Abstract
Apraxia following left hemisphere stroke disrupts pantomimed tool use (PTU), a task that requires the integrity of a number of cognitive and motor processes. Although previous studies have identified that apraxics have deficits in (1) the integrity of/access to stored tool-use gesture representations, (2) deficits in intrinsic (body-based) coordinate control, and (3) abnormal reliance on visual feedback, no study to date has simultaneously tested the relative contribution of these three deficits to poor PTU performance. In this study we assessed 38 chronic left hemisphere stroke survivors on tests of PTU and the 3 component processes. We then attempted to predict PTU with the component scores using hierarchical regression to control for overall stroke severity and the possibility of correlated component scores. Results showed that over half of the variability in PTU was predictable, with the strongest independent predictor being a test of intrinsic coordinate control without visual feedback. A test of the integrity of/access to stored representations also predicted PTU. These results confirm and extend previous claims that conceptual- and production-related factors affect PTU, even after considering that deficits in both factors are commonly observed to varying degrees in apraxic patients.
Keywords: Apraxia, imitation, pantomime, tool use, intrinsic coordinate frame, visual feedback
1. INTRODUCTION
Limb apraxia is a common disorder following left hemisphere stroke that is characterized by deficits in gesture imitation, pantomimed tool use, and actual tool use. Of these tasks, pantomimed tool use (PTU) is perhaps the most sensitive test of apraxia. On several accounts, PTU requires the integrity of a number of cognitive and motor processes, any or all of which may be deficient in the apraxia syndrome (Binkofski & Buxbaum, 2012; Buxbaum, 2001; Stamenova, Black, & Roy, 2012). Because these processes tend to be highly correlated, understanding their relative contributions to PTU has been a challenge.
On most accounts, one of the processes required for PTU is the activation of a tool-use gesture representation that specifies the spatio-temporal properties of the movement in a relatively abstract or multi-modal form (e.g. that using a hammer is associated with a movement of a certain shape, size, and temporal characteristics; Buxbaum, 2001; Geschwind, 1965; Gonzalez Rothi, Ochipa, & Heilman, 1991; Liepmann, 1920). Evidence that apraxics have poor access to (or impaired integrity of) such gesture representations comes from simple tests of gesture recognition, in which the patient is given the name of a tool-use gesture (e.g. “hammering”) and is then asked to identify from a set of gestures the one that matches the named gesture (e.g., Kalénine, Buxbaum, & Coslett, 2010; Mozaz et al., 2006). Consistent with the claim that gesture representations are disrupted in apraxia, we have previously shown a strong relationship between object-related pantomime imitation and object-related pantomime recognition (Buxbaum, Kyle, & Menon, 2005, but see Negri et al., 2007)
A second process required for PTU is the ability to implement gesture representations with the actor’s own body. Previous studies have demonstrated that apraxics exhibit spatiomotor deficits in a range of gesture production tasks. This is true even for production tasks that do not require access to stored gesture representations, such as the imitation of meaningless postures (e.g. placing a vertically oriented hand to the side of the head; De Renzi, 1985; Goldenberg & Hagmann, 1997). We have previously linked such deficits to a disruption of the motor system’s control of movement in an intrinsic coordinate frame (Buxbaum, Giovannetti, & Libon, 2000; Buxbaum, 2001; Jax, Buxbaum, & Moll, 2006). Intrinsic coordinate control specifies movement plans in terms of body position (e.g. the change in elbow and shoulder angles needed to reach a target; Orban de Xivry et al., 2011; Reina, Moran, & Schwartz, 2001; Rosenbaum, Meulenbroek, Vaughan, & Jansen, 2001). This contrasts with extrinsic coordinate control, in which movement paths are specified with respect to locations in the world (e.g., a hand trajectory’s amplitude and direction; Gordon, Ghilardi, Cooper, & Ghez, 1994). Evidence from a range of tasks indicates that the nervous system may use both forms of control in a flexible task-dependent manner (Brayanov, Press, & Smith, 2012; Meghani, Burgess, & Patton, 2009; Parmar, Huang, & Patton, 2011). On our previous account (Jax et al., 2006), apraxics may be impaired at imitating meaningless body postures because such postures are defined by body positions and would logically be performed most efficiently using body-based intrinsic coordinate control. In contrast, tasks that are less impaired in patients with apraxia, such as reaching and grasping for an object in the workspace (Buxbaum, Johnson-Frey, & Bartlett-Williams, 2005), would logically be more efficiently controlled using extrinsic coordinates (e.g. a vector specifying the hand’s amplitude and direction to the object). Deficits in intrinsic coordinate control would be predicted to disrupt PTU as well because tool use pantomime movements are frequently related to the form of the body’s movement irrespective of the movement’s location in the workspace (e.g. hammering a nail into a table or a wall requires similar coordinated joint rotations even though they occur in different parts of space).
A third process that may impact performance during PTU is the absence of critical visual feedback about the tool’s movement. Apraxics have been reported to exhibit deficits in multiple forms of movement planning, including end posture selection (Buxbaum, Johnson-Frey, et al., 2005), the time required to complete movements (Sirigu et al., 1996), and accounting for intersegmental dynamics (Mutha, Sainburg, & Haaland, 2010). These planning deficits may be partially compensated by an over-reliance on visual feedback (Buxbaum, Johnson-Frey, et al., 2005; Haaland, Harrington, & Knight, 1999; Jax et al., 2006). Because visual feedback of a tool’s motion is absent, PTU may rely relatively strongly on feedforward movement planning, rendering it particularly challenging for apraxics. Consistent with this claim, pantomimed actions are improved in apraxics when the tool is held in the hand, even if the full tool-use action is not completed (e.g. swinging a hammer without contacting nails; Hermsdörfer, Li, Randerath, Roby-Brami, & Goldenberg, 2013). Similar improvements are not observed when a neutral object is held in the hand, which provides similar tactile but not visual feedback, indicating that visual feedback is particular is important for PTU (Goldenberg, Hentze, & Hermsdörfer, 2004; Hermsdörfer, Hentze, & Goldenberg, 2006).
Although previous studies have identified that apraxics have deficits in the integrity of/access to stored representations, deficits in intrinsic coordinate control, and abnormal reliance on visual feedback, no study to date has tested the relative contribution of these three deficits to poor PTU performance. The present study was completed to address this limitation. We took a case series approach (Schwartz & Dell, 2010), in which we tested a broad group of chronic left hemisphere stroke survivors to examine the relationship between accuracy on PTU, on the one hand, and tests measuring the three hypothesized underlying deficits, on the other. We predicted that (1) tests of stored representation integrity/access would predict PTU, (2) tests of intrinsic coordinate control would more strongly predict PTU than tests of extrinsic coordinate control, (3) tests of movement production without visual feedback would more strongly predict PTU than tests of movement production with visual feedback.
2. MATERIALS AND METHODS
2.1 Participants
A group of 38 chronic (> 6 months post stroke) left hemisphere stroke participants (17 female) completed the study. All participants were right-handed and were recruited without consideration of apraxia status from a research registry at the Moss Rehabilitation Research Institute (Schwartz, Brecher, Whyte, & Klein, 2005). Participants were excluded for evidence of major psychiatric disorders, drug/alcohol abuse, co-morbid neurological conditions, or severe language comprehension deficits (as assessed using the Auditory Comprehension Subtest of the Western Aphasia Battery; Kertesz, 1982). A separate group of 11 age- and education-matched neurologically intact control were recruited for norming of the intrinsic/extrinsic tests (Control Group A). A third group of 10 neurologically intact controls (Control Group B) previously provided norming data for the PTU and conceptual action knowledge tasks (Buxbaum, Kyle, et al., 2005). Table 1 summarizes the demographic data for all three groups. All participants consented to the study in accordance with the guidelines of Einstein Healthcare Network Institutional Review Board and were paid for their participation.
Table 1.
Descriptive statistics
| Variable | Patient Mean (s.d.) | Control A Mean (s.d.) | Control B Mean (s.d.) |
|---|---|---|---|
| Age (years) | 59.8 (9.1) | 59.6 (14.3) | 64.7 (12.1) |
| Education (years) | 13.9 (2.8) | 14.4 (2.8) | 14.0 (3.2) |
| Time post-stroke (months) | 65.3 (47.5) | N/A | N/A |
| WAB auditory comprehension (/10) | 9.2 (1.1) | - | - |
| Lesion volume (mm3) | 86,678 (75,022) | N/A | N/A |
| PTU (% correct) | 83.0 (12.4) | - | 90.3 (2.5) |
| Semantic gesture recognition (% correct) | 88.1 (10.5) | - | 97.5 (3.0) |
| Spatial gesture recognition (% correct) | 85.3 (10.7) | - | 93.3 (3.5) |
| Visible intrinsic (% correct) | 79.5 (11.1) | 93.6 (4.5) | - |
| Visible extrinsic (% correct) | 85.8 (14.1) | 97.7 (4.1) | - |
| Blindfolded intrinsic (% correct) | 76.3 (14.0) | 88.6 (7.4) | - |
| Blindfolded extrinsic (% correct) | 80.6 (13.1) | 95.0 (5.0) | - |
2.2 Materials and Procedure
2.2.1 Pantomimed Tool Use Task
In the 10 trials of pantomimed tool use (PTU), tools were presented one at a time to the participant, whose task was to use the ipsilesional left arm to demonstrate how they would hold and use the object, but without touching it. All movements were videotaped and analyzed offline. Movements were scored as being correct or incorrect on four components (amplitude, timing, hand posture, and arm posture; see Buxbaum, Kyle, et al., 2005, for scoring details). Two independent raters scored a subset of videos and achieved high reliability (90% agreement or higher for all four criteria).
2.2.2 Conceptual Action Knowledge Task (Gesture recognition)
In the conceptual action knowledge test, which tested the integrity of and access to stored gesture representations without a motor production component, participants completed 24 trials in which they heard and saw an action verb involving an object (e.g., “sawing”) and selected from two video clips the one illustrating the correct action pantomimed without an object. The correct video was the first or second video an equal number of times. On half of the trials, the foil illustrated a semantically-related error (e.g., pantomimed hammering when the target was sawing). On the other half of trials, the foil illustrated a spatially-related error (e.g., pantomimed shaking of a salt shaker with excessive movement amplitude when the target was salt shaker; Buxbaum, Kyle, et al., 2005). Accuracy on the semantically- and spatially-related trials was analyzed separately. Prior to the conceptual action knowledge test, a verb comprehension control task was administered to confirm patients understood all verbs used in the conceptual action knowledge test. In this control task, participants heard and viewed the verb and were required to select from three pictures which object matched the verb. Accuracy on the conceptual action knowledge test was computed only for trials in which participants correctly matched the verb to the picture in the control task.
2.2.3 Intrinsic/Extrinsic Task
The intrinsic/extrinsic (IE) task was an abbreviated version of the meaningless imitation task used by Jax et al. (2006), and was used to test predictions about both deficits in intrinsic coordinate control and abnormal reliance on visual feedback. In the task, videos of an actor making a movement to a static end position were presented on a computer screen approximately 150 cm in front of the seated participant. Two identical 3.5 second videos were presented on each trial, with a 1 second delay between presentations. Repetition of identical videos was used to insure that participants had ample opportunity to visually process the actions. Immediately after watching the pair of videos, participants imitated the movement in the video using their ipsilesional left arms in one of two Feedback conditions. In the visible condition, participants executed movements with full visual feedback throughout the movement. In the blindfolded condition, a blindfold was placed over the participants’ eyes at the end of the second video presentation. Participants were instructed to withhold their response until a tone sounded 5 seconds after the end of the second video, at which point they imitated the movement without visual feedback1. After producing the movement, participants returned to the start position (arms on the chair’s arm rests) before the blindfold was removed.
The 20 movements in the videos were of one of two movement types (see Figure S1 in the supplementary material for a depiction of all movements). The first, which we propose primarily relies upon extrinsic coordinate control, involved imitating the grasping of a common object (e.g. pen, phone). The second, which we propose primarily relies upon intrinsic coordinate control, involved imitating a meaningless posture whose end position was defined relative to another body part (e.g. left fist with palm facing forward 10 cm from side of the right cheek). One set of 10 movements (5 intrinsic, 5 extrinsic) was always paired with the visible feedback condition for all participants, and a second set of 10 movements was always paired with the blindfolded condition. Because of the small number of trials, and the desire to minimize switching between levels of the Feedback factor, the 10 visible trials always preceded the 10 blindfolded trials. Movement type (intrinsic, extrinsic) was randomized within Feedback condition blocks.
All movements were videotaped and analyzed offline. To improve the reliability of scoring, each movement was scored on 4 components (hand configuration, wrist angle, hand orientation, hand location) and each component was scored as correct or incorrect for the left arm only. The hand configuration component was scored as correct if the hand was not “flagrantly misconfigured”. The wrist angle component was scored as correct if the hand would have to be rotated less than a total of 30 degrees along any of the wrist’s axes to be correct. The hand orientation component was scored as correct if the orientation of the hand posture could be made correct with a rotation of less than 30 degrees. The hand location component was scored as correct if the center of the hand would be in the correct end location with a movement that was less than 30% of the length of the target body part or object. Two independent raters scored a subset of videos and achieved high reliability (94% agreement or higher for all four criteria).
2.2.4 Lesion identification
Lesion identification utilized T1–weighted MRI brain scans (23 patients) or, if MRI was contraindicated, CT scans without contrast (15 patients). Lesions were segmented under the supervision of a neurologist who was blind to the study predictions. Details of the lesion identification and registration procedure can be found in Kalénine et al. (2010). Total lesion volume was then calculated (see Table 1 for descriptive statistics) and used as a control for overall stroke severity. 2 We control for the effects of overall stroke severity because stroke disrupts many perceptual, motor, and cognitive functions, and in general a larger stroke will affect more of these functions. Therefore, in a group of stroke patients with a wide range of damage there will tend to be correlations between ANY two tests of functioning, with poor performance on one task generally associated with poor performance on the other task. The association between behavioral performance and lesion volume is likely to be especially strong when the behavioral test requires the integration of multiple functions, as we propose is true for our test of apraxia (PTU). In order to provide evidence that our hypothesized processes underlie apraxia, it is important for us not only to show that our component tests predict PTU, but also to reduce the possibility that tests of other functions predict PTU. Unfortunately, it is impractical for participants to complete tests of all other possible perceptual, motor, and cognitive functions that might affect PTU performance. But, we can begin to address this issue by controlling for lesion volume, which will partially control for the effects of other untested functions that are correlated with lesion volume.
3. RESULTS
3.1 Analysis approach
The overall goal of the study was to understand between-patient variability in apraxia (as measured by PTU) and whether that variability was predicable by deficits in 3 component processes (deficits in integrity of/access to stored representations, deficits in intrinsic coordinate control, and abnormal reliance on visual feedback). Rather than selecting patients who were either clearly apraxic or clearly not apraxic (as we did in Jax et al., 2006), we recruited a set of left hemisphere stroke patients who exhibited a wide and continuous range of PTU scores. Accordingly, we chose to use a regression analysis approach so that we could retain the full range of scores when testing the study’s central prediction that PTU would be predicted by deficits in the three component processes. Because scores on the component-process tests were likely to be correlated with one another, and all correlated with overall stroke severity, we needed to address the potential problem of predictor multicollinearity. To do so, we used hierarchical regression (Cohen & Cohen, 1983) to test whether individual component-process tests could predict PTU after considering the influence of other predictors of PTU. For each of these regressions, we began with a set of predictors including one predictor of theoretical interest (the “target predictor”). Next, we calculated the variance in PTU accounted for (as measured by R2) with one model that included all of the predictors except the target predictor (the “reduced” model). Then, we calculated the variance in PTU accounted for with all of the predictors including the target predictor (the “full” model). Finally, we computed the differences in R2 between the two models, which yielded a measure of how strongly the target predictor was able to predict PTU above and beyond the non-target predictors in the reduced model. In several pairs of these regressions we varied the target predictor in order to test whether that predictor significantly improved the prediction of PTU. Critically, any shared variance between the target predictor and other predictors is accounted for in the reduced model, so that if prediction is improved by including the target predictor in the full model, that improvement can only come from the unique variance from the target predictor. Specific uses of this approach are described in the following sections.
3.2 Individual task performance
Descriptive statistics for the PTU test, conceptual action knowledge tests, IE tests, and lesion volume are shown in Table 1. Examples of errors produced in the PTU and IE tasks by the same apraxic participant are shown in Figures 1 and 2, respectively.
Figure 1.
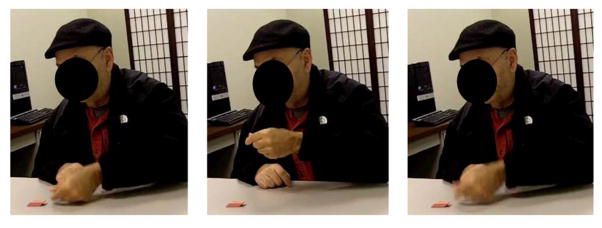
Example of an error when attempting to pantomime the use of an eraser (shown in the lower left corner of each panel). The participant accurately pantomimed the hand posture needed to use the eraser, but made the movement in the vertical rather than the horizontal plane (incorrect arm posture) and with an excessive amplitude.
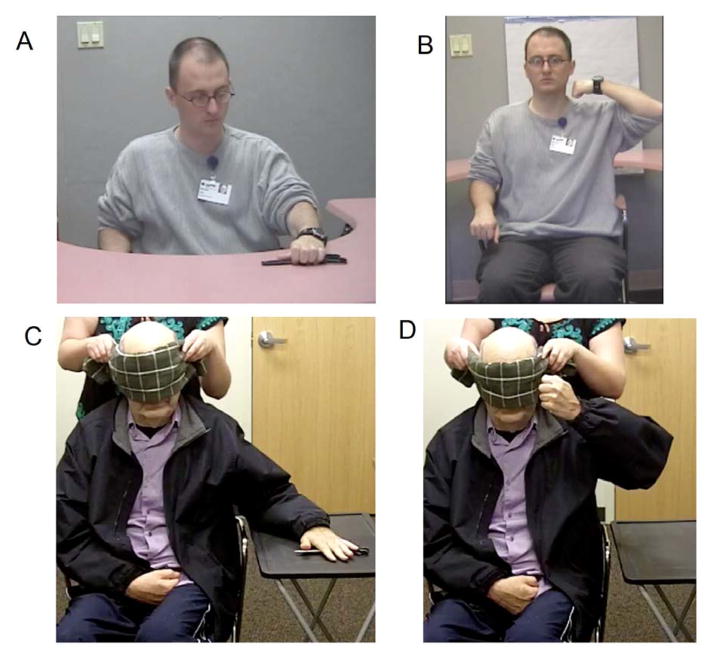
Figure 2.
Examples of left arm errors in the blindfolded extrinsic (target posture shown in A; incorrect hand configuration in C) and blindfolded intrinsic (target posture shown in B; incorrect hand orientation in D) tasks.
We first examined whether PTU could be predicted by individual tests of overall stroke severity (as assessed by lesion volume), accuracy (in percent correct) on the 2 conceptual action knowledge tests (spatial gesture recognition, semantic gesture recognition), and scores on the 4 IE subtests (visible intrinsic, visible extrinsic, blindfolded intrinsic, blindfolded extrinsic). Scatterplots showing the relationship between PTU and each of these individual tests are shown in Figure S2 of the supplementary material. As we predicted, total lesion volume was a significant predictor of PTU (R2 = .22, p =.003). Therefore, we first created a reduced model using only lesion volume to predict PTU, and then compared that model to 6 different full models, each predicting PTU with lesion volume and one of the remaining six tests (which were the target predictors). The change in R2 between the reduced and full models indicated how well each target predictor was able to predict PTU above and beyond overall stroke severity. The results of these regressions are shown in Table 2. Only scores on the spatial gesture recognition, blindfolded intrinsic, and blindfolded extrinsic tests significantly predicted PTU above and beyond total lesion volume. Of those three variables, the change in R2 was largest when adding the blindfolded intrinsic test (R2 change = .248), second largest when including the spatial gesture recognition test (.140), and smallest when including the blindfolded extrinsic test (.088).
Table 2.
Results of 6 hierarchical regressions predicting PTU with individual tests after controlling for lesion volume
| Target predictor | ΔR2 | p for ΔR2 |
|---|---|---|
| Semantic gesture recognition | .008 | .558 |
| Spatial gesture recognition | .140 | .009 |
| Visible intrinsic | .028 | .266 |
| Visible extrinsic | .023 | .309 |
| Blindfolded intrinsic | .248 | < .001 |
| Blindfolded extrinsic | .088 | .042 |
The finding that the R2 change when adding the blindfolded intrinsic test was larger than when adding the blindfolded extrinsic test support our previous claim (Jax et al., 2006) that apraxia may result in part from deficits in intrinsic coordinate control. That is, apraxia was better predicted by a test of intrinsic coordinate control than extrinsic coordinate control. In addition, our prior claim linking apraxia to an over-reliance on visual feedback was supported by the finding that larger R2 changes were observed for the two blindfolded conditions than for the corresponding two visible conditions. That is, apraxia was better predicted by tests without visual feedback than tests when visual feedback was present.
3.3. Unique contributions to predicting PTU
Next, we performed a more rigorous test of the individual test’s contribution to PTU prediction by extending the single-component regression analyses described in section 3.2 to include the possible effects of shared variance between components. That is, we focused on the 3 tests that significantly predicted PTU individually (spatial gesture recognition, blindfolded intrinsic, and blindfolded extrinsic) and used hierarchical regressions to examine whether any single test predicted PTU above and beyond the other two (as well as lesion volume). To do so, we compared the amount of variance accounted for with a single full model (which included lesion volume, spatial gesture recognition, blindfolded intrinsic, and blindfolded extrinsic; see Table 3) with the amount of variance accounted for with one of three reduced models: one with spatial gesture recognition removed, one with blindfolded intrinsic removed, and one with blindfolded extrinsic removed. The differences in R2 between the reduced and full models indicated how strongly the removed test was able to predict PTU above and beyond the other predictors. For example, to test whether spatial gesture recognition uniquely contributed to PTU, we compared the variance accounted for with the full model to a reduced model including only lesion volume, blindfolded intrinsic, and blindfolded extrinsic scores (the “Spatial gesture recognition removed” model in Table 3). We created analogous reduced models that excluded the blindfolded intrinsic test (“Blindfolded intrinsic removed” model in Table 3) and the blindfolded extrinsic test (“Blindfolded extrinsic removed” in Table 3) to test their unique contribution to PTU prediction.
Table 3.
Results of multiple regressions predicting PTU with component tests found to be individually predictive of PTU.
| Model | Predictors | β | model R2 | ΔR2 from full model | p for ΔR2 change |
|---|---|---|---|---|---|
| Full | Lesion volume | −.39 | .527 | ||
| Blindfolded intrinsic | .33 | ||||
| Blindfolded extrinsic | .19 | ||||
| Spatial gesture recognition | .25 | ||||
|
| |||||
| Spatial gesture recognition removed | Lesion volume | −.44 | .481 | .047 | .081 |
| Blindfolded intrinsic | .45 | ||||
| Blindfolded extrinsic | .41 | ||||
|
| |||||
| Blindfolded intrinsic removed | Lesion volume | −.37 | .452 | .075 | .029 |
| Blindfolded extrinsic | .31 | ||||
| Spatial gesture recognition | .39 | ||||
|
| |||||
| Blindfolded extrinsic removed | Lesion volume | −.42 | .497 | .030 | .154 |
| Blindfolded intrinsic | .41 | ||||
| Spatial gesture recognition | .20 | ||||
Overall, the full model with all four predictors accounted for a large amount of variance in PTU score, R2 = .527, p < .001. In addition, effects of multicollinearity were minor, as the VIF (variance inflation factor) coefficients in the full model ranged from 1.05 – 1.45 (where a coefficient of 1 indicates no collinearity and coefficients greater than 10 are considered problematic; Kutner, Nachtsheim, & Neter, 2004). Only scores on the blindfolded intrinsic task contributed uniquely to PTU prediction above and beyond the other two tests (p = .029), although the spatial gesture recognition tests did so at a trend level (p = .081). Scatterplots illustrating the relationship between (1) residuals in PTU score for the reduced models and (2) raw scores for the test added to the full model indicated that outliers did not drive the improved full model predictions (see Figure S3 in the supplementary material).
Finally, we confirmed the results reported in Table 3 with a data-driven, rather than theory-driven, approach. Specifically, we started with a full regression model predicting PTU with all 7 predictor scores we considered (lesion volume, spatial gesture recognition, semantic gesture recognition, visible intrinsic, visible extrinsic, blindfolded intrinsic, and blindfolded extrinsic). Next, we used automated backward elimination (as implemented in SPSS 20.0) to sequentially remove predictors that accounted for the least amount of PTU variance until a single model was reached for which removal of any predictor resulted in a significant (p < .05) reduction in R2. The results of these regressions (see Table 4) mirrored the results reported in Table 3. The order of removal (from least predictive to more predictive) was semantic gesture recognition, visible extrinsic, visible intrinsic, blindfolded extrinsic, and then spatial gesture recognition, with the final model containing only lesion volume and the blindfolded intrinsic task. These results further confirm that the blindfolded intrinsic task was an important predictor of PTU.
Table 4.
Results of backward elimination regressions predicting PTU.
| Model | Predictors | Predictor removed from previous model | R2 |
|---|---|---|---|
| 1 | LV, SpGR, SemGR, VE, VI, BE, BI | - | .531 |
| 2 | LV, SpGR, VE, VI, BE, BI | SemGR | .530 |
| 3 | LV, SpGR, VI, BE, BI | VE | .530 |
| 4 | LV, SpGR, BE, BI | VI | .527 |
| 5 | LV, SpGR, BI | BE | .497 |
| 6 | LV, BI | SpGR | .464 |
Abbreviations: LV, lesion volume; SpGR, spatial gesture recognition; SemGR, semantic gesture recognition; VE, visible extrinsic; VI, visible intrinsic; BE, blindfolded extrinsic; BI, blindfolded intrinsic
4. DISCUSSION
In this study we tested whether variability in pantomimed tool use would be predicted by performance on tests of integrity of/access to stored gesture representations, intrinsic coordinate control (as measured by meaningless imitation), and/or visual feedback over-reliance. When all three deficits were considered, along with a measure of overall stroke severity (lesion volume), over half of the variability in PTU could be explained. Moreover, the present study is the first of which we are aware that has attempted to predict PTU performance while controlling for the issues of overall stroke severity and the possibility of correlated predictors. Controlling for these issues allowed us to more precisely specify the perceptual-motor processes that are predictive of PTU performance, and thus enable us to refine a model of the mechanisms underlying apraxic gesture production.
Our analyses indicated that accuracy on the blindfolded intrinsic test predicted PTU accuracy even after considering the effects of lesion volume, spatial gesture recognition accuracy, and blindfolded extrinsic task accuracy. Thus, we were able to exclude from consideration any processing requirements that the blindfolded intrinsic test shared with the other tests. For instance, the blindfolded intrinsic and blindfolded extrinsic tests shared several features: both had similar perceptual input requirements, both required control of the ipsilesional limb, and both required that movements be made without visual feedback. The variance in PTU that could be explained by these shared features, however, was accounted for before the blindfolded intrinsic test was considered. Thus, the additional variance in PTU that was accounted for with the blindfolded intrinsic scores must have come from that test’s unique processing requirements. We propose that the need to specify a movement in body-defined intrinsic coordinates is the primary characteristic that differentiates the blindfolded intrinsic task from the other tasks, although future research is needed to confirm that the coordinate frame difference is the only factor that differentiates the tasks.
Although the slightly lower accuracy for the blindfolded intrinsic (76.3%) than the blindfolded extrinsic task (80.6%), along with a related increase in variability (standard deviations of 14.0% vs. 13.1%), may have allowed for slightly more PTU variance to be accounted for with the blindfolded intrinsic task, we find it unlikely that these small differences could account for the magnitude of the predictive differences between the two tests. For example, after accounting for lesion volume, the blindfolded extrinsic task accounted for only 8.8% of PTU variance (see Table 2), whereas the blindfolded intrinsic task accounted for 24.8% of PTU variance. We believe this near-tripling of variance accounted for is unlikely to have been due to the small differences between the distribution of scores for the two tests. That the blindfolded intrinsic test predicted PTU substantially more accurately than the blindfolded extrinsic test confirms our previous claim that intact intrinsic coordinate control is a critical for accurate tool use pantomime, and that deficits in intrinsic coordinate control play an important role in apraxia (Buxbaum et al., 2000; Buxbaum, 2001; Jax et al., 2006).
It is important to note that only performance on the blindfolded intrinsic test (but not the visible intrinsic test) independently predicted PTU above and beyond other variables (Table 2). In addition, performance on the blindfolded extrinsic test (but not the visible extrinsic test) predicted PTU, albeit not as strongly as performance on the blindfolded intrinsic test. These two findings highlight the importance of visual feedback over-reliance in apraxia, a deficit that occurs in addition to the intrinsic coordinate control deficit. That is, consistent with a recent study of the role of feedback in apraxia (Hermsdörfer et al., 2013), apraxics appear to be particularly affected by the removal of visual feedback during movement. Admittedly, our blindfolded tasks removed vision of the arm during movement and did not involve tools, whereas PTU retains vision of the arm during movement but removes potential visual feedback from tool movement. Although the specific visual information removed in our blindfolded tasks and absent in PTU differ, they do share a more general feature of reduced visual feedback. Our observation that deficits stemming from removing arm feedback in the IE task predict deficits on a task where tool feedback is removed lends support to this more general mechanism of visual feedback reliance.
While the present study indicates that visual feedback about the tool’s movement in PTU is important in apraxia, the specific information gained from this feedback remains unclear. One likely possibility is that the tool feedback provides an error signal for the perceptual-motor system. If the system was able to visually recognize that an incorrect movement were being produced, it could lead the system to attempt an alternative response in order to more closely approximate the correct action. Anecdotally, we have observed such behavior in some of our apraxic patients. When asked to pantomime, a patient may initially produce an incorrect movement and then change that movement after seeing that it was incorrect. In some instances, this incorrect initial movement is accompanied by a clear indication the patient recognizes the error (e.g. saying “no” while producing the movement). This behavior, known as conduite d’approche, is commonly observed in conduction aphasia (Bartha & Benke, 2003) and has been reported in apraxia (Gurd, Kischka, & Marshall, 2010; Luzzi, Piccirilli, Pesallaccia, Fabi, & Provinciali, 2010; Smania, Girardi, Domenicali, Lora, & Aglioti, 2000). To test these possibilities, future studies could be conducted in which apraxics are asked to continue to pantomime an action over an extended period of time, with the prediction that accuracy would increase with multiple repetitions.
Critically, PTU performance was not wholly accounted for by the movement production components described above. Variance in the spatial recognition test, which measured access to and integrity of the long-term stored gesture information without any movement production, also uniquely accounted for performance on the PTU test above and beyond lesion volume and performance on both blindfolded IE tests. Although this effect was only a statistical trend, it was observed after controlling for several other factors including overall stroke severity (something that is rarely done in the field of apraxia), indicating that it is a relatively important component of PTU. In addition, our failure to observe a statistically significant effect may have been affected by regression attenuation, the phenomenon in which measurement error in the outcome variable (PTU) leads to a systematic underestimate of the absolute value of a regression slope (Cheng & Van Ness, 1999). Nonetheless, our findings are consistent with a long-held belief that apraxic patients have damage to “gesture engrams” (Geschwind, 1965; Gonzalez Rothi et al., 1991; Liepmann, 1920). However, the present study was not designed to differentiate this account concerning stored gesture representation from other accounts in which gesture representations are constructed “on demand” based on mechanical reasoning and general functional knowledge (Goldenberg, 2013; Osiurak, Jarry, & Le Gallb, 2011). The present results also broadly confirm and extend models that posit two major components of the praxis system: a conceptual (also termed representational) and a production (or kinematic) component (Buxbaum, 2001; Gonzalez Rothi et al., 1991; Roy & Square, 1985). These models have, for the most part, been developed to explain patterns of dissociation among various gesture tasks. For example, patients who are able to recognize gestures but not produce them have been characterized as deficient in the production component. The present data enable characterization of the roles that both conceptual and production-related factors play within a single task.
Although the present study confirmed the contribution of intrinsic coordinate control, visual feedback, and gesture engrams to PTU performance, several results were not anticipated and are worthy of future research. First, in contrast to the results of our previous study (Jax et al., 2006), scores on both the visible intrinsic and visible extrinsic tasks were similarly poor at predicting PTU in the present study (Table 2). There were a number of methodological differences between the two studies, however, the most significant being that in the present study we accounted for the effects of stroke severity (by first controlling statistically for lesion volume), whereas in the previous study we did not. Thus, the previously-identified relationship between PTU and the intrinsic task (when visual feedback is present) may have been affected by stroke severity, a limitation we addressed in the present study.
The present study was also unable to contribute to understanding of the neural correlates of PTU deficits because the VLSM analyses did not reach statistical significance after correction for multiple comparisons. The most likely cause of this negative finding is that VLSM’s focus on voxel-level prediction of behavioral deficits is less effective at identifying critical brain regions when a network of multiple brain regions subserves a common function. If damage to any nodes of the network could lead to disrupted behavior, it is less likely that damage to an individual voxel within that network would be predictive of behavioral deficits. Currently, there are no methods of which we are aware that allow researchers to take such a network-analysis approach with static lesion data, although such methods have been developed for functional imaging and are increasingly used to understand post-stroke plasticity (e.g., functional connectivity; Carter, Shulman, & Corbetta, 2012). In future studies, we hope to augment our static lesion analysis approach (Bates et al., 2003; Buxbaum, Shapiro, & Coslett, 2014; Kalénine et al., 2010) with such functional imaging measures to further explore the neural correlates of the perceptual-motor processes under investigation in this study.
A better understanding of the underlying processing deficits in apraxia will hopefully lead to improved rehabilitation of the disorder, an area where few therapies exist (Sathian et al., 2011) even though it significantly impacts stroke survivors’ levels of disability (Donkervoort, Dekker, & Deelman, 2006). For example, along with colleagues we have developed a virtual feedback system that incorporates both visual and vibrotactile feedback to improve motor skill learning (Bark et al., 2011, in press; Kapur, Jensen, Buxbaum, Jax, & Kuchenbecker, 2010). The inclusion of vibrotactile feedback may be particularly beneficial for apraxic patients with deficits in intrinsic coordinate control because the feedback is provided within intrinsic coordinates (on the body) rather than in extrinsic, spatial, coordinates. We are currently testing this hypothesis. In addition, giving apraxic participants practice in producing movements with progressively less visual feedback may help reduce their over-reliance on visual feedback, and given the observed relationship between feedback reliance and PTU, may in turn lead to improved functioning in activities of daily living.
Supplementary Material
Highlights.
Apraxia following left hemisphere stroke disrupts pantomimed tool use (PTU)
We attempted to predict PTU with tests of 3 component processes
A test of intrinsic coordinate control without visual feedback predicted PTU
Deficits in the integrity of/access to stored spatial representations predicted PTU
These data allow refinement of models of apraxia
Acknowledgments
This study was funded by NIH grant R01-NS065049 to Laurel J. Buxbaum. The authors would like to thank H. Branch Coslett for his assistance with lesion identification, as well as Daniel Mirman for his statistical consultation. Data used in all regressions are available for download at osf.io/7kz2b.
Footnotes
The 5 second delay was required for the experimenter to position the blindfold. Although the visible and blindfolded conditions differed in both the available visual feedback (the primary manipulation of interest) and temporal delay between video presentation and production, our previous study (Jax et al., 2006) indicated that this delay had no effect on either controls or a similar group of left hemisphere stroke patients when visual feedback was available. Therefore, we did not include a condition that matched the blindfolded condition’s delay but allowed for visual feedback during production.
Lesions were also used for whole-brain voxel-based lesion-symptom mapping (VLSM; Bates et al., 2003) analyses to better understand the relationships between performance on the behavioral tests and lesion locations. However, none of these analyses reached statistical significance after correction for multiple comparisons except for those pertaining to the two conceptual action knowledge tests. Because these results were similar to a previous study of post-stroke action recognition deficits from our laboratory (Kalénine et al., 2010), which included many of the patients in the present study, we will not report the results of these VLSM analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bark K, Hyman E, Tan F, Cha E, Jax SA, Buxbaum LJ, Kuchenbecker KJ. Effects of vibrotactile feedback on human learning of arm motions. IEEE Transactions on Neural Systems and Rehabilitation Engineering. doi: 10.1109/TNSRE.2014.2327229. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bark K, Khanna P, Irwin R, Kapur P, Jax SA, Buxbaum LJ, Kuchenbecker KJ. Lessons in using vibrotactile feedback to guide fast arm motions. Proceedings of the 2011 IEEE World Haptics Conference; IEEE; 2011. pp. 355–360. [DOI] [Google Scholar]
- Bartha L, Benke T. Acute conduction aphasia: An analysis of 20 cases. Brain and Language. 2003;85(1):93–108. doi: 10.1016/s0093-934x(02)00502-3. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buxbaum LJ. Two action systems in the human brain. Brain and Language. 2012 doi: 10.1016/j.bandl.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayanov JB, Press DZ, Smith MA. Motor memory is encoded as a gain-field combination of intrinsic and extrinsic action representations. Journal of Neuroscience. 2012;32(43):14951–14965. doi: 10.1523/JNEUROSCI.1928-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum LJ. Ideomotor apraxia: A call to action. Neurocase. 2001;7(6):445–458. doi: 10.1093/neucas/7.6.445. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Giovannetti T, Libon D. The role of the dynamic body schema in praxis: Evidence from primary progressive apraxia. Brain and Cognition. 2000;44(2):166–191. doi: 10.1006/brcg.2000.1227. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Johnson-Frey SH, Bartlett-Williams M. Deficient internal models for planning hand-object interactions in apraxia. Neuropsychologia. 2005;43(6):917–929. doi: 10.1016/j.neuropsychologia.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: Internal representations subserving imitation and recognition of skilled object-related actions in humans. Cognitive Brain Research. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Shapiro AD, Coslett HB. Critical brain regions for tool-related and imitative actions: a componential analysis. Brain. 2014;137(7):1971–1985. doi: 10.1093/brain/awu111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? NeuroImage. 2012;62(4):2271–2280. doi: 10.1016/j.neuroimage.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CL, Van Ness JW. Statistical regression with measurement error. John Wiley & Sons; 1999. [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1983. [Google Scholar]
- De Renzi E. Methods of limb apraxia examination and their bearing on the interpretation of the disorder. Advances in Psychology. 1985;23:45–64. http://dx.doi.org/10.1016/S0166-4115(08)61135-8. [Google Scholar]
- Donkervoort M, Dekker J, Deelman B. The course of apraxia and ADL functioning in left hemisphere stroke patients treated in rehabilitation centres and nursing homes. Clinical Rehabilitation. 2006;20(12):1085–1093. doi: 10.1177/0269215506071257. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88(3):585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S. The meaning of meaningless gestures: A study of visuo-imitative apraxia. Neuropsychologia. 1997;35(3):333–341. doi: 10.1016/s0028-3932(96)00085-1. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hentze S, Hermsdörfer J. The effect of tactile feedback on pantomime of tool use in apraxia. Neurology. 2004;63(10):1863–1867. doi: 10.1212/01.wnl.0000144283.38174.07. [DOI] [PubMed] [Google Scholar]
- Gonzalez Rothi LJ, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cognitive Neuropsychology. 1991;8(6):443–458. doi: 10.1080/02643299108253382. [DOI] [Google Scholar]
- Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. Experimental Brain Research. 1994;99(1):112–130. doi: 10.1007/BF00241416. [DOI] [PubMed] [Google Scholar]
- Gurd JM, Kischka U, Marshall JC, editors. Handbook of clinical neuropsychology. Oxford University Press; 2010. [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Spatial deficits in ideomotor limb apraxia: A kinematic analysis of aiming movements. Brain. 1999;122(6):1169–1182. doi: 10.1093/brain/122.6.1169. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Hentze S, Goldenberg G. Spatial and kinematic features of apraxic movement depend on the mode of execution. Neuropsychologia. 2006;44(10):1642–1652. doi: 10.1016/j.neuropsychologia.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Hermsdörfer J, Li Y, Randerath J, Roby-Brami A, Goldenberg G. Tool use kinematics across different modes of execution: Implications for action representation and apraxia. Cortex. 2013;49(1):184–199. doi: 10.1016/j.cortex.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Jax SA, Buxbaum LJ, Moll A. Deficits in movement planning and intrinsic coordinate control in ideomotor apraxia. Journal of Cognitive Neuroscience. 2006;18:2063–2076. doi: 10.1162/jocn.2006.18.12.2063. [DOI] [PubMed] [Google Scholar]
- Kalénine S, Buxbaum LJ, Coslett HB. Critical brain regions for action recognition: Lesion symptom mapping in left hemisphere stroke. Brain. 2010;133(11):3269–3280. doi: 10.1093/brain/awq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur P, Jensen M, Buxbaum LJ, Jax SA, Kuchenbecker KJ. Spatially distributed tactile feedback for kinesthetic motion guidance. Proceedings of the 2010 IEEE Haptics Symposium; IEEE; 2010. pp. 519–526. [DOI] [Google Scholar]
- Kertesz A. Western aphasia battery test manual. Psychological Corp; 1982. [Google Scholar]
- Kutner MH, Nachtsheim CJ, Neter J. Applied Linear Regression Models. 4. McGraw-Hill Irwin; 2004. [Google Scholar]
- Liepmann H. Apraxie. In: Brugsch H, editor. Ergbnisse der Gesamten Medizin. Vol. 1. Wien Berlin: Urban & Schwarzenberg; 1920. pp. 516–543. [Google Scholar]
- Luzzi S, Piccirilli M, Pesallaccia M, Fabi K, Provinciali L. Dissociation apraxia secondary to right premotor stroke. Neuropsychologia. 2010;48(1):68–76. doi: 10.1016/j.neuropsychologia.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Meghani AB, Burgess JK, Patton JL. Intermanual transfer of learning reveals representations in simultaneous extrinsic and intrinsic coordinate systems. IEEE International Conference on Rehabilitation Robotics; 2009. pp. 40–45. [DOI] [Google Scholar]
- Mozaz M, Garaigordobil M, Gonzalez Rothi LJ, Anderson J, Crucian GP, Heilman KM. Posture recognition in Alzheimer’s disease. Brain and Cognition. 2006;62(3):241–245. doi: 10.1016/j.bandc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010;48(13):3855–3867. doi: 10.1016/j.neuropsychologia.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cognitive Neuropsychology. 2007;24(8):795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Marko MK, Pekny SE, Pastor D, Izawa J, Celnik P, Shadmehr R. Stimulation of the human motor cortex alters generalization patterns of motor learning. Journal of Neuroscience. 2011;31(19):7102–7110. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiurak F, Jarry C, Le Gall D. Re-examining the gesture engram hypothesis: new perspectives on apraxia of tool use. Neuropsychologia. 2011;49:299–312. doi: 10.1016/j.neuropsychologia.2010.12.041. [DOI] [PubMed] [Google Scholar]
- Parmar PN, Huang FC, Patton JL. Simultaneous coordinate representations are influenced by visual feedback in a motor learning task. Annual International Conference of the IEEE Engineering in Medicine and Biology Society.; 2011. pp. 6762–6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina GA, Moran DW, Schwartz AB. On the relationship between joint angular velocity and motor cortical discharge during reaching. Journal of Neurophysiology. 2001;85(6):2576–2589. doi: 10.1152/jn.2001.85.6.2576. Retrieved from http://jn.physiology.org/content/85/6/2576.short. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DA, Meulenbroek RJ, Vaughan J, Jansen C. Posture-based motion planning: Applications to grasping. Psychological Review. 2001;108(4):709–734. doi: 10.1037/0033-295x.108.4.709. [DOI] [PubMed] [Google Scholar]
- Roy EA, Square PA. Common considerations in the study of limb, verbal and oral apraxia. Advances in Psychology. 1985;23:111–161. [Google Scholar]
- Sathian K, Buxbaum LJ, Cohen LG, Krakauer JW, Lang CE, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: Common clinical deficits. Neurorehabilitation and Neural Repair. 2011;25(5 Suppl):21S–32S. doi: 10.1177/1545968311410941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Brecher AR, Whyte J, Klein MG. A patient registry for cognitive rehabilitation research: a strategy for balancing patients’ privacy rights with researchers’ need for access. Archives of Physical Medicine and Rehabilitation. 2005;86(9):1807–1814. doi: 10.1016/j.apmr.2005.03.009. S0003-9993(05)00315-1 [pii] [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Dell GS. Case series investigations in cognitive neuropsychology. Cognitive Neuropsychology. 2010;27(6):477–494. doi: 10.1080/02643294.2011.574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273(5281):1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Smania N, Girardi F, Domenicali C, Lora E, Aglioti S. The rehabilitation of limb apraxia: A study in left-brain-damaged patients. Archives of Physical Medicine and Rehabilitation. 2000;81(4):379–388. doi: 10.1053/mr.2000.6921. [DOI] [PubMed] [Google Scholar]
- Stamenova V, Black SE, Roy EA. An update on the Conceptual-Production Systems model of apraxia: Evidence from stroke. Brain and Cognition. 2012;80(1):53–63. doi: 10.1016/j.bandc.2012.03.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.