Abstract
Aims
Simultaneous positron emission tomography/MRI has recently been introduced to the clinic and dual positron emission tomography/MRI probes are rare and of growing interest. We have developed a strategy for producing multimodal probes based on a carbon nanotube platform without the use of chelating ligands.
Materials & methods
Gd3+ and 64Cu2+ ions were loaded into ultra-short single-walled carbon nanotubes by sonication. Normal, tumor-free athymic nude mice were injected intravenously with the probe and imaged over 48 h.
Results & conclusion
The probe was stable for up to 24 h when challenged with phosphate-buffered saline and mouse serum. Positron emission tomography imaging also confirmed the stability of the probe in vivo for up to 48 h. The probe was quickly cleared from circulation, with enhanced accumulation in the lungs. Stable encapsulation of contrast agents within ultra-short single-walled carbon nanotubes represents a new strategy for the design of advanced imaging probes with variable multimodal imaging capabilities.
Keywords: carbon nanotube, contrast agent, MRI, multimodal imaging, positron emission tomography
Recent studies have demonstrated the continued progress in the development of nanoparticle-based systems for medical applications [1–3] . Among these, single-walled carbon nanotubes (SWCNTs) are of special interest due to their unique combination of notable physical and chemical properties such as high tensile strength, high aspect ratio, electrical conductivity, limited chemical reactivity and near-infrared fluorescence [4,5]. Importantly, SWCNTs have several advantages over other nanoparticle delivery systems, which make them uniquely suited for delivery of medical imaging agents. For example, unlike fullerenes, nanotubes may be filled postsynthesis, allowing for significant reduction in cost and time during preparation of the probe. Carbon nanotubes also allow intracellular delivery of imaging agents due to their ability to cross the cell membrane [6].
A key attribute of SWCNTs involves the potential to use their interior space for encapsulation of small molecules or ions [7, 8] . Encapsulation offers better protection from transmetallation than metal chelation and also avoids the necessity of developing new ligands for different imaging agents and applications. For example, there are nine different commercialized gadolinium-based contrast agents (CAs) for MRI [9]. Since stable encapsulation shields the payload from biological interaction with surrounding tissue, large quantities of payload can theoretically be administered without systemic toxicities or other unwanted interactions. This structural property renders SWCNTs as a potential delivery vehicle for medical imaging agents that are otherwise toxic, such as heavy metal ions or radionuclides. Furthermore, the large, external surface area of SWCNT materials can also be used as a scaffold to which targeting or therapeutic moieties may be attached [10–12], allowing for targeted delivery of imaging agents and their theranostic counterparts. Compared with liposomes, which are actively used for delivery of therapeutics [13], nanotubes are better able to retain encapsulated materials, making them more effective for imaging applications in which the delivered materials should not be released.
Imaging of SWCNTs can be achieved by exploiting the intrinsic properties of the nanotube by using Raman spectroscopy, near-infrared fluorescence spectroscopy or photoacoustic tomography [14] . However, such techniques may have limited signal penetration in vivo and lack the widespread clinical utility observed with more conventional diagnostic imaging modalities. Thus far, there have been only a few reports of SWCNT-based probes for clinically relevant imaging modalities with full-body penetration such as computed tomography (CT), single-photon emission CT, positron emission tomography (PET) and MRI [15–17] . Both T1- and T2-weighted SWCNT-based magnetic resonance (MR) CAs have been prepared, frequently through attachment of chelated Gd3+ or iron oxide on the external surface of the SWCNT material [18, 19] . Radionuclides have similarly been attached to the SWCNT exterior through the use of chelating ligands [15,16]. There is also one report of molten-phase filling of SWCNTs with a radionuclide [20], but the extensive procedure required for preparation of the probe is likely prohibitive for clinical applications.
In this work, we demonstrate the synthesis of a bimodal SWCNT-based probe for PET/MRI by stable encapsulation of imaging agents (Gd3+ and 64Cu) within ultra-short SWCNTs (US-tubes, Figure 1C). Therefore, the present study explores whether encapsulation of metal-ion-based imaging agents can offer a viable alternative to metal-ion chelation chemistry in medical imaging technology. A probe of SWCNT design (a US-tube) was chosen for these initial studies to take advantage of the fact that Gd3+-ion-loaded US-tubes (gadonanotubes [GNTs]) are the highest-performing T1-weighted MRI CA material known, with a R1 relaxivity up to 40-times greater than current clinical agents such as Magnevist® (Bayer AG [Leverkusen, Germany]; gadopentetate dimeglumine; R1: 3.4 mM−1 s−1) [21, 2 2]. For this reason, MRI CAs and other multimodal imaging agents that incorporate a GNT structural element would be highly desirable in the clinic. Furthermore, a successful encapsulation strategy as opposed to chelation chemistry would allow for production of imaging agents with any or all whole-body imaging modalities in a single chemical entity.
Figure 1. Production of the bimodal 64Cu2+/Gd3+ imaging probe.
(A) Full-length single-walled carbon nanotubes are (B) fluorinated, pyrolyzed under argon and purified with concentrated HCI to produce (C) ultra-short single-walled carbon nanotubes with sidewall defect sites. Gd3+ and 64Cu2+ are then internally coloaded by aqueous sonication to produce (D)64Cu@GNTs. (E) Excess Gd3+ and 64Cu2+ are removed by serum challenge and washing. (F) Purified 64Cu@GNTs are suspended in 0.1% Tween® 20 (Aurion [Wageningen, The Netherlands]) to produce surfactant-wrapped 64Cu@GNTs for in vivo experiments.
64Cu@GNT: Ultra-short single-walled carbon nanotube filled with 64Cu2+ and Gd3+.
To our knowledge, the present work is the first report of a SWCNT-based bimodal PET/MR probe of any nature. Since clinical instrumentation for simultaneous PET/MRI has only recently been developed [23], only a few probes utilizing both modalities are known [24, 25]. As such, production of new bimodal PET/MR probes where maximized sensitivity (PET) and resolution (MRI) in medical imaging are simultaneously achieved is currently of special interest.
Materials & methods
General procedures
All reagents and solvents were analytical grade, purchased from commercial suppliers, and used without further purification unless otherwise stated. 64Cu was produced by Washington University School of Medicine (MO, USA) in 0.1 M HCl 0.1 M NaOAc (pH 6) for labeling reactions.
Preparation of US-tubes
Full-length SWCNTs (Carbon Solutions Inc. [CA, USA]) produced by the electric arc discharge method (Figure 1A) were used to prepare US-tubes by fluorination and pyrolysis (Figure 1B), as previously reported [26]. After preparation of the US-tubes, they were purified of amorphous carbon and metal impurities by sonication with concentrated HCl. The resulting purified US-tubes were then activated for internal ion loading by a brief treatment with nitric acid (6 M; 15 min reflux). The US-tubes were then reduced using Na0 (sodium metal) in tetrahydrofuran to further debundle them into individual US-tubes (Figure 1C) [27].
Preparation of Cu@US-tubes
US-tubes (15 mg) were bath sonicated in a solution of aqueous CuCl2 (10 ml; 5 mM) for 60 min. The Cu@ US-tube product was isolated by filtration with a coarse glass filter frit and washed with deionized water until the filtrate showed no detectable levels of Cu2+, as determined by inductively coupled plasma optical emission spectroscopy (ICP-OES; limit of detection: 0.01 ppm).
Preparation of US-tubes filled with Cu2+ & Gd3+
US-tubes (15 mg) were sonicated in an aqueous solution of CuCl2 and GdCl3 (10 ml 2.5 mM CuCl2, 2.5 mM GdCl3; or 10 ml, 1 mM CuCl2 4 mM GdCl3) and bath sonicated for 60 min. NaOH (1 M; 0.6 ml) was added to adjust to neutral pH to trap the Cu2+ ions within the Gd3+ clusters within the tubes. The US-tubes filled with Cu2+ and Gd3+ (Cu@GNT) product was isolated by filtration with a coarse glass filter frit and washed with deionized water until the filtrate showed no detectable levels of Cu2+ or Gd3+, as determined by ICP-OES.
Particle size & charge measurements
Empty US-tubes and Cu@GNTs were dispersed in 0 . 1 % Tween® 20 (Aurion [Wageningen, The Netherlands]) and particle size and zeta-potential were determined using a Malvern Instruments™ (Worcestershire, UK) Zen 3600 Zetasizer®.
Preparation of US-tubes filled with 64Cu2+ & Gd3+
US-tubes filled with 64Cu2+ and Gd3+ (64Cu@GNTs) production employed the same procedures as nonradioactive Cu2+ and was accomplished by adding GdCl3 (0.5 ml; 1 mM) and 64CuCl2 (25 µl; 155.4 MBq) to a scintillation vial containing US-tubes (2.2 mg). After 30 min of bath sonication, NaOH was added to reach neutral pH. The product was isolated by ultrafiltration (Amicon® Ultra [Sigma-Aldrich; MO, USA]; 10 kD molecular weight cut-off) to remove nonencapsulated metal ions, then washed three times with deionized water (Figure 1D). Radiochemical purity was assessed by radio-thin-layer chromatography with Whatman® chromatography paper (Sigma-Aldrich) and 0.1 M ammonium acetate/0.05 M EDTA (pH 6) as the mobile phase and analyzed with an AR-2000 scanner purchased from Bioscan (Washington DC, USA).
Removal of nonspecifically bound 64Cu2+ from 64Cu@GNTs & aqueous suspension of 64Cu@GNTs
After preparation, purified 64Cu@GNT was dispersed in 0.1% Tween 20 in deionized water by brief sonication to yield surfactant-wrapped 64Cu@GNT. Nonspecifically bound 64Cu2+ was removed by incubation with 10 µM EDTA (40°C; 15 min) followed by incubation in 50% mouse serum (37°C; 15 min; Figure 1E). The product was isolated by ultrafiltration and washed with deionized water until the filtrate had minimal activity. The product was resuspended in 0.1% Tween 20 in phosphate-buffered saline (PBS) for the in vivo experiments (Figure 1F).
Stability studies
In vitro stability studies were performed on dry samples of Cu@US-tubes and Cu@GNTs to simulate in vivo pH, salinity and serum interaction in order to determine the retention properties of the US-tubes. Samples were incubated in 50% (w/v) bovine serum albumin (BSA) and PBS at 37°C for 12 h, 24 h or 7 days. At each time-point, samples were purified by filtration on a coarse fritted glass filter. The recovered sample and the filtrate were analyzed for Cu2+ and Gd3+ by ICP-OES. Water, PBS and BSA used in the experiment were also analyzed for Cu2+ and Gd3+ for determination of background corrections. For the 64Cu@GNTs, 50% mouse serum was used instead of BSA with an incubation period of 24 h at 40°C in order to provide better biocompatibility for in vivo studies. Ultrafiltration was performed in 100 kD molecular weight cut-off centrifuge filters. All experiments were performed in triplicate.
Bimodal imaging of an aqueous suspension of 64Cu@GNTs
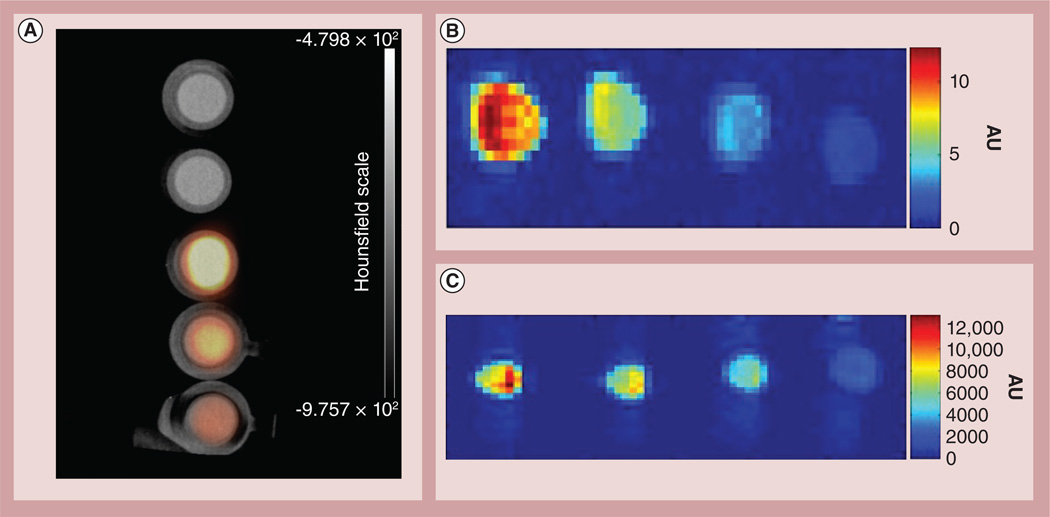
Images were acquired on a Siemens (TN, USA) Inveon™ MicroPET/CT scanner using the instrument parameters previously reported [28]. Phantoms were prepared at concentrations of 1.85, 3.7 and 7.4 MBq/ml with control wells containing water and Tween 20, and PET imaging was performed for 20 min (Figure 2A). The radioactivity of surfactant-wrapped 64Cu@GNT was allowed to decay sufficiently (ten half-lives) and phantom MRIs were obtained on Philips (Amsterdam, The Netherlands) Achieva™ 1.5 T and 3 T systems. An inversion recovery sequence was used for image acquisition (repetition time: 6500 ms; echo time: 10 ms at 1.5 T; repetition time: 9000 ms, echo time: 20 ms at 3 T; Figures 2B & 2C).
Figure 2. Phantom images of purified US-tubes filled with 64Cu2+ and Gd3+ obtained by positron emission tomography/computed tomography and T1-weighted MRI.
(A) Micro PET/CT image of purified US-tubes filled with 64Cu2+ and Gd3+ at varying concentrations. From top to bottom: Milli-Q® water (Millipore Corp. [MA, USA]), 1% Tween® 20 (Aurion [Wageningen, The Netherlands]), 7.4 MBq/ml, 3.7 MBq/ml and 1.85 MBq/ml. (B) MRI of purified US-tubes filled with 64Cu2+ and Gd3+ at varying concentrations for a 1.5-T field strength. From left to right: 7.4 MBq/ml, 3.7 MBq/ml, 1.85 MBq/ml and 1% Tween 20. (C) MRI of purified US-tubes filled with 64Cu2+ and Gd3+ at varying concentrations for a 3.0-T field strength. From left to right: 7.4 MBq/ml, 3.7 MBq/ml, 1.85 MBq/ml, and 1% Tween 20.
CT: Computed tomography; PET: Positron emission tomography.
In vivo PET/CT imaging
In vivo imaging studies were carried out in accordance with the standards of the University of Texas Health Science Center at Houston (USA), Center for Molecular Imaging, after review and approval of the protocol by respective Institutional Animal Care and Use or Animal Welfare Committees. Female athymic nude mice (3–4 week old; 18–22 g; Harlan Sprague– Dawley Inc. [IN, USA]) were housed five per cage in a pathogen-free mouse colony and provided with sterilized pellet chow and sterilized water. Initially, the mice were anesthetized with 1% isoflurane and a CT scan was performed. The bed position was then moved into the PET field-of-view where the mice received 7.4–11.1 MBq of surfactant-wrapped 64Cu@GNTs or free 64Cu2+ through the tail vein (n = 3 per group). Dynamic PET imaging was performed and region-of-interest analysis was used to generate time activity curves (TACs) for the liver, kidneys, heart, lung, muscle and bladder according to prior methods [29]. Static PET/CT images were acquired at 4, 24 and 48 h postinjection (p.i.) for each mouse and processed with the vendor software package (Inveon™ Research Workplace [Siemens]) to quantify tracer uptake in selected organs.
Results
Preliminary studies
Studies to determine the viability of encapsulating 64Cu2+ within US-tubes were successful. Nonradioactive CuCl2 was loaded by aqueous sonication, yielding the Cu@US-tube product, which was determined to contain 2.05% (w/w) Cu2+ by ICP-OES. However, challenge with PBS or BSA resulted in significant Cu2+ leakage (>45%) within 12 h. Simultaneous loading of Cu2+ and Gd3+ was attempted using Cu2+:Gd3+ ratios of 1:1 and 1:4 and analysis of the Cu@GNT products by ICP-OES indicated Cu2+ contents of 0.7% (w/w) and 0.4% (w/w), respectively. Challenge with PBS or BSA over a 7-day period resulted in no detectable Cu2+ or Gd3+ leakage. T1 relaxivitiy values for the Cu@GNT product and GNTs produced from the same batch of US-tubes were determined to be 52.7 and 53.4 mM−1s−1, respectively. Particle size analysis by dynamic light scattering indicated Cu@GNTs are slightly larger than empty US-tubes, with an average diameter of 98 nm compared with 70 nm for empty US-tubes. Zeta-potentials for suspensions of Cu@GNTs and empty US-tubes in 0.1% Tween 20 were 23.7 and 24.6 mV, respectively, indicating stable suspensions of the probe.
Synthesis & characterization of bimodal probe
Synthesis of the bimodal probe was accomplished by replacing the nonradioactive CuCl2 with 64CuCl2. After washing, the product was determined to be radiochemically pure by radio-thin-layer chromatography with a specific activity of 19.69 MBq/mg. 64Cu2+ labeling was found to be stable to PBS challenge over 24 h at room temperature. However, a 37% loss in activity was observed after 24 h exposure to 50% mouse serum. Further exposure of the sample to fresh serum at room temperature for an additional 24 h did not result in additional loss of 64Cu2+, indicating that only a limited population of 64Cu2+ can be removed by serum challenge. Phantom images of the probe indicated both PET and MRI contrast separately (Figure 2).
In vivo imaging
Dynamic imaging of mice receiving surfactant-wrapped 64Cu@GNTs or free 64Cu2+ resulted in the generation of TACs for the heart, lungs, liver, kidneys, bladder and muscle. The major site of accumulation of surfactant-wrapped 64Cu@GNTs was in the lungs with a peak value of 21.4 % injected dose (ID)/g at 2-min p.i. and persistent signal that was significantly higher (p< 0.001) than the control group throughout the scan (Figure3A). Heart uptake was also higher in mice injected with surfactant-wrapped 64Cu@GNTs, indicating prolonged blood pool activity of the bimodal agent compared with free 64Cu2+ (Figure 3B). TAC profiles for liver, kidneys and muscle revealed no significant differences between agent distribution in these organs (Figures 3C–3E). Bladder signal was approximately threefold higher in mice injected with free 64Cu2+, suggesting increased renal clearance of this agent compared with surfactant-wrapped 64Cu@GNTs, although significance was not achieved (data not shown).
Figure 3. Time-activity curves generated from dynamic positron emission tomograpy imaging of mice receiving surfactant-wrapped US-tubes filled with 64Cu2+ and Gd3+ and free 64Cu2+.
(A) Lungs, (B) heart, (C), liver, (D) kidneys and (E) muscle. The histograms show quantification of agent uptake as % injected dose/g tissue (n = 3). Error bars represent mean ± SD.
*p ≤ 0.001.
**p ≤ 0.01.
64Cu@GNT: Ultra-short single-walled carbon nanotube filled with 64Cu2+ and Gd3+.
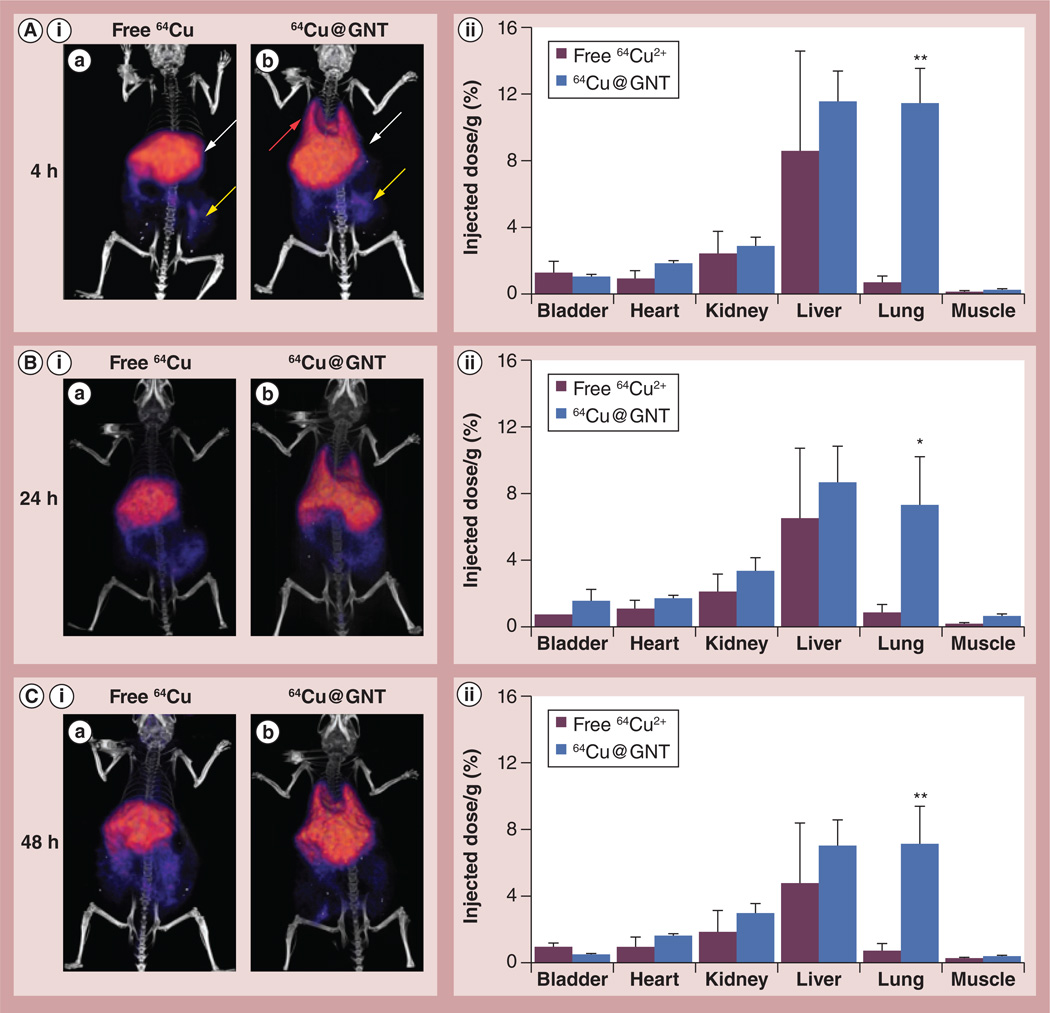
Delayed PET/CT imaging results correlated with the dynamic imaging data. Scans at 4 h p.i. revealed accumulation of surfactant-wrapped 64Cu@GN Ts primarily in the liver (11.4 ± 1.9% ID/g tissue) and lungs (11.2 ± 2.2% ID/g tissue) with minor uptake in the kidneys and GI tract, whereas free 64Cu2+ showed strong liver signal and minor gastrointestinal uptake (Figure 4A). Region-of-interest values from the heart were no longer significant in mice injected with surfactant-wrapped 64Cu@GNTs as a result of continued clearance of the agent from systemic circulation. No notable differences in kidney and muscle uptake were observed between the groups. At 24 h, both groups continued to exhibit high liver signal, while the mice injected with surfactant-wrapped 64Cu@GNTs also showed persistent lung uptake that was significantly higher than in control mice (p< 0.05; Figure 4B). Further imaging at 48 h revealed no significant changes in tissue distribution (Figure4C). Elimination of both agents either by hepatobiliary or renal routes appeared to be slow according to the small change in % ID per gram values of the liver, kidneys and bladder from 4–48 h p.i.
Figure 4. Longitudinal micro-positron emission tomography/computed tomography imaging of normal mice injected intravenously with surfactant-wrapped US-tubes filled with 64Cu2+ and Gd3+ or free 64Cu2+.
Representative images are shown from acquisitions at (A,i) 4, (B,i) 24 and (C,i) 48 h postinjection of radiotracers. (A,ii–C,ii) Histograms show quantification of agent uptake as % injected dose/g tissue (n = 3). (A,ia) The arrows highlight the presence of 64Cu in the liver (white arrow) and GI tract (yellow arrow) for free 64Cu, (A,ib) and in the lungs (red arrow), liver and GI tract for US-tubes filled with 64Cu2+ and Gd3+. Error bars represent mean ± SD.
*p ≤ 0.05.
**p ≤ 0.01.
64Cu@GNT: Ultra-short single-walled carbon nanotube filled with 64Cu2+ and Gd3+.
Discussion
Our choice of SWCNTs as a platform for developing a bimodal PET/MR probe is based on several factors. First, we aimed to take advantage of the capability of SWNCTs to encapsulate materials for imaging or therapy [7]. Although methods exist for attaching radionuclides to the outer surface of carbon nanotubes [10], functionalization is achieved through chelators such as 1,4,7,10-tetra-azacyclododecane-1,4,7,10-tetraacetic acid, which may still permit loss of label through transmetallation [30]. Furthermore, many nanoparticle systems may be surface functionalized, but few afford the option of encapsulation, which offers better protection from biological interaction. Second, although there is much debate about the toxicity of carbon nanotube materials, the US-tubes used in this work are known to be well tolerated both in vitro and in vivo at doses up to 0.5 g/kg [31,32]. Finally, previous work has shown that US-tubes, which are produced by pyrolysis of fluorinated SWCNTs [26], can be internally loaded with aqueous solutions of Gd3+. The resulting material, Gd@US-tubes or GNTs, has a remarkably high T1-weighted relaxivity, which is up to 40-times greater than clinically available Gd3+-based CAs under magnetic fields of 1.5 T [21,33]. Therefore, using this platform as a starting point for the development of a bimodal probe ensures a significant MR contrast component of the probe.
Preliminary studies
We initially attempted to load aqueous Cu2+ ions into US-tubes by aqueous sonication. Loading was successful, with 2.05% (w/w) Cu2+ detected. However, upon challenge with PBS or BSA, significant Cu2+ leakage was observed within 12 h. We then attempted to trap Cu2+ within the nanotube by simultaneously loading Gd3+ and Cu2+. Although the resulting Cu2+ retention was reduced, no detectable Cu2+ or Gd3+ was found to leak over a 7-day period upon PBS or BSA challenge. This suggested that the pH-dependent hydrolysis chemistry of the Gd3+ ion is essential for retaining the Cu2+. Gd3+ can enter the US-tubes through the sidewall defects under acidic conditions, but forms insoluble µ-oxo or µ-hydroxy clusters at a physiologic pH of 7.4, rendering the ions unable to exit through the same sidewall defects through which they entered [34,35]. The retained Cu2+ is likely either incorporated into the Gd3+ clusters or simply trapped within the US-tubes by the Gd3+ clusters at the defect sites that prevent their exit.
Next, it was demonstrated that 64Cu2+ can be retained within US-tubes in quantities relevant for PET imaging. Experiments with 64Cu2+ validated the findings we observed with cold Cu2+ for which the loading protocol was optimized. PBS and mouse serum challenges were performed in preparation for in vivo experiments to examine the stability of the purified 64Cu@GNTs (Figure 1E). Although some 64Cu2+ was removed by serum challenge, the remaining population was found to be stable to further challenge. Further characterization of the bimodal probe was not attempted due to the radioactive nature of the agent and the fact that similar US-tube materials have been well studied previously [21,32,33,35].
T1-weighted relaxivity values were determined for the cold Cu@GNTs and for GNTs prepared from the same batch of US-tubes and were found to be 52.7 and 53.4 mM−1s−1, respectively. With so few Cu2+ ions present in a Cu@GNT sample, it is not surprising that the relaxivity per Gd3+ ion is not dramatically different from that of the parent GNTs. In both cases, the T1-weighted relaxivity values are still greatly superior to the most widely used clinical agent, Magnevist, which has an R1 of approximately 3.4 mM−1s−1 [22].
Phantom & in vivo imaging
In vivo biodistribution of a surfactant-suspended 64Cu@GNT sample (Figure 1F) could be investigated by PET due to the extreme sensitivity of the technique; however, in vivo MRI was found to be impractical using the protocol of these studies due to the high dose requirements of MRI (~1 mM) and the relative low solubility of the 64Cu@GNT/Tween 20 agent. New functionalization chemistry, such as that recently reported to yield a solubility of 50 mg/ml for SWCNT-based agents like US-tubes [36] will need to be developed for 64Cu@GNT to achieve the solubility necessary for in vivo MRI. Nonetheless, the phantom images in Figure 2 clearly demonstrate the bimodal imaging capacity of the 64Cu@GNT probe.
There have been several reports on the in vivo biodistribution of radiolabeled SWCNTs with surface functionalization; however, to the best of our knowledge, similar characterization of a bimodal MRI/PET SWCNT construct has yet to be performed. In our study, we developed a loading procedure with 64Cu2+ and Gd3+ that provided sufficient stability and radioactivity, and allowed us to examine agent distribution and clearance by PET. Dynamic and static imaging studies were performed in normal mice injected with surfactant-wrapped 64Cu@GNTs and free 64Cu2+ at 4, 24 and 48 h p.i., and showed similar liver uptake for both agents at each timepoint. In the case of free 64Cu2+, liver signal can be explained by chelation of the radiometal by copper-binding proteins in the liver [37], whereas the hepatic uptake of surfactant-wrapped 64Cu@GNT occurs as a result of opsonization and is consistent with other reports showing high reticuloendothelial system uptake of radiolabeled SWCNTs [15, 16] . Importantly, there was no increase in liver signal in mice injected with surfactant-wrapped 64Cu@GNTs. It should be mentioned that since both free 64Cu2+ and surfactant-wrapped 64Cu@GNTs undergo hepatic clearance, it is difficult to distinguish if the liver signal is due to nanoparticle localization, degradation of the probe, or a combination of both. However, given the data from the serum challenge experiments that suggest that encapsulated 64Cu2+ is not expected to undergo further transchelation by serum proteins after injection, and the persistence of radioactivity in other organs such as the lungs (without notable reduction even after 48 h), we concluded that surfactant-wrapped 64Cu@GNTs do not undergo significant degradation and its biodistribution profile reflects the in vivo stability of the probe.
A major difference in agent distribution was evident in the lungs where mice receiving surfactant-wrapped 64Cu@GNTs showed prominent accumulation, which was detectable at 2 min p.i. and persisted up to 48 h p.i. In the lungs, pulmonary surfactant proteins are present in the air–liquid interface of the lungs and play an important role in defending the lungs against infection. Specifically, surfactant proteins A and D, which have been shown to selectively bind to carbon nanotubes due to the presence of surface modifications, may have played a role in the high lung accumulation observed in our study [38]. Similar lung uptake has been reported by others with radiolabeled SWCNTs and attributed to the interaction of lung-localized proteins known to interact with the N-acetylglucosamine molecules on the surface of the SWCNTs [20]. Although we did not perform any chemical modifications to the surface of the SWCNTs, it is conceivable that the -OH groups of Tween 20 may have reacted similarly to the -OH groups on N-acetylglucosamine to trigger an interaction with the pulmonary surfactant proteins. The size and shape of surfactant-wrapped 64Cu@GNTs may also play a role in its capture and retention within the pulmonary beds, and ultimately contribute to the slow clearance of the agent from the body. From a therapeutic perspective, the robust lung uptake of surfactant-wrapped 64Cu@GNTs is promising as it could allow for a novel approach for imaging-guided drug delivery using hybrid PET/MRI detection. For example, the loading procedure for 64Cu2+ and Gd3+ within our probe could be modified to include a cytotoxic moiety to capitalize on lung localization and enable the use of a theranostic analog of surfactant-wrapped 64Cu@GNTs for locoregional drug delivery for lung cancer. Noninvasive monitoring by PET/MRI could be used to examine agent biodistribution and determine the optimal dose for treatment.
Although blood was not collected from the mice, the TACs for the heart may be used as a noninvasive surrogate for blood pool retention of the probe. Images from the delayed time points revealed heart uptake values for 64Cu@GNTs that were similar to free 64Cu2+, indicating that the agent does not localize in the myocardial tissue over time and that the early radioactive signal in the heart observed during dynamic imaging is likely due to perfusion of the organ. This observation demonstrates that the bimodal construct is effectively cleared from systemic circulation in a reasonable time frame. Conceivably, alternative coating materials could be tested with our agent to reduce lung localization, while maintaining the efficient blood clearance properties, thus creating a ‘stealth’ version of surfactant-wrapped 64Cu@GNTs, which could be useful not only for diagnostic purposes where it permits increased signal-to-noise ratios, but also for therapeutic use by minimizing the interaction of a cytotoxic payload (i.e., therapeutic radionuclide or chemotherapy agent) with healthy tissues.
Conclusion
The growing use of hybrid imaging instrumentation such as PET/MRI encourages development of corresponding imaging probes that are capable of maximizing the diagnostic utility of each modality. In this work, we have developed a new bimodal imaging agent for T1-weighted MRI and PET by sequestering Gd3+ and 64Cu2+ within US-tubes to produce a SWCNT-based, biologically stable, bimodal imaging probe without the use of chelating ligands. We observed passive targeting to the lung in vivo, suggesting a potential use for US-tubes as a drug-delivery vehicle against various lung diseases including lung cancer [7]. Active targeting of the bimodal agent can also be achieved using proven strategies for surface modification without altering the encapsulation approach described, thus enabling increased target specificity with minimal off-target effects. The combination of these and other advances in carbon nanotube design have the potential to produce a family of imaging probes capable of targeted, multimodal imaging with any desired combination of CAs that are compatible with clinically used whole-body imaging modalities.
Future perspective
A large amount of current research is devoted to potential diagnostic and therapeutic applications of various nanoparticle platforms. Within the next decade, some of these platforms likely will gain US FDA approval for clinical use. The US-tubes described in this work are one such platform, given that they can be functionalized to render them biocompatible. Due to their ability to encapsulate small molecules and ions, US-tubes are uniquely suitable for providing contrast for virtually any medical imaging technology, as well as supporting mixed diagnostic/therapeutic applications. Further advances in high-resolution, high-sensitivity probes should lead to advanced applications such as single-cell imaging and tracking.
Executive summary.
Preliminary studies
A bimodal positron emission tomography/MRI probe was produced by loading ultra-short single-walled carbon nanotubes with 64Cu2+ and Gd3+.
The bimodal probe was produced without the use of chelating agents.
The external surface of the probe remains unmodified, allowing for the future attachment of targeting agents.
The probe was stable to in vitro challenges with EDTA, phosphate-buffered saline and serum.
Phantom & in vivo imaging
Separate phantom imaging indicated the probe was both positron emission tomography and MRI active.
In vivo positron emission tomography imaging indicated high lung uptake of the probe.
Acknowledgements
The authors wish to thank N Wilganowski and H Robinson for performing the in vivo imaging studies.
This work was supported by the Welch Foundation (Rice University [TX, USA], C-0627; LJ Wilson), the National Science Foundation’s Graduate Research Fellowship Program 0940902 (Rice University; JJ Law), and NIH U54 CA136404 (University of Texas Health Science Center [USA]; EM Sevick-Muraca).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Hahn MA, Singh AK, Sharma P, Brown SC, Moudgil BM. Nanoparticles as contrast agents for in-vivo bioimaging: current status and future perspectives. Anal. Bioanal. Chem. 2011;399(1):3–27. doi: 10.1007/s00216-010-4207-5. [DOI] [PubMed] [Google Scholar]
- 2.Lee DE, Koo H, Sun IC, Ryu JH, Kim K, Kwon IC. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012;41(7):2656. doi: 10.1039/c2cs15261d. [DOI] [PubMed] [Google Scholar]
- 3.Sailor MJ, Park JH. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012;24(28):3779–3802. doi: 10.1002/adma.201200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treacy MMJ, Ebbesen TW, Gibson JM. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature. 1996;381(6584):678–680. [Google Scholar]
- 5.Ouyang M, Huang JL, Lieber CM. Fundamental electronic properties and applications of single-walled carbon nanotubes. Acc. Chem. Res. 2002;35(12):1018–1025. doi: 10.1021/ar0101685. [DOI] [PubMed] [Google Scholar]
- 6.Pantarotto D, Briand JP, Prato M, Bianco A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. 2004;1:16–17. doi: 10.1039/b311254c. [DOI] [PubMed] [Google Scholar]
- 7.Guven A, Rusakova IA, Lewis MT, Wilson LJ. Cisplatin@ US-tube carbon nanocapsules for enhanced chemotherapeutic delivery. Biomaterials. 2012;33(5):1455–1461. doi: 10.1016/j.biomaterials.2011.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gautam UK, Costa PMFJ, Bando Y, et al. Recent developments in inorganically filled carbon nanotubes: successes and challenges. Sci. Technol. Adv. Mater. 2010;11:054501. doi: 10.1088/1468-6996/11/5/054501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M. MRI contrast agents: basic chemistry and safety. J. Magn. Reson. Imaging. 2012;36(5):1060–1071. doi: 10.1002/jmri.23725. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed. 2002;41(11):1853–1859. doi: 10.1002/1521-3773(20020603)41:11<1853::aid-anie1853>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Mackeyev Y, Hartman KB, Ananta JS, Lee AV, Wilson LJ. Catalytic synthesis of amino acid and peptide derivatized gadonanotubes. J. Am. Chem. Soc. 2009;131(24):8342–8343. doi: 10.1021/ja900918x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco A, Kostarelos K, Partidos CD, Prato M. Biomedical applications of functionalised carbon nanotubes. Chem Commun. 2005;5:571–577. doi: 10.1039/b410943k. [DOI] [PubMed] [Google Scholar]
- 13.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Yang K, Lee ST. Single-walled carbon nanotubes in biomedical imaging. J. Mater. Chem. 2011;21(3):586. [Google Scholar]
- 15.McDevitt MR, Chattopadhyay D, Jaggi JS, et al. PET imaging of soluble yttrium-86-labeled carbon nanotubes in mice. PLoS ONE. 2007;2(9):e907. doi: 10.1371/journal.pone.0000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Z, Cai W, He L, et al. In vivo biodistribution and highly efficient tumour targeting of carbon nanotubes in mice. Nat. Nanotechnol. 2007;2(1):47–52. doi: 10.1038/nnano.2006.170.. • 64Cu-labeled single-walled carbon nanotubes target tumors.
- 17.Luo J, Wilson JD, Zhang J, et al. A dual PET/MR imaging nanoprobe: 124I labeled Gd3N@C80. Appl. Sci. 2012;2(4):465–478. [Google Scholar]
- 18.Richard C, Doan BT, Beloeil JC, Bessodes M, Toth E, Scherman D. Noncovalent functionalizatsion of carbon nanotubes with amphiphilic Gd3+ chelates: toward powerful T-1 and T-2 MRI contrast agents. Nano Lett. 2008;8(1):232–236. doi: 10.1021/nl072509z. [DOI] [PubMed] [Google Scholar]
- 19.Choi JH, Nguyen FT, Barone PW, et al. Multimodal biomedical imaging with asymmetric single-walled carbon nanotube/iron oxide nanoparticle complexes. Nano Lett. 2007;7(4):861–867. doi: 10.1021/nl062306v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong SY, Tobias G, Al-Jamal KT, et al. Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging. Nat. Mater. 2010;9(6):485–490. doi: 10.1038/nmat2766.. • Shortened single-walled carbon nanotube accumulates in the lungs.
- 21. Sitharaman B, Kissell KR, Hartman KB, et al. Superparamagnetic gadonanotubes are high-performance MRI contrast agents. Chem. Commun. 2005;31:3915–3917. doi: 10.1039/b504435a.. • • First report of gadonanotubes.
- 22.Laurent S, Elst LV, Muller RN. Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents. Contrast Media Mol. Imaging. 2006;1(3):128–137. doi: 10.1002/cmmi.100. [DOI] [PubMed] [Google Scholar]
- 23. Judenhofer MS, Wehrl HF, Newport DF, et al. Simultaneous PET-MRI: a new approach for functional and morphological imaging. Nat. Med. 2008;14(4):459–465. doi: 10.1038/nm1700.. • First report of simultaneous positron emission tomography/MRI.
- 24.Jarrett BR, Gustafsson B, Kukis DL, Louie AY. Synthesis of 64Cu-labeled magnetic nanoparticles for multimodal imaging. Bioconjug Chem. 2008;19(7):1496–1504. doi: 10.1021/bc800108v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Comes Franchini M, Pucci A, Locatelli E, et al. Biocompatible nanocomposite for PET/MRI hybrid imaging. Int. J. Nanomedicine. 2012;7:6021–6033. doi: 10.2147/IJN.S38107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Z, Peng H, Hauge RH, Smalley RE, Margrave JL. Cutting single-wall carbon nanotubes through fuorination. Nano Lett. 2002;2(9):1009–1013. [Google Scholar]
- 27.Ashcroft JM, Hartman KB, Mackeyev Y, et al. Functionalization of individual ultra-short single-walled carbon nanotubes. Nanotechnology. 2006;17(20):5033–5037. [Google Scholar]
- 28.Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. Detection of cancer metastases with a dual-labeled near-infrared/positron emission tomography imaging agent. Transl. Oncol. 2010;3(5):307–317. doi: 10.1593/tlo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh SC, Ghosh P, Wilganowski N, et al. Multimodal chelation platform for near-infrared fluorescence/nuclear imaging. J. Med. Chem. 2013;56(2):406–416. doi: 10.1021/jm300906g. [DOI] [PubMed] [Google Scholar]
- 30.Idée J-M, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C. Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam. Clin. Pharmacol. 2006;20(6):563–576. doi: 10.1111/j.1472-8206.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 31.Tran LA, Krishnamurthy R, Muthupillai R, et al. Gadonanotubes as magnetic nanolabels for stem cell detection. Biomaterials. 2010;31(36):9482–9491. doi: 10.1016/j.biomaterials.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, et al. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to Swiss mice. ACS Nano. 2012;4(3):1481–1492. doi: 10.1021/nn901573w.. • Single-walled carbon nanotubes are nontoxic in vivo.
- 33.Hartman KB, Laus S, Bolskar RD, et al. Gadonanotubes as ultrasensitive pH-smart probes for magnetic resonance imaging. Nano Lett. 2012;8(2):415–419. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- 34.Wang R, Carducci MD, Zheng Z. Direct hydrolytic route to molecular oxo-hydroxo lanthanide clusters. Inorg. Chem. 2000;39(9):1836–1837. doi: 10.1021/ic991391p. [DOI] [PubMed] [Google Scholar]
- 35. Sethi R, Mackeyev Y, Wilson LJ. The gadonanotubes revisited: a new frontier in MRI contrast agent design. Inorganica Chim. Acta. 2012;393:165–172.. • • Review of gadonanotubes.
- 36.Gizzatov A, Dimiev A, Mackeyev Y, Tour JM, Wilson LJ. Highly water soluble multi-layer graphene nanoribbons and related honey-comb carbon nanostructures. Chem. Commun. 2012;48(45):5602–5604. doi: 10.1039/c2cc31407j. [DOI] [PubMed] [Google Scholar]
- 37.Rogers BE, Anderson CJ, Connett JM, et al. Comparison of four bifunctional chelates for radiolabeling monoclonal antibodies with copper radioisotopes: biodistribution and metabolism. Bioconjug Chem. 1996;7(4):511–522. doi: 10.1021/bc9600372. [DOI] [PubMed] [Google Scholar]
- 38.Salvador-Morales C, Townsend P, Flahaut E, et al. Binding of pulmonary surfactant proteins to carbon nanotubes; potential for damage to lung immune defense mechanisms. Carbon. 2007;45(3):607–617. [Google Scholar]