowel ischemia, lack of retinal vascularization, retinal hemorrhages, and intracranial hemorrhages, occurring both pre- and post-natally. Conversely, the classic findings of AOS, terminal transverse limb defects and aplasia cutis congenita, for this patient have not been sources of great morbidity. Profound intellectual impairment and her complex cardiac defect are central features of her condition, and the mechanisms by which DOCK6 dysfunction leads to these developmental problems remain poorly understood. The patient sustained a perinatal ischemic event as evidenced by meconium-stained amniotic fluid, low Apgar scores, acidemia, perinatal hypoglycemia, and transient thrombocytopenia. This may have contributed to her neurological impairment, and it is possible that the observed anomalous vascular development of the placenta may have been the cause of the event, in addition to causing her intrauterine growth restriction.
DOCK6 likely has a direct role in brain development as well. Knockdown of endogenous Dock6 by small interfering RNA has been shown in a murine neuroblastoma cell line to reduce activation of Rac1 and Cdc42 and neurite outgrowth [Miyamoto et al., 2007]. A mouse model transgenic for Dock6 shRNA shows shortened peripheral neuronal fibers by embryonic day 11 [Miyamoto et al., 2013]. The mechanism for the vasculopathy in AOS remains to be elucidated. It may occur through decreased Rac1 activity. The most informative Rac1 mouse models have been conditional knock-outs because fully null embryos have early lethality with gastrulation defects [Sugihara et al., 1999]. An endothelial cell targeted knock-out Rac1 mouse has a dramatic lack of sprouting vessel branches, indicating severely impaired angiogenesis, as well as cardiac defects, and does not survive beyond mid-gestation Tan et al., 2008]. A second group studied endothelial-specific Rac1 haploinsufficient mice and found impaired vascular tone with depressed nitric oxide release, as well as impaired post-ischemic angiogenesis [Sawada et al., 2008]. Our patient demonstrated a striking lack of vascularization as was visible in her retina. Other Rac1 conditional knock-out mouse models also mimic the features of AOS. The loss of Rac1 from limb bud ectoderm or limb bud mesenchyme leads to severe limb truncation or syndactyly, respectively [Suzuki et al., 2013].
A vasculopathy has been recognized in AOS for many years. In fact, some have suggested it was the direct mediator of the limb and skull defects, via inadequate blood supply [Toriello et al., 1988; Swartz et al., 1999]. Persistent cutis marmorata is one of the most common vascular abnormalities seen in AOS. A less common, but major cause of mortality in the severe forms of AOS, is pulmonary hypertension [Patel et al., 2004]. Pulmonary venous stenosis has been observed in multiple patients as well as loss of intrahepatic portal veins, and tortuous, dilated scalp veins [Patel et al., 2004; Dadzie et al., 2007; Pouessel et al., 2006]. Incomplete retinal vascularization has been reported previously in two patients with AOS, plus we have noted it a third, unreported time. In two of these patients [Peralta-Calvo et al., 2012; Patel et al., 2004 - patient 1] DOCK6 was analyzed by our group and no mutations were found (data unpublished). Normal retinal vascularization requires intact pericyte function, supporting the hypothesis that pericyte dysfunction plays a key role in the pathogenesis of the AOS vasculopathy [Patel et al., 2004; Liu et al., 2010]. From these data, we can infer there is likely a critical role for CDC42/RAC signaling in the development of small to medium blood vessels.
A published estimate of the incidence for all sub-types of AOS was 0.44 per 100,000 live births [Martínez-Frías et al., 1996]. Our tertiary pediatric care center serves a population base of 4.6 million people in British Columbia with 44,000 annual births, but the Medical Genetics department has diagnosed only 3 patients with AOS in the last 15 years (unpublished data) supporting an incidence of ~0.5 per 100,000 live births. Contrary to this low incidence, probable detrimental variants in DOCK6 are observed more frequently than expected for highly penetrant variants causing AOS. For example, one of the homozygous frameshift mutations in DOCK6 previously reported as a cause of AOS [Shaheen et al., 2011a], p.T455Sfs*24, has an allele frequency of 0.34% in the European-American ESP data. Therefore, considering just this single variant alone, about 1.2 per 100,000 European-American individuals would be homozygous, and thus expected to have AOS. We used Kaviar to calculate the combined frequency of stop-gain and splice-site variants in DOCK6 and, for comparison, we assessed 19 other genes that are known to cause rare autosomal recessive diseases. We did not make any direct comparisons of carrier frequency, given the multiple differences among these diseases: variance of the relative proportions of the different classes of mutation in each disease; different disease frequencies despite our effort to find similarly rare diseases (Orphanet Prevalence of Rare Diseases, June 2013, www.orpha.net); and different ranges of disease expressivity. For these reasons, we did not calculate a difference, but simply present the data to show that Adams Oliver syndrome caused by DOCK6 stands out.
We did not assess indels because of the high false positive rate from next generation sequencing for this type of variant. Although the theoretical incidence for DOCK6-related AOS is lower than the observed AOS incidence, it is still much higher than expected, given that DOCK6 is only an infrequent cause of AOS and the known AOS-causing DOCK6 frameshift mutation is not taken into consideration [Shaheen et al., 2011a]. We found that the frequency of stop-gain and splice site variants is higher than expected not only in DOCK6 but also in a few other recessive disease genes. There could be a number of explanations for these discrepancies. The frequency and deleteriousness of the variants might be overestimated. Technical errors in the sequencing data could lead to increased frequencies, or the data sets included in Kaviar could by chance alone have sampled more carriers than expected from the general population. Also, not all splice-site and stop gain variants are pathogenic. Embryonic lethality could be increased with bi-allelic loss of DOCK6. However, three of the four families thus far reported had more than one sibling affected, suggesting a preserved Mendelian ratio (although there would be ascertainment bias for studying families with multiple affected siblings). It is possible that the penetrance of DOCK6-related AOS is affected by additional, yet to be resolved factors. For example, it has been reported for Krabbe disease, one of the classic recessive disorders we found to have an unexpectedly high rate of mutation carriers, that the number of infants with biallelic GALC mutations and deficient enzyme activity was more than twice the expected number in a New York state newborn screening program [Salveson, 2011]. Furthermore, it was recently found that this disease, previously considered to primarily affect infants, may more often have later onset with a variable range of severity [Hossain et al., 2014]. Clearly, these findings indicate a more complex disease etiology than previously thought. Other autosomal recessive conditions are known to have notably incomplete penetrance, e.g. familial Mediterranean fever, Schimke immuno-osseous dysplasia, and autosomal recessive deafness type 26 [Riazuddin et al., 2000; Baradaran-Heravi et al., 2012; Mikula et al., 2008]. However, it is not possible to conclude that DOCK6-related AOS truly features decreased penetrance without direct observation of an unaffected individual with bi-allelic mutations.
Acknowledgments
This work was supported in part by the Rare Disease Foundation, the BC Children’s Hospital Foundation and the University of Luxembourg – Institute for Systems Biology Program. ABS was supported by the German Academic Exchange Service (DAAD).
References
- Baradaran-Heravi A, Cho KS, Tolhuis B, Sanyal M, Morozova O, Morimoto M, Elizondo LI, Bridgewater D, Lubieniecka J, Beirnes K, Myung C, Leung D, Fam HK, Choi K, Huang Y, Dionis KY, Zonana J, Keller K, Stenzel P, Mayfield C, Lücke T, Bokenkamp A, Marra MA, van Lohuizen M, Lewis DB, Shaw C, Boerkoel CF. Penetrance of biallelic SMARCAL1 mutations is associated with environmental and genetic disturbances of gene expression. Human Molecular Genetics. 2012;21:2572–2587. doi: 10.1093/hmg/dds083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I, Silberstein E, Perez Y, Landau D, Elbedour K, Langer Y, Kadir R, Volodarsky M, Sivan S, Narkis G, Birk OS. Autosomal recessive Adams-Oliver syndrome caused by homozygous mutation in EOGT, encoding an EGF domain-specific O-GlcNAc transferase. Eur J Hum Genet. 2013 doi: 10.1038/ejhg.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadzie OE, Tyszczuk L, Holder SE, Teixeira F, Charakida A, Scarisbrick J, Chu A. Adams-Oliver syndrome with widespread CMTC and fatal pulmonary vascular disease. Pediatr Dermatol. 2007;24:651–653. doi: 10.1111/j.1525-1470.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- EVS: Exome Variant Server. NHLBI GO Exome Sequencing Project (ESP), Seattle, Washingtion, USA. EVS: Exome Variant Server. NHLBI GO Exome Sequencing Project (ESP), Seattle, Washingtion, USA.
- Glusman G, Caballero J, Mauldin DE, Hood L, Roach JC. Kaviar: an accessible system for testing SNV novelty. Bioinformatics. 2011;27:3216–3217. doi: 10.1093/bioinformatics/btr540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassed SJ, Wiley GB, Wang S, Lee J-Y, Li S, Xu W, Zhao ZJ, Mulvihill JJ, Robertson J, Warner J, Gaffney PM. RBPJ mutations identified in two families affected by Adams-Oliver syndrome. Am J Hum Genet. 2012;91:391–395. doi: 10.1016/j.ajhg.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MA, Otomo T, Saito S, Ohno K, Sakuraba H, Hamada Y, Sakai N. Late-onset Krabbe disease is predominant in Japan and its mutant precursor protein undergoes more effective processing than the infantile-onset form. Gene. 2014;534:144–154. doi: 10.1016/j.gene.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Liu HH, Zhang WW, Kennard SS, Caldwell RBR, Lilly BB. Notch3 is critical for proper angiogenesis and mural cell investment. Circ Res. 2010;107:860–870. doi: 10.1161/CIRCRESAHA.110.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Frías ML, Arroyo Carrera I, Muñoz-Delgado NJ, Nieto Conde C, Rodríguez-Pinilla E, Urioste Azcorra M, Omeñaca Teres F, García Alix A. The Adams-Oliver syndrome in Spain: the epidemiological aspects. An Esp Pediatr. 1996;45:57–61. [PubMed] [Google Scholar]
- Mikula M, Buller A, Sun W, Strom CM. Prevalence of known mutations in the familial Mediterranean fever gene (MEFV) in various carrier screening populations. Genet Med. 2008;10:349–352. doi: 10.1097/GIM.0b013e3181723cc8. [DOI] [PubMed] [Google Scholar]
- Mitchell GA, Grompe M, Lambert M, Tanguay RM. Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Disease. NY: McGraw Hill; 2001. pp. 1777–806. [Google Scholar]
- Miyamoto Y, Torii T, Yamamori N, Ogata T, Tanoue A, Yamauchi J. Akt and PP2A reciprocally regulate the guanine nucleotide exchange factor Dock6 to control axon growth of sensory neurons. Sci Signal. 2013;6:ra15. doi: 10.1126/scisignal.2003661. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Sanbe A, Tanoue A. Dock6, a Dock-C subfamily guanine nucleotide exchanger, has the dual specificity for Rac1 and Cdc42 and regulates neurite outgrowth. Exp Cell Res. 2007;313:791–804. doi: 10.1016/j.yexcr.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Patel MS, Taylor GP, Bharya S, Al-Sanna’a N, Adatia I, Chitayat D, Lewis SME, Human DG. Abnormal pericyte recruitment as a cause for pulmonary hypertension in Adams-Oliver syndrome. Am J Med Genet. 2004;129A:294–299. doi: 10.1002/ajmg.a.30221. [DOI] [PubMed] [Google Scholar]
- Peralta-Calvo J, Pastora N, Casa-Ventura YG, Hernandez-Serrano R, Abelairas J. Peripheral Ischemic Retinopathy in Adams-Oliver Syndrome. Arch Ophthalmol. 2012;130:1078–1080. doi: 10.1001/archophthalmol.2012.531. [DOI] [PubMed] [Google Scholar]
- Pouessel G, Dieux-Coeslier A, Wacrenier A, Fabre M, Gottrand F. Association of Adams-Oliver syndrome and hepatoportal sclerosis: an additional case. Am J Med Genet. 2006;140:1028–1029. doi: 10.1002/ajmg.a.31192. [DOI] [PubMed] [Google Scholar]
- Riazuddin S, Castelein CM, Ahmed ZM, Lalwani AK, Mastroianni MA, Naz S, Smith TN, Liburd NA, Friedman TB, Griffith AJ, Riazuddin S, Wilcox ER. Dominant modifier DFNM1 suppresses recessive deafness DFNB26. Nat Genet. 2000;26:431–434. doi: 10.1038/82558. [DOI] [PubMed] [Google Scholar]
- Salveson R. Expansion of the New York State Newborn Screening Panel and Krabbe Disease: A Systematic Program Evaluation. New York: Dissertation, Columbia University; 2011. [Google Scholar]
- Sawada N, Salomone S, Kim H-H, Kwiatkowski DJ, Liao JK. Regulation of Endothelial Nitric Oxide Synthase and Postnatal Angiogenesis by Rac1. Circ Res. 2008;103:360–368. doi: 10.1161/CIRCRESAHA.108.178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Aglan M, Keppler-Noreuil K, Faqeih E, Ansari S, Horton K, Ashour A, Zaki MS, Al-Zahrani F, Cueto-González AM, Abdel-Salam G, Temtamy S, Alkuraya FS. Mutations in EOGT confirm the genetic heterogeneity of autosomal-recessive Adams-Oliver syndrome. Am J Hum Genet. 2013;92:598–604. doi: 10.1016/j.ajhg.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R, Al-Dirbashi OY, Al-Hassnan ZN, Al-Owain M, Makhsheed N, Basheeri F, Seidahmed MZ, Salih MAM, Faqih E, Zaidan H, Al-Sayed M, Rahbeeni Z, Al-Sheddi T, Hashem M, Kurdi W, Shimozawa N, Alkuraya FS. Clinical, biochemical and molecular characterization of peroxisomal diseases in Arabs. Clin Genet. 2011a;79:60–70. doi: 10.1111/j.1399-0004.2010.01498.x. [DOI] [PubMed] [Google Scholar]
- Shaheen R, Faqeih E, Sunker A, Morsy H, Al-Sheddi T, Shamseldin HE, Adly N, Hashem M, Alkuraya FS. Recessive mutations in DOCK6, encoding the guanidine nucleotide exchange factor DOCK6, lead to abnormal actin cytoskeleton organization and Adams-Oliver syndrome. Am J Hum Genet. 2011b;89:328–333. doi: 10.1016/j.ajhg.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape KMG, Ruddy D, Zenker M, Wuyts W, Whiteford M, Johnson D, Lam W, Trembath RC. The spectra of clinical phenotypes in aplasia cutis congenita and terminal transverse limb defects. Am J Med Genet. 2009;149A:1860–1881. doi: 10.1002/ajmg.a.32708. [DOI] [PubMed] [Google Scholar]
- Southgate L, Machado RD, Snape KM, Primeau M, Dafou D, Ruddy DM, Branney PA, Fisher M, Lee GJ, Simpson MA, He Y, Bradshaw TY, Blaumeiser B, Winship WS, Reardon W, Maher ER, Fitzpatrick DR, Wuyts W, Zenker M, Lamarche-Vane N, Trembath RC. Gain-of-function mutations of ARHGAP31, a Cdc42/Rac1 GTPase regulator, cause syndromic cutis aplasia and limb anomalies. Am J Hum Genet. 2011;88:574–585. doi: 10.1016/j.ajhg.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara K, Nakatsuji N, Nakamura K, Nakao K, Hashimoto R, Otani H, Sakagami H, Kondo H, Nozawa S, Aiba A, Katsuki M. Rac1 is required for the formation of three germ layers during gastrulation. Oncogene. 1999;17:3427–3433. doi: 10.1038/sj.onc.1202595. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Yamada A, Kamijo R. The essential roles of the small GTPase Rac1 in limb development. Journal of Oral Biosciences. 2013;55:116–121. [Google Scholar]
- Suzuki Y, Oshima A, Nanba E. The metabolic and molecular bases of inherited disease. NY: McGraw Hill; 2001. β-Galactosidase deficiency (β-galactosidosis): GM1 gangliosidosis and Morquio B disease; pp. 3775–3809. [Google Scholar]
- Swartz EN, Sanatani S, Sandor GG, Schreiber RA. Vascular abnormalities in Adams-Oliver syndrome: cause or effect? Am J Med Genet. 1999;82:49–52. doi: 10.1002/(sici)1096-8628(19990101)82:1<49::aid-ajmg10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- Toriello HV, Graff RG, Florentine MF, Lacina S, Moore WD, Optiz JM, Reynolds JF. Scalp and limb defects with cutis marmorata telangiectatica congenita: Adams-Oliver syndrome? Am J Med Genet. 1988;29:269–276. doi: 10.1002/ajmg.1320290204. [DOI] [PubMed] [Google Scholar]
- David Wenger. Krabbe disease. Gene Reviews. 2011 http://www.ncbi.nlm.nih.gov/books/NBK1238/