Abstract
Objective
The relationship between acute care clinical indicators in the first severe Pediatric traumatic brain injury (TBI) Guidelines and outcomes have not been examined. We aimed to develop a set of acute care guideline-influenced clinical indicators of adherence and tested the relationship between these indicators during the first 72 hours after hospital admission and discharge outcomes.
Design
Retrospective multicenter cohort study
Setting
Five regional pediatric trauma centers affiliated with academic medical centers.
Patients
Children under 17 years with severe TBI (admission Glasgow coma scale (GCS) score ≤ 8, ICD-9 diagnosis codes of 800.0-801.9, 803.0-804.9, 850.0-854.1, 959.01, 950.1-950.3, 995.55, maximum head abbreviated injury severity score ≥ 3) who received tracheal intubation for at-least 48 hours in the intensive care unit (ICU) between 2007 -2011 were examined.
Interventions
None
Measurements and Main Results
Total percent adherence to the clinical indicators across all treatment locations (pre-hospital [PH], emergency department [ED], operating room [OR], and intensive care unit [ICU]) during the first 72 hours after admission to study center were determined. Main outcomes were discharge survival and Glasgow outcome scale (GOS) score.
Total adherence rate across all locations and all centers ranged from 68-78%. Clinical indicators of adherence were associated with survival (aHR 0.94; 95 % CI 0.91, 0.96). Three indicators were associated with survival: absence of PH hypoxia (aHR 0.20; 95% CI 0.08, 0.46), early ICU start of nutrition (aHR 0.06; 95% CI 0.01, 0.26), and ICU PaCO2 >30 mm Hg in the absence of radiographic or clinical signs of cerebral herniation (aHR 0.22; 95% CI 0.06, 0.8). Clinical indicators of adherence were associated with favorable GOS among survivors, (aHR 0.99; 95% CI 0.98, 0.99). Three indicators were associated with favorable discharge GOS: all OR CPP >40 mm Hg (aRR 0.64; 95% CI 0.55, 0.75), all ICU CPP > 40mm Hg (aRR 0.74; 95% CI 0.63, 0.87), and no surgery (any type; aRR 0.72; 95% CI 0.53, 0.97).
Conclusions
Acute care clinical indicators of adherence to the Pediatric Guidelines were associated with significantly higher discharge survival and improved discharge GOS. Some indicators were protective, regardless of treatment location, suggesting the need for an interdisciplinary approach to the care of children with severe TBI.
Keywords: pediatrics, trauma, brain injury, indicators, outcomes, injury
INTRODUCTION
Traumatic brain injury (TBI) is a leading cause of morbidity and mortality and is responsible for more than 630,000 emergency department visits, 60,000 hospitalizations, and 6,000 deaths in the United States annually among children and youth 0 to 19 years of age.1 Outcomes after pediatric TBI depend on factors such as age, injury severity and intent of injury2-4 but may also depend on timely and high quality acute care5-9. Publication of the 2003 Pediatric TBI Guidelines represents the first interdisciplinary effort to provide guidance on provision of evidence-based acute care for infants and children with severe TBI 10 but the association between adherence and outcomes has not been examined. The majority of the original topics were retained in the 2012 second edition11.
Eighteen of the nineteen chapters of the 2003 Guidelines contain fourteen intensive care unit (ICU) treatment recommendations based on strength of evidence. Since the majority of the recommendations offer clinicians options to consider clinical indicators rather than being prescriptive about their use, clinicians may choose not to implement these recommendations and measures of effectiveness and adherence may be difficult to evaluate. Yet, these guidelines provide parameters believed by experts to be associated with outcomes and provide an opportunity to test the association between indicators of adherence and outcomes. Process and flow characteristics in treatment locations such as the intensive care unit (ICU), pre-hospital (PH) setting, emergency department (ED) and operating room (OR) add to the complexity of implementing guideline recommendations and yet inform the development of adherence benchmarks where children with severe TBI receive care.10,12-13
We developed a set of clinical indicators of adherence to the 2003 Pediatric Guidelines and then tested the relationship between these indicators and outcomes at 5 pediatric trauma centers (PTCs). Due to the retrospective nature of the planned analysis, the close study timeline in relation to publication of the 2012 Pediatric Guidelines, and the lag between its publication and implementation, we used the 2003 Guidelines. We hypothesized that adherence to acute care clinical indicators during the first 72 hours after hospital admission would be associated with improved discharge survival and neurological outcome.
MATERIALS AND METHODS
Study Center Selection and Data Sources
Five geographically dispersed PTCs affiliated with academic medical centers were recruited based on willingness to participate, a priori ability to contribute data from 30-50 children with severe TBI annually, electronic medical records, representation in the Pediatric Neurocritical Care Research Group (http://www.pncrg.org/), PTC publications, regional PTC status, and recognized expertise severe TBI care. Study centers were the University of Washington's Harborview Medical Center, Seattle, WA (lead and data coordinating center); Children's Hospital of Pittsburgh, Pittsburgh, PA; Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL; Harbor-University of California, Los Angeles Medical Center, Torrance, CA; and Nationwide Children's Hospital, Columbus, OH. During the study period (1/1/2007-12/31/2011), all considered themselves adherent to the Pediatric Guidelines but adherence rates were unknown. Human subjects’ committee approval was obtained at each site.
Center training and Reliability Testing
Data abstraction training modules were to each data abstractor who underwent training followed by data abstraction from two test cases. A third case was used to determine baseline inter-abstractor reliability with the lead abstractor (NK). After pooled abstraction of the first 100 medical records, another two test cases were abstracted for reliability testing. A kappa of 0.8 was a priori considered adequate between center reliability16-18. Supplementary trainings were conducted for kappa values <0.8 until the goal was achieved.
Study Population
Eligible participants were subjects 0 to 17 year old with severe TBI, defined as having ≥ one ICD-9 discharge diagnosis code: 800.0-801.9, 803.0-804.9, 850.0-854.1, 959.01, 950.1-950.3, and 995.55, consistent with the Centers for Disease Control and Prevention definition14. Criteria for severe TBI required: a minimum head abbreviated injury severity (AIS) score ≥ 3, post-resuscitation Glasgow Coma Scale (GCS) score ≤ 8, alive with tracheal intubation in the ICU ≥ 48 hours from the time of ICU admission, trauma history, and abnormal admission head computed tomography. We excluded patients who died within the first 48 hours after ICU admission because we wanted to examine adherence which meant that patients had to be alive for some period of time to allow for adherence to be examined. We included subjects with extracranial injuries and those who were transferred from scene to an outside hospital before admission to the study center.
Medical Record Abstraction
At each site, data were abstracted from electronic medical records and included data elements from four treatment locations (PH, ED, OR and ICU). Data were remotely and securely entered into a web based data entry system. All centers achieved excellent inter-rater agreement (kappa > 0.8)15-17.
Measures of Pediatric Guideline Adherence
Sixteen clinical indicators were created to represent measures of adherence with recommendations within the Pediatric Guidelines (Supplemental Digital Content - Table 1). The number and type of study indicators for each treatment location was determined a priori by the study group. While we initially derived indicators from the Pediatric guidelines 2003, we had to operationalize some variables. This was an iterative process involving the project investigators. The effect of adherence was unable to be examined for the subset of indicators with close to 100% adherence (Supplemental Digital Content - Table 4). The indicators examined at each treatment location are given in Supplemental Digital Content - Table 1.
The number of indicators does not map directly to the Guidelines due to duplication of indicators across some chapters and involvement of multiple indicators in others (Supplemental Digital Content - Table 1). Since patients may undergo surgery either before or after admission to the ICU, intracranial pressure (ICP) monitoring and cerebral perfusion pressure (CPP) indicators were collected for both the OR and ICU. Five indicators were collected for the PH setting, 5 for the ED, 10 for the OR, and 14 for the ICU. For some indicators, we added a time component based on time-dependent effect on patient outcomes. Each clinical indicator was examined for conditionality; clinical indicators were considered relevant for patients who had underlying conditions that would have qualified for given treatments. We examined ICP in a number of ways. First, we have defined high ICP (intracranial hypertension) as presence of cerebral herniation (unequal pupils, or hypertension & bradycardia, as determined clinically) or administration of mannitol and/or hypertonic saline, or ICP > 20mmHg if ICP monitor was placed. These definitions were used in each treatment location (Prehospital [PH; includes EMS & Index hospital], ED, OR and ICU; Supplemental Digital Content - Table 1). We also examined the effect of ICP on CPP (MAP – ICP) as part of examining hyperventilation as opposed to normoventilation in the presence of cerebral herniation, and as part of the decompressive craniectomy indicator. Lastly, we examined ICP effects in the context of barbiturate use in the absence of hypotension for refractory high ICP and use of hypertonic saline to treat high ICP.
The main outcome was the association of adherence across all treatment locations with mortality and discharge Glasgow Outcome Scale (GOS) score. Secondary outcomes were the association with the location-specific adherence to indicators. For both summary measures, we included only those indicators with a protective point risk estimate after adjustment for all confounding variables. Summary measures were defined as the sum of indicators to which care was adherent divided by the number of relevant adherence indicators for a given patient at a given treatment location or across all locations.
Patient Outcomes
Outcomes included in-hospital mortality and discharge GOS. Deaths occurring within 48 hours after ICU admission were excluded. A dichotomous measure of discharge GOS was used (baseline, minor-moderate impairment vs. major impairment-vegetative state). We also conducted a sensitivity analysis in which death was part of GOS (combined with major impairment/vegetative state category)18.
Statistical Analyses
Patient and injury characteristics were examined in bivariate analyses by discharge GOS using χ2 tests for categorical variables and t-tests for continuous variables. All analyses were clustered within hospital. Statistical significance was defined as p < 0.05. All multiple regression analyses were adjusted for age, sex, maximum head AIS, maximum non-head AIS, and motor GCS at admission. Multivariable time-dependent extended Cox regression (survival analysis) was performed to examine the effect of adherence at each treatment location (and across all locations using the adherence rate as the independent variable and adjusting for any adherence indicators with a significantly adverse effect and all confounders) on in-hospital mortality after adjustment for age, sex, maximum head AIS, maximum non-head AIS, and motor GCS at admission. Adherence measures were modeled as time-dependent variables corresponding to the interval in each treatment location. Multivariable modified Poisson regression (Poisson regression with a robust error variance to estimate the relative risk directly)19,20 was used for examining the effect of adherence at each treatment location (and across all locations) on GOS with additional adjustment for length of stay. For both outcomes, adherence indicators were first examined singly with adjustment for confounders. Adherence variables with a p < 0.2 were entered into a final model. The final model was trimmed by successively dropping adherence variables with a p > 0.05 beginning with the variable with the highest p value and continuing until all adherence variables remaining were significant at p < 0.05. The purpose of the final model was to examine if each indicator still exhibited a unique effect beyond that explained by other adherence indicators.
All study analyses accounted for clustering by participating site. Because missing data were rare for variables used in the regression analyses, we conducted complete case analysis. Data were analyzed using Stata v. 1121 and SAS 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
Severe Pediatric TBI Sample Characteristics
Subjects had a mean age of 8.0 (SD 6.3) years, the majority was male (69%), and the most common mechanism of injury was abusive head trauma (25%). The majority (53%) had a head AIS score of 5 with mean ISS of 28.3 (SD 12.8). Seventy two percent had extracranial injuries; mostly to the extremities (48%) and face (45%). Subdural hematoma occurred frequently (67%), 82% developed high ICP (clinical signs of intracranial hypertension or ICP > 20 mm Hg) within 72 hours of admission, and 54% had radiographic evidence of cerebral edema at some point during hospitalization. Decompressive craniectomy was performed with patients with various lesions and pathologies, such as high ICP, EDH, SDH and cerebral edema. The majority 58% (71/122) of patients who underwent decompressive craniectomy had their procedures performed within 48hrs of admission to trauma center and had one or more of the above 4 conditions as an indication, specifically, 82% (58/71) had 2 or more conditions; 2 had only EDH, 6 had only SDH, 4 had only edema and 1 had only high ICP.
Outcome Characteristics
Median ICU length of stay was 11 (IQR 14) days, in-hospital mortality was 13 % (range 2.1-20) and median GOS was 3.0 (IQR 1.0, range 1-5). Among survivors, 48% were discharged alive with major impairment. Nearly half 14/30 (47%) of children who died in hospital had abusive TBI. Outcomes varied by ISS scores (p<0.0001), type of head CT diagnosis (intraventricular hemorrhage [p=0.004], EDH [p=0.03] or cerebral edema [p<0.001]) and whether or not patients were directly transferred from scene to a study hospital (p=0.03; Supplemental Digital Content - Tables 2 and 3).
Adherence to Clinical Indicators and Mortality
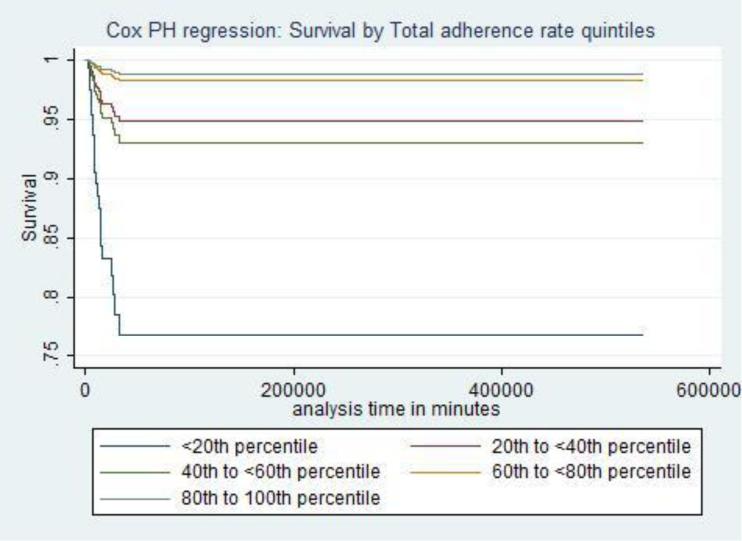
The average overall adherence rate across all centers and treatment locations was 72.8 (SD 8.9).The clinical characteristics by center and GOS are given in Supplemental Digital Content - Tables 2 and 3, respectively. Across all locations and study centers, clinical indicators of adherence were associated with better survival (aHR 0.94; 95 % CI 0.91, 0.96), indicating a 6% lower hazard of death for every percentage point increase in adherence. Higher quintiles of percent adherence were associated with significantly higher survival (Table 5, Figure 1). Supplemental Digital Content - Table 6 shows that after adjustment for confounders, some clinical indicators emerged as either protective or harmful. Three indicators were associated with lower mortality: 1) absence of PH hypoxia (aHR 0.20; 95% CI 0.08, 0.46); 2) early ICU start of nutrition (aHR 0.06; 95% CI 0.01, 0.26); and 3) ICU PaCO2 > 30 mm Hg in the absence of radiographic or clinical signs of cerebral herniation (aHR 0.22; 95% CI 0.06, 0.8). Early nutrition was associated with lower mortality, regardless of presence or absence of abdominal injuries. Two indicators were associated with higher mortality. The first was absence of surgical intervention compared to receipt of surgical intervention other than decompressive craniectomy among patients with high ICP or/and EDH or/and SDH or/and cerebral edema (aHR 5.33; 95% CI 3.44, 8.44). The second was PaCO2 ≤ 30 mm Hg (aHR 11.8; 95% CI 3.7, 37.9) compared to PaCO2 > 30 mm Hg among patients with cerebral herniation. Clinical indicators associated with discharge survival at each treatment location are presented in Table 7.
Table 5.
Association of Overall Adherence Rate by Quintile on Discharge Mortality and Glasgow Outcome Scale (GOS) score. Risk estimates are adjusted Hazard Ratios (aHR) for in-hospital mortality and adjusted Relative Risks (aRR) for discharge GOS.
| Overall Adherence Rate Quintile (Percentile) | aHR (95% CI) Mortality* | aRR (95% CI) GOS*** |
|---|---|---|
| < 20th | 1.00 (Ref) | 1.00(Ref) |
| 20th to <40th | 0.20 (0.05, 0.80) | 0.93(0.67, 1.29) |
| 40th to <60th | 0.27 (0.12, 0.61) | 0.75(0.54, 1.04) |
| 60th to <80th | 0.06 (0.02, 0.17) | 0.61(0.36, 1.05) |
| 80th to 100th | 0.04 (0.003, 0.57) | 0.66(0.43, 1.01) |
Data adjusted for age, sex, head Abbreviated Injury Score (AIS), maximum non-head AIS, and Glasgow coma scale score motor.
p <0.0001 and model only includes protective indicators. Adjusted for decompressive craniectomy and PaCO2 (significant adverse indicators)
Model only includes protective indicators. Adjusted for Intensive Care Unit (ICU) Length of Stay (LOS), Operating room neuromuscular blockade monitored and ICU neuromuscular blockade monitored (significant adverse indicators)
Figure 1.
Discharge Survival by Quintiles of Total Adherence Rate to Clinical Indicators of Adherence to 2003 Severe Pediatric Traumatic Brain Injury Guidelines for Cohort of 236 Subjects across Five Pediatric Trauma Study Centers
Table 7.
Association between Clinical Indicators of Adherence and Discharge Survival by Each Treatment Location.
| Adherence Indicators Affecting Mortality | Prehospital 5 indicators* | Emergency Department 5 indicators* | Operating room 10 indicators* | Intensive Care Unit 14 indicators* |
|---|---|---|---|---|
| aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | aHR (95% CI) | |
| Direct transport to pediatric trauma center from scene | ||||
| No; transport to outside hospital then pediatric trauma center | 1.00 (---) | |||
| Yes; direct transport from scene | 0.14 (0.03, 0.73) | |||
| Decompressive craniectomy | ||||
| No DC for high ICP or/and SDH or/and EDH or/and cerebral edema | 1.0 (---) | |||
| DC for high ICP or/and SDH or/and EDH or/and cerebral edema | 4.3 (3.3, 5.6) | |||
| No high ICP or/and SDH or/and EDH or/and cerebral edema | not included in model*** | |||
| Hypoxia treated within 30 minutes after onset | ||||
| Hypoxia present & untreated or treated > 30 minutes | 1.00 (---) | |||
| Hypoxia present & treated in 30 minutes | 0.02 (0.001, 0.31) | |||
| No hypoxia present | 0.006 (0.005, 0.071) | |||
| Early nutrition start | ||||
| Start after 72hours of TBI | 1.00 (---) | |||
| Start within 72 hours of TBI | 0.06 (0.02, 0.17)** | |||
| PaCO2 > 30mmHg in absence of herniation & PaCO2 ≤30mmHg in presence of herniation (abnormal pupil or CT evidence) | ||||
| HERNIATION PRESENT | ||||
| PaCO2 > 30 mmHg | 1.00 (---) | |||
| PaCO2 ≤ 30 mmHg | 18.3 (5.69, 58.9) | |||
| NO HERNIATION | ||||
| PaCO2 ≤ 30 mmHg | 1.0 (---) | |||
| PaCO2 > 30 mmHg | 0.26 (0.06, 1.13) | |||
| Hypertonic saline or Mannitol used for high ICP | ||||
| High ICP and neither used | 1.00 (---) | |||
| High ICP and either/both used | 0.14 (0.01, 1.58) | |||
| No high ICP present | 0.25 (0.07, 0.99) | |||
| Barbiturates used in high ICP | ||||
| No barbiturates used with high ICP | 1.00 (---) | |||
| Barbiturates used with high ICP | 0.22 (0.18-0.26) | |||
| No high ICP | 0.78 (0.43, 1.45) |
See Table 1 for Location specific indicators examined.
No difference when model run excluding subjects with abdominal injuries.;
n = 7 for this group and no deaths occurred
All models adjusted for age, sex, head abbreviated injury severity (AIS) score, motor Glasgow coma scale score, and maximum non-head AIS.
Each location is a separate model.
---Indicator not in model.
Adherence to Clinical Indicators and Discharge Neurological Outcome
Across all treatment locations and study centers, clinical indicators of adherence were associated with favorable GOS (aHR 0.99; 95% CI 0.98, 0.99 among survivors), indicating a 1% increase in adherence with a 1% decrease in the chance of poor (vegetative or major impairment) functional outcome of survivors. The risk estimates for poor outcome decreased with increasing quintiles of adherence but these results were not significant (Supplemental Digital Content - Table 2). The 3rd, 4th and 5th percentiles for adherence were associated with significantly lower poor GOS (death, vegetative or major impairment) compared with 1st quintile when deaths were included in the GOS model.
Supplemental Digital Content - Table 6 shows that, after adjustments for confounders, some clinical indicators were associated with discharge GOS. Among survivors, three protective indicators were: all OR CPP > 40 mm Hg (aRR 0.61; 95% CI 0.58, 0.64), all ICU CPP > 40 mm Hg (aRR 0.73; 95% CI 0.63, 0.84), and no surgery (any type; aRR 0.68; 95% CI 0.53, 0.86). One indicator increased the risk of poor GOS: lack of OR neuromuscular blockade administration (aRR 1.37; 95% CI 1.24, 1.53). We defined neuromuscular blockade monitoring as documentation of the use of a twitch monitor (example train of four). First, 112 of 122 patients undergoing surgery/anesthesia received neuromuscular blockade (only 10 did not) and, reflective of the clinical practice of providing a balanced anesthetic regimen in TBI patients. However, only 21/112(18.8%) documented NMB monitoring in the OR. Since the indicator examined was NMB monitoring, the comparison/reference group was the group who received NMB but who were not monitored (Supplemental Digital Content - Table 6 and Supplemental Digital Content - Table 1: Group 0 is the reference group). The clinical indicators associated with discharge GOS at each treatment location are displayed in Table 8.
Table 8.
Association between Clinical Indicators of Adherence and Discharge Glasgow Outcome Scale (GOS) score by Each Treatment Location.
| Adherence Indicators Affecting Discharge GOS (Vegetative/Major vs. Baseline/Minor/Moderate Impairment | Prehospital 5 Indicators* | Emergency Department 5 Indicators* | Operating Room 10 Indicators* | Intensive Care Unit 14 Indicators* |
|---|---|---|---|---|
| aRR (95% CI) | aRR(95% CI) | aRR(95% CI) | aRR(95% CI) | |
| Maintenance of CPP > 40mmHg | ||||
| Any CPP < 40mmHg | 1.00 (--) | 1.00 (--) | ||
| All CPP > 40 mmHg | 0.57 (0.51,0.63) | 0.72 (0.59, 0.87) | ||
| Neuromuscular blockade | ||||
| NMB not monitored when NMB administered | 1.00 (--) | |||
| NMB monitored when NMB administered | 1.33 (0.93, 1.9) | |||
| No neuromuscular blockade given | 1.42 (1.28, 1.58) | |||
| Hypertonic saline/Mannitol used for high ICP | ||||
| High ICP and neither used | 1.00 (--) | |||
| High ICP and either/both used | 0.62 (0.42, 0.93) | |||
| No high ICP present | 0.59 (0.41, 0.84) |
See Table1 for Location specific indicators examined.
All models adjusted for age, sex, head abbreviated injury severity (AIS) score, motor Glasgow coma scale score, and maximum non-head AIS, ICU LOS.
Each location is a separate model.
--- indicates not in the model.
We performed a sensitivity analysis with and without TBI due to child abuse. and there was no significant difference between adherence and outcome between these groups.
DISCUSSION
The main findings of our study are that for children with severe TBI who received care at five regional PTCs: 1) Adherence to acute care clinical indicators in the 2003 Pediatric TBI Guidelines during the first 72 hours after hospital admission was associated with increased discharge survival and favorable neurological outcome and 2) Some clinical indicators of adherence were protective in more than one treatment location where TBI care is provided. These findings suggest that early adherence to the Pediatric Guidelines may be important for achieving favorable discharge outcomes for children with severe TBI and support guideline based acute care in these children.
There are some advantages to our study. This study represents the experience and practice of 5 leading academic PTCs and benchmarks quality of acute care22. We used retrospective data collection procedures because the combination of prospective protocols that would incorporate the 16 total clinical indicators embodied within our study would render a prospective study challenging. Moreover, prospective studies may be affected by trial effects23 whereas in this study, alteration of clinical practice for data collection did not occur. Finally, while the Pediatric Guidelines were not intended to be limited to ICU care, treatment location was not clearly specified. Small scale studies suggest an association between second insults, such as hypotension occurring in non ICU treatment locations, and poor outcomes13, 227. The Pediatric Guidelines provided us with an opportunity to examine interventions at different treatment locations within a hospital. Future work will need to examine the generalizability of our findings at other centers which care for this population.
In comparison to the 2003 Pediatric Guidelines, 13 topics were retained, 5 were dropped, and 2 were added in the 2012 edition. Of the 13 retained topics, the level of recommendations was retained in 8 and the level of evidence changed in the other 5. For those acute care clinical indicators which were present in the 2003, but not in the 2012 Guidelines, we found a benefit for direct transfer to a PTC and for rapid correction of hypoxia. Across analyses, retained recommendations for barbiturate use in the absence of hypotension for refractory high ICP, use of hypertonic saline to treat high ICP, avoidance of high ICP, and avoidance of hypocarbia in absence of herniation and CPP management, are supported by our findings.
The range of optimal CPP in children with severe TBI is unknown. In this study, maintenance of all CPP > 40 mm Hg during the first 72 hours after TBI in both the ICU and OR was associated with improved discharge GOS, suggesting that maintenance of CPP above this minimum threshold should be a high priority for management in both treatment locations. As reported recently in adult TBI28, ICP monitoring within 4 hours of admission in this study was not protective against mortality or poor discharge GOS. However, the effect of ICP monitoring has to be considered in the context of preserving CPP. Therefore, while not individually significant, and we cannot comment on a safe upper limit for CPP, these data suggest that timely placement of ICP monitoring may, in-fact, be important for ensuring a minimum CPP > 40 mmHg. In this study, fewer subjects had surgery/anesthesia than might have been needed to show a relationship between intraoperative CPP management and discharge survival. Few ICP monitors were placed in the ED, and hence, the association between CPP and outcomes in the ED could not be sufficiently examined. Moreover, high ICP and herniation were harmful, suggesting further evaluation of ICP monitoring in pediatric TBI and examination of a safe upper limit of CPP is warranted.
We also found that avoidance of PH hypoxia to be significant for reducing discharge mortality, suggesting that this should be a high priority strategy for clinicians who render emergency services at the scene, during transport, or at an outside hospital prior to transfer to a PTC11. While we cannot comment on the specifics of neither diet nor glycemic control, and while provision of early parenteral nutrition to critically ill patients may not always reduce mortality29, early nutrition within 72 hours of severe TBI may be beneficial in severe pediatric TBI. Finally, hyperventilation (as opposed to normoventilation) in the absence of cerebral herniation was protective but in the presence of cerebral herniation was associated with mortality. Unwanted decrease in cerebral blood flow could be associated with poor outcome in both cases, suggesting the need for preservation of cerebral perfusion even in the presence of very high ICP. Alternately, our findings may also reflect the underlying harmful effect of cerebral herniation, rather than a hyperventilation effect given that despite hyperventilation, cerebral herniation was associated with poor outcome. Our findings also suggest that absence of surgical intervention compared to receipt of surgical intervention other than decompressive craniectomy among patients with high ICP or/and EDH or/and SDH or/and cerebral edema is associated with higher mortality. We used “non-adherence” as the reference group, and other groups were compared with this group, the other groups being decompressive craniectomy for those indicated to receive it; the effect of the absence of surgery relative to those indicated for decompressive craniectomy (high ICP or EDH or SDH); and lastly the effect of no surgery for those not indicated for decompressive craniectomy (those with no ICP or no EDH or no SDH; Supplemental Digital Content - Table 6). The use of neuromuscular blockade after severe pediatric TBI is not well-studied, and findings should be considered hypothesis-generating.
There are some study limitations. We could not collect the time (minutes) to achieving adherence since we had to operationalize some clinical indicators based on feasibility of data collection and on best available evidence. We may have lost some details due to the data collection burden for this study, given the complexity of the analysis. However, in the majority of cases, the absence of such granularity reflects the lack of available data. For example, we could not evaluate all clinical indicators, duration of indicator events or fluctuations in indicators/ treatment paradigms or all treatment facilities that may be important to patient outcomes30. The study population excluded patients who failed to survive for more than 48 hours in the ICU. Early deaths could reflect differences in PH adherence, which we were unable to capture. However, our rationale for using this criterion was to allow patients the opportunity to experience adherence, so as to be able to examine its association with outcomes.
Despite adjustment for potential confounders and reassuring sensitivity analyses, there may still be residual confounding by patient or treatment factors. Survival bias could have influenced selected indicators. We could not identify those subjects who did not receive the protective indicator care due to intangibles such as perceptions of futility for whom indicators could not be delivered or achieved. Subjects with major impairment at discharge may have recovered later but we were limited by existing data; this study preceded publication of the latest National Institute of Neurological Disease and Stroke TBI Common Data Elements recommendation for the use of the GOS-Extended score31. Finally, we cannot conclude which protective indicator is more effective than another. The magnitude of the observed effects suggests a beneficial effect of select acute care clinical indicators of adherence to the 2003 Pediatric Guidelines. While we could not retrospectively examine process flow measures, these results support current clinical practice that focuses on provision of these treatments, and research which examines the overall treatment effect of these protective indicators.
CONCLUSIONS
In summary, select acute clinical indicators of adherence that we believe represent the intent of the 2003 Pediatric Guidelines for the acute care management of severe TBI across treatment locations were associated with significantly higher discharge survival and improved discharge GOS. This study provides evidence regarding protective and harmful indicators in severe pediatric TBI across 5 leading pediatric trauma centers that can be benchmarked as quality indicators in severe TBI. These findings also suggest that clinical indicators are relevant to health care providers across the continuum of acute care.
Supplementary Material
ACKNOWLEDGEMENTS
Monica S. Vavilala, MD had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Mary Kernic, PhD, Department of Epidemiology and Jin Wang, PhD, Staff biostatistician of the University of Washington are responsible for the data analysis.
There are no conflicts of interests, including relevant financial interests, activities, relationships, and affiliations.
The source of support for this work including design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript was NINDS R01 NS072308-03.
The following staff have contributed to data collection reported in the manuscript and were compensated as project staff and we have obtained permission from them, and they have been sent the manuscript under review: Rachelle Bell, RN University of Pittsburgh Medical Center, Pittsburgh, PA; Kristi Schmidt, MD, Ann & Robert H. Lurie Children's Hospital, Chicago, IL; Alma Ramirez, Los Angeles BioMedical Research Institute, Harbor-UCLA Medical Center, Torrance, CA; Sheila Giles, RN, Nationwide Children's Hospital, Columbus, OH.
The work was performed at University of Washington's Harborview Medical Center, Seattle, WA (lead and data coordinating center); Children's Hospital of Pittsburgh, Pittsburgh, PA; Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL; Harbor University of California, Los Angeles Medical Center, Torrance, CA; and Nationwide Children's Hospital, Columbus, OH
Footnotes
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Faul M, Xu L, Wald MM, et al. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002-2006. Centers for Disease Control, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- 2.Kouznetsov E, Brennan M, Vassilyadi M. Towards development of a survival prediction tool for pediatric head injury. Pediatr Neurosurg. 2012;48(1):1–5. doi: 10.1159/000340068. doi: 10.1159/000340068. Epub 2012 Aug 21. [DOI] [PubMed] [Google Scholar]
- 3.Rivara FP, Koepsell TD, Wang J, et al. Disability 3, 12, and 24 months after traumatic brain injury among children and adolescents. Pediatrics. 2011 Nov;128(5):e1129–1138. doi: 10.1542/peds.2011-0840. doi: 10.1542/peds.2011-0840. Epub 2011 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sills MR, Libby AM, Orton HD. Prehospital and in-hospital mortality: a comparison of intentional and unintentional traumatic brain injuries in Colorado children. Arch Pediatr Adolesc Med. 2005 Jul;159(7):665–670. doi: 10.1001/archpedi.159.7.665. [DOI] [PubMed] [Google Scholar]
- 5.Zebrack M, Dandoy C, Hansen K, Scaife E, Mann NC, Bratton SL. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009 Jul;124(1):56–64. doi: 10.1542/peds.2008-1006. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 6.Franschman G, Peerdeman SM, Greuters S, et al. Prehospital endotracheal intubation in subjects with severe traumatic brain injury: guidelines versus reality. Resuscitation. 2009 Oct;80(10):1147–51. doi: 10.1016/j.resuscitation.2009.06.029. doi: 10.1016/j.resuscitation.2009.06.029. Epub 2009 Jul 25. [DOI] [PubMed] [Google Scholar]
- 7.Rusnak M, Janciak I, Majdan M, Wilbacher I, Mauritz W. Austrian Severe TBI Study Investigators: Severe traumatic brain injury in Austria VI: effects of guideline-based management. Wien Klin Wochenschr. 2007 Feb;119(1-2):64–71. doi: 10.1007/s00508-006-0765-0. [DOI] [PubMed] [Google Scholar]
- 8.Pronovost PJ, Rinke ML, Emery K, Dennison C, Blackledge C, Berenholtz SM. Interventions to reduce mortality among subjects treated in intensive care units. J Crit Care. 2004;19(3):158–164. doi: 10.1016/j.jcrc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Hesdorffer DC, Ghajar J. Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J Trauma. 2007 Oct;63(4):841–848. doi: 10.1097/TA.0b013e318123fc21. discussion 847-8. [DOI] [PubMed] [Google Scholar]
- 10.Adelson PD, Bratton SL, Carney NA, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Pediatr Crit Care Med. 2003 Jul;4(3 Suppl):S1–75. doi: 10.1097/01.CCM.0000067635.95882.24. [DOI] [PubMed] [Google Scholar]
- 11.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012 Jan;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 12.Pollack MM, Dean JM, Butler J, et al. The Ideal Time Interval for Critical Care Severity-of-Illness Assessment. Pediatr Crit Care Med. 2013 Jun;14(5):1–6. doi: 10.1097/PCC.0b013e31828a7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samant UB, IV, Mack CD, Koepsell T, Rivara FP, Vavilala MS. Time of hypotension and discharge outcome in children with severe traumatic brain injury. J Neurotrauma. 2008;25:495–502. doi: 10.1089/neu.2007.0491. [DOI] [PubMed] [Google Scholar]
- 14.International Classification of Diseases 9th Revision Clinical Modification for Physicians. Contexo Media. 2010:v.2. [Google Scholar]
- 15.Altman DG. Practical statistics for medical research. Chapman and Hall; London: 1991. [Google Scholar]
- 16.Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20:37–46. [Google Scholar]
- 17.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. Third Edition. John Wiley & Sons; Hoboken, NJ: 2003. [Google Scholar]
- 18.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual subjects after severe head injury. Lancet. 1976 May 15;1(7968):1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 19.Zhou G. A Modified Poisoon Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 20.McNutt LA, Wu C, Xue X, et al. Estimating the Relative Risk in Cohort Studies and Clinical Trials in Common Outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 21.Stata Statistical Software (computer program). Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 22.Rogers EM, et al. Diffusion of Innovation. Fourth Edition. The Free Press; New York, NY: 1995. p. 113. [Google Scholar]
- 23.Peppercorn JM, Weeks J, Cook E, Joffe S. Comparison of outcomes in cancer subjects treated within and outside clinical trials: conceptual framework and structured review. Lancet. 2004;363(9405):263–270. doi: 10.1016/S0140-6736(03)15383-4. [DOI] [PubMed] [Google Scholar]
- 24.Manley G, Knudson MM, Morabito D, Damron S, Erickson V, Pitts L. Hypotension, hypoxia, and head injury -frequency, duration, and consequences. Arch Surg. 2001;136:1118–1123. doi: 10.1001/archsurg.136.10.1118. [DOI] [PubMed] [Google Scholar]
- 25.Pigula FA, Wald SL, Shackford SR, Vane DW. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg. 1993 Mar;28(3):310–314. doi: 10.1016/0022-3468(93)90223-8. discussion 315-316. [DOI] [PubMed] [Google Scholar]
- 26.Chi JH, Knudson M, Vassar MJ, et al. Prehospital hypoxia affects outcome in subjects with traumatic brain injury: a prospective multicenter study. J Trauma. 2006;61:1134–1141. doi: 10.1097/01.ta.0000196644.64653.d8. [DOI] [PubMed] [Google Scholar]
- 27.Kokoska ER, Smith GS, Pittman T, Weber TR. Early hypotension worsens neurological outcome in pediatric subjects with moderately severe head trauma. J Pediatr Surg. 1998 Feb;33(2):333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 28.Chesnut RM, Temkin N, Carney N, et al. A Trial of Intracranial-Pressure Monitoring in Traumatic Brain Injury. N Engl J Med. 2012 Dec 27;367(26):2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill subjects with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. JAMA. 2013 May 22;309(20):2130–213821. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 30.Kernic MA, Rivara FP, Zatzick DF, et al. Triage of Children with Moderate and Severe Traumatic Brain Injury to Trauma Centers. J Neurotrauma. 2013 Jul 1;30(13):1129–1136. doi: 10.1089/neu.2012.2716. doi: 10.1089/neu.2012.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Institutes of Health (NIH) [10/01/13];NINDS Common Data Elements website. Available at: http://www.commondataelements.ninds.nih.gov/TBI.aspx#tab=Data_Standards.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.