Abstract
Purpose
Phase 1 clinical trials are generally conducted to identify the maximum tolerated dose (MTD) or the biologically active dose (BAD) using a traditional dose escalation design. This design may not be applied to cancer vaccines, given their unique mechanism of action. The FDA recently published “Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines.” However, many questions about the design of cancer vaccine studies remain unanswered.
Experimental Design
We analyzed the toxicity profile in 239 phase 1 therapeutic cancer vaccine trials. We addressed the ability of dose escalation to determine the MTD or the BAD in trials that used a dose escalation design.
Results
The rate of grade 3/4 vaccine-related systemic toxicities was 1.25 adverse event per 100 patients and 2 per 1000 vaccines. Only 2 out of the 127 dose escalation trials reported vaccine-related dose limiting toxicities, both of which used bacterial vector vaccines. Out of the 116 trials analyzed for the dose-immune response relationship, we found a statistically significant dose-immune response correlation only when the immune response was measured by antibodies (p<0.001) or delayed type hypersensitivity (p<0.05). However, the increase in cellular immune response did not appear further sustainable with the continued increase in dose.
Conclusions
Our analysis suggests that the risks of serious toxicities with therapeutic cancer vaccines are extremely low and that toxicities do not correlate with dose levels. Accordingly, the conventional dose escalation design is not suitable for cancer vaccines with few exceptions. Here we propose an alternative design for therapeutic cancer vaccine development.
Keywords: Cancer, Vaccine, Phase I, Trial, Design
Introduction
Traditionally the first-in-human clinical trials conducted in oncology drug development are phase 1 safe-dose-seeking trials. Since the establishment of the Federal Food, Drug, and Cosmetic Act in 1938, all drug manufacturers are required to prove drug safety prior to marketing (1). A later amendment passed in 1962 required that not only safety must be demonstrated but efficacy as well (2). These regulations created a strong motivation for the design of clinical trials. In the late 1970s the guidelines for phase 1 trials of anti-cancer drugs were established, and they have remained largely unchanged to this day (3). Finding the maximum tolerated dose (MTD) was set as the primary goal for phase 1 trials, because at that time, most anti-cancer drugs were cytotoxic agents. These drugs exhibit a dose-toxicity relationship that was also considered to hold for efficacy. Therefore, the highest tolerated dose was assumed to be the most efficacious (4). Although toxicity is an important endpoint in phase 1 trials testing cytotoxic agents, newer drugs such as molecular targeted agents (MTAs) are often characterized by a dose-response curve that plateaus at a dose less than the MTD (5–7). In such cases, the preferred effect can be provided without considerable toxicity. Furthermore, the anti-tumor effects of most successful recently developed targeted agents have been mediated via measurable pharmacodynamic effects on their targets. Therefore, identifying a biologically active dose (BAD) has become an important goal for phase 1 trials testing MTAs (6). Cancer vaccines have emerged as a promising novel modality and identifying their MTD and BAD using the traditional phase 1 design has been a challenge (8). Cancer vaccines produce their effect by modulating the immune system to target specific antigens on cancer cells. This mechanism of action is not expected to be dose-related or to induce severe toxicity. Accordingly, the FDA realized the need to change the paradigm in early cancer vaccine development (9). To date there has been no extensive analysis of cancer vaccine toxicity or the dose-safety and dose-immune response relationships. In this paper, we have attempted to provide such analyses. We also describe an alternative design strategy for the conduct of early therapeutic cancer vaccine clinical trials, and we identify the circumstances in which a traditional dose escalation design is appropriate.
Materials and Methods
Inclusion criteria and trials classification
In order to establish our database for analysis we searched PubMed for all phase 1, phase1/2, and pilot studies for therapeutic cancer vaccines conducted from 1990 through 2011, excluding all trials for combination treatments and studies that did not use the NCI Common Toxicity Criteria. The vaccine studies were classified according to vaccine type. Some studies used vaccines that fell under more than one category. To simplify the analysis, the method of delivery (e.g., dendritic cells) was used as the main guideline for classification.
Toxicity analysis
We reported the number of systemic vaccine-related grade 3/4 toxicities, number of treated patients and number of administered vaccines by vaccine category. We then examined the dose-toxicity relationship in the dose escalation trials, specifically looking for dose limiting toxicities (DLTs). Vaccine-related toxicities are defined as grade 3/4 toxicities that are possibly, probably, or definitely related to the vaccine, excluding local reactions, constitutional symptoms, and adjuvant-related events.
Dose-immune response meta-analysis
To address the question of whether dose escalation could lead to a better immune response, we performed a meta-analysis on all dose escalation trials that reported the number of immune responders per dose level. Trials included in the meta-analysis were those dose escalation trials analyzed earlier for toxicity in addition to phase 2 trials that used dose escalation to determine the dose that induces the best immune response as a primary endpoint.
Furthermore, we analyzed the correlation of immunological response with dose level for each of the immune assay endpoints. This analysis included all studies that reported results with a given endpoint regardless of the type of vaccine. Statistical significance was based on a test for trend, which is essentially equivalent to an evaluation of whether the response (0 for no immunological response vs. 1 for immunological response) is linearly correlated with dose level (1, 2, etc.). For each study s, a value yidi was computed where yi denotes the response for the i'th patient in the study, di denotes the dose level (1, 2, etc.) of the i'th patient in the study and n is the number of patients in the study. A large value of Ts indicates a relationship of response to dose, but the size of Ts depends on the number of patients in the study, the number of dose levels, and the distribution of patients per dose level. A standardized statistic was calculated for each study by subtracting off the mean and dividing by the square root of the variance under the null hypothesis of no trend of response to dose; i.e., . The studies were combined by computing an unweighted average of the Ts values, i.e., . The statistical significance of was evaluated by computing its exact permutation distribution. That is, for each study, the assignment of doses to patients was permuted randomly; new values of Ts and were calculated for the permuted data. This was repeated thousands of times resulting in the distribution of under the null hypothesis. A one-tailed significance level is the area of the tail of the null distribution beyond the actual value of for the real un-permuted data. A two-tailed significance level is taken as twice the one-sided value. The calculations were programmed in the R statistical programming language.
Results
Therapeutic Cancer Vaccine Trials
We reviewed 239 phase 1, phase1/2, and pilot therapeutic cancer vaccine studies published between 1990 and 2011. We classified these trials into cellular (autologous or allogeneic), and synthetic vaccines based on the type of vaccination. Cellular-based vaccines (autologous or allogeneic tumor cell–based vaccines) utilize the whole cells or cell lysates as the source of antigens, which allows multiple antigens to be simultaneously targeted without being prospectively identified. Autologous vaccines were sub-classified into dendritic and tumor cell vaccines. Synthetic vaccines are administered directly to the patients or utilize a vector to deliver the antigen. Synthetic vaccines were sub-classified into peptide, DNA, RNA, viral, bacterial, anti-idiotypic, and liposomal vaccines. A full list of the 239 analyzed trials is reported in supplemental table 1, in addition to the range of the administered vaccine doses. An aggregate total of 4,952 patients were enrolled in these trials. Trials using synthetic vaccines accounted for the greatest number (135 trials), enrolling 2,853 patients, constituting more than half the total patients (57.61%). Autologous vaccines constituted 87 trials and 34.17% of the total patients (1,692 patients). There were 17 allogeneic vaccine trials with 407 treated patients (8.22% of the total patients) (Table 1).
Table 1.
Vaccine-related toxicities based on the number of treated patients
| Vaccine trials | All Grade 3/4 AE | *Systemic Vaccine-Related Grade 3/4 AE | ||||
|---|---|---|---|---|---|---|
| Vaccine Category | No. Trials | No. Patients | No. Events | % Events | No. Events | % Events |
| Autologous | 87 | 1692 | 37 | 2.19 | 23 | 1.36 |
| DC | 51 | 922 | 9 | 0.98 | 3 | 0.33 |
| Tumor | 30 | 770 | 28 | 3.64 | 20 | 2.60 |
| Allogeneic | 17 | 407 | 22 | 5.41 | 5 | 1.23 |
| Synthetic | 135 | 2853 | 108 | 3.79 | 35 | 1.23 |
| Peptide | 68 | 1333 | 40 | 3.00 | 11 | 0.83 |
| DNA | 17 | 311 | 1 | 0.32 | 1 | 0.32 |
| RNA | 2 | 36 | 0 | 0 | 0 | 0 |
| Virus | 30 | 662 | 23 | 3.47 | 13 | 1.96 |
| Bacteria | 6 | 126 | 27 | 21.43 | 7 | 3.97 |
| Anti-idiotypic | 10 | 362 | 15 | 4.14 | 0 | 0 |
| Liposomal | 2 | 23 | 2 | 8.70 | 2 | 8.70 |
| Total | 239 | 4952 | 167 | 3.37 | 62 | 1.25 |
Vaccine-related toxicity
Vaccine-related toxicity in relation to the number of treated patients
Here, we report the incidence of vaccine-related toxicity in relation to the number of treated patients (Table 1). We found that amongst the 4,952 patients assessed, a total of 162 grade three and 5 grade four treatment-related toxicities were reported. Of these toxicities, 60 were local reactions, 40 were constitutional symptoms, and 5 were related to the adjuvants used in the vaccines. The rest, 62 systemic adverse events, were reported by the investigators to be at least “possibly related” to the vaccines. This constitutes 1.25 adverse events per 100 patients. Of these 62 events 23 were reported to be “possibly related,” 1 “probably related,” and 16 “definitely related” to the vaccines. The relationship of the remaining 22 events to the vaccines could not be determined because of the advanced stage of disease. We further analyzed these adverse events based on the type of cancer vaccines. We found that autologous vaccine trials had a vaccine-related toxicity rate of 1.36 events per 100 patients (23 events of the total 62 events). Five toxicities were reported in the allogeneic and 35 in the synthetic vaccine trials, giving a vaccine-related toxicity rate of 1.23 events per 100 patients in each group. The highest vaccine-related toxicity rate among the synthetic vaccines was reported in the bacterial vector trials (a total of 7 events, 3.97 events per 100 patients).
Vaccine-related toxicity in relation to the number of administered vaccines
We also evaluated the incidence of toxicities in relation to the total number of administered vaccines (Table 2). Only 206 of the 239 trials stated the total number of administered vaccines, and were included in this analysis. These 206 trials treated in aggregate a total of 4,024 patients who received a total of 21,835 vaccines and experienced a total of 120 grade 3/4 toxicities. Of these 120 toxicities, 43 were systemic vaccine-related grade 3/4, accounting for 2 adverse events per 1000 vaccines. Autologous vaccines had the lowest systemic vaccine-related toxicity rate (1.4 adverse events per 1000 vaccines), followed by the synthetic vaccines (2.1 adverse events per 1000 vaccines), and the allogeneic vaccines (2.6 adverse events per 1000 vaccines).
Table 2.
Vaccine-related toxicities based on the number of administered vaccmes
| Vaccine Trials | All Grade 3/4 AE | *Systemic Vaccine-Related Grade 3/4 AE | |||||
|---|---|---|---|---|---|---|---|
| Vaccine Category | No. Trials | No. Patients | No. Vaccines | No. Events | % Events | No. Events | % Events |
| Autologous | 73 | 1301 | 5722 | 20 | 0.35 | 8 | 0.14 |
| DC | 51 | 796 | 3424 | 9 | 0.26 | 3 | 0.09 |
| Tumor | 22 | 505 | 2298 | 11 | 0.48 | 5 | 0.22 |
| Allogeneic | 16 | 347 | 1874 | 22 | 1.17 | 5 | 0.26 |
| Synthetic | 117 | 2376 | 14239 | 78 | 0.55 | 30 | 0.21 |
| Peptide | 61 | 1183 | 7637 | 37 | 0.48 | 9 | 0.12 |
| DNA | 15 | 259 | 1388 | 1 | 0.07 | 1 | 0.07 |
| RNA | 2 | 36 | 335 | 0 | 0 | 0 | 0 |
| Virus | 27 | 535 | 2365 | 22 | 0.93 | 13 | 0.55 |
| Bacteria | 4 | 80 | 530 | 9 | 1.70 | 5 | 0.94 |
| Anti-idiotypic | 7 | 266 | 1938 | 7 | 0.36 | 0 | 0 |
| Liposomal | 1 | 17 | 46 | 2 | 4.35 | 2 | 4.35 |
| Total | 206 | 4024 | 21835 | 120 | 0.55 | 43 | 0.20 |
Dose-related toxicity
To determine whether there is any relationship between dose and toxicity, we analyzed the dose-adverse event relationship in the trials that used a dose escalation design (Table 3). Of the 239 trials, 127 used a vaccine dose escalation design, treating a total of 2,985 patients. Amongst these 127 dose escalation trials, only 22 (17%) reported toxicities of grade 3/4, a total of 98 events. Forty of these 98 events were systemic, non-constitutional, and vaccine-related. To further investigate whether a dose-toxicity correlation existed, we performed two analyses:
-
1)
Vaccine-related toxicity in relation to the dose where it occurred
In the first analysis, we identified the dose level where these 40 systemic vaccine-related toxicities occurred in each of the 22 trials. Seven of these 22 trials did not specify the dose at which the toxicities occurred (24 systemic toxicities) (10–16). In the remaining 15 trials, only 6 reported the toxicities at the highest dose level (10 systemic toxicities). Two out of these 6 trials that reported toxicities at the highest dose level used bacterial vaccines (17, 18), and the rest used autologous (19), DNA (20), viral (21), or liposomal vaccines (22). Two toxicities occurred in the middle dose level in one trial that used a bacterial vaccine, with no further toxicity occurring when the dose was escalated (23). Four toxicities occurred at the lowest dose level in 3 trials, 2 with peptide vaccines (24, 25) and one with a liposomal vaccine (22). Interestingly, none of these trials reported further toxicities when the dose was escalated (Supplemental Table 2).
-
2)
Trials with vaccine-related DLTs
In the second analysis, we evaluated whether dose escalation resulted in a DLT. Accordingly, we analyzed the 22 dose escalation trials that reported grade 3/4 systemic vaccine-related toxicities for evidence of DLT. We found that only 3 of these 22 trials (15, 21, 26) reported DLTs (Tables 3 and 4). Amongst these, one trial, reported by Dols et al., used allogeneic vaccine and attributed the DLT of nausea and vomiting to the adjuvant (GM-CSF). There were no life-threatening side effects on this trial (26). The two other trials, reported by Maciag et al. and Guthmann et al., attributed the DLTs to the vaccines, both of which used bacterial vectors of live-attenuated Listeria monocytogenes and Neisseria meningitidis, respectively (15, 21). In both trials the DLT was consisted of hypotension that was successfully controlled with IV fluids and supporting medications. All the trials that used bacterial vector vaccines are described in supplemental table 3.
Table 3.
Dose-toxicity relationship
| Vaccine Category | No. Trials | No. Patients | Grade 3/4 | No. Trials with DLT | ||
|---|---|---|---|---|---|---|
| No. Trials | No. Events | *No. Systemic Vaccine-Related AE | ||||
| Autologous | 40 | 847 | 2 | 11 | 8 | 0 |
| DC | 27 | 466 | 0 | 0 | 0 | 0 |
| Tumor | 13 | 381 | 2 | 11 | 8 | 0 |
| Allogeneic | 5 | 130 | 3 | 20 | 5 | 1 |
| Synthetic | 83 | 2008 | 17 | 67 | 27 | 2 |
| Peptide | 36 | 852 | 7 | 10 | 7 | 0 |
| DNA | 12 | 208 | 1 | 1 | 1 | 0 |
| Virus | 26 | 592 | 7 | 47 | 17 | 0 |
| Bacteria | 4 | 81 | 4 | 27 | 7 | 2 |
| Anti-idiotypic | 8 | 339 | 1 | 7 | 0 | 0 |
| Liposomal | 1 | 17 | 1 | 2 | 2 | 0 |
| Total | 127 | 2985 | 22 | 98 | 40 | 3 |
Table 4.
Trials with Dose Limiting Toxicities (DLTs)
| Trial | Vaccine used | Toxicity | DLT |
|---|---|---|---|
| Dols, 2003 | Allogenic HER2/neu+ breast cancer cells (SC) with GM-CSF or BCG | Nausea Vomiting | 1 patient due to GM-CSF |
| Maciag, 2009 | Listeria monocytogenes secreting HPV-16 E7 fused to Lm listeriolysin O (IV) | Hypotension | 3 patients at highest dose level |
| Guthmann, 2004 | GM3 ganglioside with Neisseria meningitidis outer membrane (IM) | Hypotension | 1 patient at highest dose level |
Dose-immune response analysis
In order to determine whether vaccine dose affects the resultant immune response, we reviewed the dose escalation trials that reported the number of immune responders at each dose level. Only 106 out of the 127 dose escalation trials reported the number of immune responders at each dose level. We also included 10 additional phase 2 trials that used a dose escalation design and tested for immune response as a primary endpoint. Accordingly, we identified 116 trials that could be analyzed for correlation between dose of the vaccine and immune response.
Dose-immune response relationship based on the trials' reports
Out of these 116 trials, only 2 reported a statistically significant dose-immune response correlation. The immune response was measured by antibodies in both of these trials. One used an allogeneic vaccine (11) and the other used an anti-idiotypic vaccine (27).
Dose-immune response relationship based on the meta-analysis results
Furthermore, we analyzed the relationship of immunological response with dose level based on the assay that was used to measure the immune response. These assays included delayed-type hypersensitivity (DTH), T-cell proliferation, interferon-γ ELISPOT, and tetramer assay in addition, at times, to humoral immune response. We found a statistically significant correlation between the vaccine dose and the immune response only when the immune response was measured by antibodies (p<0.001) or DTH (p <0.05). None of the other assays showed a trend that was statistically significant at a two-tailed 5% level. The results for tetramer assay and T-cell proliferation were of borderline significance, with a two-tailed significance level of about 10%. The borderline significance of the tetramer result was driven by a single study reported by Dangoor et al. that used plasmid DNA and a recombinant modified Vaccinia Virus Ankara (MVA), both expressing epitopes from five melanoma antigens (18). Without the Dangoor et al. study, the two-sided p value for the remaining 10 studies did not approach significance. The borderline significance for T-cell proliferation was similarly non-robust. Only 5 studies were available that reported results for T-cell proliferation, all were small, and none showed strong evidence of correlation between the vaccine dose and the immune response. Removal of the study by Pinilla-Ibarz et al. (28), which used a bcr-abl fusion peptide vaccine, resulted in a two-tailed p value of 0.26, which did not approach significance.
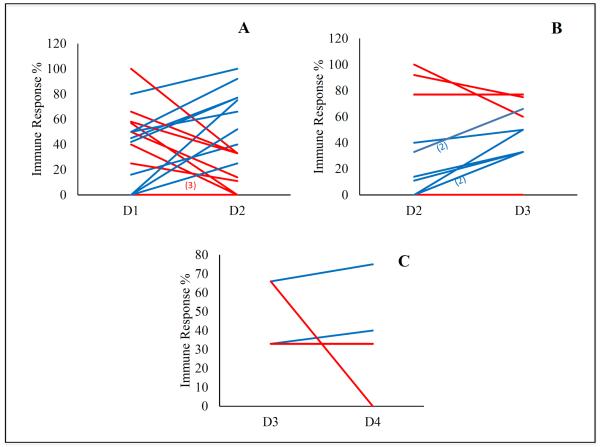
Since DTH was the only assay that showed a significant dose-cellular immune response correlation, we examined whether the percentage of DTH immune responders, on a specific trial, is increased with increasing the dose level (Figure 1). A total of 19 trials reported the immune response by DTH. We found that the percentage of responders between dose level 1 and dose level 2 increased or maintained the same in 12 out of these 19 trials, while it trended downward in 7 (Figure1a). Amongst the 12 trials that showed an increase or no change in the percentage of responders, 8 trials continued to show an increase when the dose was escalated from dose level 2 to dose level 3 (Figure 1b). However, only 4 out of these 8 trials continued to increase the dose to level 4 with only 2 out of these 4 trials reporting an increased immune response by DTH (Figure 1c). Although the number of patients per dose level in individual trials is limited, the data indicate the relationship between dose and DTH immune response, although statistically significant, was variable and inconsistent.
Figure 1.
The change in the percentage of immune responders on each trial measured by DTH from dose level 1 (D1) to 2 (D2) (Figure 1a); dose level 2 (D2) to 3 (D3) (Figure. 1b) and dose level 3 (D3) to 4 (D4) (Figure. 1c). Trials that showed an increase in the percentage of immune responders are marked in blue while trials that showed no change or decrease in the percentage of responders are marked in red.
Discussion
The traditional phase 1 dose escalation clinical trial design has been implemented in the development of cancer vaccines for more than two decades. However, the utility of this design has been questioned by many investigators and regulatory agencies, including the FDA and the European Medicines Agency (EMA) (9, 29). Although therapeutic cancer vaccines are generally expected to have a relatively safe profile and dose-independent efficacy, to date there has been no systemic evaluation that provides objective evidence to address this issue. Here, and for the first time, we provide evidence-based proof for limited serious adverse events and lack of dose-related toxicity for therapeutic cancer vaccines. We found the incidence of grade 3/4 vaccine-related systemic toxicities to be 1.25 per 100 treated patients and 2 per 1000 administered vaccines. Moreover, vaccine-related DLTs were found in only 2 of the 127 dose escalation toxicity-seeking clinical trials reviewed. Both of these clinical trials used bacterial vaccine vectors. Accordingly, we propose that, with the exception of bacterial vector vaccines, the traditional dose escalation design to determine MTD is not warranted in the development of cancer vaccines. Noteworthy, all the cancer vaccine trials that we reviewed here were in early development and had no clinical efficacy. In addition, there is a possibility that there may be a correlation between dose escalation and local or constitutional symptoms since these were not taken into account in our dose-toxicity relationship analyses. However, these symptoms are not relevant to the determination of the DLT since they are all manageable toxicities.
Phase 1 trials may also be used to determine the BAD (6, 8). Our analysis of the included trials in this meta-analysis suggested that the vaccine dose does not increase the rate of the cellular immune response as measured by some immune assays. Although increasing the vaccine dose was associated with an increase in immune response rate, as measured by DTH in some trials, the increase in immune response did not appear consistent or sustainable with further dose escalation. Our analysis is limited by the fact that most of the trials used experimental immune assays that have not been validated in order to accurately evaluate the induced cellular immune response (30). It was also limited by the fact that these phase I trials were not designed with the objective of characterizing the relationship between dose and immunological response or even determining whether there is a dose response. Furthermore, the relationship between dose escalation and the degree of the induced immune response was not addressed in this manuscript since only a minority of the reviewed trials addressed this point. Detecting a dose that is biologically active is different from detecting the minimal dose that is biologically active or determining whether there is a relationship between dose and immunological response. We suggest that the standard 3+3 phase I design is not generally adequate in detecting a minimal BAD or the relationship between dose and immunological response in therapeutic cancer vaccines.
Need for alternative designs for early development of therapeutic cancer vaccines
The FDA has recently recognized the need for changing the way therapeutic cancer vaccine trials are being conducted (9). Our results, described above, support that conclusion. The incidence of dose limiting toxicity in such trials has been very low and consequently the use of designs developed for the detection of MTD is generally not appropriate.
A “Guidance for Industry: Clinical Considerations for Therapeutic Cancer Vaccines” has been recently issued by the FDA (9). That Guidance recognizes the inapplicability of the traditional 3+3 design, but indicates that a dose escalation design be used to determine the BAD and that the selection of the starting “safe” dose for cancer vaccines should be supported by preclinical and/or prior human safety data. Developing a safe starting dose based on preclinical testing is, however, not a feasible option in the majority of cancer vaccines due to the species-specific presentation and recognition of such antigens and variation in immune response activity. In addition, the results of our review suggest that the dose-escalation approach is problematic for most cancer vaccines since neither toxicity nor cellular immune response appears consistently related to dose.
Suggested alternative designs by others
Few alternative designs have been considered to replace the traditional dose escalation 3+3 phase 1 design for cancer vaccines. Messer et al. described a combined phase 1/2 design where a phase 1 dose escalation trial serves as an interim safety analysis prior to proceeding to phase 2 (31). On the other hand, Korn et al. described an initial accelerated phase design where one patient per dose level is treated until a biological response occurs. After the first response is seen, cohorts of 3–6 patients are treated per dose level in a traditional dose escalation phase (32). Hunsberger et al. described two stages of dose escalation in order to reach a molecular targeted endpoint. The first stage uses a 3+3 traditional escalation and the second is developed to continue to escalate the dose as long as the biological response rate is increasing (33). Simon et al. described alternative phase 2 designs for early cancer vaccine trial development (34). The cancer vaccine clinical trial working group (CVCTWG) has proposed a proof-of-principal trial design that combines some aspects of phase 1 and 2 trials in patients “without rapidly progressive disease” (35). Importantly, all of these suggested alternative designs are using a dose escalation method, which, as we have shown, rarely determines toxicity or biological activity measured by cellular immune response.
New purposed alternative design
Assumptions in early cancer vaccines development
Here, we suggest an alternative design for cancer vaccine development. To determine the starting dose of the vaccine, we need to take into account the following assumptions: 1) A cellular immune response is necessary to induce clinical efficacy. Immune response had been used as a surrogate for cancer vaccine efficacy in clinical trials (36–39). Many investigators have shown that patients who generate a T-cell immune response are more likely to have longer survival compared to non-immune responders (40–42). 2) Cancer vaccine dose does not consistently correlate with either toxicity or with the prevalence of induction of cellular immune response. 3) A vaccine as a single agent isn't enough to induce clinical efficacy, and combination therapy is needed (43). Accordingly, it is crucial to optimize the cellular immune response generated by a cancer vaccine when designing an early phase clinical trial by combining the vaccine with other agents such as immune modulators. Based on the above, our suggested design proposes to define a dose that can induce an immune response and then combine the selected dose with the appropriate immune modulating agents or other therapeutic modalities in order to obtain a better outcome.
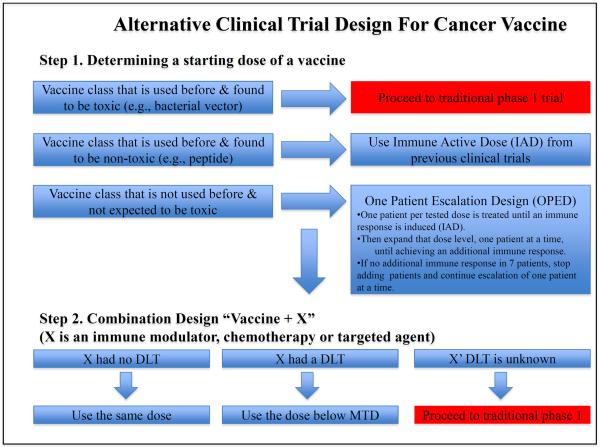
Step 1. Determining a starting dose of a vaccine
We propose a 2-step design (Figure 2). The first step is to determine an immune response-inducing dose. This would be used in step 2, a phase 2 trial testing the combination of cancer vaccine and immune modulating agents or other therapeutic modalities. Accordingly, the intention, first, is to determine a dose that can generate an immune response of a cancer vaccine, or immune active dose (IAD). Since local adjuvant is often used in combination with the vaccine, both the vaccine and the adjuvant are considered one entity in our design. The method of determining the IAD will be dependent on whether the vaccine belongs to a class of vaccines that has been previously tested in humans. If the vaccine class has been administered in humans and found to be non-toxic (e.g., a peptide), then we propose using the same IAD shown in previous trials in a phase 2 combination approach with immune modulators and/or other therapies. On the other hand, if the vaccine belongs to a class that had been used before and found to be toxic in humans (e.g., bacterial vectors, as shown above), or a new self-antigen that is crucial for a vital organ or function (e.g., angiogenesis antigens), then we recommend using the traditional 3+3 dose escalation design.
Figure 2.
The suggested alternative design for early cancer vaccine development
Finally, if the tested vaccine is considered “novel” or belongs to a class that has not been tested before and is expected to be non-toxic, we recommend using a “One Patient Escalation Design (OPED)” to determine the dose of the antigen that is active in at least one patient. By using the OPED, one patient per tested dose is treated until an immune response is induced in order to determine the IAD. To confirm the dose as the IAD, the same dose will be administered to an expanded group of patients. Having a precise estimate of the immunological response rate at that dose is not the goal; rather the goal is to confirm that there is some biological activity at that dose. We propose the following approach: if the expected cellular immune response rate, with the vaccine alone at that dose is 30%, the probability of obtaining no responses in an expansion cohort of 7 patients is .082. So from this perspective, in order to make sure that the initial response was real, it would be appropriate to expand the cohort at that dose level, one patient at a time, until achieving an additional response. If no additional response is seen in 7 patients, then we will stop adding patients and will continue escalation of one patient at a time until we obtain an immunological response with a confirmatory expansion cohort. However, if an additional immune response is observed in the expanded cohort, no more patients will be enrolled since the purpose of adding more patients is to confirm the first immune response.
If the probability of immune response is denoted by p and is independent of dose, then the number of patients treated until the first response is observed has a geometric distribution; the probability that the first response occurs at the n'th patient is pqn−1 where q=(1−p). The probability that the IAD will be confirmed by studying up to 7 patients at the dose level giving a response is 1 − q7. When p=0.33, the probability that the IAD occurs at the first dose level is 0.31 and the mean dose level at which it occurs is 3. The probability that the IAD is confirmed is 0.94. When p=0.5, the probability that the IAD occurs at the first dose level is 0.496 and the mean dose level at which it occurs is 2. The probability that the IAD is confirmed is 0.99. If there is a dose-immunological response relation, then the probability that the first response occurs at the n'th patient is q1 q2 qn−1 pn.
Step 2. Combining the vaccine with immune modulating agents or other therapeutic agents
The second step is to combine the vaccine with immune modulating agent, chemotherapeutic agent or other cancer therapy agent referred to as “X” in order to enhance the cancer vaccine efficacy. During this step the vaccine and the adjuvant are considered one entity and referred to as “vaccine”. During this step, we recommend using the vaccine's IAD that is identified in the first step and setting the “X” dose based on its safety profile. If “X” had no known DLT, then we recommend combining the vaccine with the same “X” dose that was used before. On the other hand, if “X” has a known DLT then we recommend combining the vaccine with an “X” dose that is below its MTD. However, if the “X” DLT is unknown (testing a novel agent), then we recommend proceeding to a traditional phase 1 (3+3) design by escalating the “X” dose in combination with a fixed dose of the vaccine (IAD). Enhancing the cancer vaccine efficacy further may require adding another agent “Y” (e.g., immune modulator, chemotherapy or targeted agent) to the combination of (vaccine + X). In this case, the combination of vaccine and X should be treated as one entity (vaccine + X) and the “Y” dose could be changed based on its safety profile in a similar method that is used when combing the vaccine with “X”. More agents could be added to the combination of (vaccine + X + Y) in a similar fashion (Figure 2). Simon et al. has described a variety of phase 2 designs that can be used for optimizing a vaccine-based regimen (34).
Advantages and limitations of the new purposed design
Our suggested design has many advantages including the ability to identify the safe and immune active dose without the need to enroll a large number of patients unless the study is testing a vaccine class that is known to be toxic. Our design also allows for enhancing the vaccine efficacy by combining the vaccine with other agents. However, our recommendation may have some limitations since it does not take into account the possible cumulative toxicities of cancer vaccines and the lack of validation of immunological assays. Nevertheless, our suggested design may represent a step forward in therapeutic cancer vaccine development and needs to be tested in the future.
Supplementary Material
Translational Relevance.
The traditional phase I dose escalation design has been used by investigators in early therapeutic cancer vaccine development for decades. However, in contrast to cytotoxic agents, this design may not be applicable for therapeutic cancer vaccines given their unique mechanism of action and their relatively limited toxicities. The data presented in this report support the lack of correlation between dose escalation and cancer vaccines' toxicities or cellular immune responses except for certain cases. In addition, this current report presents a novel design for therapeutic cancer vaccines early development. This design may allow investigators to conduct early phase clinical trials in cancer vaccines without the need to enroll large number of patients in order to identify the safe and immune active dose. Subsequently, the identified safe and immune active dose could be used in combination with other agents in phase II/III clinical trials. This would provide clinical investigators with a new tool to conduct clinical trials in cancer immunotherapy.
Acknowledgments
Financial support: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The material submitted in this manuscript for publication has not been previously reported nor is it under consideration for publication elsewhere.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.FDA . The 1938 Food, Drug, and Cosmetic Act. FDA; 1938. p. 93. [Google Scholar]
- 2.FDA The Kefauver-Harris Amendments of 1962. 1962 cited; Available from: http://www.fda.gov/RegulatoryInformation/Legislation/ucm2005639.htm.
- 3.Arbuck SG. Workshop on phase I study design. Ninth NCI/EORTC New Drug Development Symposium, Amsterdam, March 12, 1996. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 1996;7:567–73. doi: 10.1093/oxfordjournals.annonc.a010672. [DOI] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, O'Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000;18:684–92. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 5.Kummar S, Gutierrez M, Doroshow JH, Murgo AJ. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br J Clin Pharmacol. 2006;62:15–26. doi: 10.1111/j.1365-2125.2006.02713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox E, Curt GA, Balis FM. Clinical trial design for target-based therapy. Oncologist. 2002;7:401–9. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK, Lee JJ, Hong D, Markman M, Gong J, Naing A, et al. Phase I oncology studies: evidence that in the era of targeted therapies patients on lower doses do not fare worse. Clin Cancer Res. 2010;16:1289–97. doi: 10.1158/1078-0432.CCR-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horstmann E, McCabe MS, Grochow L, Yamamoto S, Rubinstein L, Budd T, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. The New England journal of medicine. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 9.FDA USDoHaHS. Administration FaD. Research CfBEa Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines. 2011 cited 2011 October 2011]; Available from: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm.
- 10.Nemunaitis J, Sterman D, Jablons D, Smith JW, 2nd, Fox B, Maples P, et al. Granulocyte-macrophage colony-stimulating factor gene-modified autologous tumor vaccines in non-small-cell lung cancer. Journal of the National Cancer Institute. 2004;96:326–31. doi: 10.1093/jnci/djh028. [DOI] [PubMed] [Google Scholar]
- 11.Higano CS, Corman JM, Smith DC, Centeno AS, Steidle CP, Gittleman M, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113:975–84. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 12.Kaumaya PT, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:5270–7. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Mehren M, Arlen P, Tsang KY, Rogatko A, Meropol N, Cooper HS, et al. Pilot study of a dual gene recombinant avipox vaccine containing both carcinoembryonic antigen (CEA) and B7.1 transgenes in patients with recurrent CEA-expressing adenocarcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:2219–28. [PubMed] [Google Scholar]
- 14.Bertinetti C, Zirlik K, Heining-Mikesch K, Ihorst G, Dierbach H, Waller CF, et al. Phase I trial of a novel intradermal idiotype vaccine in patients with advanced B-cell lymphoma: specific immune responses despite profound immunosuppression. Cancer research. 2006;66:4496–502. doi: 10.1158/0008-5472.CAN-05-4233. [DOI] [PubMed] [Google Scholar]
- 15.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27:3975–83. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Smith AM, Justin T, Michaeli D, Watson SA. Phase I/II study of G17-DT, an anti-gastrin immunogen, in advanced colorectal cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:4719–24. [PubMed] [Google Scholar]
- 17.Osorio M, Gracia E, Rodriguez E, Saurez G, Arango Mdel C, Noris E, et al. Heterophilic NeuGcGM3 ganglioside cancer vaccine in advanced melanoma patients: results of a Phase Ib/IIa study. Cancer biology & therapy. 2008;7:488–95. doi: 10.4161/cbt.7.4.5476. [DOI] [PubMed] [Google Scholar]
- 18.Dangoor A, Lorigan P, Keilholz U, Schadendorf D, Harris A, Ottensmeier C, et al. Clinical and immunological responses in metastatic melanoma patients vaccinated with a high-dose poly-epitope vaccine. Cancer immunology, immunotherapy : CII. 2010;59:863–73. doi: 10.1007/s00262-009-0811-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell HV, Strother D, Mei Z, Rill D, Popek E, Biagi E, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. J Immunother. 2007;30:227–33. doi: 10.1097/01.cji.0000211335.14385.57. [DOI] [PubMed] [Google Scholar]
- 20.Weber J, Boswell W, Smith J, Hersh E, Snively J, Diaz M, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–23. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 21.Guthmann MD, Bitton RJ, Carnero AJ, Gabri MR, Cinat G, Koliren L, et al. Active specific immunotherapy of melanoma with a GM3 ganglioside-based vaccine: a report on safety and immunogenicity. J Immunother. 2004;27:442–51. doi: 10.1097/00002371-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Palmer M, Parker J, Modi S, Butts C, Smylie M, Meikle A, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer. 2001;3:49–57. doi: 10.3816/clc.2001.n.018. discussion 8. [DOI] [PubMed] [Google Scholar]
- 23.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer research. 2010;70:875–82. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterston AM, Gumbrell L, Bratt T, Waller S, Gustav-Aspland J, L'Hermenier C, et al. Phase I study of TNFalpha AutoVaccIne in patients with metastatic cancer. Cancer immunology, immunotherapy : CII. 2005;54:848–57. doi: 10.1007/s00262-005-0661-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oka Y, Tsuboi A, Taguchi T, Osaki T, Kyo T, Nakajima H, et al. Induction of WT1 (Wilms' tumor gene)-specific cytotoxic T lymphocytes by WT1 peptide vaccine and the resultant cancer regression. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13885–90. doi: 10.1073/pnas.0405884101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dols A, Smith JW, 2nd, Meijer SL, Fox BA, Hu HM, Walker E, et al. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Human gene therapy. 2003;14:1117–23. doi: 10.1089/104303403322124828. [DOI] [PubMed] [Google Scholar]
- 27.Brett BT, Smith SC, Bouvier CV, Michaeli D, Hochhauser D, Davidson BR, et al. Phase II study of anti-gastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:4225–31. doi: 10.1200/JCO.2002.11.151. [DOI] [PubMed] [Google Scholar]
- 28.Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J, et al. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95:1781–7. [PubMed] [Google Scholar]
- 29.(EMEA) EMA GUIDELINE ON THE CLINICAL DEVELOPMENT OF PRODUCTS FOR SPECIFIC IMMUNOTHERAPY. 2008 cited; Available from: www.ema.europa.eu/docs/en_GB/document…/WC500003605.pdf.
- 30.Keilholz U, Weber J, Finke JH, Gabrilovich DI, Kast WM, Disis ML, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Messer K, Natarajan L, Ball ED, Lane TA. Toxicity-evaluation designs for phase I/II cancer immunotherapy trials. Statistics in medicine. 2010;29:712–20. doi: 10.1002/sim.3799. [DOI] [PubMed] [Google Scholar]
- 32.Korn EL, Arbuck SG, Pluda JM, Simon R, Kaplan RS, Christian MC, et al. Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Oncol. 2001;19:265–72. doi: 10.1200/JCO.2001.19.1.265. [DOI] [PubMed] [Google Scholar]
- 33.Hunsberger S, Rubinstein LV, Dancey J, Korn EL. Dose escalation trial designs based on a molecularly targeted endpoint. Statistics in medicine. 2005;24:2171–81. doi: 10.1002/sim.2102. [DOI] [PubMed] [Google Scholar]
- 34.Simon R. Clinical trial designs for therapeutic cancer vaccines. Cancer treatment and research. 2005;123:339–50. doi: 10.1007/0-387-27545-2_14. [DOI] [PubMed] [Google Scholar]
- 35.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 36.Disis ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer immunology, immunotherapy : CII. 2011;60:433–42. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Meara MM, Disis ML. Therapeutic cancer vaccines and translating vaccinomics science to the global health clinic: emerging applications toward proof of concept. OMICS. 2011;15:579–88. doi: 10.1089/omi.2010.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slota M, Lim JB, Dang Y, Disis ML. ELISpot for measuring human immune responses to vaccines. Expert Rev Vaccines. 2011;10:299–306. doi: 10.1586/erv.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broussard EK, Disis ML. TNM staging in colorectal cancer: T is for T cell and M is for memory. J Clin Oncol. 2011;29:601–3. doi: 10.1200/JCO.2010.32.9078. [DOI] [PubMed] [Google Scholar]
- 40.Slingluff CL, Jr., Petroni GR, Olson W, Czarkowski A, Grosh WW, Smolkin M, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:4973–80. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkwood JM, Lee S, Moschos SJ, Albertini MR, Michalak JC, Sander C, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/−granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–51. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunol Immunother. 2012;61:373–84. doi: 10.1007/s00262-011-1100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR, et al. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol. 2012;39:323–39. doi: 10.1053/j.seminoncol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.