Abstract
The EGFR antibody cetuximab is used to treat numerous cancers, but intrinsic and acquired resistance to this agent is a common clinical problem. In this study we show that overexpression of the oncogenic receptor kinase AXL is sufficient to mediate acquired resistance to cetuximab in models of non-small cell lung cancer (NSCLC) and head and neck squamous cell carcinoma (HNSCC), where AXL was overexpressed, activated and tightly associated with EGFR expression in cells resistant to cetuximab (CtxR cells). Using RNAi methods and novel AXL targeting agents, we found that AXL activation stimulated cell proliferation, EGFR activation and MAPK signaling in CtxR cells. Notably, EGFR directly regulated the expression of AXL mRNA through MAPK signaling and the transcription factor c-Jun in CtxR cells, creating a positive feedback loop that maintained EGFR activation by AXL. Cetuximab-sensitive parental cells were rendered resistant to cetuximab by stable overexpression of AXL or stimulation with EGFR ligands, the latter of which increased AXL activity and association with the EGFR. In tumor xenograft assays, the development of resistance following prolonged treatment with cetuximab was associated with AXL hyperactivation and EGFR association. Furthermore, in an examination of patient-derived xenografts established from surgically resected HNSCCs, AXL was overexpressed and activated in tumors that displayed intrinsic resistance to cetuximab. Collectively, our results identify AXL as a key mediator of cetuximab resistance, providing a rationale for clinical evaluation of AXL targeting drugs to treat cetuximab-resistant cancers.
Keywords: AXL, EGFR, Cetuximab, Resistance
Introduction
The TAM family of receptor tyrosine kinases is comprised of three family members: Tyro-3 (Sky), AXL (Ark or Ufo), and MerTK. Cognate ligand binding to TAM receptors on the cell surface leads to receptor dimerization, kinase domain activation, and auto/trans-phosphorylation of tyrosine residues located on each receptor's cytoplasmic tail (1). The activation of TAM receptors stimulates PI3K/AKT and Ras/Raf/Mek/Erk (MAPK) signaling cascades leading to increased cell survival, proliferation, migration, invasion, and angiogenesis (1-4).
TAM family overexpression and activation have been observed in many human cancers (1-11). Recently, the AXL receptor has been particularly implicated in cancer cell resistance to anti-EGFR tyrosine kinase inhibitors (TKI) (12-17) and other chemotherapeutics (10, 15, 18). Collectively, this data indicates that AXL functions as a potent oncogene that can modulate resistance to conventional and targeted cancer therapies.
Cetuximab is an anti-EGFR monoclonal antibody therapy that has shown efficacy in treating head and neck squamous cell carcinoma (HNSCC), metastatic colorectal cancer (mCRC) and non-small cell lung cancer (NSCLC) (19-26). Unfortunately, clinical studies indicate that most patients whom initially respond to cetuximab eventually acquire resistance (27-29). To understand mechanisms of acquired resistance, we previously created a model in which the cetuximab sensitive (CtxS) NSCLC cell line NCI-H226 was treated with increasing doses of cetuximab for a period of six months until resistant single cell clones emerged (30). Analysis of cetuximab resistant (CtxR) clones demonstrated the expression of EGFR and its activation was dramatically increased due to dysregulated EGFR internalization and degradation without mutation of the receptor (30). Overall, CtxR cells remained highly addicted to the EGFR signaling network (30-32).
Based on these previous findings, we investigated if the AXL receptor played a role in cetuximab resistance. Examination of in vitro NSCLC and HNSCC models of acquired resistance indicated AXL was highly overexpressed and activated in CtxR cells. Further analysis indicated CtxR cells had increased dependency on AXL for cellular proliferation, EGFR activation and MAPK signaling. AXL activity was also examined in tumors harvested from de novo acquired CtxR NCI-H226 xenografts, where AXL was highly activated and associated with the EGFR. Finally, AXL was overexpressed and hyperactivated in HNSCC patient derived xenografts (PDXs) that were intrinsically resistant to cetuximab therapy. Collectively, this work indicates that AXL plays a role in cetuximab resistance and provides rationale for the clinical evaluation of anti-AXL therapeutics for the treatment of cetuximab resistant cancers.
Materials and Methods
Cell lines and development of acquired resistance
The human NSCLC cell line NCIH226 was purchased from ATCC (Manassas, VA, USA) and maintained in 10% FBS in RPMI-1640 (Mediatech Inc., Manassas, VA, USA) with 1% penicillin and streptomycin. The HNSCC cell line UM-SCC1 was provided by Dr. Thomas E. Carey (University of Michigan, Ann Harbor, MI) and maintained in 10% FBS in Dulbecco's modified Eagle's medium (DMEM) with 1% penicillin and streptomycin. The development of CtxR cells has been previously described (30-32). All CtxR cell lines were validated to express wild type (WT) EGFR by sequencing.
Materials
R428 was purchased from Selleckchem (Houston, TX, USA) and MAb173 was produced in the laboratory of Dr. Parkash Gill (Department of Medicine and Pathology, University of Southern California, Los Angeles, USA). Cetuximab (ICM-225, Erbitux™) was purchased from University of Wisconsin Pharmacy. EGF was purchased from Millipore (Billerica, MA, USA) and TGFα was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Antibodies
All antibodies were purchased from commercial sources as indicated below: R&D Systems (Minneapolis, MN, USA): AXL (for IB) and pAXL-Y779. Cell Signaling Technology (Danvers, MA, USA): pAXL-Y702, pEGFR-Y1068, pMAPK (T202/Y204), MAPK, p-cRAF (S289/296/301), cRAF, p-AKT (S473), AKT, p-rpS6 (S240/244), rpS6, p-c-Jun (S73), c-Jun and GAPDH. Santa Cruz Biotechnology Inc. (Dallas, TX, USA): pEGFR-Y1173, AXL (for IP), and HRP-conjugated goat-anti-rabbit IgG, goat-anti-mouse IgG, donkey-anti-goat IgG. Life Technologies (Carlsbad, CA, USA): AXL (For IF). Abcam (Cambridge, MA, USA): EGFR. Calbiochem (Billerica, MA, USA): α-Tubulin.
Small interfering RNA and transfection
CtxR cells were transiently transfected with siAXL (ON-TARGETplus, SMARTpool #L-003104, Dharmacon, Lafayette, CO, USA), siEGFR (ON-TARGETplus, SMARTpool #L-003114, Dharmacon), siHER2 (ONTARGETplus, SMARTpool #L-003126, Dharmacon), siHER3 (ON-TARGETplus, SMARTpool #L-003127, Dharmacon), p44/42 MAPK (ERK1/2) siRNA (Cell Signaling Technology #6560), AKT1 siRNA (ON-TARGETplus, SMARTpool #L-003000, Dharmacon), c-Jun siRNA (ON-TARGETplus, SMARTpool #L-003268, Dharmacon) or non-targeting siRNA (ON-TARGETplus Non-targeting Pool, #D-001810, Dharmacon) using Lipofectamine RNAiMAX according to the manufacture's instructions (Life Technologies).
Immunoblot analysis
Whole cell lysis was performed as previously described (31, 33). ECL chemiluminescence detection system was used to visualize proteins. For detection of phosphorylated AXL, cells were treated with pervanadate (0.12 mM Na3VO4 in 0.002 % H2O2) for 2 minutes prior to cell lysis, a method previously described (10). EGF and TGFα ligands were added to growth media 30 min prior to lysis.
Immunoprecipitation
Cells were processed for IP as previously described (34). Five hundred μg of protein and 2 μg of anti-AXL (Santa Cruz Biotechnology), cetuximab, or IgG antibody (Santa Cruz Biotechnology) were used.
Cell proliferation assay
Crystal violet assay and Cell Counting Kit-8 (Dojindo Molecular Technologies, Rockville, MD, USA) were performed as previously described (31, 35). Cellular proliferation was measured 72 hr post siRNA or drug treatment.
Flow cytometric analysis
Cells were processed as previously described (36) and analyzed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Propidium iodide was added to each sample at a final concentration of 5 mg/ml. Histogram analysis was performed using FlowJo software (Tree Star Inc. Ashland, OR, USA).
Plasmids, transfection, and stable cell line construction
pDONR223-AXL (Plasmid 23945) was purchased from Addgene (Cambridge, MA, USA) and subcloned into the BamH1/EcoR1 restriction sites of the pCDNA6.0 expression vector (Life Technologies). Stable transfection was performed using Lipofectamine LTX and Opti-MEM I (Life Technology) commencing 48 hr post-transfection via 6 μg/mL blasticidin to the growth media. Single cell clones were chosen for expansion and validation for AXL expression.
cDNA synthesis and quantitative PCR
Total RNA and cDNA synthesis were prepared as previously described (34). All reactions were performed in triplicate. To determine the normalized value, 2ΔΔCt values were compared between AXL and 18S, where the change in crossing threshold (ΔCt) =CtAXL –Ct18S and ΔΔCt = ΔCt(HC1, HC4, or HC8) -ΔCt(HP).
Cetuximab resistant cell line xenografts and PDXs
CtxR cell line xenografts were established as previously described (31), and HNSCC PDXs were established and evaluated for cetuximab response as described in the Supplemental Materials and Methods.
Statistical analysis
Student t-tests were employed to evaluate differences in proliferation, AXL mRNA expression, and pAXL-Y779 expression levels by IHC. Differences were considered statistically significant if *p<0.05.
Results
AXL is overexpressed and activated in a model of acquired resistance to cetuximab
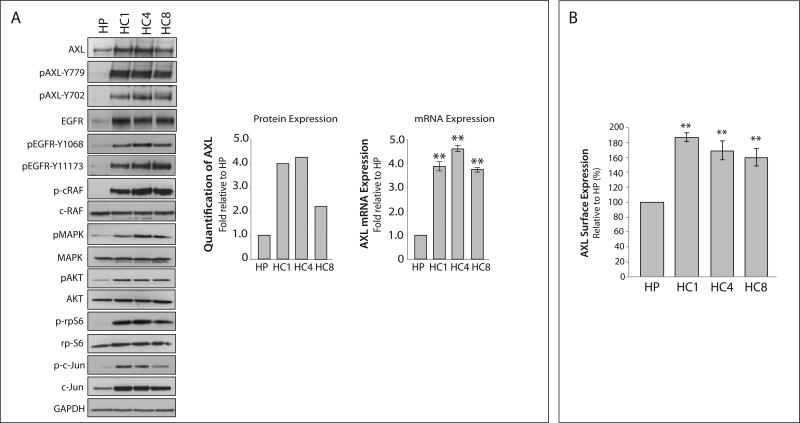
The NSCLC CtxR clones HC1, HC4, and HC8 have been previously shown to be resistant to increasing doses of cetuximab as compared to the CtxS NCI-H226 parental cell line HP (30, 31). Analysis of CtxR clones HC1, HC4 and HC8 demonstrated all clones expressed increased AXL mRNA and protein as compared to HP cells (Figure 1A). Further, AXL exhibited increased phosphorylation on tyrosine 702 and 779 in all CtxR clones. Additionally, MAPK and AKT pathways were hyperactivated and there was increased expression and phosphorylation of the transcription factor c-Jun in CtxR clones. Moreover, plasma membrane levels of AXL were detected via flow cytometry, where CtxR cells had approximately 50–80% more surface AXL expression as compared to HP cells (Figure 1B). Collectively, these data demonstrate that AXL is overexpressed and activated in established clones with acquired resistance to cetuximab.
Figure 1. The receptor tyrosine kinase AXL and its downstream effector molecules are overexpressed in cetuximab resistant cells.
(A) Whole cell lysate was harvested from the CtxS parental cell line (HP) and three CtxR cell clones (HC1, HC4, and HC8) followed by immunoblotting for the indicated proteins. GAPDH was used as a loading control. Total AXL protein expression was quantitated using Image J software. AXL mRNA expression was detected by qPCR and normalized to AXL expression in HP cells (n=3 in 3 independent experiments). 18S was used as an endogenous control. (B) Surface level AXL expression was detected by flow cytometry and normalized to HP. IgG stained cells were used as a background control (n=3 in 2 independent experiments). Data points are represented as mean± SEM. **p<0.01.
AXL and EGFR cooperate in CtxR clones to sustain proliferation via MAPK and c-Jun
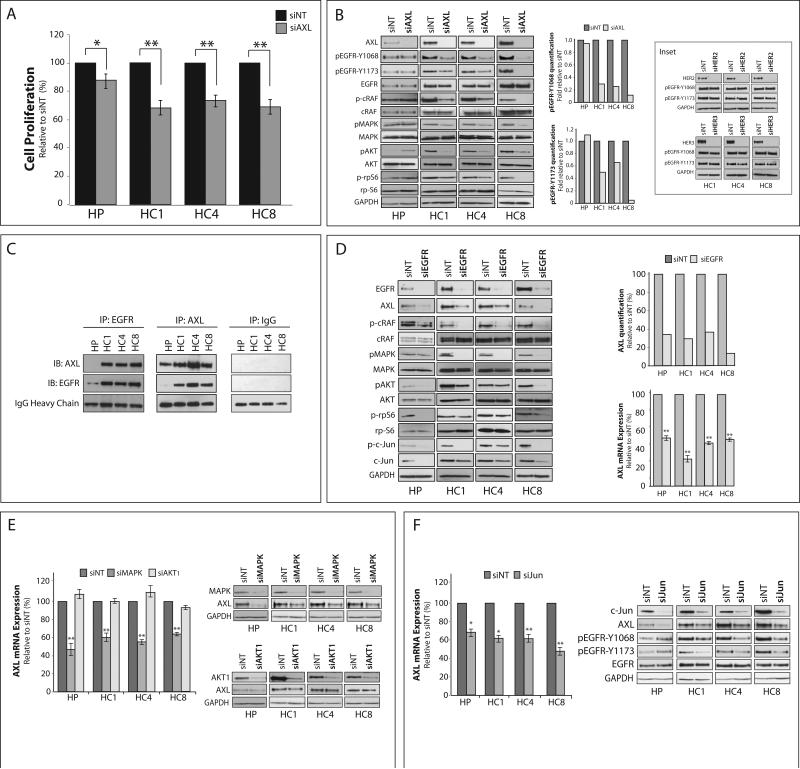
CtxR clones are known to be highly dependent on EGFR for proliferation (30-32). To determine if AXL also plays a role in CtxR cell proliferation, proliferation assays were performed 72 hours post-transfection with a pooled AXL-siRNA (siAXL) or non-targeting siRNA (siNT) (Figure 2A). Loss of AXL expression resulted in statistically significant inhibition of proliferation (25–35%) in all three CtxR clones. As compared to parental HP cells, the CtxR clones demonstrated significantly greater decreases in proliferation post AXL knockdown (p<0.01). Analysis of CtxR clones post AXL knockdown demonstrated EGFR activation was severely diminished at both tyrosine 1068 and 1173, autophosphorylation sites responsible for recruiting Grb2 and Shc (37) (Figure 2B). Additionally, the activation of c-Raf, p44/42 MAPK, AKT and ribosomal protein S6 (rpS6) were diminished in all CtxR clones upon AXL knockdown, while the activation of these molecules were relatively unchanged or slightly increased in HP cells (Figure 2B). Interestingly, ablation of HER2 or HER3 receptors, previously shown to be hyperactivated in CtxR cells (30), did not affect the phosphorylation of EGFR at either tyrosine site (Figure 2B, Inset). Collectively, these data demonstrate CtxR clones are dependent on AXL for cellular proliferation via EGFR activation and downstream signaling.
Figure 2. Cetuximab resistant cells depend on AXL and its cooperation with EGFR.
(A) Cells were transfected with AXL siRNA (siAXL) or non-targeting siRNA (siNT) for 72 hr prior to performing proliferation assays. Proliferation is plotted as percentage of growth relative to NT transfected cells (n=6 in 3 independent experiments). (B) Cells were incubated with AXL siRNA or NT siRNA for 72 hr prior to harvesting whole cell lysate and immunoblotting for the indicated proteins. GAPDH was used as a loading control. Phosphorylation of EGFR on tyrosine 1068 and 1173 were quantitated using Image J software. Inset: Cells were transfected with siRNA against HER2, HER3, or NT siRNA for 72 hr prior to harvesting whole cell lysate. GAPDH was used as a loading control. (C) 500 μg of whole cell lysate was subjected to immunoprecipitation analysis with cetuximab (IP:EGFR), anti-AXL (IP:AXL), or anti-IgG (IP:IgG) antibody followed by immunoblotting (IB) for either AXL or EGFR. IgG heavy chain staining from the IB:AXL blot was used as a loading control. (D, E, F) Whole cell lysate and mRNA were harvested from CtxR clones 72 hr post-transfection with EGFR siRNA (D), MAPK and AKT1 siRNAs (E), c-Jun siRNA (F), or NT siRNA. GAPDH was used as loading control for protein. In (D), AXL protein expression was quantitated using Image J software. AXL mRNA expression was detected by qPCR and normalized to AXL expression in siNT transfected cells (n=3 in 3 independent experiments). 18S was used as an endogenous control Data points are represented as mean± SEM. *p<0.05, **p<0.01.
To determine if AXL and EGFR were physically associated in CtxR clones, coimmunoprecipitation (IP) experiments were performed and indicated AXL was associated with EGFR in all CtxR clones but not parental cells (Figure 2C). EGFR and AXL cooperation was further analyzed by reciprocally knocking down EGFR expression with siRNA (Figure 2D). EGFR knockdown led to a loss of total AXL protein and mRNA expression in CtxR clones and parental HP cells, as well as diminished activation of c-Raf, p44/42 MAPK, AKT, rpS6, and c-Jun. To examine if EGFR regulation of AXL was contingent on MAPK or AKT signaling directly, we alternatively knocked down p44/42 MAPK or AKT1 with siRNA (Figure 2E). This experiment indicated that knockdown of p44/42 MAPK led to a loss of AXL mRNA and protein expression, while AKT1 did not regulate AXL expression. These results suggest EGFR regulates AXL expression specifically through MAPK signaling.
Previous studies indicated the AXL promoter contains binding motifs for AP-1 family transcription factors, in which phorbol myristate acetate (PMA) stimulation of leukemia cells led to increased AXL expression through MAPK signaling to the transcription factor c-Jun (38). Since CtxR clones were found to overexpress c-Jun (Figure 1A) (39), we hypothesized c-Jun may function downstream of MAPK to regulate AXL mRNA expression. To investigate this, c-Jun was knocked down with siRNA (Figure 2F), leading to an approximate 35–55% decrease in AXL mRNA levels. Moreover, there was a loss of AXL protein expression, which appeared similar to the levels detected post EGFR or MAPK knockdown (Figure 2D and 2E). Importantly, this led to a loss of EGFR activation in CtxR clones, but not in parental HP cells, indicating AXL is required for EGFR activation and subsequent signaling in the resistant setting. Collectively, these data indicate AXL expression and subsequent EGFR activation are regulated through the MAPK/c-Jun signaling pathway in CtxR clones.
CtxR cells are sensitive to anti-AXL monoclonal antibody and tyrosine kinase inhibitor therapies
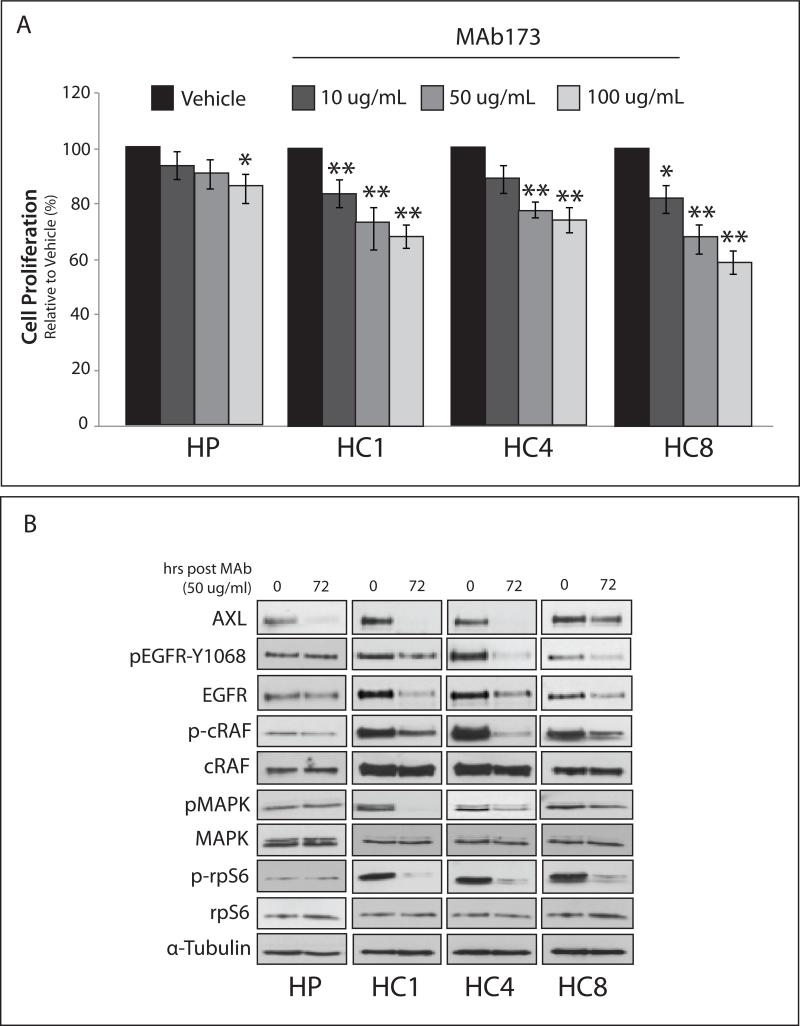
Since CtxR clones were sensitive to AXL knockdown by siRNA, we hypothesized these cells would also be sensitive to anti-AXL therapeutics. First, we tested the ability for the anti-AXL monoclonal antibody MAb173 to inhibit CtxR cell proliferation (Figure 3A). CtxR clones were significantly growth inhibited upon treatment with increasing doses of MAb173, while CtxS HP cells were less sensitive. Additionally, the growth inhibitory effects of CtxR clones were statistically decreased from the effect on HP cells when treated with 50 μg/mL and 100 μg/mL of MAb173 (p<0.01). Consistent with previous studies (9), MAb173 induced AXL degradation (Figure 3B). Interestingly, total EGFR protein levels were reduced upon MAb173 treatment of CtxR clones, in addition to loss of MAPK signaling. MAb173 did not affect the activation of EGFR or MAPK signaling in HP cells.
Figure 3. Cetuximab resistant cells are sensitive to therapeutic degradation of AXL with the monoclonal antibody MAb173.
(A) Cells were subjected to increasing doses of MAb173 (10, 50, and 100 μg/mL) for 72 hr prior to performing proliferation assays. Proliferation is plotted as a percentage of growth relative to vehicle treated cells (n=6 in 3 independent experiments). Data points are represented as mean± SEM. *p<0.05, **p<0.01. (B) Cells were subjected to 50 μg/mL of MAb173 for 72 hr prior to harvesting whole cell lysate and immunoblotting for the indicated proteins. α-Tubulin was used as a loading control.
Next, the small molecule tyrosine kinase inhibitor (TKI) R428, which has greater than 100 fold selectivity for AXL as compared to EGFR or Tyro and 50 fold greater affinity than Mer (40), was tested for therapeutic benefit in CtxR clones (Figure 4A). All CtxR clones demonstrated robust anti-proliferative effects upon treatment with 0.8 μM and 1 μM of R428, while HP cells were less sensitive at these concentrations. Additionally, the growth inhibitory effects of CtxR clones were statistically decreased from the effect on HP cells when treated with 0.8 μM and 1 μM of R428 (p<0.01). Analysis of CtxR clones post-treatment, via pan-tyrosine, demonstrated that AXL phosphorylation was inhibited with 1.0 μM of R428, the same dose that elicited anti-proliferative responses (Figure 4B). Additionally, R428 treatment led to a loss of EGFR phosphorylation on tyrosine 1068 and MAPK signaling, while these targets were relatively unaffected in HP cells (Figure 4C). Interestingly, both MAb173 and R428 did not influence the apoptosis pathway in CtxR clones (data not shown), indicating that AXL more predominantly activates growth-promoting pathways in resistant cells.
Figure 4. Cetuximab resistant cells are sensitive to therapeutic blockade of AXL activity with the AXL tyrosine kinase inhibitor R428.
(A) Cells were subjected to increasing doses of R428 (.01 – 1 μM) for 72 hr prior to performing proliferation assays. Proliferation is plotted as a percentage of growth relative to vehicle treated cells (n=6 in 4 independent experiments). Data points are represented as mean± SEM. *p<0.05, **p<0.01. (B) Cells were treated with vehicle (-) or indicated doses of R428 for 24 hr. 500 μg of whole cell lysate was subjected to IP analysis with an anti-AXL antibody followed by immunoblotting for either AXL or pan-tyrosine (panTyr). IgG heavy chain staining from the IB:AXL blot was used as a loading control. (C) Cells were treated with vehicle (-) or indicated doses of R428 for 24 hr prior to harvesting whole cell lysate and immunoblotting for the indicated proteins. α-Tubulin was used as a loading control.
AXL activation and overexpression confers cetuximab resistance in vitro and in vivo mouse xenograft models
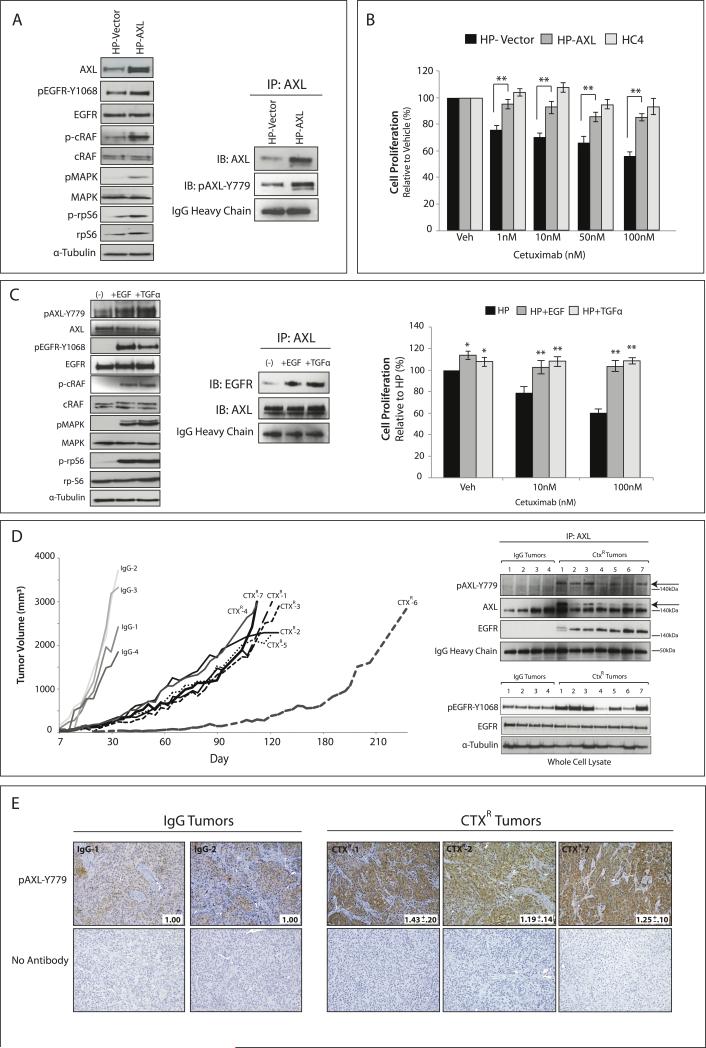
To confirm the role of AXL in cetuximab resistance, AXL was stably overexpressed in the CtxS parental cell line HP (Figure 5A). IP analysis of HP-AXL stable cells indicated AXL was phosphorylated on tyrosine 779, resulting in increased phosphorylation of EGFR and downstream MAPK signaling. Cetuximab dose response proliferation assays demonstrated HP-AXL cells were statistically more resistant to cetuximab as compared to HP-Vector cells (p<0.01) (Figure 5B). HC4 cells served as a cetuximab resistant control in these experiments. These data demonstrate that the stable overexpression of AXL can confer resistance to cetuximab in a CtxS cell line, supporting a putative role for AXL in the development of cetuximab resistance.
Figure 5. AXL overexpression and activity results in cetuximab resistance in CtxS cells in vitro and in de novo models of CtxR in vivo.
(A) HP cells were made to stably express either PCNDA6.0-AXL (HP-AXL) or PCDNA6.0-Vector (HP-Vector). Whole cell lysate was harvested and subjected to immunoblot analysis. α-Tubulin was used as a loading control. 500 μg of protein was subjected to IP with an anti-AXL antibody for analysis of pAXL-Y779. IgG heavy chain staining from the IB:AXL blot was used as a loading control. (B) HP-AXL, HP-Vector, or HC4 cells were treated with increasing doses of cetuximab (1-100 nM) for 72 hr prior to performing proliferation assays. Proliferation is plotted as a percentage of growth relative to vehicle treated cells (n=6 for 4 independent experiments). Data points are represented as mean± SEM. **p<0.01. (C) HP cells were stimulated with 50 ng/mL of EGF or TGFα for 45 min prior to harvesting whole cell lysate. 500 μg of protein was subjected to IP with an anti-AXL antibody for analysis of EGFR association. Proliferation assays were performed 72 hr post-treatment with increasing doses of cetuximab and either 50 ng/mL EGF or TGFα. Proliferation is plotted as a percentage of growth relative to vehicle treated HP cells (n=8 for 3 independent experiments). Data points are represented as mean± SEM. **p<0.01. (D, E) Established CtxS NCI-H226 xenografts were treated with cetuximab (40 mg/kg) or IgG twice weekly. IgG treated tumors grew uninhibited (IgG1-IgG4), while acquired resistance to cetuximab was observed after day 30 in 6 of 7 treated mice (CtxR-1 to CtxR-5, CtxR-7). CtxR-6 acquired resistance after 90 days of treatment. (D) 500 μg of tumor cell lysate was subjected to IP with an anti-AXL antibody followed by immunoblotting for pAXL-Y779, AXL, or EGFR. IgG heavy chain staining from the IB:AXL blot was used as a loading control. pEGFR-Y1068 status was defined by western blot. α-Tubulin was used as a loading control. (E) IHC analysis of pAXL-Y779 in tumor samples (20X). Quantitation of IHC was performed via Image J software (average of 5 independent fields of view per tumor); values were normalized to the average staining in IgG tumor sections.
We previously reported that CtxR clones overexpressed EGFR ligands (36), however, whether EGFR ligands influenced cetuximab resistance through regulating AXL activity and/or association with the EGFR was not investigated. Therefore, HP cells were stimulated with two EGFR ligands, EGF or TGFα, and subsequently measured for AXL activation, association with the EGFR, and cetuximab response (Figure 5C). Analysis of HP cells post ligand stimulation indicated that both ligands led to increased AXL activation and association with the EGFR (detected by IP analysis). Additionally, incubation with either ligand resulted in increased resistance to cetuximab. Interestingly, the ligand for AXL, Gas6, was not overexpressed in CtxR clones and did not drive resistance in HP cells (data not shown). Collectively, these data suggest EGFR ligands may influence cetuximab resistance through stimulating AXL activation and association with the EGFR.
To further analyze the role of AXL in cetuximab resistance, we developed de novo tumors with acquired resistance to cetuximab in vivo (31, 32). To develop de novo acquired resistance, the CtxS cell line NCI-H226 was inoculated unilaterally into the dorsal flank of 11 athymic nude mice (Figure 5D). Once tumors reached approximately 100 mm3, four mice were treated with IgG control antibody (1 mg/mouse) and 7 mice were treated with cetuximab (1 mg/mouse) by intraperitoneal injection twice weekly. Tumors treated with IgG grew rapidly (tumors denoted as IgG-1 to IgG-4 in Figure 5D), while all cetuximab treated tumors displayed initial growth control. Acquired resistance was observed after approximately 30 days post cetuximab exposure in 6 of the cetuximab treated mice (tumors denoted as CtxR-1 to CtxR-5, and CtxR-7), at which point there was marked tumor growth in the presence of continued cetuximab therapy (Figure 5D). One mouse was continued on cetuximab for 90 days until a significant increase in tumor growth was observed (CtxR-6). Once tumors reached 2000 mm3, they were harvested and processed for immunoblot analysis (Figure 5D) and IHC (Figure 5E). To detect the levels of total and activated AXL (Y779), IP analysis was performed from tumor lysates. Strikingly, a double banding pattern for total AXL was observed in all CtxR tumors, while a single AXL band was observed in the IgG treated tumors. The upper band corresponds to a shift in AXL molecular weight due to the presence of phosphorylated AXL, which was detected by the phospho AXL-Y779 antibody (Figure 5D, arrows). Additionally, AXL was associated with EGFR only in the CtxR tumors by IP (Figure 5D). Analysis of whole cell lysate indicated EGFR was also highly activated (indicated by tyrosine 1068 phosphorylation) in the CtxR tumors that expressed the highest levels of pAXL-Y779. IHC analysis of IgG versus CtxR tumors revealed CtxR tumors had statistically significant increases in pAXL-Y779 staining (Figure 5E). Collectively, these data demonstrate AXL overexpression and/or activation plays a role in acquired resistance to cetuximab in vitro and in vivo.
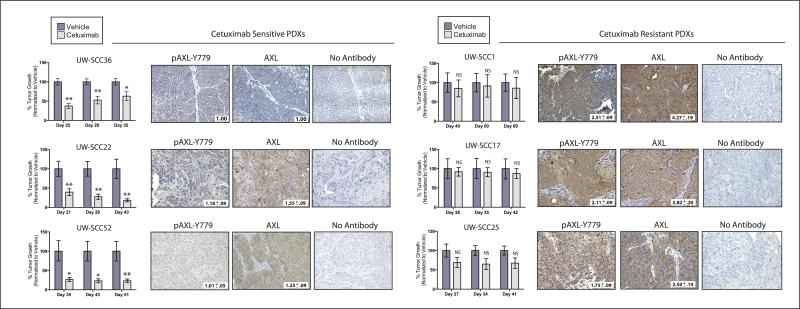
To expand these findings to a more clinically relevant model system, we determined if there was a correlation between cetuximab response and AXL expression in PDXs established directly from surgically removed HNSCC. Six PDXs were established from patients whom had not received prior cetuximab therapy (see Supplemental Table 1 for clinical characteristics of patients prior to surgery). For each PDX, dual flank tumors were established in 16 athymic nude mice. When tumors reached approximately 200 mm3, the mice were stratified into two treatment groups: control (vehicle treated) and cetuximab (n=8 mice/16 tumors per group). After completing the treatment regimen, tumor growth was monitored to evaluate response to therapy. Overall, there were three cetuximab sensitive PDXs (UW-SCC36, UW-SCC22, and UW-SCC52) and three cetuximab resistant PDXs (UW-SCC1, UW-SCC17, and UW-SCC25) (Figure 6).
Figure 6. AXL is overexpressed and activated in cetuximab resistant HNSCC PDXs.
PDXs were evaluated for cetuximab response as described in the supplemental materials and methods. Tumor growth was plotted as a percentage of averaged vehicle treated tumor volumes at the last three time points of the study; *p<0.05, **p<0.01. Representative images of IHC analysis of AXL and pAXL-Y779 staining in early passaged PDXs are shown (20X). Quantitation of IHC was performed via Image J software (average of 2-5 independent tumors were stained and imaged); values were normalized to the average staining of UW-SCC36.
PDXs harvested from early passaged tumors prior to treatment were evaluated for AXL expression and activation by IHC analysis (Figure 6). The cetuximab sensitive PDXs had low levels of AXL and pAXL-Y779 staining, with UW-SCC36 having nearly absent expression of both markers. In comparison, the three cetuximab resistant PDXs expressed 1.8–2.5 fold increases in pAXL-Y779 expression, and 2.5–4.3 fold increases in total AXL expression as compared to the staining intensity detected in UW-SCC36 tumors. Collectively, these data demonstrate AXL is overexpressed and activated in PDXs that are intrinsically resistant to cetuximab therapy.
AXL plays a role in acquired resistance to cetuximab in HNSCC
To further investigate whether AXL plays a more global role in acquired resistance to cetuximab, we developed a model of acquired resistance to cetuximab using the CtxS parental cell line UM-SCC1 (30). This resulted in a parental SCC1 cell line (SP) and three cetuximab resistant clones (SP7, SP8 and SP11). SP cell growth was inhibited upon treatment with increasing doses of cetuximab, while the three HNSCC CtxR clones remained resistant (Figure 7A). Analysis of HNSCC CtxR clones indicated all clones had increased steady state expression of AXL as compared to SP (Figure 7B). Additionally, each clone demonstrated increased activation of c-Raf, p44/42 MAPK, AKT, rpS6, and c-Jun (Figure 7B). To determine if AXL influenced HNSCC CtxR cell proliferation, cells were transfected with siAXL or NT siRNA and proliferation assays were performed. Loss of AXL expression resulted in a significant inhibition in cellular proliferation (20%–25%) in HNSCC CtxR clones, while parental SP cells were non-responsive (Figure 7C). The growth inhibitory effects of siAXL in HNSCC CtxR clones were statistically decreased compared to the effect on SP cells (p<0.01). Further, all HNSCC CtxR clones expressed diminished activation of EGFR (by tyrosine 1068 phosphorylation) as well as MAPK and AKT signaling pathways upon AXL knockdown, while the activation of these molecules were relatively unchanged or slightly increased in SP cells. Collectively, these data suggest AXL plays a role in acquired resistance to cetuximab in HNSCC.
Figure 7. AXL mediates acquired resistance to cetuximab in HNSCC.
(A) CtxR cell clones (SP7, SP8, and SP11) and the CtxS parental cell line (SP) were treated with increasing doses of cetuximab (1 nM, 10 nM, and 100nM) for 72 hr prior to performing proliferation assays. Proliferation is plotted as a percentage of growth relative to vehicle treated cells (n=5 for 3 independent experiments). (B) Whole cell lysate was harvested from cells followed by immunoblotting for the indicated proteins. α-Tubulin was used as a loading control. Total AXL protein expression was quantitated using Image J software. (C) Cells were incubated with AXL siRNA (siAXL) or non-targeting (NT) siRNA for 72 hr prior to performing proliferation assays or isolation of whole cell lysate and immunoblotting for indicated proteins. α-Tubulin was used as a loading control. Proliferation is plotted as a percentage of growth relative to NT transfected cells (n=3 for 3 independent experiments). Data points are represented as mean± SEM. *p<0.05, **p<0.01.
Discussion
Cetuximab is a commonly used anti-EGFR monoclonal antibody therapy that has shown efficacy in HNSCC, mCRC, and NSCLC (19-26). While cetuximab treatment has yielded clinical benefit, both intrinsic and acquired resistance are common outcomes. Recently, a novel mutation was identified in the EGFR (S492R) that mediates resistance to cetuximab (41), however, resistance also occurs in the wild-type (WT) setting. Multiple mechanisms of cetuximab resistance exist, including upregulation of EGFR ligands (42), nuclear translocation of EGFR (36), oncogenic shift to vascular endothelial growth factor receptor-1 (VEGFR-1) (43), and constitutive activation of downstream signaling molecules such as KRAS (44) and c-Src (45). The study herein is the first to describe a role for AXL in resistance to cetuximab in the setting of WT EGFR, and thus provides rationale for the development and use of anti-AXL therapeutics for treatment of CtxR tumors.
Cetuximab resistance is challenging to study due to the lack of access to patient tissue upon relapse. To model CtxR mechanisms that may occur in humans, several models of acquired resistance were established via prolonged exposure of CtxS cells to cetuximab (30-32). These models indicated CtxR clones and tumors had increased expression and dependency on the EGFR (30-32). In the current study, AXL was found to activate EGFR in CtxR clones, while HER2 and HER3 receptors did not, suggesting that AXL is a key mediator of EGFR activity in the resistant setting. Furthermore, EGFR and AXL were associated in CtxR clones and tumors (Figures 2C and 5D), a finding previously reported in triple-negative breast cancers (TNBCs) (14), tumors that are intrinsically resistant to cetuximab. Interestingly, EGF mediated AXL induced signaling pathways in TNBC while Gas6 did not (14), similar to our findings in Figure 5C. Another novel finding was EGFR signaling led to increased AXL mRNA expression in CtxR clones. The regulation of AXL mRNA was contingent on MAPK and c-Jun since knockdown of either decreased AXL expression (Figures 2E and 2F).
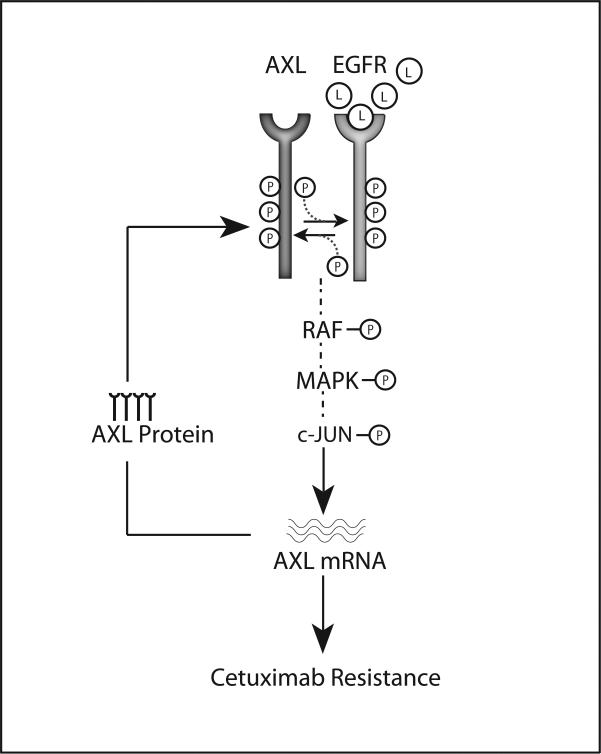
These data support a positive-feedback loop that occurs in EGFR-dependent CtxR cells (Figure 8). In this model, resistance is characterized by increased EGFR ligand production, dimerization and transactivation of AXL and EGFR. This interaction results in hyperactivated MAPK/c-Jun signaling, upregulation of AXL mRNA expression, and maintenance of constitutive EGFR activation and cetuximab resistance. The de novo CtxR cell line xenografts support this model, as CtxR tumors expressed increased total and activated AXL (especially as compared to IgG-1 and IgG-2). While MAPK was capable of regulating AXL mRNA expression in CtxS parental cells, this regulation did not reduce EGFR activity (Figure 2F), suggesting EGFR and AXL are not coupled in CtxS cells.
Figure 8. Model for AXL and EGFR cooperation in cetuximab resistance.
Cetuximab resistance is characterized by increased AXL mRNA and protein expression, EGFR activation, and MAPK pathway signaling. In CtxR cells, increased EGFR ligand (L) production leads to AXL and EGFR association and transactivation. This results in MAPK and c-Jun signaling and subsequent increases in AXL transcription. Increases in AXL mRNA result in elevated AXL protein levels and maintenance of EGFR activation and signaling. This positive feedback loop results in the constitutive activation of both AXL and EGFR in CtxR cells and thereby mediates cetuximab resistance.
Due to limited availability of patient tissue post cetuximab failure, the expression status of AXL and pAXL-Y779 was evaluated in intrinsically resistant HNSCC PDXs. PDXs are clinically relevant cancer models because they accurately maintain many aspects of the parental tumor including its histology, gene expression profile and copy number variance, and metastatic patterns (46, 47). In this study, total and activated AXL were highly overexpressed in HNSCC PDXs that were resistant to cetuximab (Figure 6). The strong correlation between AXL and cetuximab resistance observed in the PDXs support the mechanistic work performed in this study and suggest AXL may mediate both intrinsic and acquired resistance to cetuximab in patients.
To date, AXL has been identified to play a role in resistance to EGFR TKIs in NSCLC (16), HNSCC (13), and TNBC (14). In NSCLC, AXL was overexpressed and activated in EGFR mutant erlotinib resistant cells, where AXL inhibition resensitized tumor cells to erlotinib (13, 16). In the current study, AXL inhibition was sufficient to inhibit the growth of CtxR clones, but did not resensitize CtxR clones to cetuximab (data not shown). Cetuximab sensitivity was not restored upon AXL inhibition because AXL inhibition robustly decreased EGFR activation; thus adding cetuximab provided no further benefit. While AXL inhibition led to robust anti-proliferative effects in CtxR clones, cell growth was not completely arrested, suggesting other RTKs may influence resistance. Previous work from our laboratory and others suggests signaling emanating from HER2:HER3 heterodimers play a role in resistance to anti-EGFR agents (30, 48). Thus, targeting AXL and either HER2 or HER3 may result in even more robust anti-proliferative responses since EGFR signaling could be abrogated through AXL inhibition and HER2:HER3 signaling could be abrogated with anti-HER2 or HER3 agents. Ultimately, this approach may lead to a complete loss of HER family signaling capabilities and serve as a powerful strategy for the treatment of CtxR tumors.
With increasing evidence supporting the role of AXL in resistance to anti-EGFR agents, the development of anti-AXL therapeutics is essential. In this study, two novel anti-AXL therapeutics were tested: MAb173, an anti-AXL neutralizing monoclonal antibody, and R428, a selective small molecule AXL TKI. In previous studies, researchers demonstrated AXL was hyperactivated in Karposi Sarcomas and that MAb173 induced AXL endocytosis and degradation (9). In addition to AXL, total EGFR expression was decreased upon MAb173 treatment of CtxR cells (Figure 3B) supporting the existence of AXL and EGFR heterodimers and the utility of this antibody in the setting of cetuximab resistance. Furthermore, EGFR was not degraded in MAb173 treated HP cells which lack AXL and EGFR association (Figure 2C). The anti-AXL TKI R428 has also shown anti-tumorigenic effects in multiple cancer models, including breast cancer (14, 40) and HNSCC (13). The differences in growth inhibition observed between MAb173 and R428 may result from off–target effects of R428 leading to more robust anti-proliferative responses. R428 has now entered phase I clinical trials, while MAb173 is still undergoing preclinical testing.
Overall, AXL plays a key role in tumor growth, metastasis, angiogenesis, and resistance to anti-EGFR agents (12-17). Additionally, AXL inhibition has been shown to enhance the efficacy of standard chemotherapy regimens (10, 15, 18). With AXL at the forefront, Tyro and Mer receptors also influence parameters of tumor biology (1, 4). In fact, both Tyro and Mer receptors were differentially overexpressed in the current CtxR models (unpublished data) promoting further research on the global role of TAM receptors in cetuximab resistance. Collectively, the studies herein have strong potential for translation into future clinical trials and therapies for patients with cetuximab resistant tumors.
Supplementary Material
Acknowledgments
Financial Support: grant UL1TR000427 from the Clinical and Translational Science Award program (D.L.Wheeler), through the NIH National Center for Advancing Translational Sciences, grant RSG-10-193-01-TBG from the American Cancer Society (D.L.Wheeler), grant W81XWH-12-1-0467 from United States Army Medical Research and Materiel Command (D.L.Wheeler), and Mary Kay Foundation grant MSN152261 (D.L.Wheeler).
Footnotes
Conflicts of Interest: None
References
- 1.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Advances in cancer research. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma A, Warner SL, Vankayalapati H, Bearss DJ, Sharma S. Targeting AXL and Mer kinases in cancer. Mol Cancer Ther. 2011;10:1763–73. doi: 10.1158/1535-7163.MCT-11-0116. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Ye X, Tan C, Hongo JA, Zha J, Liu J, et al. AXL as a potential therapeutic target in cancer: role of AXL in tumor growth, metastasis and angiogenesis. Oncogene. 2009;28:3442–55. doi: 10.1038/onc.2009.212. [DOI] [PubMed] [Google Scholar]
- 4.Linger RM, Keating AK, Earp HS, Graham DK. Taking aim at Mer and AXL receptor tyrosine kinases as novel therapeutic targets in solid tumors. Expert opinion on therapeutic targets. 2010;14:1073–90. doi: 10.1517/14728222.2010.515980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paccez JD, Vogelsang M, Parker MI, Zerbini LF. The receptor tyrosine kinase AXL in cancer: Biological functions and therapeutic implications. Int J Cancer. 2014;134:1024–33. doi: 10.1002/ijc.28246. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YX, Knyazev PG, Cheburkin YV, Sharma K, Knyazev YP, Orfi L, et al. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–15. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 7.Rankin EB, Fuh KC, Taylor TE, Krieg AJ, Musser M, Yuan J, et al. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70:7570–9. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paccez JD, Vasques GJ, Correa RG, Vasconcellos JF, Duncan K, Gu X, et al. The receptor tyrosine kinase AXL is an essential regulator of prostate cancer proliferation and tumor growth and represents a new therapeutic target. Oncogene. 2013;32:689–98. doi: 10.1038/onc.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu R, Gong M, Li X, Zhou Y, Gao W, Tulpule A, et al. Induction, regulation, and biologic function of AXL receptor tyrosine kinase in Kaposi sarcoma. Blood. 2010;116:297–305. doi: 10.1182/blood-2009-12-257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linger RM, Cohen RA, Cummings CT, Sather S, Migdall-Wilson J, Middleton DH, et al. Mer or AXL receptor tyrosine kinase inhibition promotes apoptosis, blocks growth and enhances chemosensitivity of human non-small cell lung cancer. Oncogene. 2013;32:3420–31. doi: 10.1038/onc.2012.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Tian R, Yong B, Luo C, Tan P, Shen J, et al. Gas6/AXL mediates tumor cell apoptosis, migration and invasion and predicts the clinical outcome of osteosarcoma patients. Biochem Biophys Res Commun. 2013;435:493–500. doi: 10.1016/j.bbrc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies AXL as a therapeutic target for overcoming EGFR inhibitor resistance. Clin Cancer Res. 2013;19:279–90. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giles KM, Kalinowski FC, Candy PA, Epis MR, Zhang PM, Redfern AD, et al. AXL mediates acquired resistance of head and neck cancer cells to the epidermal growth factor receptor inhibitor erlotinib. Mol Cancer Ther. 2013;12:2541–58. doi: 10.1158/1535-7163.MCT-13-0170. [DOI] [PubMed] [Google Scholar]
- 14.Meyer AS, Miller MA, Gertler FB, Lauffenburger DA. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye X, Li Y, Stawicki S, Couto S, Eastham-Anderson J, Kallop D, et al. An anti-AXL monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–64. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Lee JC, Lin L, Olivas V, Au V, LaFramboise T, et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature genetics. 2012;44:852–60. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rho JK, Choi YJ, Kim SY, Kim TW, Choi EK, Yoon SJ, et al. MET and AXL Inhibitor NPS-1034 Exerts Efficacy against Lung Cancer Cells Resistant to EGFR Kinase Inhibitors Because of MET or AXL Activation. Cancer Res. 2014;74:253–62. doi: 10.1158/0008-5472.CAN-13-1103. [DOI] [PubMed] [Google Scholar]
- 18.Dunne PD, McArt DG, Blayney JK, Kalimutho M, Greer S, Wang T, et al. AXL Is a Key Regulator of Inherent and Chemotherapy-Induced Invasion and Predicts a Poor Clinical Outcome in Early-Stage Colon Cancer. Clin Cancer Res. 2014;20:164–75. doi: 10.1158/1078-0432.CCR-13-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baselga J. The EGFR as a target for anticancer therapy--focus on cetuximab. Eur J Cancer. 2001;37(Suppl 4):S16–22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 20.Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 21.Govindan R. Cetuximab in advanced non-small cell lung cancer. Clinical Cancer Research. 2004;10:4241S–4S. doi: 10.1158/1078-0432.CCR-040015. [DOI] [PubMed] [Google Scholar]
- 22.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 23.Thienelt CD, Bunn PA, Hanna N, Rosenberg A, Needle MN, Long ME, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. Journal of Clinical Oncology. 2005;23:8786–93. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 24.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 25.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 26.Lynch TJ, Patel T, Dreisbach L, McCleod M, Heim WJ, Hermann RC, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol. 2010;28:911–7. doi: 10.1200/JCO.2009.21.9618. [DOI] [PubMed] [Google Scholar]
- 27.Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol. 2010;28:1254–61. doi: 10.1200/JCO.2009.24.6116. [DOI] [PubMed] [Google Scholar]
- 28.Brand TM, Iida M, Wheeler DL. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol Ther. 2011;11:777–92. doi: 10.4161/cbt.11.9.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diaz LA, Jr., Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537–40. doi: 10.1038/nature11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler DL, Huang S, Kruser TJ, Nechrebecki MM, Armstrong EA, Benavente S, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–56. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iida M, Brand TM, Starr MM, Li C, Huppert EJ, Luthar N, et al. Sym004, a Novel EGFR Antibody Mixture, Can Overcome Acquired Resistance to Cetuximab. Neoplasia. 2013;15:1196–206. doi: 10.1593/neo.131584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brand TM, Dunn EF, Iida M, Myers RA, Kostopoulos KT, Li C, et al. Erlotinib is a viable treatment for tumors with acquired resistance to cetuximab. Cancer Biol Ther. 2011;12:436–46. doi: 10.4161/cbt.12.5.16394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iida M, Brand TM, Campbell DA, Li C, Wheeler DL. Yes and Lyn play a role in nuclear translocation of the epidermal growth factor receptor. Oncogene. 2013;32:759–67. doi: 10.1038/onc.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brand TM, Iida M, Luthar N, Wleklinski MJ, Starr MM, Wheeler DL. Mapping C-terminal transactivation domains of the nuclear HER family receptor tyrosine kinase HER3. PLoS One. 2013;8:e71518. doi: 10.1371/journal.pone.0071518. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Li C, Brand TM, Iida M, Huang S, Armstrong EA, van der Kogel A, et al. Human epidermal growth factor receptor 3 (HER3) blockade with U3-1287/AMG888 enhances the efficacy of radiation therapy in lung and head and neck carcinoma. Discov Med. 2013;16:79–92. [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Iida M, Dunn EF, Ghia AJ, Wheeler DL. Nuclear EGFR contributes to acquired resistance to cetuximab. Oncogene. 2009;28:3801–13. doi: 10.1038/onc.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarden Y, Pines G. The ERBB network: at last, cancer therapy meets systems biology. Nat Rev Cancer. 2012;12:553–63. doi: 10.1038/nrc3309. [DOI] [PubMed] [Google Scholar]
- 38.Mudduluru G, Leupold JH, Stroebel P, Allgayer H. PMA up-regulates the transcription of AXL by AP-1 transcription factor binding to TRE sequences via the MAPK cascade in leukaemia cells. Biology of the cell / under the auspices of the European Cell Biology Organization. 2010;103:21–33. doi: 10.1042/BC20100094. [DOI] [PubMed] [Google Scholar]
- 39.Pei DQ, Shih CH. An “attenuator domain” is sandwiched by two distinct transactivation domains in the transcription factor C/EBP. Molecular and Cellular Biology. 1991;11:1480–7. doi: 10.1128/mcb.11.3.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland SJ, Pan A, Franci C, Hu Y, Chang B, Li W, et al. R428, a selective small molecule inhibitor of AXL kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–54. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 41.Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221–3. doi: 10.1038/nm.2609. [DOI] [PubMed] [Google Scholar]
- 42.Hatakeyama H, Cheng HX, Wirth P, Counsell A, Marcrom SR, Wood CB, et al. Regulation of Heparin-Binding EGF-Like Growth Factor by miR-212 and Acquired Cetuximab-Resistance in Head and Neck Squamous Cell Carcinoma. Plos One. 2010;5 doi: 10.1371/journal.pone.0012702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianco R, Rosa R, Damiano V, Daniele G, Gelardi T, Garofalo S, et al. Vascular endothelial growth factor receptor-1 contributes to resistance to anti-epidermal growth factor receptor drugs in human cancer cells. Clin Cancer Res. 2008;14:5069–80. doi: 10.1158/1078-0432.CCR-07-4905. [DOI] [PubMed] [Google Scholar]
- 44.Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519–27. doi: 10.1038/nrclinonc.2009.111. [DOI] [PubMed] [Google Scholar]
- 45.Wheeler DL, Iida M, Kruser TJ, Nechrebecki MM, Dunn EF, Armstrong EA, et al. Epidermal growth factor receptor cooperates with Src family kinases in acquired resistance to cetuximab. Cancer Biol Ther. 2009;8:696–703. doi: 10.4161/cbt.8.8.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–9. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimple RJ, Harari PM, Torres AD, Yang RZ, Soriano BJ, Yu M, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clin Cancer Res. 2013;19:855–64. doi: 10.1158/1078-0432.CCR-12-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L, Castanaro C, Luan B, Yang K, Fan L, Fairhurst JL, et al. ERBB3/HER2 Signaling Promotes Resistance to EGFR Blockade in Head and Neck and Colorectal Cancer Models. Mol Cancer Ther. 2014;13:1345–55. doi: 10.1158/1535-7163.MCT-13-1033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.