Abstract
Elevated levels of TGF-β are a negative prognostic indicator for patients diagnosed with pancreatic cancer; as a result the TGF-β pathway is an attractive target for therapy. However, clinical application of pharmacologic inhibition of TGF-β remains challenging because TGF-β has tumor suppressor functions in many epithelial malignancies including pancreatic cancer. In fact, direct neutralization of TGF-β promotes tumor progression of genetic murine models of pancreatic cancer. Here we report that neutralizing the activity of murine TGF-β receptor 2 using a monoclonal antibody (2G8) has potent anti-metastatic activity in orthotopic human tumor xenografts, syngenic tumors and a genetic model of pancreatic cancer. 2G8 reduced activated fibroblasts, collagen deposition, microvessel density and vascular function. These stromal specific changes resulted in tumor cell epithelial differentiation and a potent reduction in metastases. We conclude that TGF-β signaling within stromal cells participates directly in tumor cell phenotype and pancreatic cancer progression. Thus, strategies that inhibit TGF-β dependent effector functions of stromal cells could be efficacious for the therapy of pancreatic tumors.
Keywords: pancreatic cancer, TGF-beta, tumor-stroma interactions, epithelial to mesenchymal transition
Introduction
Pancreatic cancer, the fourth leading cause of cancer-related mortality, presents a formidable challenge for treatment (1). Early metastasis, aggressive tumor biology and a stromal rich microenvironment provide potential mechanisms for the resistance of pancreatic cancer to conventional chemotherapy. Recent work suggests that stromal cells within the tumor microenvironment are critical determinants of tumor development, progression and metastasis (2,3). Therefore, there is heightened interest in strategies that target stromal cells including cancer-associated fibroblasts, immune cells and vascular cells.
Numerous cytokines participate in the progression of pancreatic malignancies. In particular, transforming growth factor β (TGF-β) has a complex function in pancreatic ductal adenocarcinoma (PDA). TGF-β is known to inhibit tumor progression in early stages of PDA development yet, at later stages TGF-β functions as a tumor promoter (4). The features that underlie the switch of TGF-β from a tumor suppressor to a tumor promoter in PDA are unclear. Mutations in the TGF-β signaling pathway occur in a large percentage (>50%) of human PDA (5) and likely contribute to the TGF-β functional switch. This has been modeled in mice where alterations of the TGF-β pathway (e.g, deletion of Smad4 or Tgfβr2) cooperated with activated Kras to promote disease progression (6–8). Furthermore, elevated expression of TGF-β, which is frequent in PDA can promote tumor development, tumor cell Epithelial to Mesenchymal Transition (EMT) and tumor cell survival and motility (9–12). TGF-β also induces immunosuppression, activation of fibroblasts, angiogenesis and collagen deposition (13,14). Therefore, specifically targeting the pro-tumoral aspects of TGF-β might provide therapeutic efficacy. Pharmacological strategies that block TGF-β activity have been explored in preclinical models of pancreatic cancer (reviewed in (15)). As discussed by Achyut and Yang (15) targeting the TGF-β pathway alters the tumor microenvironment and the outcome of therapy might be more dependent upon microenvironmental actions than on direct tumor cell effects.
Previously, 2G8 (aka MT1), a monoclonal antibody against mouse TGF-β receptor 2 (Tgfβr2), reduced primary tumor growth and metastasis in several syngenic models of breast cancer, primarily through its effects on tumor cells and tumor-associated immune cells (16). Given the clinical importance of TGF-β dysregulation in PDA (15), we hypothesized that stromal Tgfβr2 inhibition could be effective in mouse models of PDA. Given that 2G8 is mouse-specific, we implemented this antibody in human xenograft models of PDA to specifically target stromal Tgfβr2 without interrupting TGF-β signaling in the xenografted human tumor cells. We found that inhibition of the stromal TGF-β signaling promoted epithelial differentiation in tumor cells and inhibited metastasis, results that were recapitulated in immunocompetent models of PDA. The findings in xenografts highlight that TGF-β induction of stromal cell function is critical for its impact on tumor cell behavior and PDA progression.
Methods
Further information can be found in Supplementary Methods.
Cell lines
Murine pancreatic cancer cell line Pan02 (Panc02) was obtained from the Developmental Therapeutics Program at the NCI. The development of primary murine PDA cell lines (mPLR) is discussed in the supplement. Human pancreatic cancer cell lines (Capan-1 and MiaPaCa-2) were obtained from American Type Culture Collection (ATCC). Colo357 cells were obtained from Dr. Jason Fleming (MD Anderson Cancer Center). C5LM2 is a cell line derived from liver metastasis from a Panc-1 tumor bearing mouse isolated by our lab. RAW 264.7 (TIB-71) cells and NIH 3T3 cells were obtained from ATCC. Cell lines were confirmed to be pathogen-free and human cell lines were authenticated to confirm origin before use. Cells were cultured in DMEM (Invitrogen) or RPMI (Invitrogen) containing 10% fetal bovine serum and maintained at 37°C in a humidified incubator with 5% CO2 and 95% air.
In vivo models
Animals were housed in pathogen free facility and all animal studies were performed on a protocol approved by the IACUC at University of Texas Southwestern Medical Center. Animals were treated with 2G8 (provided by Imclone Systems), a rat IgG2a anti-mouse Tgfβr2 that does not bind human TGFβR2 (Supplemental Figure 1), AFRC Mac 48 (Mac 48, a rat IgG2a specific for phytochrome; European Collection of Animal Cell Cultures, Salisbury, United Kingdom), or gemcitabine (Eli Lilly and Company) purchased from the clinical pharmacy at UT Southwestern.
Orthotopic Pan02 Model
Pan02 cells (5×105) were injected orthotopically in 6–8 week old C57BL/6 mice. Tumor implantation was confirmed using ultrasound measurements. Ten days after tumor cell implantation, mice were randomized to receive Saline, Gemcitabine (25 mg/kg/wk), 2G8 (60 mg/kg/wk) or 2G8 + Gemcitabine. After 4 weeks of therapy, mice were sacrificed. Gross metastatic burden was assessed at necropsy.
Genetic Endpoint Model
LSL-KrasG12D; Cdkn2alox/lox; p48Cre (KIC) mice were generated as previously described (17). Mice 28–30 days old were randomized to receive Saline, Gemcitabine (12.5 mg/kg three times a week), 2G8 (120 mg/kg once weekly) or 2G8 + Gemcitabine. At the conclusion of 4 weeks of therapy, mice were sacrificed and organs harvested for tissue analysis. Liver micrometastasis was assessed by hemotoxylin and eosin on the anterior lobes of the liver.
Intrasplenic injection model
Pan02 cells (2.5×105) were injected using splenic parenchyma of 6–8 week old C57BL/6 mice. Groups were randomized to receive 2G8 (30 mg/kg/wk) 1 day prior to splenic injection, post injection day 1, post injection day 7, post injection day 14 or Mac48 (European Collection of Animal Cell Cultures), a rat isotyped matched IgG specific for phytochrome (30 mg/kg/wk). Mice were sacrificed after 5 weeks post tumor cell injection.
Xenograft studies
Eight week old SCID mice were injected orthotopically with 1×106 cells (Capan-1, Colo357, MiaPaCa-2 and C5LM2). One week after tumor cell injection mice were randomized to receive 2G8 (30 mg/kg/ wk) or Saline. Mice were sacrificed after 8–10 weeks of treatment. At the time of sacrifice, gross metastases were counted and primary tumors were snap frozen in liquid nitrogen or fixed in 10% formalin.
Histology
Formalin-fixed tissues were embedded in paraffin, sectioned and stained with Masson’s Trichrome and PAS-Alcian Blue by the Molecular Pathology Core (UT Southwestern). Immunohistochemistry was performed with the following antibodies: phospho-Histone H3 (Millipore, 06-570), cleaved caspase-3 (Cell Signaling, 9664), E-cadherin (Santa Cruz, sc-7870), vimentin (PhosphoSolution, 2105-VIM), β-catenin (Cell Signaling, 9582), Zeb1 (Santa Cruz, sc 25388), α-smooth muscle actin (Neomarkers, RB9010-P), S100A4 (abcam, ab27957), F4/80 (Santa Cruz, sc-26642), MCP-1 (Santa Cruz, sc1304), CD206-FITC conjugated (Biolegend, 123006), CD11b-FITC conjugated (Biolegend, 101206), Gr1-PE conjugated (Biolegend, 108408), and NK 1.1 (Wako, 986-10001). DeadEnd Flourometric TUNEL staining was performed according to manufacturer’s instructions (Promega, G3250). Flourescent images were captured with Photometric Coolsnap HQ camera using NIS Elements AR 2.3 Software (Nikon). Color images were obtained Nikon Eclipse E600 microscope using Nikon Digital Dx1200me camera and ACT1 software (Universal Imaging Corporation). Pictures were analyzed using NIS Elements (Nikon).
Study Statistics
Data were analyzed using GraphPad software (GraphPad Prism version 5 for Windows). All values are expressed as mean +/− SEM. For all statistical analysis ANOVA or where appropriate unpaired t-test was performed and results were considered significant at p<0.05.
Results
Pharmacologic blockade of stromal Tgfβr2 reduces metastasis
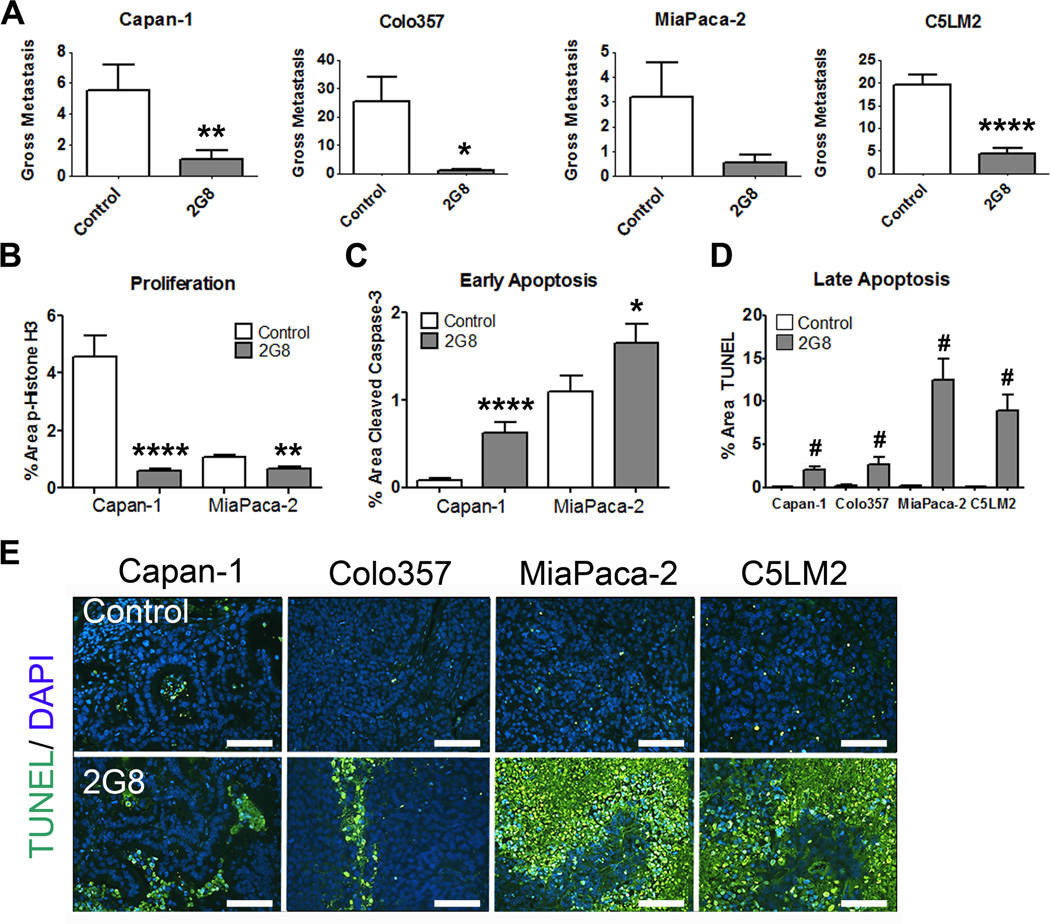
To explore the relationship between stromal TGF-β signaling and the phenotype of primary tumor cells in vivo, we established human xenograft models of PDA in which we specifically inhibited stromal Tgfβr2 with the mouse-specific antibody 2G8 (Supplemental Figure 1). Mice bearing established human pancreatic tumor orthotopic xenografts (Capan-1, Colo357, MiaPaCa-2 and C5LM2) were treated systemically with 2G8. In this setting, 2G8 specifically targets stromal Tgfβr2 where it blocks ligation by TGF-β, inhibits canonical Smad signaling and induces internalization of the receptor (16). Mice were randomized to receive control or 2G8 weekly 7–10 days after tumor cell injection. We found that inhibition of stromal Tgfβr2 significantly reduced surface metastases on the liver and other visceral organs (Figure 1A), reduced cell proliferation (Figure 1B) and elevated apoptosis (Figure 1C-E) in all four tumor models.
Figure 1. Inhibition of stromal Tgfβr2 reduces metastasis in vivo.
Human pancreatic cancer cell lines Capan-1, Colo357, MiaPaCa-2 and C5LM2 were orthotopically implanted into NOD-SCID mice. After tumor establishment, mice were randomized to receive saline (control) or 2G8 (30 mg/kg/week, n= 7–12/group). Total gross metastases were determined by evaluation of liver, diaphragm, GI lymph nodes, and lung at the time of sacrifice (A). B and C, Cell proliferation (B) and apoptosis (C) in control and 2G8 treated Capan-1 and MiaPaCa-2 tumors were assessed by immunohistochemistry for phospho-histone H3 (B) or cleaved caspase 3 (C), respectively. D and E, Control and 2G8 treated tumors from each model were evaluated for apoptosis using TUNEL. (TUNEL, green; scale bar, 100 µm). 5 animals/group were analyzed with 5 representative pictures taken and analyzed per animal. Results are expressed as mean+/−SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001; #, p<0.0001 vs. control.
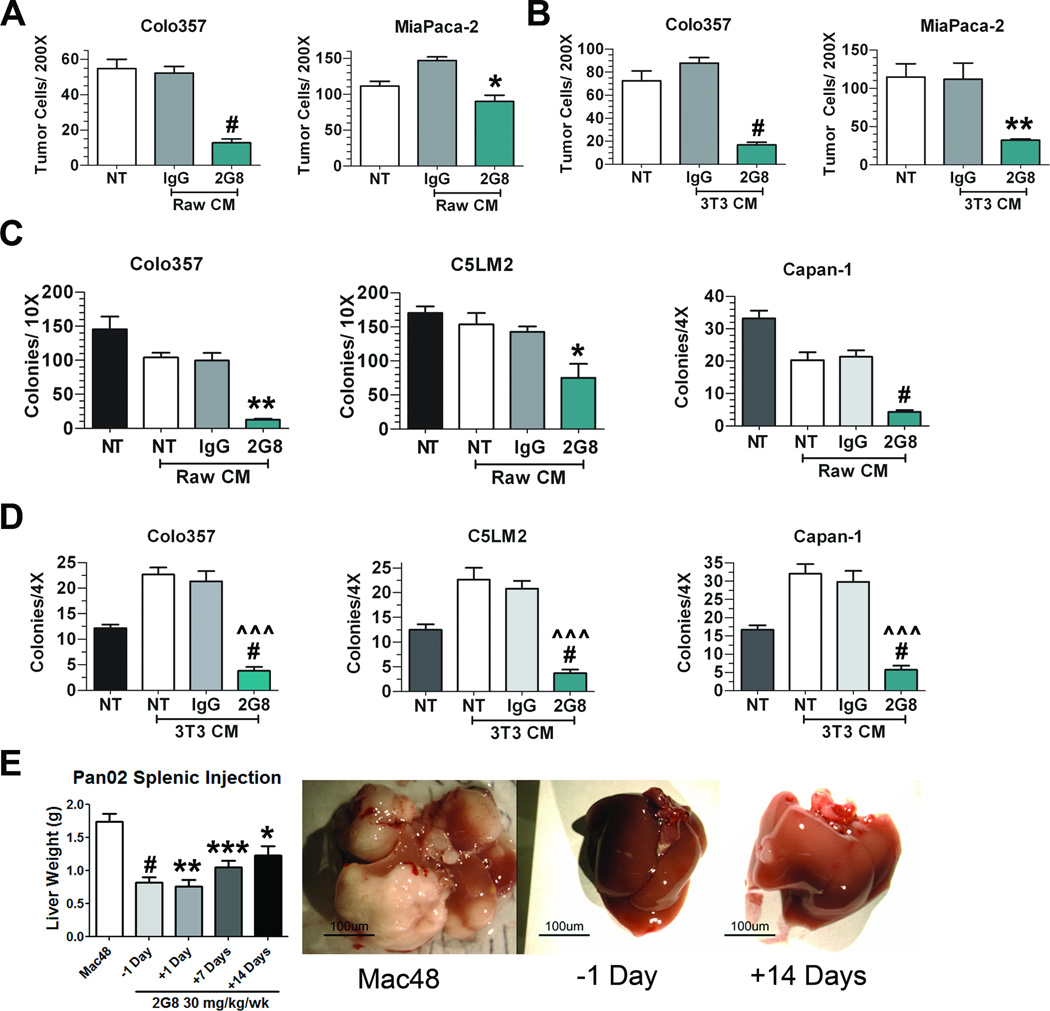
To investigate if 2G8-treated stromal cells secrete paracrine factors that affect tumor cell viability, migration and colony formation, conditioned media from cultured mouse stromal cells (RAW 264.7 and NIH 3T3 cells) treated with 2G8 was collected and incubated with human pancreatic tumor cells. Supplemental Figure 2 demonstrates that conditioned media from 2G8-treated stromal cells did not alter tumor cell viability; however, 2G8 blunted the ability of stromal-derived media to promote tumor cell migration towards macrophages (Figure 2A) and 3T3 cells (Figure 2B). Furthermore, when we evaluated anchorage independent growth conditioned media from 2G8-treated RAW 264.7 cells (Figure 2C) and 3T3 cells (Figure 2D) showed significantly reduced colony formation compared to other conditions.
Figure 2. Inhibition of Tgfβr2 on stromal cells limits tumor cell migration and colony formation in vitro and in vivo.
A and B, Transwell migration of tumor cells (Colo357 and MiaPaCa-2) towards RAW 264.7 (A) or NIH 3T3 (B) cells treated with serum free media (NT), control Rat IgG (IgG) or 2G8 for 24 hours. After removing treatment conditions, Colo357 and MiaPaCa-2 cells were plated in transwell chambers and allowed to migrate overnight towards previously treated stromal cells. Bar graphs represent number of cells/200× field. The assays were performed in triplicate in two independent experiments. *, p<0.05; **, p<0.01; #, p<0.0001 vs. Rat IgG. C and D, Anchorage independent growth of tumor cells (Colo357, C5LM2, and Capan-1) in the presence of growth media with 10% serum (NT), conditioned media from RAW 264.7 (C) or NIH 3T3 (D) cells grown in media with 10% serum with or without control Rat IgG (IgG) or 2G8. Colony formation was quantified by number of colonies per well at 4× or 10× magnification. Bar graph represents mean +/− SEM of a single experiment performed in triplicate with similar results found upon 2 independent experiments. *, p<0.05; **, p<0.01; **, p<0.01; #, p<0.0001 vs. NT; ^^^, p<0.0001 vs Rat IgG. E, Pan02 cells were injected intrasplenically and liver weight used as a surrogate for tumor burden in the liver. Mice were treated with a control rat IgG (Mac48, n=14) or 2G8 initiated at -1 (n=10), +1 (n=3), +7 (n=8) or +14 (n=7) days post splenic injection. Representative images of livers from mice are shown. Results are expressed as mean+/−SEM. *, p<0.05; **, p<0.01; ***, p<0.001; #, p<0.0001 vs. control
To further define the effect of Tgfβr2 inhibition on the development of liver metastases, we performed a splenic injection model with Pan02 cells. Mice were randomized to receive a control antibody (Mac48) or 2G8 the day prior to splenic tumor cell injection (-1 Day), or 1, 7, or 14 days post injection. Liver weight at experiment completion was used as a surrogate for metastatic burden. Tgfβr2 inhibition significantly reduced metastatic burden, with livers from 2G8-treated mice appearing normal while livers from Mac48-treated mice were replaced with tumor (Figure 2E). Unlike agents that target TGFβR1 and TGFβR2 (12), timing of the treatment did not influence the tumor burden. Treatment of mice with 2G8 the day before injection or the day after injection did not alter its ability to limit the metastatic burden in the liver. Similarly, there was no significant difference between mice treated 1 week or 2 weeks after tumor cell inoculation. These data support the functional importance of stromal Tgfβr2 in the metastasis of pancreatic cancer.
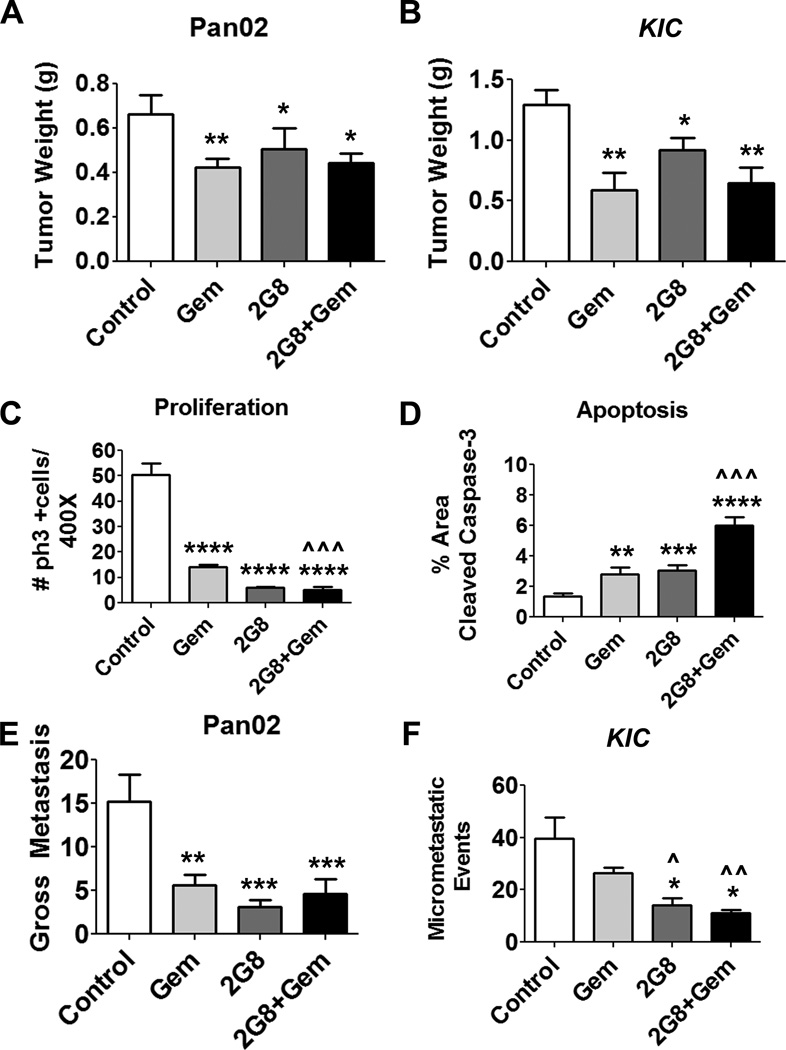
These results suggest that stromal TGF-β signaling is critical for acute tumor development and metastasis of established tumor cell lines. To determine if the therapeutic efficacy of 2G8 extended to syngenic immunocompetent models we explored its activity in orthotopic Pan02 tumors and LSL-KrasG12D; Cdkn2alox/lox; p48Cre (KIC) mice (18). Importantly, Pan02 and KIC cells express Tgfβr2, active TGF-β and are sensitive to 2G8 in vitro (Supplemental Figure 3). To test 2G8 in vivo, animals with established primary tumor burden were randomized to receive saline, gemcitabine, 2G8, or a combination of 2G8 and gemcitabine. Inhibition of Tgfβr2 alone (2G8 treatment) or in combination with gemcitabine modestly attenuated the weight of Pan02 (Figure 3A) and KIC (Figure 3B) tumors. However, consistent with the human xenograft results, 2G8 alone or in combination with gemcitabine significantly decreased tumor cell viability as evidenced by the changes in cell proliferation and apoptosis shown in Figure 3C, D and Supplemental Figure 4A-C.
Figure 3. Inhibition of tumor and stromal Tgfβr2 results in reduced primary pancreatic tumor growth and metastasis in murine models.
A, Orthotopic Pan02 tumors were established and mice randomized and treated for 4 weeks with vehicle (control), gemcitabine (Gem, 25 mg/kg/week), 2G8 (60 mg/kg/week) or 2G8+Gem. 2G8 alone and in combination with gemcitabine reduced primary tumor growth (n=7–10/group). B, LSL-KrasG12D; Cdkn2alox/lox; p48Cre (KIC) mice (n = 8–12/group) were randomized when 4 weeks old and treated for 4 weeks as above with Gem (12.5 mg/kg 3×/week), 2G8 (120 mg/kg/week) or the combination. At sacrifice, mice treated with 2G8 and 2G8+Gem had reduced tumor growth. C and D, Pan02 tumors were harvested and sections were evaluated by immunohistochemistry for proliferation (phospho-histone H3 (ph3+), C) and apoptosis (cleaved caspase-3, D). 2G8 reduced the number of ph3+ cells and increased the number of cells positive for cleaved caspase 3 compared to control. E and F, Total gross metastases in Pan02 bearing animals were determined by evaluation of liver, diaphragm, GI lymph nodes, and lung at the time of sacrifice. Metastatic burden in the KIC model was determined by histologic evaluation of H&E stained liver tissue. 2–3 sections of the anterior lobe of the liver (n=at least 5/group) were scored for lesions. 2G8 alone suppressed metastasis in each tumor model. Results are expressed as mean+/−SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001 vs. control; ^, p<0.05; ^^, p<0.01; ^^^, p<0.0001 vs. Gem.
Strikingly 2G8, as a single agent, was very effective at reducing metastasis (3–5 fold, Figure 3E, F). In fact, inhibition of TGF-β signaling was more effective than gemcitabine at reducing metastases in mice bearing Pan02 tumors (Figure 3E) and in KIC mice (Figure 3F). However, co-treatment with gemcitabine was not additive with 2G8. Interestingly, 2G8 and gemcitabine reduced perfusion and permeability in KIC tumors (Supplemental Figure 5), partially explaining the lack of additivity in the combination treated groups. The results in syngenic, immunocompetent models implicate stromal Tgfβr2 as a critical driver of PDA dissemination.
Blockade of Tgfβr2 reduces collagen deposition and fibroblast activation
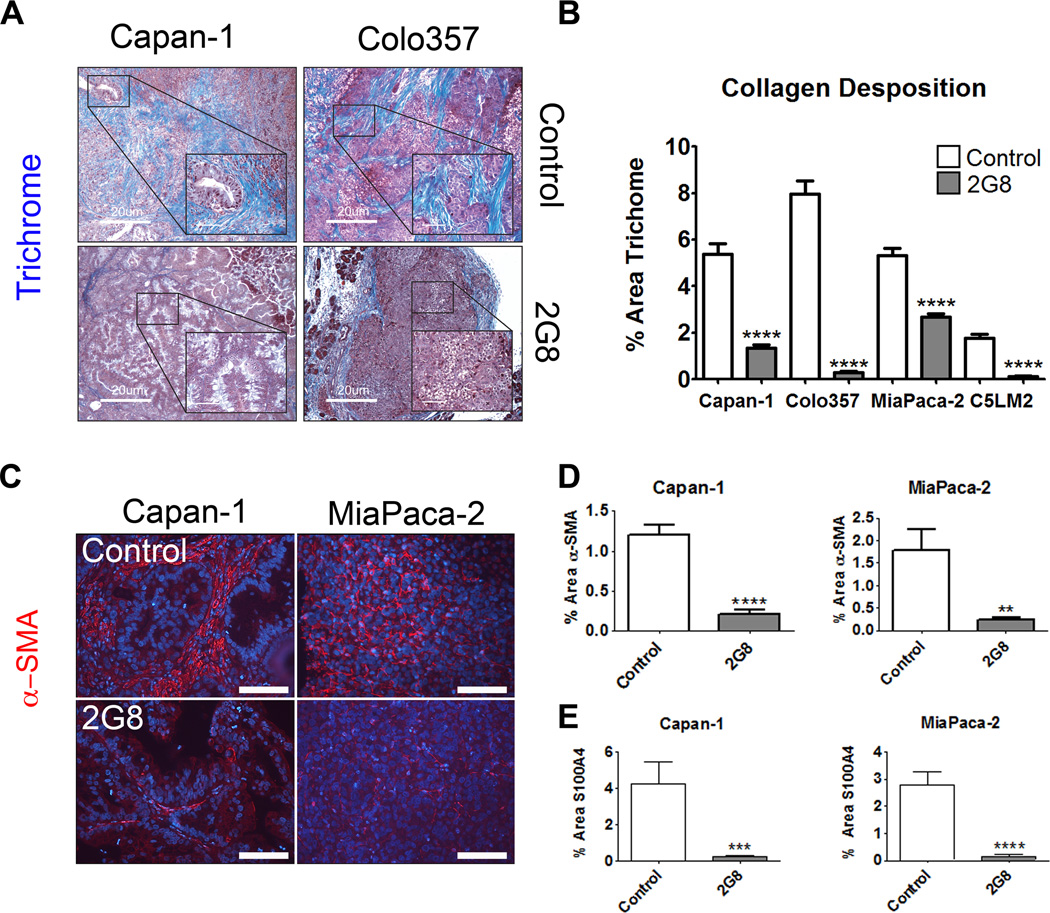
Stromal cells are key participants in the construction and remodeling of the tumor microenvironment, activities that are regulated in part by TGF-β (14,19–21). PDA is a desmoplastic disease that consists of high levels of collagen (19,22), which facilitates tumor cell survival and may impede the delivery of chemotherapy to tumor cells (23–25). We assessed collagen deposition by Masson’s trichrome staining and found that human xenografts (Figure 4A and B) and syngeneic murine tumors (Supplemental Figure 6) from mice treated with 2G8 had significantly reduced collagen deposition. We also found a concordant and significant 2G8-mediated reduction in mature fibroblasts as evidenced by α-smooth muscle actin (Figure 4C and D) and S100A4 (Figure 4E) immunoreactivity in Capan-1 and MiaPaCa-2 xenografts and Pan02 tumors (Supplemental Figures 6C, 4D). These findings implicate Tgfβr2 regulation of ECM deposition and fibroblast phenotype as critical features of the PDA microenvironment.
Figure 4. Inhibition of mouse Tgfβr2 blunts collagen deposition within xenografts.
A and B, The level of fibrillar collagen deposited in human tumor xenografts from mice treated with saline (Control) or 2G8 was determined by trichrome histology (Trichrome, blue; scale bar, 20 µm, insets 5 µm A). B, The intensity of trichrome staining was quantified and shows that 2G8 significantly reduced collagen deposition within each tumor (5 animals/group, 5 pictures/200× field). C-E, To determine the level of fibroblast investment, xenografts from control and 2G8 treated animals were stained for α-smooth muscle actin (C and D) and S100A4 (E). Results are expressed as mean+/−SEM. **, p<0.01; ***, p<0.001; #, p<0.0001 vs. control.
2G8 promotes a proinflammatory immune phenotype in pancreatic tumors
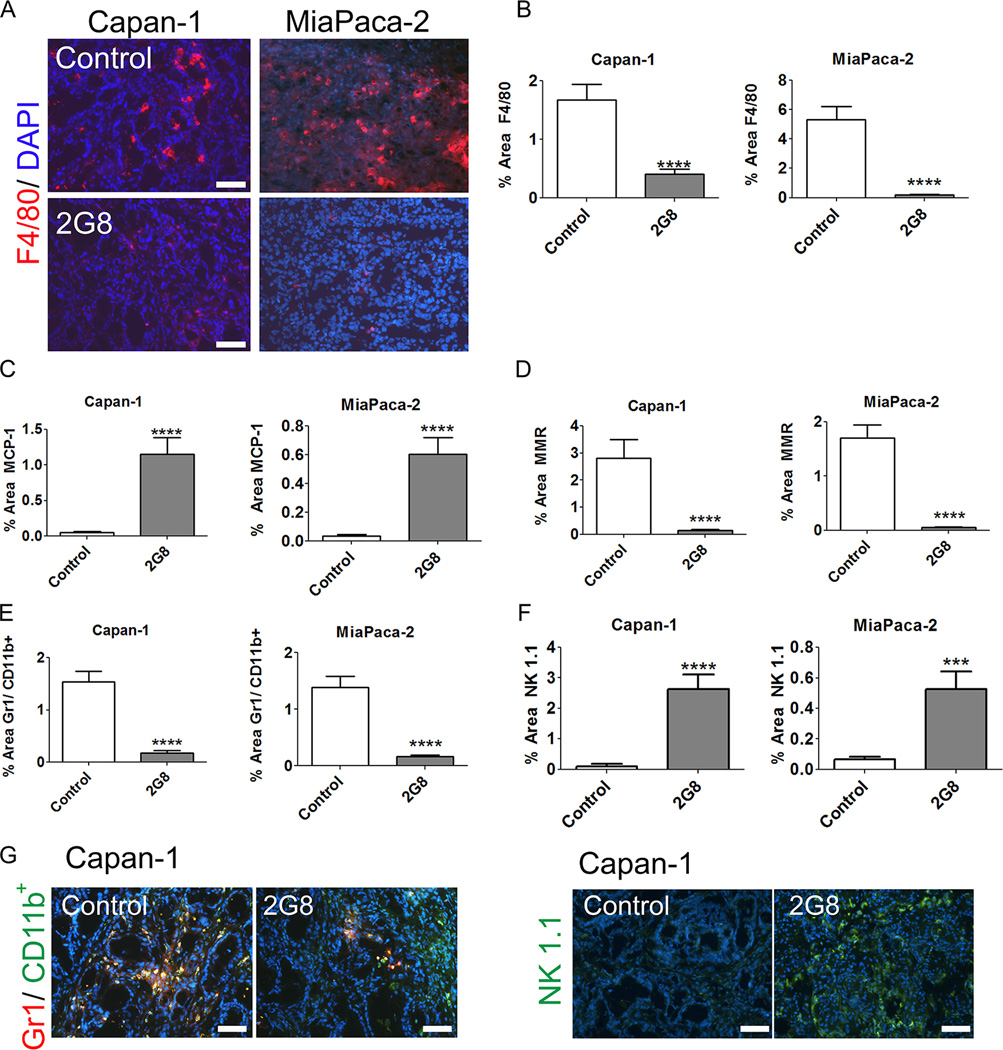
Metastasis is facilitated by an anti-inflammatory (M2) immune cell phenotype, which TGF-β is known to drive (4,26–29). In support of this, we found that blocking Tgfβr2 in RAW 264.7 cells in the presence or absence of TGF-β stimulated an M1 (pro-inflammatory) phenotype in vitro (Supplemental Figure 7). We also evaluated the immune status of xenografts treated with 2G8. 2G8 reduced the number of F4/80+ (Figure 5A-B) and CD68+ (data not shown) macrophages, increased the number of macrophages positive for MCP-1 (a marker of M1 macrophages, Figure 5C) and decreased the number of macrophages expressing MMR (a marker of M2 macrophages, Figure 5D) in Capan-1 and MiaPaCa-2 tumors. Furthermore, 2G8 therapy decreased myeloid derived suppressor cells (MDSCs, Gr1+CD11b+ cells, Figure 5F) while significantly increasing NK cells (NK 1.1+ cells, Figure 5G) in Capan-1 and MiaPaCa-2 tumors.
Figure 5. Inhibition of mouse Tgfβr2 promotes a pro-inflammatory immune cell phenotype.
A-D, The level of F4/80 (A and B), MCP-1 (C), and MMR (D) expressing macrophages in Capan-1 and MiaPaCa-2 xenografts was determined by immunohistochemistry. The effect of 2G8 on the level of MDSC (E) and NK cells (F) in Capan-1 and MiaPaCa-2 xenografts was also evaluated by immunohistochemistry with example images shown in panel G. Graphs represent 5 animals/group with 5/pictures per animal. Results are expressed as mean+/−SEM. ***, p<0.001; #, p<0.0001 vs. control.
We also assessed the effect of Tgfβr2 inhibition on the immune landscape in the immunocompetent models. As displayed in Supplemental Figure 8, inhibition of Tgfβr2 with 2G8 dramatically altered the immune cell phenotype in Pan02 tumors. These changes included a reduction in total macrophage number (F4/80, Supplemental Figure 8A), an increase in the ratio of M1:M2 macrophages (Supplemental Figure 8B, C), a reduction in MDSCs (Gr1+CD11b+, Supplemental Figure 8D) and T regulatory cells (Treg, CD4+FoxP3+, Supplemental Figure 8E) and an increase in NK cell recruitment (NK 1.1, Supplemental Figure 8F). These results indicate that stromal Tgfβr2 functions to promote an immunosuppressive environment while blockade of Tgfβr2 function with 2G8 stimulates recruitment and retention of immune cells that can combat the tumor.
Targeting Tgfβr2 on tumor cells and stroma inhibits EMT in vivo
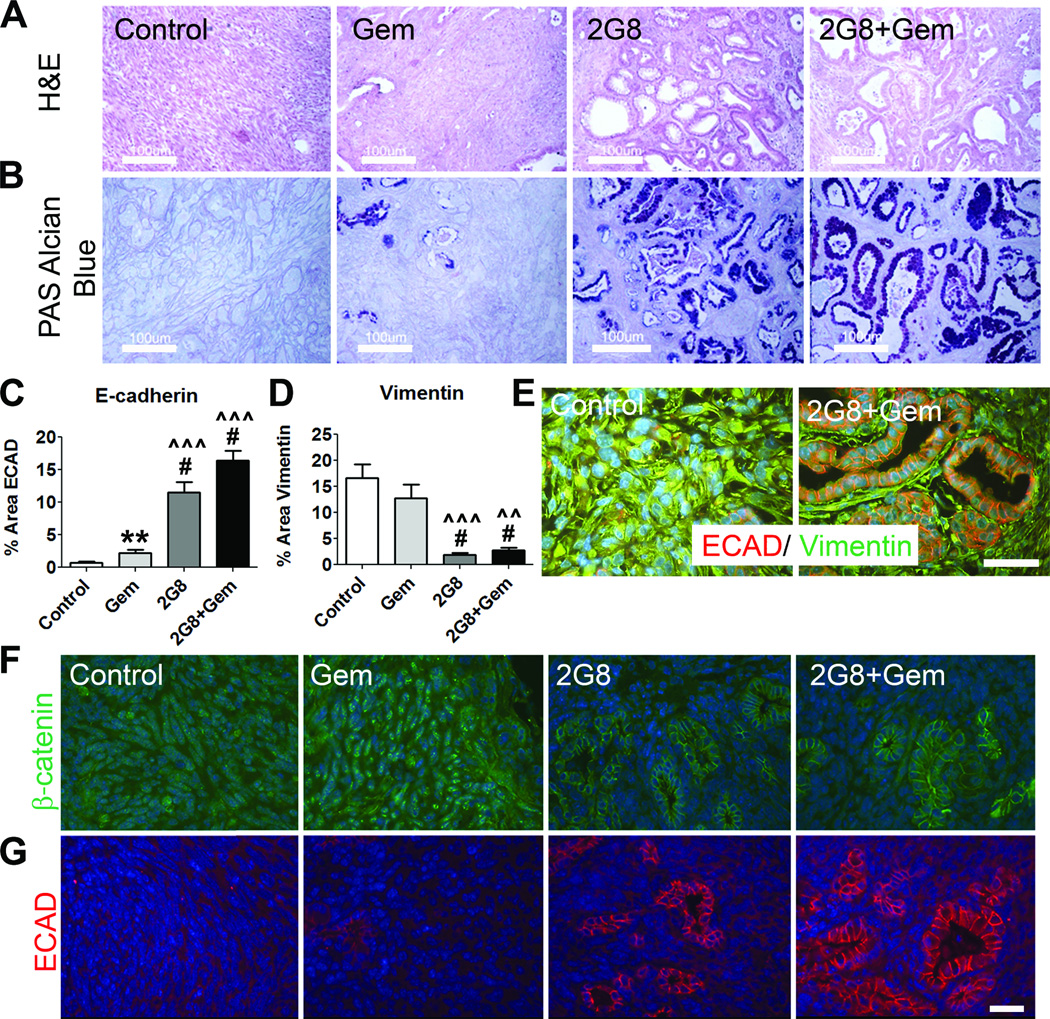
TGF-β can drive tumor cells to adopt a mesenchymal-like phenotype that promotes tumor cell invasion and metastasis (30–34). We hypothesized that targeted inhibition of Tgfβr2 would prevent or reverse the induction of EMT in vivo. Figure 6A displays general histology (H&E) of spontaneous KIC tumors from each treatment group at the end of therapy. Mice receiving 2G8 alone or in combination with gemcitabine showed an increase in a ductal histologic phenotype that resembles PanIN lesions. This was confirmed by staining with PAS-Alcian Blue, which revealed a significant increase in mucin-secreting cells in 2G8 treated tumors (Figure 6B). Primary tumors and metastases in the KIC model exhibited pathologic evidence of ductal differentiation and were classified as pancreatic ductal adenocarcinomas (Supplemental Figure 9). These features included distinct gland formation and ductal-type mucinous epithelium. Furthermore, tumors from mice treated with 2G8 displayed a significant increase in E-cadherin expression (Figure 6C, E) and a significant decrease in vimentin expression (Figure 6D-E).
Figure 6. 2G8 promotes an epithelial phenotype in murine tumors in vivo.
A-E, LSL-KrasG12D; Cdkn2alox/lox; p48Cre (KIC) mice establish tumors and precursor PanIN lesions by 4 weeks old. Mice at this timepoint were randomized to receive saline (Control), gemcitabine (Gem), 2G8 or 2G8 + Gem for 4 weeks. A, Analysis of tumor architecture using Hemotoxylin and Eosin staining (scale bar, 100 µm). 2G8 treated tumors were noted to have significantly more PanIN and epithelial lesions than the mice treated with Gem or Control. B, This was confirmed with PAS-Alcian Blue staining that marks mucin-secreting PanIN lesions but not invasive lesions (PAS-Alcian Blue, purple; scale bar, 100 µm). Additionally, 2G8 tumors had significantly increased Ecad expression (C) and decreased vimentin expression (D) by immunohistochemistry. E, Representative images of these tumors demonstrate the predominant epithelial phenotype of 2G8-treated tumors (Ecad red, vimentin green; scale bar, 50 µm). F and G, 2G8 induces a similar epithelial phenotype in Pan02 tumors. Immunohistochemical analysis of β-catenin (F) and E-cadherin (Ecad, G) in Pan02 tumors treated with control, Gem, 2G8 or 2G8+GEM. Bar graphs represent mean+/−SEM. **, p<0.01; #, p<0.0001 vs. control; ^^, p<0.001; ^^^, p<0.0001 vs. Gem.
To extend these observations, we evaluated epithelial and mesenchymal markers in the highly mesenchymal Pan02 model (35). At baseline, Pan02 cells express nuclear β-catenin and high levels of Zeb1 (35). β-catenin is expressed on the membrane of epithelial cells and translocates into the nucleus during the process of EMT. We observed that while tumors from control and gemcitabine treated animals expressed nuclear β-catenin, tumors from mice receiving 2G8 expressed membranous β-catenin with increased prevalence of pseudoducts (Figure 6F,G). Furthermore we found that ECAD was seen most prominently in 2G8 treated tumors (Figure 6G). This indicates that TgfβR2 inhibition drives an epithelial, differentiated phenotype in Pan02 tumors in vivo.
Inhibition of Tgfβr2 on tumor cells is insufficient to inhibit EMT in vitro
Given the significant changes seen in stromal and primary tumor cells in vivo, we evaluated the effect of 2G8 on TGF-β-induced changes in mPLR and Pan02 cells in vitro. Cells were plated on collagen (mPLR) or plastic (Pan02) and treated with serum free media (SFM), TGF-β, 2G8 or TGF-β + 2G8 for 24–72 hrs and analyzed by immunocytochemistry. mPLR2D cells treated with SFM expressed ECAD and NCAD, a mesenchymal marker, at detectable levels. Treatment with 2G8 reduced NCAD and increased ECAD expression levels while TGF-β had the opposite effect (Supplemental Figure 10). Pan02 cells treated in a similar fashion had a mild decrease in zeb1 and vimentin expression after exposure to 2G8 (Supplemental Figure 10). However, Pan02 did not express ECAD in vitro at any time point under any condition (data not shown). Overall, our in vitro studies did not fully recapitulate the dramatic phenotypic changes induced by 2G8 in vivo. This led us to hypothesize that stromal cells participate in the 2G8–driven changes in tumor cell phenotype and reduction in metastases seen in vivo.
Inhibition of stromal TGF-β signaling promotes epithelial differentiation
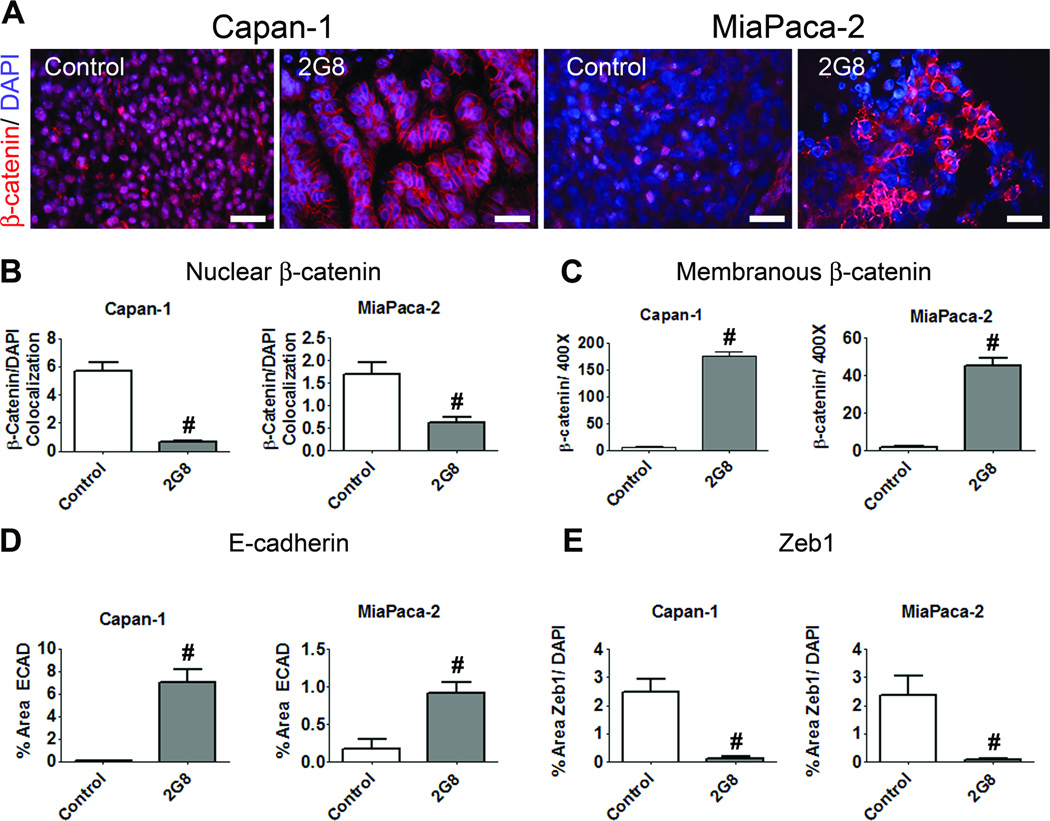
TGF-β, collagen, fibroblasts and immune cells all contribute to tumor cell phenotype. Tumor cells that adopt a mesenchymal phenotype are more aggressive and metastatic (36). Capan-1 and Colo357 are epithelial while MiaPaCa-2 and C5LM2 cells have a mesenchymal-like phenotype in vitro (37,38). 2G8 treatment in vivo promoted an epithelial phenotype in each xenograft analyzed (Figure 7 and Supplemental Figure 11). Inhibition of Tgfβr2 induced a shift in β-catenin from the nucleus to the membrane (Figure 7A-C), increased tumor cell expression of ECAD (ECAD, Figure 7D) and decreased Zeb1 levels (Zeb1, Figure 7E) in Capan-1 and MiaPaCa-2 tumors. Similar results were found in Colo357 and C5LM2 models (Supplemental Figure 11). These data indicate that activation of stromal Tgfβr2 is critical for tumor cell adoption of a mesenchymal like phenotype.
Figure 7. Inhibition of stromal Tgfβr2 promotes an epithelial phenotype in xenograft models of pancreatic cancer.
A-C, The expression and localization of β-catenin in Capan-1 and MiaPaCa-2 xenografts treated with control or 2G8 was determined by immunohistochemistry (scale bar, 50 µm). Localization of β-catenin to the nucleus (B) or membrane (C) was quantified. D and E, The effect of 2G8 on the expression of E-cadherin (ECAD, D) and Zeb1 (E) was also determined by immunohistochemistry. Graphs represent 5–6 animals/group with 5/pictures per animal. Results are expressed as mean+/−SEM. #, p<0.0001 vs. control.
Discussion
We have shown that pharmacologic blockade of stromal Tgfβr2 can slow primary tumor growth, reduce metastasis and promote epithelial differentiation in mouse models of pancreatic cancer. The changes in tumor cell phenotype and behavior occurred in the context of microenvironmental changes that resulted in a pro-inflammatory immune cell phenotype and a reduced presence of mature/activated fibroblasts. These alterations in cellular and extracellular components of the tumor after Tgfβr2 blockade resulted in striking reductions in metastatic spread and help to functionally define the importance of stromal Tgfβr2 for primary pancreatic tumor growth and metastasis.
We employed human PDA xenografts that metastasize robustly but are often criticized for not recapitulating the progression of human PDA as well as GEMMs. Yet, we found the same pro-epithelial and anti-metastatic effects after Tgfβr2 inhibition in a well-established GEMM of PDA. Furthermore, we used established human pancreatic cell lines rather than direct human xenografts. Given that we specifically wanted to target mouse stromal cells, direct xenografts would not have been appropriate because they are a mixed human tumor and stromal cell population (39). Our xenograft models utilized NOD-SCID mice that lack B and T cell immunity; therefore our studies do not reflect the effect of Tgfβr2 inhibition on B and T cells that can participate in anti-tumor effects. However, we demonstrate that inhibition of Tgfβr2 on stromal cells promotes a pro-inflammatory macrophage phenotype that limits tumor cell migration and colony formation in vitro and reduces metastasis in vivo. Furthermore, we observed a potent anti-metastatic effect in immunocompetent models with concordant changes in immune cells.
Our analyses focused on the effect of TGF-β on macrophage and fibroblast phenotype. However, we did identify significant changes after Tgfβr2 blockade in other cell types, including NK cells. Our results indicate that 2G8 therapy increased the level of tumor associated NK cells. These finding are consistent with the study by Zhong et al (16) who demonstrated that 2G8 increased NK cell mediated killing and secretion of IFN-γ. The effect of Tgfβr2 inhibition on other cells within the tumor microenvironment such as pancreatic stellate cells, mesenchymal stem cells or endothelial cells was not explored in depth. These cells are known to respond to TGF-β and thus inhibition of TGF-β signaling in these cell types could contribute to the reduction in tumor progression observed.
Overall, our results emphasize the impact of stromal cell function on tumor cell phenotype and metastasis. In particular, our in vitro studies suggest interruption of Tgfβr2 signaling in stromal cells alters the expression of soluble factors that impact tumor cell behavior. This is exemplified by the concordant results of our anchorage independent growth assays in vitro where conditioned media from mouse stromal cells enhanced colony formation in a Tgfβr2-depedent manner and the observed reduction in metastasis in vivo after blockade of stromal Tgfβr2. Whether 2G8 treatment prevents the expression of pro-tumor factors or induces the expression of factors that inhibit tumor cell colony formation and metastasis is not clear. We evaluated the expression of 12 cytokines by macrophages (Raw 264.7) and fibroblasts (3T3) after treatment with 2G8 (Supplemental Figure 12) and found only modest changes in a small subset of cytokines. Whether the 2G8-induced elevation in MIP-1α, MIP-1β, TNF-α and IFN-γ expressed by macrophages are sufficient to induce the dramatic alterations in tumor cell phenotype and progression remains to validated.
In a GEMM of PDA we found that suppression of Tgfβr2 resulted in an increase in PanIN lesions and an increased epithelial tumor cell phenotype compared to control treated mice. In contrast, genetic deletion of Tgfβr2 in pancreatic tumor cells (Ptf1aCre/+; KrasG12D; Tgfβr2lox/lox) resulted in elevated CTGF expression, abundant stroma and a worse overall survival compared to Tgfβr2wt/wt animals (7). Given that TGF-β functions as a tumor suppressor early in PDA development, it is not surprising that targeted ablation of Tgfβr2 during or prior to tumor development drives tumor progression. We chose to target Tgfβr2 at a time point when the mice are known to have established invasive lesions (18). Furthermore, in Ptf1aCre/+; KrasG12D; Tgfβr2lox/lox mice Tgfβr2 is ablated in tumor cells whereas in our study we targeted tumor and stromal cell Tgfβr2 within the tumor microenvironment. Overall, our studies document the critical nature of stromal TGF-β signaling to stromal cell recruitment and activity and strengthen the argument that the microenvironment is a major participant in tumor progression. The results presented here support that blockade of stromal Tgfβr2 inhibits tumor cell EMT but do not rule out that inhibition of stromal Tgfβr2 promotes tumor cell MET (mesenchymal to epithelial transition). Given the prevalence of poorly differentiated (e.g, mesenchymal phenotype) tumors cells in control treated tumors in each model system employed and the demonstration that EMT occurs early in PDA (36) it is feasible that 2G8 promotes MET in PDA cell lines in vivo.
Although the focus of our work was TGF-β dependent tumor-stromal cell interactions in PDA, several studies targeting tumor cell TGF-β pathways have observed anti-tumor effects. In xenograft models, inhibition of TGFβR1 and TGFβR2 resulted in decreased metastasis after splenic-injection of tumor cells (12). Additionally, inhibition of tumor cell TGFβR2 in subcutaneous models of human PDA resulted in reduced primary tumor growth and reduced microvessel density (40). However, neither of these studies explored stromal cell or tumor cell phenotype after TGF-β receptor inhibition.
Recently Hezel et al (41), found that direct inhibition of TGF-β or the integrin αvβ6, which is critical for activation of latent TGF-β (42) accelerated the progression of pancreatic cancer in p48CreKrasG12Dp53lox/+ animals. The tumors in the p48CreKrasG12Dp53lox/+ animals do not exhibit the pronounced EMT observed in the GEMM used in the current study. Furthermore, the study primarily evaluated survival and documented that inhibition of TGF-β or αvβ6 at early or later stages accelerated disease progression in a Smad4-dependent fashion. However, differences in stromal or tumor cell phenotype after therapy were not evaluated. Additionally, the number of animals in any treatment group with distinct metastatic lesions was too few to draw meaningful conclusions regarding the effect of these strategies on tumor dissemination. These studies taken in the context of our results suggest that there is a substantial mechanistic difference between targeting TGF-β ligand and inhibiting downstream signaling by increasing Tgfβr2 internalization.
Studies targeting stromal cells in pancreatic cancer have shown that decreased stroma results in increased drug delivery and a reduction in tumor growth (24,25). In fact, several ongoing clinical trials in pancreatic cancer aim at stromal depletion by inhibiting the hedgehog pathway (43). We found in all 6 of our mouse models that targeting stromal Tgfβr2 with 2G8 resulted in a significant reduction in tumor-associated collagen deposition. However, we found that blockade of Tgfβr2 significantly reduced perfusion and permeability of high and low molecular weight dextran (Supplemental Figure 5), thus collagen deposition is not the only factor contributing to poor drug distribution in PDA. .
Results in murine models of cancer document the complexity of targeting TGF-β pathways in vivo. Our data suggest that specific blockade of stromal Tgfβr2 has a profound anti-metastatic effect and demonstrate that stromal cell phenotype is more critical for tumor cell phenotype and metastasis than previously appreciated. TGF-β acts in a paracrine function in virtually every cell type and dissecting which stromal cell type is primarily responsible for the anti-metastatic effect of 2G8 remains a challenge. However, our results strongly implicate Tgfβr2 on stromal cells as a critical participant in pancreatic cancer metastasis and underscore the need for an improved understanding of TGF-β biology in this challenging disease.
Supplementary Material
Acknowledgements
We gratefully acknowledge the assistance of Jason E. Toombs and our collaborators at Imclone Systems. We additionally would like to thank Drs. Elisabeth Martinez, James Kim, Andries Zijlstra, Jerry Neiderkorn and Nabeel Bardeesy for advice and Dr. Diego Castrillon for pathological consultation. This work is supported in part by a sponsored research agreement from Imclone Systems (RAB), the NIH (CA118240 to RAB), the Department of Surgery, UT Southwestern (KTO) and the Effie Marie Cain Scholarship for Angiogenesis Research (RAB).
Footnotes
Conflict of Interest Statement Xiaohong Xu, Desiree Nugent, and Kyla D. Carroll are employees of Imclone Systems, a wholly-owned subsidiary of Eli Lilly and Company. This work was supported in part by a sponsored research agreement from Imclone Systems to Rolf A. Brekken.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71(14):5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31(6):220–227. doi: 10.1016/j.it.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321(5897):1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izeradjene K, Combs C, Best M, Gopinathan A, Wagner A, Grady WM, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11(3):229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20(22):3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20(22):3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, et al. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23(1):24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friess H, Yamanaka Y, Buchler M, Ebert M, Beger HG, Gold LI, et al. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105(6):1846–1856. doi: 10.1016/0016-5085(93)91084-u. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Venkatasubbarao K, Lazor JW, Sperry J, Jin C, Cao L, et al. Inhibition of STAT3 Tyr705 phosphorylation by Smad4 suppresses transforming growth factor beta-mediated invasion and metastasis in pancreatic cancer cells. Cancer Res. 2008;68(11):4221–4228. doi: 10.1158/0008-5472.CAN-07-5123. [DOI] [PubMed] [Google Scholar]
- 12.Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, et al. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7(4):829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truty MJ, Urrutia R. Basics of TGF-beta and pancreatic cancer. Pancreatology. 2007;7(5–6):423–435. doi: 10.1159/000108959. [DOI] [PubMed] [Google Scholar]
- 14.Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, et al. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61(2):550–555. [PubMed] [Google Scholar]
- 15.Achyut BR, Yang L. Transforming growth factor-beta in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141(4):1167–1178. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z, Carroll KD, Policarpio D, Osborn C, Gregory M, Bassi R, et al. Anti-transforming growth factor beta receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multieffects on cancer, stroma, and immune cells. Clin Cancer Res. 2010;16(4):1191–1205. doi: 10.1158/1078-0432.CCR-09-1634. [DOI] [PubMed] [Google Scholar]
- 17.Dineen SP, Roland CL, Greer R, Carbon JG, Toombs JE, Gupta P, et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010;70(7):2852–2861. doi: 10.1158/0008-5472.CAN-09-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aguirre AJ, Bardeesy N, Sinha M, Lopez L, Tuveson DA, Horner J, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krantz SB, Shields MA, Dangi-Garimella S, Cheon EC, Barron MR, Hwang RF, et al. MT1-MMP cooperates with Kras(G12D) to promote pancreatic fibrosis through increased TGF-beta signaling. Mol Cancer Res. 2011;9(10):1294–1304. doi: 10.1158/1541-7786.MCR-11-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 21.Taipale J, Saharinen J, Keski-Oja J. Extracellular matrix-associated transforming growth factor-beta: role in cancer cell growth and invasion. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/s0065-230x(08)60740-x. [DOI] [PubMed] [Google Scholar]
- 22.Shields MA, Dangi-Garimella S, Redig AJ, Munshi HG. Biochemical role of the collagen-rich tumour microenvironment in pancreatic cancer progression. Biochem J. 2012;441(2):541–552. doi: 10.1042/BJ20111240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dangi-Garimella S, Krantz SB, Barron MR, Shields MA, Heiferman MJ, Grippo PJ, et al. Three-dimensional collagen I promotes gemcitabine resistance in pancreatic cancer through MT1-MMP-mediated expression of HMGA2. Cancer Res. 2011;71(3):1019–1028. doi: 10.1158/0008-5472.CAN-10-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324(5933):1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinson DM, LeClaire RD, Lorsbach RB, Parmely MJ, Russell SW. Regulation by transforming growth factor-beta 1 of expression and function of the receptor for IFN-gamma on mouse macrophages. J Immunol. 1992;149(6):2028–2034. [PubMed] [Google Scholar]
- 28.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107(5):2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 29.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Ellenrieder V, Hendler SF, Boeck W, Seufferlein T, Menke A, Ruhland C, et al. Transforming growth factor beta1 treatment leads to an epithelial-mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal-regulated kinase 2 activation. Cancer Res. 2001;61(10):4222–4228. [PubMed] [Google Scholar]
- 31.Tsuji T, Ibaragi S, Hu GF. Epithelial-mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 2009;69(18):7135–7139. doi: 10.1158/0008-5472.CAN-09-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen L, Chan TH, Yuan YF, Hu L, Huang J, Ma S, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120(4):1178–1191. doi: 10.1172/JCI40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teraoka H, Sawada T, Nishihara T, Yashiro M, Ohira M, Ishikawa T, et al. Enhanced VEGF production and decreased immunogenicity induced by TGF-beta 1 promote liver metastasis of pancreatic cancer. Br J Cancer. 2001;85(4):612–617. doi: 10.1054/bjoc.2001.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teraoka H, Sawada T, Yamashita Y, Nakata B, Ohira M, Ishikawa T, et al. TGF-beta1 promotes liver metastasis of pancreatic cancer by modulating the capacity of cellular invasion. Int J Oncol. 2001;19(4):709–715. doi: 10.3892/ijo.19.4.709. [DOI] [PubMed] [Google Scholar]
- 35.Arnold SA, Rivera LB, Carbon JG, Toombs JE, Chang CL, Bradshaw AD, et al. Losartan Slows Pancreatic Tumor Progression and Extends Survival of SPARC-Null Mice by Abrogating Aberrant TGFbeta Activation. PLoS One. 2012;7(2):e31384. doi: 10.1371/journal.pone.0031384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Q, Yoshimitsu M, Kuwahata T, Maeda K, Hayashi T, Obara T, et al. Establishment of a highly migratory subclone reveals that CD133 contributes to migration and invasion through epithelial-mesenchymal transition in pancreatic cancer. Hum Cell. 2012;25(1):1–8. doi: 10.1007/s13577-011-0037-9. [DOI] [PubMed] [Google Scholar]
- 39.Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE. Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc. 2009;4(11):1670–1680. doi: 10.1038/nprot.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medicherla S, Li L, Ma JY, Kapoun AM, Gaspar NJ, Liu YW, et al. Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res. 2007;27(6B):4149–4157. [PubMed] [Google Scholar]
- 41.Hezel AF, Deshpande V, Zimmerman SM, Contino G, Alagesan B, O'Dell MR, et al. TGF-beta and alphavbeta6 integrin act in a common pathway to suppress pancreatic cancer progression. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-12-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96(3):319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 43.Garber K. Stromal depletion goes on trial in pancreatic cancer. J Natl Cancer Inst. 2010;102(7):448–450. doi: 10.1093/jnci/djq113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.