Abstract
The clinical efficacy of chimeric antigen receptor (CAR)-redirected T cells remains marginal in solid tumors compared to leukemias. Failures have been attributed to insufficient T-cell migration and to the highly immunosuppressive milieu of solid tumors. To overcome these obstacles, we have combined CAR-T cells with an oncolytic virus (OV) armed with the chemokine RANTES and the cytokine IL-15, reasoning that the modified OV will have both a direct lytic effect on infected malignant cells and facilitate migration and survival of CAR-T cells. Using neuroblastoma (NB) as a tumor model we found that the adenovirus Ad5Δ24 exerted a potent, dose-dependent, cytotoxic effect on tumor cells, while CAR-T cells specific for the tumor antigen GD2 (GD2.CAR-T cells) were not damaged. When used in combination, Ad5Δ24 directly accelerated the caspase pathways in tumor cells exposed to CAR-T cells, while the intratumoral release of both RANTES and IL-15 attracted CAR-T cells and promoted their local survival, respectively, increasing the overall survival of tumor bearing mice. These preclinical data support the use of this innovative biological platform of immunotherapy for solid tumors.
Keywords: chimeric antigen receptor, oncolytic virus, GD2, RANTES, IL-15
Introduction
Adoptive transfer of T cells genetically modified to express a CD19-specific chimeric antigen receptor (CAR) coupled with costimulatory endodomains has shown significant clinical impact in lymphoid leukemias (1–3). In contrast, the clinical relevance of this approach has been more limited for solid tumors (4–6).
Insufficient migration of CAR-T cells to the tumor site and suboptimal persistence within the immune suppressive tumor environment are all critical factors that limit the impact of T-cell immunotherapies in solid tumors. Insufficient migration of tumor-specific T cells may result from unfavorable chemokine gradients as tumor-specific T cells may lack the appropriate chemokine receptors for chemokines secreted by tumor cells(7). Alternatively, tumor cells or stromal cells produce chemokines that preferentially attract T cells with regulatory function rather than T cells with antitumor activity(8, 9). Even if tumor-specific T cells reach the tumor environment, multiple mechanisms exploited by tumor cells themselves or by surrounding stromal cells can block an effective immune response. Among these mechanisms, downregulation of costimulatory molecules (CD28 and CD86) by tumor cells(10), abundant TGF-β production(11) and infiltration by regulatory T cells (Tregs)(9) significantly impair activation and proliferation of tumor-specific T cells, while over-expression of death receptor ligands by tumor cells including FasL and PD-L1 may directly favor premature apoptosis of T lymphocytes(12, 13).
Engineering of CAR-T cells to include costimulatory endodomains can rescue T-cell activation through the autocrine production of IL-2(14–17), but this may still be insufficient to reverse the pro-anergic properties of the tumor environment(18, 19). CAR-T cells will also need to be further modified not only to migrate to the tumor site through co-expression of specific chemokine receptors matching relevant tumor-secreted chemokines such as Gro-α(20), CCL17(21) and CCL2(22), but also to specifically counter the local inhibitory milieu, for example due to TGF-β(23) and Fas-L(24), and to make use of autocrine or administered growth factors(25–27). Since tumor cells likely use multiple inhibitory strategies, it is challenging to construct vectors able to accommodate and express in T lymphocytes all the immunomodulatory genes required to overcome tumor inhibition while increasing T-cell trafficking, survival and safety(28).
To solve this complex task, we exploited OVs that selectively infect, lyse and replicate in malignant cells whilst leaving non-malignant cells unaffected(29). OVs have sufficient cargo capacity to insert ectopic genes, and have produced clinical responses in patients with solid tumors(30). We therefore armed an oncolytic adenovirus (Ad5Δ24) with the chemokine RANTES and the cytokine IL-15 to enhance the subsequent trafficking and survival of T cells expressing a tumor-directed CAR.
Materials and Methods
Tumor cell lines
The NB cell lines IMR-32, LAN-1, SKNLP, SK-N-SH, SH-SY5Y and the lung carcinoma cell line A549 were obtained from the American Type Culture Collection, while NB cell lines LAN-5 and CHLA-255 were gifts from Dr. LS. Metelitsa (Baylor College of Medicine, TX), and we verified that these lines retain the surface expression of the target antigen GD2. Cells were maintained in RPMI-1640 (IMR-32, LAN-1, LAN-5, SKNLP, SK-N-SH, and SH-SY5Y) or IMDM (CHLA-255 and A549). All media was supplemented with 10% fetal bovine serum (FBS) and 2mM L-glutamine (Invitrogen), with the exception of CHLA-255, which was supplemented with 20% FBS. Tumor cell lines were transduced with a gamma retroviral vector encoding enhanced green fluorescent protein (eGFP) to obtain GFP positive tumor cells.
Oncolytic virus
Oncolytic adenovirus Ad5Δ24 and non-replicable control adenovirus Ad5-Luc1 were kindly provided by Dr. Akseli Hemminki (University of Helsinki, Finland). Ad5Δ24.RANTES, Ad5Δ24.IL-15 and Ad5Δ24.RANTES.IL-15 were generated and amplified according to standard procedures(31). For Ad5Δ24.RANTES.IL-15, the two genes were linked together using a 2A-like sequence(25).
Flow cytometry
The following monoclonal antibodies conjugated with fluorochromes were used: Coxackie-adenovirus receptor, GD2, CD95 (Fas), CD80, CD86, CD40L, OX40L, CD25, CD69, IFN, CD3, granzyme B (BD Biosciences), TRAIL, TRAIL-R1 and TRAIL-R2 (Biolegend). Expression of GD2.CAR by T cells was detected using a specific anti-idiotype, 1A7 (TriGem, Titan), followed by staining with secondary antibody RAM-IgG1 (BD Biosciences). FACS data were collected with a FACSCalibur (Becton Dickinson) and analyzed using FloJo software version 9.3 (Tree Star). For the caspase assay, CHLA-255 cells were seeded in 24-well plates (1 × 105/well), infected with Ad5Δ24 or mock (100 vp/cell), and cultured for 4 days. Control and GD2.CAR-T cells (2.5 × 105/well) were then added to the tumor cells. Active caspases in CHLA-255 cells were measured at 0, 2 and 4 hrs by FACS. The apoptotic cells were stained using Vybrant FAM Poly Caspases Assay Kit (Molecular Probes) according to manufacture’s instructions. The frequency of early apoptotic cells was determined as percentage of FAM+ (carboxyfluorescein group as a reporter) cells excluding CD3+ and PI+ cells from the analysis(32).
MTS assay
Cells were seeded into 96-well plates (104 cells per well), infected with or without viruses at the indicated doses and incubated at 37°C for 96 hrs. Cell viability was analyzed by MTS assay according to manufacture’s instruction (CellTiter 96 AQueous One Solution Cell Proliferation Assay, Promega). Values of virus-infected cells were normalized to those of mock-infected cells (percent cell viability). The experiments were performed in triplicate and repeated three times.
Retroviral production and CAR-T cell generation
The vectors encoding the GD2 specific CAR (GD2.CAR), incorporating the CD28 and OX40 costimulatory endodomains, the fusion protein eGFP-firefly luciferase (FFluc), and the methodology for the production of retroviral supernatant and CAR-T cells have been described previously(15, 16, 33). For the T-cell proliferation assay in vitro we used a first generation GD2.CAR that lacks both CD28 and OX40 signaling domains.
Co-culture experiments
Tumor cells were seeded in 24-well plates (5 × 104/well for cytotoxicity assay and 1 × 105/well for T-cell proliferation assay), infected with Ad5Δ24 (50 – 100 vp/cell) and then cultured for 3 days. Control and GD2.CAR-T cells (3 × 104/well for cytotoxicity assay and 5 × 104/well for T-cell proliferation assay) were then added and cultured for additional 3 days. Residual GFP+ NB cells and T cells were then counted based on GFP and CD3 expression, respectively, using microbeads (CountBright Absolute Counting Beads, Invitrogen). Normalized residual tumor cells were calculated as 100 × tumor cell counts with treatment/tumor cell counts without treatment (%).
Confocal microscopic video imaging
GFP-labeled CHLA-255 cells were seeded into 8-well chamber slide (Lab-TekII, Thermo scientific) (104 cells/well), infected with Ad5Δ24 (100 vp/cell) and cultured for 3 days. Control and GD2.CAR-T cells were then added to the well (105 cells/well). GFP+ NB cells stained with Annexin-V (Invitrogen) were imaged using a spinning disk confocal microscope for 16 hrs. Imaging data were acquired and analyzed using Zen software (Zeiss).
Migration assay
Migration assays were conducted as previously described(21) with minor modifications using 5 µm pore 24-well transwell plates (Corning Life Science). The percentage of migrating cells was calculated as follows: 100×[cell count of experimental sample – cell count of negative control] / [cell count of positive control – cell count of negative control].
ELISA and Milliplex assay
To measure the in vitro production of chemokines and cytokines, tumor cells were plated at 5 × 105 cells/ml in 24-well plates and infected with viruses (50–100 vp/cell). Supernatants were collected 72 hrs later and analyzed for the production of RANTES, MIP-1α, MIP-1β, MCP-1, IP-10, and IL-15. To measure the in vivo production of RANTES and IL-15, tumor and blood samples were collected 14 – 18 days after virus inoculation. Tumor homogenates and serum were separated and finally assayed using specific ELISA kits (R&D Systems). Human IL-17F, GM-CSF, IFN, IL-10, CCL-20, IL-12p70, IL-13, IL-17α, IL-22, IL-9, IL-1β, IL-33, IL-2, IL-21, IL-4, IL-23, IL-5, IL-6, IL-25, IL-27, IL-31, TNFα, TNFβ and IL-28α, and mouse G-CSF, GM-CSF, IFN, IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-15, IL-17 and TNFα in the serum were measured using Milliplex assay kits (Millipore) following manufacture’s protocols.
NB xenograft animal model
To assess antitumor effects and persistence of GD2.CAR-T cells, we used NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice (Jackson Laboratory). All mouse experiments were performed in accordance with Baylor College of Medicine Animal Husbandry and Institutional Animal Care and Use Committee guidelines. Mice were engrafted with CHLA-255 cells (3 × 106) s.c. and after 10 – 14 days, mice were inoculated i.t. with PBS or OVs (106 – 109 vp/tumor). Mice were then infused i.v. with either control or GD2.CAR-T cells (107 cells) 3 – 4 days after virus inoculation. Tumor volumes were monitored twice a week by caliper measurement (volume = length × width2/2). To track the migration and survival of GD2.CAR-T cells in vivo, control and GD2.CAR-T cells were labeled with eGFP.FFLuc(15). Biodistribution was assessed using the in vivo imaging system (Xenogen) as previously described(15).
Immunohistochemistry
Tumor samples were fixed, processed and stained according to standard procedures. We performed Hematoxylin and Eosin staining and labeling of human T cells using polyclonal rabbit anti-human CD3 mAb (A0452, Dako). For detection we used Dako LSAB + System-HRP (K0679, Dako).
Statistical analysis
Analysis of variance (ANOVA) with Bonferroni correction and the 2-sided unpaired t test were used for comparison of 3 or more groups, or 2 groups, respectively as stated in the figure legends. Mixed-model ANOVA was applied to compare tumor growth in different groups of mice. Survival curves were plotted using the Kaplan-Meier methods, and the differences in the survival between groups were assessed by log rank test. Data are presented as mean ± SD or SEM as stated in the figure legends. Statistical significance was defined at p<0.05. Statistical analysis was performed with Prism 5 (GraphPad Software).
Results
Coxackie-adenovirus receptor is functionally expressed in neuroblastoma (NB) cell lines but not by GD2.CAR-T cells
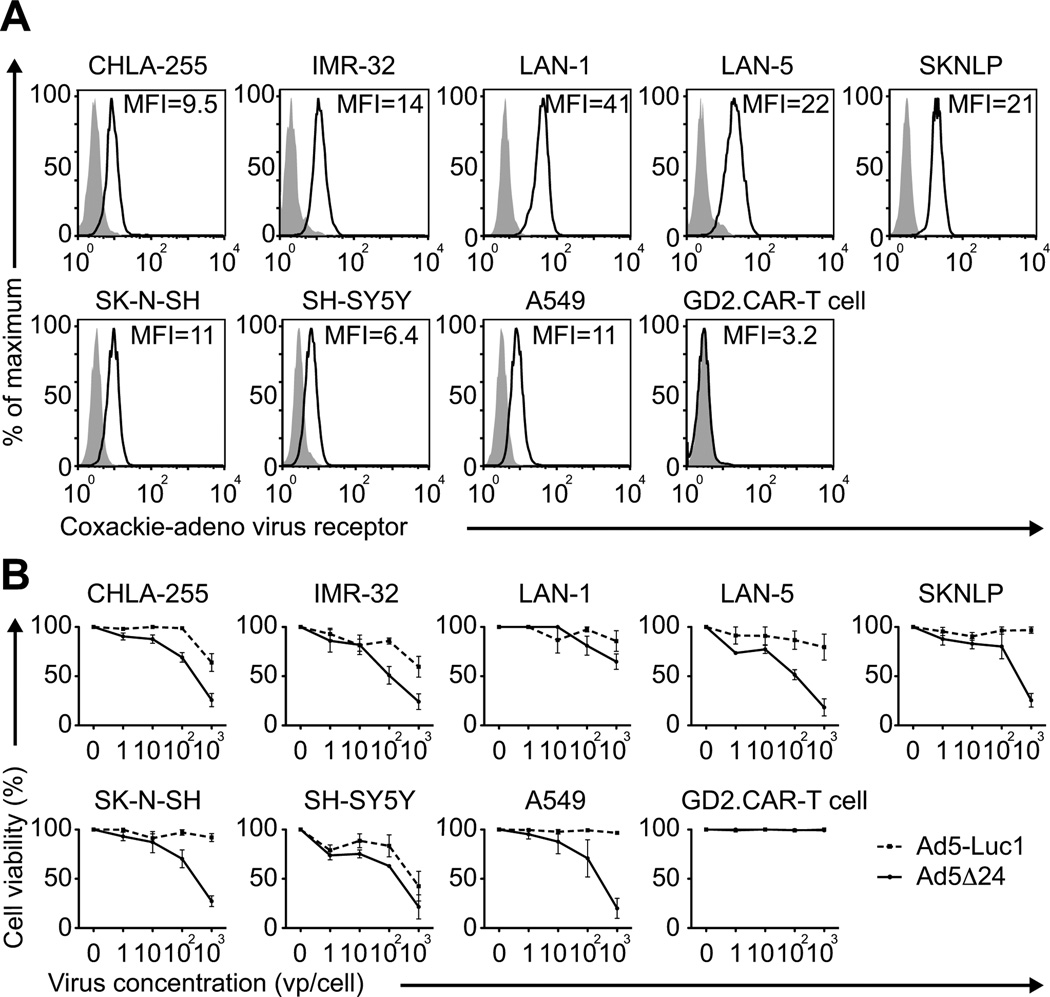
To determine if Ad5Δ24 and CAR-T cells could be combined without toxicity to T lymphocytes in a NB tumor model, we first compared the expression of the Coxackie-adenovirus receptor in 7 NB cell lines and CAR-T cells specifically targeting the GD2 antigen expressed by NB cells. As shown in Fig. 1A, all NB cell lines expressed the virus receptor as assessed by flow cytometry, while CAR-T cells did not. Incubation with the oncolytic adenovirus Ad5Δ24 induced cellular toxicity in 5/7 NB cell lines in a dose-dependent manner (Fig. 1B), while GD2.CAR-T cells were unaffected, even at the highest dose and for up to 7 days of culture (Fig. 1B). Thus Ad5Δ24 is cytopathic for NB cells but not for GD2.CAR-T cells.
Figure 1. NB cell lines but not CAR-T cells are sensitive to the OV Ad5Δ24.
(A) NB cell lines and GD2.CAR-T cells were analyzed by flow cytometry for expression of the coxackie-adenovirus receptor. Lung carcinoma cell line A549 was used as a positive control. Gray filled lines and black lines correspond to isotype control and coxackie-adenovirus receptor, respectively. (B) Tumor cell lines and GD2.CAR-T cells were incubated with increasing doses of Ad5Δ24 or with the control virus Ad5-Luc1, and analyzed 96 hrs later by MTS assay. Data are presented as mean ± SD of 3 independent experiments.
Combined therapy of GD2.CAR-T cells with Ad5Δ24 enhances apoptosis of NB cells in vitro
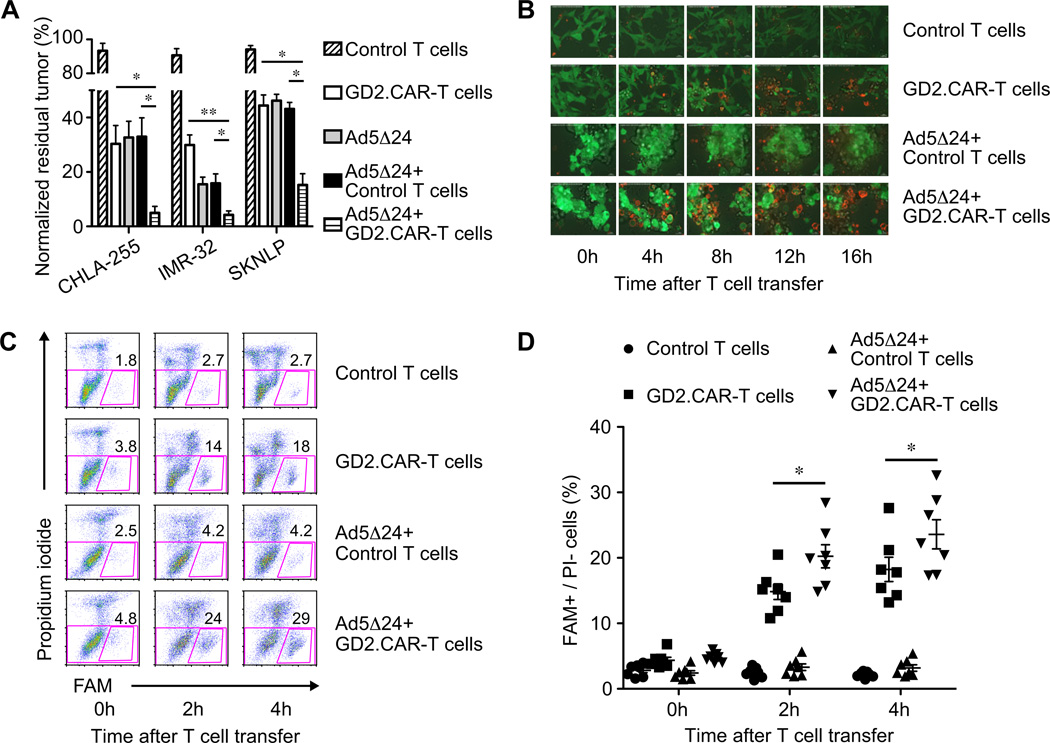
We next explored whether combining Ad5Δ24 and GD2.CAR-T cells enhanced the elimination of NB cells in vitro in co-culture experiments. When CHLA-255 cells were incubated with 75 vp/cell of Ad5Δ24 and a low ratio of GD2.CAR-T cells (Effector:Target = 3:5), tumor cells were effectively eliminated by day 7 of culture compared to tumor cells incubated with each agent alone. Residual tumor cells were significantly decreased to less than 5% in the presence of Ad5Δ24 together with GD2.CAR-T cells, compared to 30% ± 7% and 33% ± 7% in the presence of GD2.CAR-T cells (P=0.01) and Ad5Δ24 combined with control T cells (P=0.01) (Fig. 2A). Comparable results were obtained when two other NB cell lines IMR-32 and SKNLP were used (Fig. 2A). To clarify the mechanism behind the superior antitumor effects of the combined treatment, we explored whether Ad5Δ24 potentiated the function of T cells by upregulating in infected tumor cells the GD2 antigen, death receptors (Fas and TRAIL R1/R2) or costimulatory molecules and ligands (CD80, CD86, OX40L and CD40L). However, no upregulation of any of these molecules by NB cells was detected upon infection with Ad5Δ24 (Supplementary Fig. 1). We also investigated whether Ad5Δ24-infected NB cells could indirectly increase the activation or effector function of CAR-T cells. However, neither CD25, CD69, IFNγ, granzyme B nor TRAIL was significantly upregulated by GD2.CAR-T cells exposed to Ad5Δ24-infected NB cells (Supplementary Fig. 2). By contrast, we found that Ad5Δ24-infected NB cells underwent more rapid apoptosis when exposed to GD2.CAR-T cells. As illustrated in Fig. 2B and Supplementary Movie 1, we conducted sequential confocal microscope imaging of GFP+ CHLA-255 cells co-cultured with GD2.CAR-T cells. We found that NB cells preincubated for 72 hrs with Ad5Δ24 became Annexin-V+ within 4 hrs after exposure to GD2.CAR-T cells, while non infected CHLA-255 cells exposed to GD2.CAR-T cells required more than 16 hrs to bind detectable levels of Annexin-V. We quantified the active caspases in NB cells and found in response to GD2.CAR-T cells a consistent increase in the percentage of early apoptotic cells when NB cells were preincubated with Ad5Δ24 compared to NB cells that had not been exposed to Ad5Δ24 (FAM+/PI− cells were 20% ± 2% vs.15% ± 1% at 2 hrs, P < 0.05; 24% ± 2% vs.18% ± 2% at 4 hrs, P < 0.05) (Fig.2C and 2D). These results indicate that the caspase activity and apoptosis in NB cells are more pronounced after incubation with CAR-T cells if NB cells are infected with Ad5Δ24.
Figure 2. Combined Ad5Δ24 and GD2.CAR-T cells have superior proapoptotic effect on NB cells in vitro.
(A) Antitumor activity of either monotherapy or combined therapy was assessed by in vitro co-culture assay. These experiments were conducted by using 3 NB cell lines (CHLA-255, IMR-32 and SKNLP) and 6 different preparations of T cells. Data are presented as mean ± SEM. * P < 0.05; ** P < 0.01 by Student’s t test. (B) Sequential confocal microscope imaging of co-culture experiments using GFP+ CHLA-255 cells. Apoptotic CHLA-255 cells binding Annexin-V appear in orange. Data are representative of 2 independent experiments. (C) and (D) Activated caspases in CHLA-255 cells were quantified by flow cytometry and indicated as FAM+/PI− cells at time 0 and after 2 and 4 hrs of culture. Results are mean ± SEM from 7 different preparations of T cells. * P < 0.05 by ANOVA with Bonferroni correction.
Combined GD2.CAR-T cells and Ad5Δ24 have more robust antitumor activity in vivo
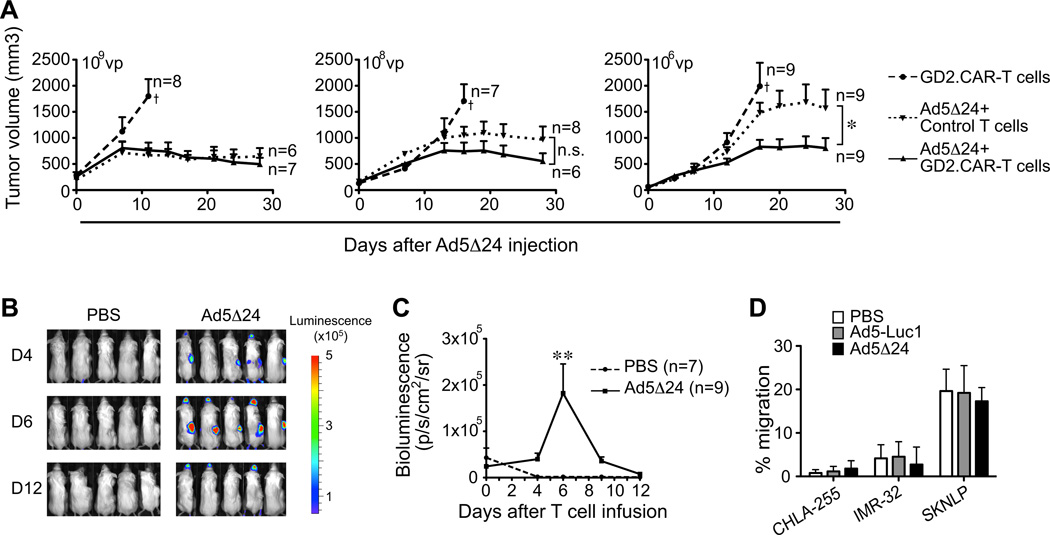
We examined whether the combination of GD2.CAR-T cells and Ad5Δ24 was also effective in a NB xenograft mouse model. Mice engrafted subcutaneously (s.c.) with the NB cell line CHLA-255 were inoculated intratumorally (i.t.) with either PBS or Ad5Δ24 and then infused intravenously (i.v.) with either control or GD2.CAR-T cells (107 cells). Control mice, inoculated with PBS and control T cells, had rapid tumor progression and were euthanized within 20 days (Supplementary Fig. 3). When mice were inoculated with a high dose of Ad5Δ24 (109 vp), tumor growth was controlled irrespective of T-cell transfer (Fig. 3A). However, when mice were inoculated with lower doses of Ad5Δ24, the addition of CAR-T cells improved tumor control compared to Ad5Δ24 plus control T cells (P=0.04 in 106 vp) (Fig. 3A).
Figure 3. Combined therapy with Ad5Δ24 and GD2.CAR-T cells has superior anti-NB effect in vivo.
(A) NSG mice were injected s.c. on the right flank with CHLA-255 cells and inoculated i.t. by day 10 – 14 with either PBS or Ad5Δ24 (106 – 109 vp) followed by i.v. infusion of either control or GD2.CAR-T cells (107 cells) 4 days later. Tumor growth was assessed by caliper measurement. Data represent mean ± SEM. *P < 0.05 by Mixed-model ANOVA. †mice euthanized. (B) and (C) Bioluminescence of GD2.CAR-T cells labeled with FFluc. Data represent mean ± SEM. ** P < 0.01 by Student’s t test. (D) In vitro migration of GD2.CAR-T cells toward the supernatant collected from control or Ad5Δ24-infected NB cells (CHLA-255, IMR-32 and SKNLP). Data represent mean ± SEM from 3 different T-cell preparations.
We also found that GD2.CAR-T cells showed enhanced, although still transient, persistence in vivo at the tumor site when tumors were inoculated with Ad5Δ24. As shown in Fig.3B and 3C, FFluc-labeled GD2.CAR-T cells localized and persisted up to day 6 in Ad5Δ24-inoculated tumors compared to PBS-inoculated tumors (1.8 × 105 ± 0.63 × 105 p/s/cm2/sr vs. 1.5 × 103 ± 0.2 × 103 p/s/cm2/sr, P < 0.01) and then became undetectable. These findings raise the possibility that Ad5Δ24-infected NB cells may release T-cell chemoattractant factors. However, measurement of the chemokines RANTES, MIP-1α, MIP-1β, MCP-1 and IP-10 in the culture of NB cells infected with Ad5Δ24 showed no significant production of these cytokines before and after infection, with the exception of the SK-N-SH NB cell line that spontaneously produced a high level of MCP-1 (Supplementary Table 1). In vitro migration assays confirmed that supernatants collected from NB cells infected with Ad5Δ24 did not promote T-cell migration, excluding the possibility that other unknown factors that favor T-cell migration are released by Ad5Δ24-infected NB cells (Fig. 3D). Thus the transient beneficial effects of the combined Ad5Δ24 and GD2.CAR-T cells support the need for the engineering of Ad5Δ24 to increase the antitumor benefits.
NB cells infected with Ad5Δ24 engineered to release RANTES and IL-15 produce functional amounts of both proteins
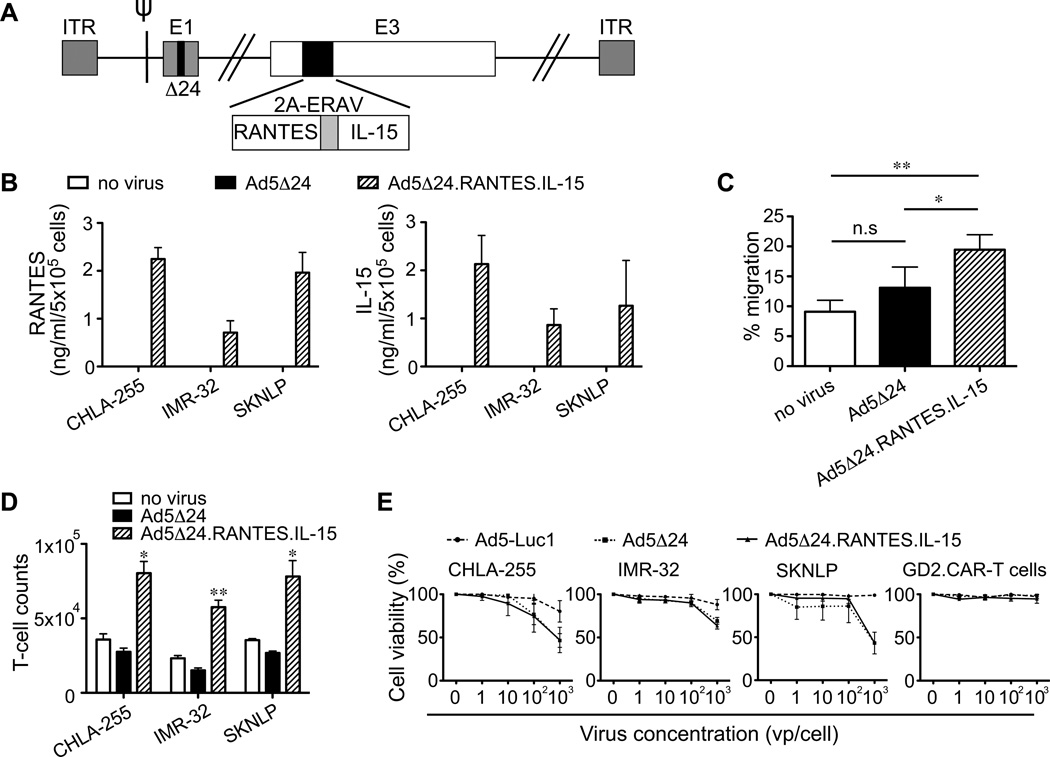
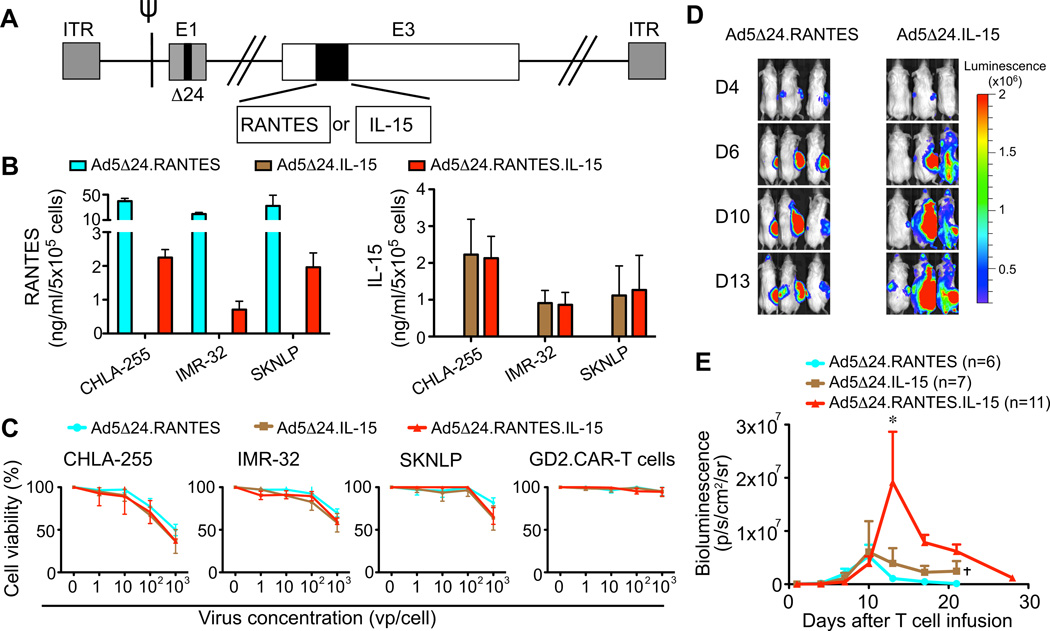
We armed Ad5Δ24 with RANTES and IL-15 (Ad5Δ24.RANTES.IL-15) to induce migration and prolong persistence of GD2.CAR-T cells at the tumor site (Fig. 4A), and demonstrated that NB cells infected with this virus release RANTES (709 – 2247 pg/ml at 72 hrs) and IL-15 (864 – 2131 pg/ml at 72 hrs) in vitro (Fig. 4B). Both factors were functional because the supernatant of NB cells infected with Ad5Δ24.RANTES.IL-15 improved the migration (19% ± 2% in Ad5Δ24.RANTES.IL-15 vs. 9% ± 2% in mock; P=0.002, and vs. 13% ± 3% in Ad5Δ24; P=0.01) and expansion (1.6 ± 0.1 fold in Ad5Δ24.RANTES.IL-15 vs. 0.71 ± 0.07 fold in mock; P=0.01, and vs. 0.55 ± 0.04 fold in Ad5Δ24; P=0.005) of GD2.CAR-T cells in vitro (Fig.4C and 4D) without impairing the oncolytic property of the virus (Fig. 4E). Thus NB cells infected with Ad5Δ24.RANTES.IL-15 produce functional levels of both proteins.
Figure 4. Ad5Δ24.RANTES.IL-15 has oncolytic effects in NB cells and release both RANTES and IL-15.
(A) Schematic representation of Ad5Δ24 encoding RANTES and IL-15. (B) RANTES and IL-15 were detected in the culture supernatant collected from infected NB cells (CHLA-255, IMR-32 and SKNLP). Data represent mean ± SD of 3 independent experiments. (C) The migration of GD2.CAR-T cells was evaluated toward the culture supernatant collected from control and infected SKNLP cells using a transwell migration assay. Data represent mean ± SEM of 4 different T-cell preparations. *P < 0.05; **P < 0.01 by Student’s t test. (D) GD2.CAR-T cell counts in co-culture experiments in which T cells were exposed to control or infected NB cells. T cells were enumerated after 3 days of culture. Data are presented as mean ± SEM of 4 different T-cell preparations. *P < 0.05; **P < 0.01 by Student’s t test. (E) Lytic activity of the viruses in NB cells and GD2.CAR-T cells as assessed by MTS assay. Data represent mean ± SD of 3 independent experiments.
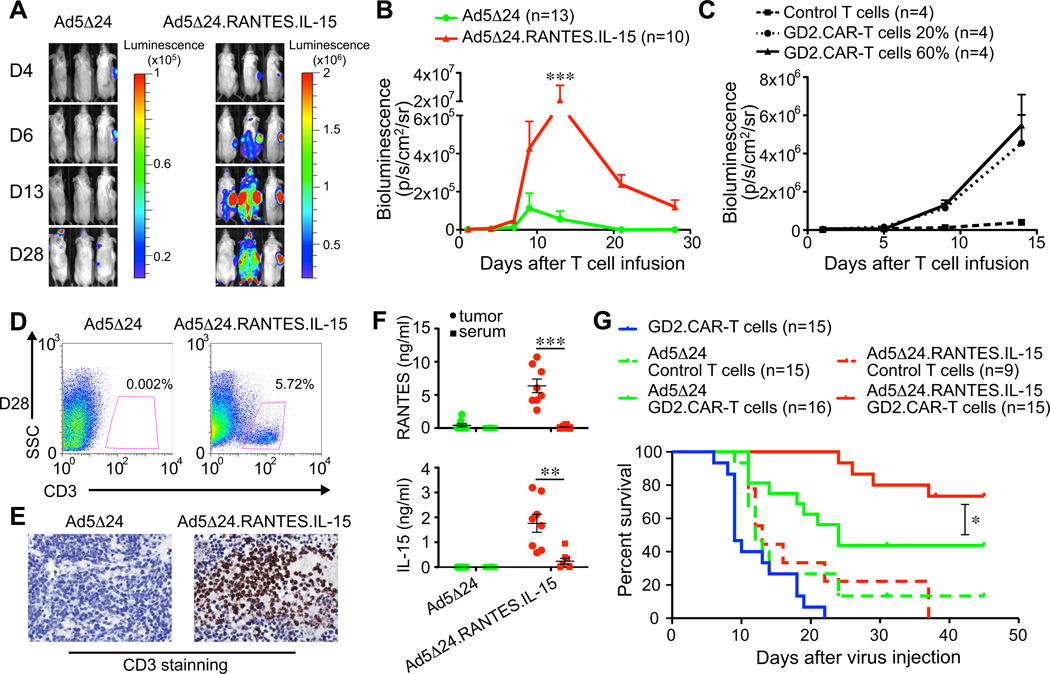
Ad5Δ24.RANTES.IL-15 enhances the persistence of GD2.CAR-T cells in vivo
Mice engrafted with CHLA-255 cells were inoculated i.t. with either Ad5Δ24 or Ad5Δ24.RANTES.IL-15 (106 – 108 vp) followed by one single i.v. infusion of FFluc-labeled GD2.CAR-T cells (107 cells). As shown in Fig.5A and 5B, bioluminescence signals of GD2.CAR-T cells were higher at the site of tumors inoculated with Ad5Δ24.RANTES.IL-15 than in tumors inoculated with unmodified Ad5Δ24 (2.1 × 107 ± 1.0 × 107 p/s/cm2/sr vs. 5.6 × 105 ± 4.1 × 105 p/s/cm2/sr at day 12, P < 0.001). RANTES and IL-15 secretion by Ad5Δ24.RANTES.IL-15 infected tumor did not promote accumulation of control T cells lacking CAR expression, indicating that antigen-specificity of T cells is also necessary for T-cell persistence at the tumor site (Fig. 5C). The increase in photon intensity corresponded to an increased numeric infiltration of human T cells within the tumor as demonstrated by both flow cytometry and immunohistochemistry (Fig.5D and 5E). Quantification of RANTES and IL-15 in the sera and tumor biopsies confirmed these two factors were produced only in mice inoculated with Ad5Δ24.RANTES.IL-15 (Fig. 5F). Moreover, both RANTES and IL-15 were predominantly detected at the tumor site (5890 ± 1055 pg/ml and 1762 ± 360 pg/ml, respectively) rather than in serum (111 ± 46 pg/ml and 357 ± 162 pg/ml, respectively) (RANTES; P < 0.001 and IL-15; P < 0.01) (Fig. 5F). Quantification of other human and mouse cytokines in serum by a Milliplex assays as indicated in the “Material and Method section” demonstrated no significant modifications except for an equal elevation of mouse granulocyte-colony stimulating factor in both Ad5Δ24 and Ad5Δ24.RANTES.IL-15 treated mouse. Combination therapy with GD2.CAR-T cells and Ad5Δ24.RANTES.IL-15 significantly enhanced the survival of mice compared with GD2.CAR-T cells and Ad5Δ24 (73% vs. 44% at day 45, P=0.03) (Fig. 5G). Finally, we found that the intratumor inoculation of the Ad5Δ24.RANTES.IL-15 is essential to sustain the persistence of CAR-T cells. When mice were indeed engrafted with tumor cells in both flanks, CAR-T cells significantly persisted and promoted antitumor activity only at the virus-inoculated tumor site (Supplementary Fig. 4).
Figure 5. Ad5Δ24.RANTES.IL-15 improves persistence of GD2.CAR-T cells.
(A) and (B) NSG mice engrafted s.c. with CHLA-255 cells were inoculated i.t. with OVs (106–108 vp) by day 10 – 14. Four days later mice were infused i.v. with FFluc labeled GD2.CAR-T cells. T-cell bioluminescence was then measured. Data represent mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001 by Student’s t test. (C) NSG mice engrafted s.c. with CHLA-255 cells were inoculated i.t. with OVs (108 vp) by day 10. Four days later mice were infused i.v. with FFluc labeled control T cells or FFluc labeled GD2.CAR-T cells diluted with control T cells at 6:10 or 2:10 ratios. T-cell bioluminescence was then measured. Data represent mean ± SEM. (D) and (E) T cells infiltrating the tumors were assessed by FACS and immunohistochemistry. (F) Detection of RANTES and IL-15 by ELISA in serum and tumor homogenates collected from mice 14 – 18 days after inoculations of OVs. Data represent mean ± SEM in 8 mice for each virus. **P < 0.01; ***P < 0.001 by Student’s t test. (G) Survival curves of NSG mice bearing CHLA-255 cells and treated with single and combined agents. *P < 0.05 by log-rank test.
We also constructed Ad5Δ24 encoding either RANTES alone (Ad5Δ24.RANTES) or IL-15 alone (Ad5Δ24.IL-15) to determine the specific role of each single component (Fig. 6A). Both viruses were functional since infected NB cells produced either RANTES or IL-15 in vitro (Fig. 6B), and both viruses retained oncolytic activity (Fig. 6C). When tested in vivo, GD2.CAR-T cell persistence was superior in mice that received Ad5Δ24.RANTES.IL-15 (1.9 × 107 ± 0.9 × 107 p/s/cm2/sr) compared to those that received either Ad5Δ24.RANTES (1.0 × 106 ± 0.2 × 106 p/s/cm2/sr, P < 0.01) or Ad5Δ24.IL-15 (3.9 × 106 ± 2.8 × 105 p/s/cm2/sr, P < 0.05) (Fig.6D and 6E). Thus the combination of i.t. injection of Ad5Δ24.RANTES.IL-15 and i.v. infusion of GD2.CAR-T cells produces significant improvements in the control of tumor growth.
Figure 6. Ad5Δ24.RANTES.IL-15 is superior to Ad5Δ24.RANTES and Ad5Δ24.IL-15 in vivo.
(A) Schematic representation of Ad5Δ24.RANTES and Ad5Δ24.IL-15. (B) Measurement of RANTES and IL-15 in culture supernatant collected from control and infected NB cells. Data represent mean ± SD of 3 independent experiments. (C) Lytic activity of the viruses in NB cells and GD2.CAR-T cells as assessed by MTS assay. Data represent mean ± SD of 3 independent experiments. (D) and (E) Bioluminescence of GD2.CAR-T cells in tumor bearing NSG mice inoculated i.t. with viruses (106 – 108 vp). Data represent mean ± SEM. *P < 0.05 by Student’s t test. †mice euthanized.
Discussion
We have demonstrated that the Ad5Δ24 armed with RANTES and IL-15 increases the number of T cells infiltrating a solid tumor, likely through the enhanced trafficking and prolongation of T-cell survival that these agents respectively induce. These effects were achieved without compromising the intrinsic lytic activity of the OV or CAR-T cells, and the combination of both agents resulted in better control of the tumor growth and prolonged survival of tumor bearing animals.
The rationale for combining OVs and CAR-T cells for the treatment of solid tumors stems from two main experimental evidences. First, Ad5Δ24 engineered to accommodate genes supporting T-cell function remain toxic to tumor cells without damaging or compromising CAR-T cell activities, even when the viruses were used at high concentrations. Second, Ad5Δ24-infected NB cells become more susceptible to the lytic effects of CAR-T cells. It was previously described that oncolytic adenoviruses induce activation of caspase-3 in infected tumor cells(34). Here we support this observation and demonstrated that Ad5Δ24 further accelerates and amplifies the occurrence of caspase-induced cell death of NB cells mediated by CAR-T cells. In turn, the faster lysis of tumor cells promoted by CAR-T cells may facilitate the spread of the virus, which in solid tumors is usually limited to the surrounding area of the virus inoculation(35). As a consequence, when unmodified Ad5Δ24 and CAR-T cells are combined better control of the NB tumor growth is observed in animals.
Despite the superior cytolytic activity observed with the combined treatment described above, we found that the overall infiltration and persistence of CAR-T cells at the tumor site was not robustly improved, as Ad5Δ24-infected NB cells did not release factors that promote T-cell migration and survival. Even though the GD2-specific CAR we used is engineered to co-express two costimulatory molecules such as CD28 and OX40(16, 22), this combination is evidently insufficient to sustain the persistence of the few CAR-T cells that reach the tumor microenvironment. These limitations strongly support our strategy to engineer OV to produce not only a T-cell chemoattractant factor, but also a T-cell growth factor.
For optimized T-cell trafficking and survival, we selected the chemokine RANTES and the cytokine IL-15. While we and others have previously focused on identifying chemokines specifically produced by tumor cells and on engineering CAR-T cells with the cognate receptor(20–22), here we selected RANTES as a broadly applicable chemokine because its receptors CCR1, CCR3, and CCR5 are retained by ex vivo expanded T cells(22, 36). This assures that upon adoptive transfer T cells will migrate to multiple types of tumors if they are forced to release RANTES. We selected IL-15 as a T-cell growth factor because, in addition to its multiple beneficial effects on T cells(19, 37), this cytokine is not produced by CAR-T cells upon activation and thus the OV provides a growth factor that is generally absent in the tumor environment(25). In addition, we previously demonstrated that IL-15 is preferentially used by effector T cells rather than Tregs(19) in the unfortunate event that RANTES also attracts this cell subset (38). We found that only Ad5Δ24 co-expressing RANTES and IL-15 produced substantive increases in CAR-T cell numbers at tumor sites, which lead to improved tumor control in vivo. Of note, the detection of RANTES and IL-15 in our model was mostly confined to the tumor site indicating a preferential local expression of both factors and thereby circumventing the toxicities associated with systemic administration of cytokines(39). To further potentiate the safety of this strategy for the clinical application, antiviral agents, such as cidofovir, or the inclusion of the thymidine kinase gene of the human herpes virus type I can be used to abort any potential toxicity of OVs (40, 41).
Although the immunodeficient mouse model we have used has limits in assessing the effect of the inhibitory components of the immune system such as regulatory T cells, tumor associated macrophages and myeloid derived suppressor cells, it is nevertheless relevant for a clinical translation. It demonstrates indeed that the infection of tumor cells with RANTES- and IL-15-engineered OV is sufficient to enhance the overall antitumor activity of CAR-T cells generated following the manufacturing procedures currently applied for the production of CAR-T cells for clinical use(33). There is also additional evidence that tumors infected with OVs can trigger danger signals by dendritic cells and promote cross-antigen presentation, ultimately leading to the elicitation of innate and adaptive immune responses(42). Thus, the positive effects of armed OVs observed in our own and other models(36, 43) may be further amplified in patients due to the recruitment of other components of the immune system.
In our model we selected to administer the OV intratumorally, since this is the preferential route of infusion of oncolytic adenoviruses in clinical trials. We found that the local presence of the virus is essential in supporting the persistence of CAR-T cells. Thus to be effective for metastatic tumors, our proposed combined approach will likely require the use of OVs that can be administered systemically, such as vaccinia viruses (30, 44).
In conclusion, our preclinical studies have shown increased antitumor efficacy of combining CAR-T cell and Ad5Δ24 armed with RANTES and IL-15 to improve migration and persistence of the infused T cells. Our data support further exploration of this platform of biological agents for therapy of solid tumors.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Malcolm K. Brenner for the critical revision of the manuscript.
Grant support: This work was supported in part by R01 CA142636 National Institute of Health-NCI, W81XWH-10-10425 Department of Defense and Technology/Therapeutic Development Award and NIH/NCI P01 CA094237. NN was supported by Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation, Japan Society for the promotion of Science.
Footnotes
Conflicts of interest: The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene and Bluebird bio.
Author contributions: N.N., G.D. designed the research; N.N., I.D., V.C., I.C., V.H., L.B.H., B.S., and G.D. performed research experiments; N.N. and G.D. analyzed the data; H.L. supervised the statistical analysis; B.S. supervised the in vivo experiments. N.N. and G.D. wrote the manuscript. All the authors reviewed and approved the manuscript.
References
- 1.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz CR, Micklethwaite KP, Savoldo B, Ramos CA, Lam S, Ku S, et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–912. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 6.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarvaiya PJ, Guo D, Ulasov I, Gabikian P, Lesniak MS. Chemokines in tumor progression and metastasis. Oncotarget. 2013;4:2171–2185. doi: 10.18632/oncotarget.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 2011;475:226–230. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 9.Yakirevich E, Resnick MB. Regulatory T lymphocytes: pivotal components of the host antitumor response. J Clin Oncol. 2007;25:2506–2508. doi: 10.1200/JCO.2007.11.3191. [DOI] [PubMed] [Google Scholar]
- 10.Mapara MY, Sykes M. Tolerance and cancer: mechanisms of tumor evasion and strategies for breaking tolerance. J Clin Oncol. 2004;22:1136–1151. doi: 10.1200/JCO.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 11.Teicher BA. Malignant cells, directors of the malignant process: role of transforming growth factor-beta. Cancer Metastasis Rev. 2001;20:133–143. doi: 10.1023/a:1013177011767. [DOI] [PubMed] [Google Scholar]
- 12.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 13.Mumprecht S, Schurch C, Schwaller J, Solenthaler M, Ochsenbein AF. Programmed death 1 signaling on chronic myeloid leukemia-specific T cells results in T-cell exhaustion and disease progression. Blood. 2009;114:1528–1536. doi: 10.1182/blood-2008-09-179697. [DOI] [PubMed] [Google Scholar]
- 14.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 15.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood. 2006;108:3890–3897. doi: 10.1182/blood-2006-04-017061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pule MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui CH, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia. 2004;18:676–684. doi: 10.1038/sj.leu.2403302. [DOI] [PubMed] [Google Scholar]
- 18.Li XC, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek TR, et al. IL-15 and IL-2: a matter of life and death for T cells in vivo. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 19.Perna SK, De Angelis B, Pagliara D, Hasan ST, Zhang L, Mahendravada A, et al. Interleukin 15 provides relief to CTLs from regulatory T cell-mediated inhibition: implications for adoptive T cell-based therapies for lymphoma. Clin Cancer Res. 2013;19:106–117. doi: 10.1158/1078-0432.CCR-12-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 21.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99:3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 24.Dotti G, Savoldo B, Pule M, Straathof KC, Biagi E, Yvon E, et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood. 2005;105:4677–4684. doi: 10.1182/blood-2004-08-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–1170. doi: 10.1038/leu.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vera JF, Hoyos V, Savoldo B, Quintarelli C, Giordano Attianese GM, Leen AM, et al. Genetic manipulation of tumor-specific cytotoxic T lymphocytes to restore responsiveness to IL-7. Mol Ther. 2009;17:880–888. doi: 10.1038/mt.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–976. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 30.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG, et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther. 2003;8:449–458. doi: 10.1016/s1525-0016(03)00200-4. [DOI] [PubMed] [Google Scholar]
- 32.Darzynkiewicz Z, Pozarowski P, Lee BW, Johnson GL. Fluorochrome-labeled inhibitors of caspases: convenient in vitro and in vivo markers of apoptotic cells for cytometric analysis. Methods Mol Biol. 2011;682:103–114. doi: 10.1007/978-1-60327-409-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121:1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72:2327–2338. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 35.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, O'Malley M, Urban J, Sampath P, Guo ZS, Kalinski P, et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol Ther. 2011;19:650–657. doi: 10.1038/mt.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klebanoff CA, Finkelstein SE, Surman DR, Lichtman MK, Gattinoni L, Theoret MR, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tan MC, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182:1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaconu I, Cerullo V, Escutenaire S, Kanerva A, Bauerschmitz GJ, Hernandez-Alcoceba R, et al. Human adenovirus replication in immunocompetent Syrian hamsters can be attenuated with chlorpromazine or cidofovir. J Gene Med. 2010;12:435–445. doi: 10.1002/jgm.1453. [DOI] [PubMed] [Google Scholar]
- 41.Abate-Daga D, Andreu N, Camacho-Sanchez J, Alemany R, Herance R, Millan O, et al. Oncolytic adenoviruses armed with thymidine kinase can be traced by PET imaging and show potent antitumoural effects by ganciclovir dosing. PLoS One. 2011;6:e26142. doi: 10.1371/journal.pone.0026142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endo Y, Sakai R, Ouchi M, Onimatsu H, Hioki M, Kagawa S, et al. Virus-mediated oncolysis induces danger signal and stimulates cytotoxic T-lymphocyte activity via proteasome activator upregulation. Oncogene. 2008;27:2375–2381. doi: 10.1038/sj.onc.1210884. [DOI] [PubMed] [Google Scholar]
- 43.Stephenson KB, Barra NG, Davies E, Ashkar AA, Lichty BD. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 2012;19:238–246. doi: 10.1038/cgt.2011.81. [DOI] [PubMed] [Google Scholar]
- 44.Breitbach CJ, Burke J, Jonker D, Stephenson J, Haas AR, Chow LQ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.