Abstract
The potential for short-term training to improve cognitive and sensory function in older adults has captured the public’s interest. Initial results have been promising. For example, eight weeks of auditory-based cognitive training decreases peak latencies and peak variability in neural responses to speech presented in a background of noise and instills gains in speed of processing, speech-in-noise recognition, and short-term memory in older adults. But while previous studies have demonstrated short-term plasticity in older adults, we must consider the long-term maintenance of training gains. To evaluate training maintenance, we invited participants from an earlier training study to return for follow-up testing six months after the completion of training. We found that improvements in response peak timing to speech in noise and speed of processing were maintained, but the participants did not maintain speech-in-noise recognition or memory gains. Future studies should consider factors that are important for training maintenance, including the nature of the training, compliance with the training schedule, and the need for booster sessions after the completion of primary training.
Keywords: Aging, Training, Auditory Plasticity, Neural Timing, Speech-in-Noise Recognition, Memory, Temporal Processing
1. Introduction
In response to growing interest in the potential for training to remediate perceptual and cognitive deficits (Pichora-Fuller & Levitt, 2012), software developers have marketed a wide variety of training programs to older adults with concerns about declines in cognitive function and the ability to hear in noisy environments. The adaptability of computer technology allows for fine manipulation of training stimuli, such as consonant-vowel (CV) transition times in a speech signal, allowing users to progress from easy to challenging perceptual tasks. In a previous study, we used an auditory-based cognitive training program (described in Smith et al., 2009) that combined adaptively expanding CV transition times and memory demands to determine if training improves neural and behavioral speech-in-noise processing and cognitive functions of memory and speed of processing in older adults (ages 55 to 70) (Anderson, White-Schwoch, Parbery-Clark, & Kraus, 2013). We evaluated neural speech-in-noise processing with the frequency following response (FFR) to a CV syllable presented in quiet and noise and found that training decreased FFR peak latencies and peak variability, two putative measures of subcortical neural synchrony, and improved untrained measures of sentence recognition in noise, short-term memory, and speed of processing. We used the FFR because it reflects temporal processing deficits in older adults (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2012; Clinard & Tremblay, 2013; Vander Werff & Burns, 2011) and because it can be modulated by training in young adults (Carcagno & Plack, 2011; Song, Skoe, Banai, & Kraus, 2012; Song, Skoe, Wong, & Kraus, 2008). The FFR reflects neural transcription of stimulus properties and is highly modulated by cognitive influences (Kraus & Chandrasekaran, 2010; Krishnan, Gandour, & Bidelman, 2010). In the current study, we investigated whether or not these effects of training on FFR processing and perceptual and cognitive function were maintained in older adults six months after cessation of training. In the following paragraphs, we outline training considerations for older adults and the evidence for generalization to untrained tasks and persistence of training benefits.
Effective training should target declines in sensory and cognitive processing and other domains that are known to decline with age. Reductions in speed of processing may underlie broad changes in cognitive function, including memory (Salthouse, 1996). In the auditory domain, these declines may contribute to the older adult’s difficulties when trying to understand spoken communication, especially in challenging listening situations. Precise temporal resolution in the auditory system is required for the discrimination of speech stimuli (Phillips, Gordon-Salant, Fitzgibbons, & Yeni-Komshian, 2000), both in individuals with hearing loss and with audiometrically-normal hearing. Examples of age-related deficits in temporal resolution are seen in delayed midbrain and cortical peak latencies (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2012; Clinard & Tremblay, 2013; Lister, Maxfield, Pitt, & Gonzalez, 2011; Tremblay, Piskosz, & Souza., 2003), delayed recovery in single neurons in inferior colliculus (Walton, Frisina, & O’Neill, 1998), loss of temporal coding by fusiform cells in dorsal cochlear nucleus (Wang et al., 2009), lower response amplitudes to amplitude-modulated tones (Parthasarathy & Bartlett, 2011), and reduced post-onset suppression in auditory cortex (Hughes, Turner, Parrish, & Caspary, 2010). Age-related declines in cognitive function can further exacerbate the effects of reduced temporal processing on speech perception, especially in unfavorable listening environments that may mask redundant speech cues (Pichora-Fuller, Schneider, & Daneman, 1995). These studies suggest that training that targets both perceptual and cognitive function is likely to be most effective for addressing age-related concerns.
Mahncke, Bronstone, and Merzenich (2006) have proposed that perceptual training can enhance cognitive performance. In fact, Berry et al. (2010) found that visual discrimination gains were associated with improvement on an untrained working memory task and that the change in N1 amplitude (an evoked cortical measure associated with attention; Coch, Sanders, & Neville, 2005) was highly correlated with the extent of working memory improvement. This link between perceptual training and cognitive performance is especially important, because reduced auditory input can impede cognitive function, particularly working memory (Pichora-Fuller, Schneider, & Daneman, 1995. Just as perceptual training is associated with cognitive gains, perhaps cognitive training can improve perceptual performance, especially in light of evidence that age-related declines in sensory function can be compensated by higher-level cognitive and attentional processes (reviewed in Grady, 2012; Li & Lindenberger, 2002; Wong et al., 2009). Wong and colleagues (2010) documented this cognitive compensation in older adults using fMRI to evaluate cortical processing of words in noise. They found reduced activity in auditory cortex but increased activity in prefrontal and precuneous regions – areas associated with memory and attention – in older vs. younger adults. Increased activity in cognitive regions was associated with better performance on a word recognition in noise task, but only in older adults, providing physiological evidence for the importance of memory and attention for hearing in noise in older adults. These results are in line with the Decline Compensation Hypothesis, which states that declines in sensory processing are accompanied by recruitment of general cognitive areas (Cabeza et al., 2004). Both the Berry and the Wong studies reinforce the idea that combining perceptual and cognitive training may engender lasting benefits in communication skills.
Another important consideration is generalization. For any program to be adopted and useful, the training must generalize from trained to untrained tasks or stimuli, especially those important for real world skills (as reviewed in Fahle, 2005). Previous evidence supports generalization for both perceptual and cognitive tasks in older adults. One study that addressed perceptual generalization showed that although older adults require more sessions to improve performance on a spectromodulation detection task than younger adults, the generalization of their performance to untrained stimuli was actually better than the generalization in younger adults (Sabin, et al., 2013). In a demonstration of transfer to off-task cognitive abilities, older adults who were trained using an auditory-based cognitive software program also had gains on an untrained memory test (Smith, et al., 2009). Taken together, these studies evince the potential for the training benefits of cognitive and perceptual training to transfer to more general perceptual and cognitive skills in older adults.
The maintenance of training gains should also be considered when evaluating treatment efficacy, and there is evidence for the maintenance of cognitive gains in older adults. In older participants (i.e., 65 to 75 years), gains on untrained tasks involving fluid intelligence (e.g., pattern recognition) and speed of processing but not working memory were maintained for eight months (Borella, Carretti, Riboldi, & De Beni, 2010). In individuals of more advanced age (i.e., 75 to 86 years), however, performance on trained tasks was maintained for eight months but the generalization effects were less robust (Borella, Carretti, Zanoni, Zavagnin, & De Beni, 2013). Although no evidence as yet supports the maintenance of perceptual gains in older adults, young adults maintain gains in sentence recognition in noise and enhanced subcortical encoding of a speech syllable in noise for up to six months after using a 20-day speech-in-noise training program (Song, Skoe, Banai, & Kraus, 2012). Given this limited information, there is clearly a need for further investigation into the long-term maintenance of training. In this study, we assessed maintenance of neural, perceptual, and cognitive training benefits after six months. Based on the Song et al. (2012) findings, we hypothesize that improvements in subcortical temporal processing (decreased latencies and inter-peak variability) and speech-in-noise performance persist for several months. We also hypothesize that gains in speed of processing, but not memory, are maintained after the cessation of training based on Borella et al. (2010).
2. Methods
2.1. Participants
We invited the 67 participants from our original training study to return for a six-month follow-up visit, and 62 participants (34 female) ranging in age from 55 to 70 years returned. We report statistics from the 62 participants who participated in all three sessions, and so there are slight differences in the means and statistical comparisons at post-test from those reported in Anderson, White-Schwoch, Parbery-Clark, and Kraus (2013b). No participants had a history of neurologic conditions, and all participants had normal IQs (≥85 on the WASI (Zhu & Garcia, 1999)).
Audiometric thresholds were measured at octave intervals from 0.125 to 8 kHz, including interoctave intervals at 3 and 6 kHz, plus 10 kHz and 12.5 kHz. No changes in threshold (≥10 dB) in any participant were noted from the previous sessions. Click-evoked auditory brainstem responses were obtained bilaterally (100 μs click presented at 80 dB SPL at 31.25 Hz), and no Wave V latency changes (≥0.05 ms) from the previous sessions were found in any participant.
All procedures were reviewed and approved by the Northwestern University Institutional Review Board. Participants provided informed consent and were compensated for their time.
2.2. Participant Groups
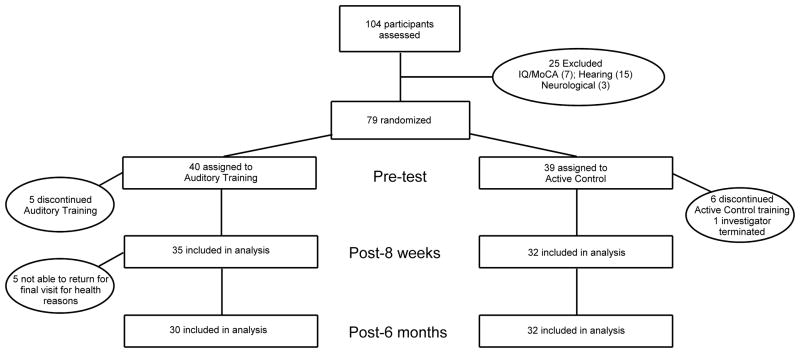
In the original study, participants were randomly assigned to one of two treatment groups, auditory training or active control (towards the end of the study, targeted enrollment was done to ensure that the groups were matched on age, sex, hearing, and IQ). Both groups completed eight weeks of in-home auditory-based activities using headphones on home computers, one hour per day, five days per week, and both groups used headphones (Koss UR/29, Koss Corporation, Milwaukee, WI) to listen to the training. Of the original 35 members of the auditory training group, 30 participated in the final testing session, whereas all 32 members of the active control returned. See Figure 1 for a schematic of the experimental design. The final groups were matched on age, sex, hearing (each frequency .125–12.5 kHz bilaterally), IQ, and test-retest interval (all p’s > 0.10).
Figure 1.
Flow of participants randomly assigned to auditory training or active control groups, with a total enrollment of 104 and a final number of 79 after participants were excluded for cognitive, hearing, or neurological reasons. The number of participants who returned for follow-up visits at 8 weeks was 67 and the number at 6 months was 62.
2.2.1. Auditory Training Group
The auditory training participants completed the Brain Fitness program (Posit Science, San Francisco, CA), consisting of six modules designed to increase the speed and accuracy of auditory processing: 1) time-order judgment of frequency-modulated sweeps, 2) discrimination between pairs of confusable syllables, 3) recognizing sequences of confusable syllables and words, 4) matching pairs of confusable syllables and words, 5) implementing sequences of commands, and 6) answering questions from stories. The participant’s speed of processing is challenged by decreasing the inter-stimulus interval in module 1 with improved performance and by adaptively expanding and contracting the rapidly changing and perceptually vulnerable formant transition of the speech stimuli used in modules 2 – 6 based on performance. Similarly, memory demands increased (remembering more words, phrases, content) with correct performance. We hypothesized that increased memory demands lead to more focused attention on the CV differences in the training stimuli and engage top-down mechanisms to strengthen sensory input. See Smith et al. (2009) for further details about the training program. Participants received daily online feedback on their performance, and completion of training was verified through online data logging.
2.2.2 Control Group
The active control group also completed 40 hours of in-home computer-based auditory activities for 8 weeks. Participants watched educational DVDs on topics of art, history, and literature and answered questions about the content, and the training was non-adaptive. Only the data of the participants who scored above chance on the questions were included. One participant’s scores were below chance level and therefore these data were excluded – indicated on Figure 1 as “Investigator terminated.”
2.3. Electrophysiology
Electrophysiology methods are identical to those described in Anderson, White-Schwoch, Parbery-Clark, et al. (2013b).
2.3.1. Stimulus
A 170-ms, six-formant speech syllable [da], synthesized in a Klatt-based synthesizer (Klatt, 1980) at a 20 kHz sampling rate was presented in two conditions: in quiet and in two-talker babble background noise (presented at a +10 dB SNR, henceforth referred to as “noise”). After an initial 5 ms stop burst, the stimulus maintained a constant fundamental frequency (F0) of 100 Hz, with peaks occurring every 10 ms. The lower 3 formants shifted (F1: 400 → 720 Hz; F2: 1700 → 1240 Hz; F3: 2580 → 2500 Hz) during the 50 ms transition from the /d/ to the /a/ but stabilized for the 120 ms steady-state vowel portion. The fourth through sixth formants (F4–F6) remained constant over 170 ms at 3300, 3750, and 4900 Hz, respectively. For participants with hearing loss (thresholds > 20 dB HL at any frequency from 0.25–6 kHz for each ear), the frequencies in the [da] were selectively amplified with the NAL-R algorithm (Byrne & Dillon, 1986) using custom routines in MATLAB (The Mathworks, Inc., Natick, MA) to create customized binaural stimuli to minimize the effects of hearing loss on the responses (Anderson, Parbery-Clark, White-Schwoch, & Kraus, 2013). Seventeen participants in the auditory training group and 23 participants in the active control received altered stimuli. As hearing thresholds did not change, each participant received the same stimulus for every testing session.
2.3.2. Recording
Stimuli were presented binaurally in alternating polarities through electrically-shielded insert earphones (ER-3; Etymotic Research) at 80 dB SPL, with an 83 ms interstimulus interval using Neuroscan Stim2 (Compumedics, Inc., Charlotte, NC). Subcortical responses were recorded with Ag-AgCl electrodes in a vertical electrode montage (Cz active, forehead ground, earlobe references) with all impedances < 5 kΩ with a 20 kHz sampling rate using Neuroscan Acquire 4.3.
2.3.3. Data reduction
Brainstem responses were offline bandpass filtered from 70–2000 Hz in Neuroscan Edit (12 dB/octave roll off, zero phase-shift), and were epoched with a −40 to 213 ms time window relative to stimulus onset at 0 ms. Responses to the two polarities were added to minimize contamination from stimulus artifact and cochlear microphonic on the response (Campbell, Kerlin, Bishop, & Miller, 2012). Responses were amplitude-baseline corrected relative to the prestimulus period (−40 to 0 ms). Final averages consisted of 6000 artifact-free responses (3000 in each polarity) in each condition.
2.3.4. Data Analysis
Peak picking
Two trained peak-pickers, blind to participant group (auditory training/active control) and test session – pre-training (pre), post-training (post), and at six months following cessation of training (post6) – manually identified the major negative-going peaks of interest in the brainstem response. An additional third peak-picker confirmed selections. Peaks of interest in the formant transition of the brainstem response occurred at approximately 34, 44, 54, and 64 ms and in the steady state region at approximately 74, 84, 94, …, 164 ms. Variability in brainstem peak latencies was determined by calculating the standard deviation of latency differences between adjacent peaks in the frequency following response (FFR) (34–44, 44–54, …,154–164) pre- and post-training.
Quiet-to-noise correlations
We used a cross-correlation technique (Skoe & Kraus, 2010) to evaluate the degree of response timing shift induced by the babble noise, because masking delays auditory brainstem responses (Anderson, Skoe, Chandrasekaran, & Kraus, 2010; Burkard & Hecox, 1987). This technique shifts the response waveform in noise relative to that in quiet (± 2 ms) until the maximum correlation coefficient (Pearson’s r) is found. Fisher z’-scores are used for statistical analyses (Cohen & Cohen, 1975). We measured this shift (lag) between the two responses before and after training in the region corresponding to the onset and transition. We expected that the training would have greater effects on the responses in noise and would therefore result in a reduced peak lag in noise relative to a response in quiet. Because this lag is automatically calculated with MATLAB software, this measure serves as an objective confirmation of decreased peak latencies determined through manual peak-picking.
2.4. Behavioural Measures
Behavioural methods are identical to those described in Anderson, White-Schwoch, Parbery-Clark, et al. (2013b).
2.4.1. Speech-in-noise recognition
Participants’ sentence recognition in noise (SIN) was evaluated with the Quick Speech-in-Noise Test (QuickSIN, Etymotic Research, Elk Grove Village, IL), a non-adaptive clinical measure. Four sets of six sentences are presented in a background of four-talker babble (three female, one male) binaurally at 70 dB HL through insert earphones (ER-2, Etymotic Research). The first sentence in each set is presented at a +25 dB SNR, and the SNR of each of the five subsequent sentences decreases by 5 dB down to a 0 dB SNR. Each sentence contains 5 target words; the total number of correctly repeated target words in each set of six sentences (maximum 30) is subtracted from 25.5 to obtain the dB SNR loss, defined as the difference between the individual’s speech-in-noise (SIN) threshold and the average SIN threshold (Killion, Niquette, Gudmundsen, Revit, & Banerjee, 2004). A composite SNR score was obtained by averaging the SNR scores from 4 lists. Different sentence sets were presented at each test session. Lower scores reflect better performance on the task.
2.4.2. Auditory short-term memory
The Woodcock-Johnson III Tests of Cognitive Abilities (Woodcock, McGrew, & Mather, 2001) Memory for Words sub-test was used to evaluate short-term memory. Participants repeat aurally-presented sequences of up to 7 words in the same order as they were presented. Because hearing loss can affect older adults’ performance on memory tasks (Baldwin & Ash, 2011), the stimuli were presented with a CD recording and each participant adjusted the volume on the CD player to a comfortable volume. Age-normed scores were used for analysis.
2.4.3. Processing speed
The Woodcock-Johnson III Tests of Cognitive Abilities Visual Matching sub-test was used to evaluate speed of cognitive processing. Participants are presented with 60 printed sets of 6 numbers, ranging from single to triple digits, and in 3 minutes have to identify (and circle) two identical numbers within each set. Age-normed scores were used for analysis.
2.5. Statistical analyses
Separate repeated measures analyses of variance (RmANOVA) were used to compare neural (inter-peak variability and quiet-to-noise timing) and behavioural measures (speech recognition in noise, short-term memory, and processing speed) across three testing sessions (within subject factor: pre/post/post6) and between groups (between subject factor: auditory training/active control). Multivariate RmANOVA were used to compare overall timing changes in the transition (34, 44, 54, 64 ms) and steady state (74, 84, 94, 104, …,164 ms) peaks of the brainstem responses. Pearson’s correlations were calculated for the auditory training group to evaluate relationships among changes in behavioral and neurophysiological variables from the first to the final visit. Normality of all variables was ensured with the Shapiro-Wilk test (all p > 0.1) and homogeneity of variance with Levene’s test. Mauchly’s test of sphericity was used in RmANOVAs, and the Greenhouse-Geisser correction was applied when the sphericity assumption was violated. Bonferroni corrections were applied for follow-up tests in the RMANOVA; p-values reflect two-tailed tests.
3. Results
3.1. Neurophysiology
3.1.1. Latency
In the original study, we found that training improved the timing of neural responses, in particular, the peak latencies in the FFR to a speech syllable. Here we find that the most robust effect on latency in our original study, improved timing in response to the consonant-vowel (CV) transition of a speech syllable presented in noise, persisted for six months. For the peak latency analysis, there was a significant group × session interaction (F(2, 61) = 2.806, p = 0.011), reflecting a reduction in latency that persisted across the three sessions in the auditory training group (F(1, 29) = 2.618, p = 0.012) but not in the active control group (F(1, 31) = 1.329, p = 0.236). Post-hoc tests demonstrated a significant reduction in latency from pre to post (F(1, 29) = 6.550, p = 0.001) and pre to post6 (F(1, 29) = 2.807, p = 0.046) that was maintained with no changes from post to post6 (F(1, 29) = 0.607, p = 0.661). There were no latency changes from either pre to post, pre to post6, or from post to post6 in the active control group (all p’s > 0.1). The stimulus and grand average responses obtained at the six-month post session in quiet and noise are presented in Figure 2. The periodicity of the stimulus [da] is seen in the response, with peaks occurring every 10 ms, corresponding to the F0 of 100 Hz. Latency changes are presented in Figure 3.
Figure 2.
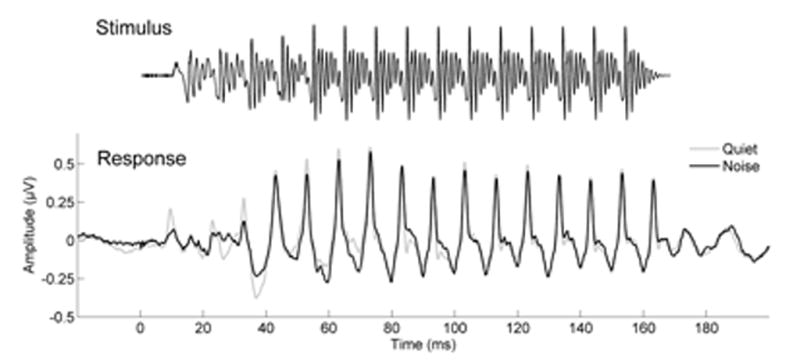
The periodicity of the evoking 170-ms stimulus [da] with a fundamental frequency of 100 Hz is represented in the grand average response obtained at the six-months post visit to the stimulus both in quiet (gray) and in noise (black), with peaks occurring at a frequency of every 10 ms.
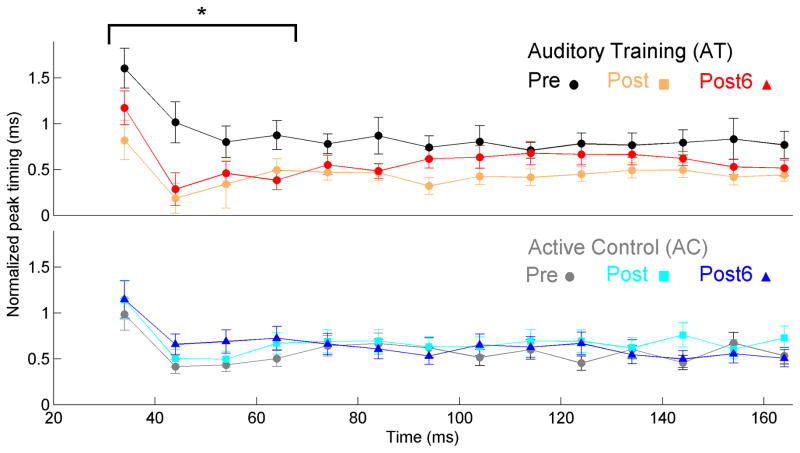
Figure 3.
In noise, the reduction in peak latencies in the region corresponding to the consonant-vowel transition (20–60 ms, marked with brackets) that was found after 8 weeks of training was maintained at the six-month follow-up visit. To facilitate visualization of the data across time, peak latency values are normalized by subtracting individual absolute latencies from the expected response latencies for each peak based on average data in young adults, taking into account stimulus characteristics and neural lag (Anderson et al., 2012). Therefore, faster timing corresponds to smaller differences between actual and expected latency values. Expected latencies were 9 ms for the onset, and 34, 44, 54, 64, etc., until 164 ms for peaks during the transition and steady-state. No latency changes were seen in the active control group. *p < 0.05 – significance value for the pre to post-6 change in latency. Error bars = +/− 1 S.E.
The original results showed a significant, but weaker, reduction in peak latencies in response to the steady-state region of the speech syllable in noise than was found in the transition region, but this training effect did not persist for 6 months – there was no group × session interaction across the three sessions (F(2, 61) = 1.542, p = 0.152). Table 3 displays mean peak latencies in the noise condition for each group across the three test sessions.
Table 3.
Means (S.E.s) are displayed for peak latencies occurring every 10 ms from 34 to 164 ms in response to the syllable [da] presented in two-talker babble obtained during the pre-, post-8 weeks, and post-6 months sessions for each group.
| Peak latencies (ms) | Auditory Training | Active Control | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Post 6 | Pre | Post | Post 6 | |
| 34 | 35.61 (0.22) | 34.90 (0.21) | 35.17 (0.18) | 35.22 (0.22) | 35.24 (0.20) | 34.95 (0.16) |
| 44 | 45.02 (0.23) | 44.23 (0.17) | 44.29 (0.18) | 46.24 (0.15) | 46.19 (0.20) | 45.96 (0.13) |
| 54 | 54.80 (0.17) | 54.41 (0.13) | 54.46 (0.13) | 54.47 (0.15) | 54.45 (0.15) | 54.34 (0.12) |
| 64 | 64.88 (0.16) | 64.52 (0.13) | 64.38 (0.10) | 64.49 (0.12) | 64.62 (0.18) | 64.52 (0.12) |
| 74 | 74.78 (0.11) | 74.53 (0.08) | 74.55 (0.10) | 74.63 (0.14) | 74.67 (0.18) | 74.46 (0.13) |
| 84 | 84.87 (0.20) | 84.51 (0.09) | 84.48 (0.08) | 84.64 (0.16) | 84.67 (0.18) | 84.44 (0.09) |
| 94 | 94.74 (0.12) | 94.35 (0.09) | 94.62 (0.10) | 94.60 (0.14) | 94.60 (0.17) | 94.43 (0.09) |
| 104 | 104.81 (0.17) | 104.48 (0.09) | 104.64 (0.10) | 104.50 (0.09) | 104.58 (0.16) | 104.42 (0.09) |
| 114 | 114.71 (0.08) | 114.48 (0.08) | 114.68 (0.13) | 114.58 (0.13) | 114.65 (0.21) | 114.41 (0.08) |
| 124 | 124.78 (0.12) | 124.50 (0.07) | 124.67 (0.12) | 124.44 (0.08) | 124.67 (0.21) | 124.44 (0.09) |
| 134 | 134.77 (0.13) | 134.52 (0.08) | 134.67 (0.11) | 134.58 (0.13) | 134.58 (0.13) | 134.47 (0.09) |
| 144 | 144.80 (0.14) | 144.55 (0.07) | 144.62 (0.08) | 144.44 (0.09) | 144.71 (0.22) | 144.38 (0.06) |
| 154 | 154.84 (0.23) | 154.46 (0.09) | 154.53 (0.09) | 154.65 (0.13) | 154.58 (0.16) | 154.42 (0.09) |
| 164 | 164.77 (0.15) | 164.45 (0.07) | 164.52 (0.09) | 164.51 (0.09) | 164.70 (0.22) | 164.43 (0.09) |
The original study also reported a latency reduction in response to the CV transition of a speech syllable presented in quiet, but this change of latency in quiet was not maintained after six months – there was no group × test session interaction (F(2, 61) = 1.563, p = 0.160). There had been no training effect in the original study in response to the steady state region in quiet, and similarly no changes were noted at 6 months (i.e., there was no group × test session interaction) – (F(2, 61) = 1.007, p = 0.474).
3.1.2. Inter-peak variability
In addition to earlier peak latencies in response to speech in noise, the initial study found that training reduced inter-peak variability in the neural responses. The reduction in inter-peak variability in the noise condition was maintained at 6 months during transition and steady-state periods. There was a group × session interaction (F(2, 61) = 5.860, p = 0.005), with long term training effects across the three sessions in the auditory training group (F(2, 29) = 3.622, p = 0.040) but not in the active control group (F(2, 31) = 2.219, p = 0.126). Post-hoc tests demonstrated a significant reduction in variability from pre to post (F(1, 29) = 9.979, p = 0.004) and pre to post6 (F(1, 29) = 4.391, p = 0.047) with no significant changes from post to post6 sessions in the auditory training group (F(1, 29) = 0.13, p = 0.910). There were no changes from either pre to post, pre to post6 or from post to post6 in the active control group (all p’s > 0.1).
3.1.3. Quiet-to-noise timing
In the original study we also observed a reduction in quiet-to-noise timing shift, or the extent to which the babble noise delayed response peaks. This reduction was not maintained - group × session interaction and paired t-tests all resulted in p’s > 0.1.
3.2. Behavioural
In our original study, we observed behavioural improvements in speed of processing, short-term memory, and speech-in-noise recognition. Group means and S.D.s for pre- and post-training and post6 behavioural measures are displayed in Table 2. There were no significant differences in auditory training and active control group performance on these measures at pre-test (SIN recognition: t(65) = 0.501, p = 0.618; short-term memory: t(65) = 1.422, p = 0.160; processing speed: t(65) = 0.678, p = 0.500).
Table 2.
Pre-, post-8 week, and post-6 month behavioural means (S.E.s) for the auditory training and active control groups. The speed of processing and short-term memory means are age-normed standard scores. The speech-in-noise (SIN) score means are in dB SNR and represent the difference between the individual’s SIN threshold and the average SIN threshold – the SNR loss (Killion et al., 2004).
| Auditory Training | Active Control | |||||
|---|---|---|---|---|---|---|
| Pre | Post | Post 6 | Pre | Post | Post 6 | |
| Speed of processing (standard score) | 107.93 (2.15) | 112.13 (2.53) | 112.57 (2.61) | 109.74 (1.86) | 107.33 (1.85) | 110.71 (2.15) |
| Speech-in-noise (dB SNR loss) | 1.08 (0.21) | 0.36 (0.25) | 0.81 (0.19) | 1.06 (0.17) | 0.65 (0.21) | 0.84 (0.20) |
| Short-term memory (standard score) | 106.90 (2.88) | 114.87 (2.35) | 110.80 (2.73) | 110.00 (1.70) | 110.85 (2.13) | 110.68 (2.21) |
The improvement in speed of processing was maintained at the six-month follow-up session in the auditory training group as evidenced by a group × session interaction (F(2, 61) = 3.192, p = 0.048) with a long-term training effect across the three test sessions in the auditory training group (F(2, 29) = 4.317, p = 0.023) but not in the active control group (F(2, 31) = 1.730, p = 0.195). Post-hoc testing indicated that for the auditory training group, there was improvement in speed of processing from pre to post (F(1, 29) = 6.556, p = 0.016) and pre to post6 (F(1, 29) = 6.940, p = 0.014) which was maintained from post to post6 (F(1, 29) = 0.043, p = 0.837). In the active control group, there were no changes in speed of processing from pre to post or from pre to post6 (all p’s > 0.1). However, improvements in speech-in-noise performance and in short-term memory were not retained. There was no group × session interaction for speech-in-noise performance (F(2, 61) = 0.613, p = 0.545). There was a significant group × session interaction for memory (F(2, 61) = 5.292, p = 0.008), because the training effect seen in the auditory training group at the post session (F(1, 29) = 8.282, p < 0.001) was not maintained at post6 (F(1, 29) = 3.937, p = 0.057), and there were no changes seen from pre to post or post to post6 in the active control group (all p’s > 0.1). Changes in behavioural performance on speech-in-noise recognition, short-term memory and speed of processing between pre and post and between pre and post6 for both groups are depicted in Figure 4.
Figure 4.
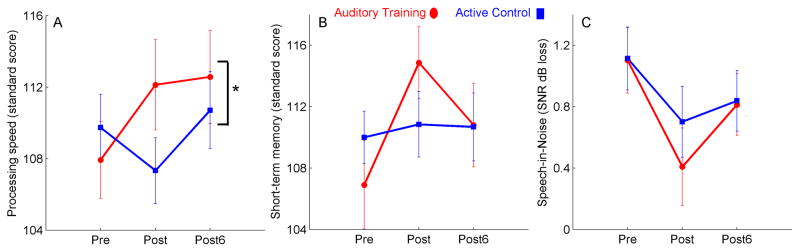
In the auditory training group (red circles), the increase in processing speed (standard score) seen at the post-training visit persisted for 6 months (A), but the training-induced enhancements in short-term memory (standard score; B) and sentence recognition in noise (dB SNR loss - lower scores indicate better performance; C) did not persist. There were no initial changes in processing speed or memory in the active control group (blue squares), and the initial change in speech-in-noise recognition did not persist. *p < 0.05 – significance value for the group × session interaction. Error bars = +/− 1 S.E.
3.3. Correlations
Using data from the auditory training group, we found that improvements in processing speed were related to with decreases in peak variability from pre to post6 (Figure 5). In addition, decreases in peak variability were related to decreases in transition peak latency. There were no other significant correlations among the speech-in-noise and short-term memory measures and neurophysiological measures from pre to post6. In addition, there were no significant correlations among any of the variables from pre to post or from post to post6. See Table 5 for correlation values for pre to post6 changes in behavioral and neurophysiological measures in the auditory training group.
Figure 5.
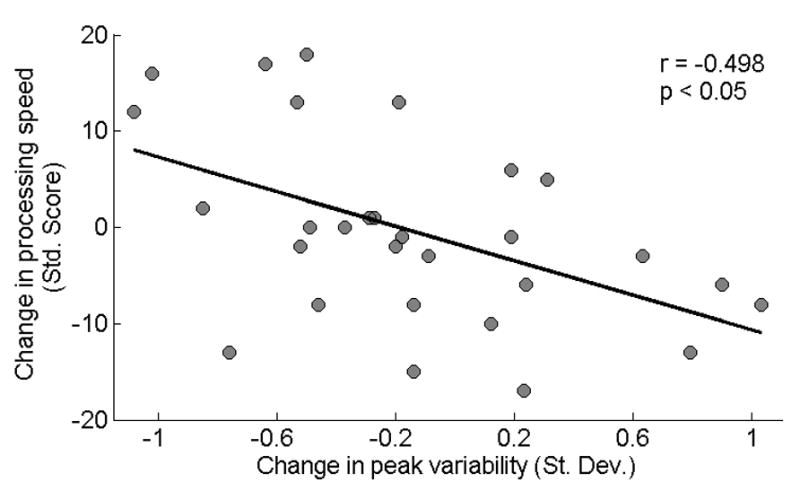
Changes in processing speed relate to changes in brainstem peak variability from pre to post6 in the auditory training group, such that individuals who experienced greater improvement in processing speed were more likely to have reduced peak variability.
Table 5.
Correlation values are listed for pre to post6 change measures in peak variability, transition latency (peak 44), speed of processing, short-term memory, and speech-in-noise recognition in the auditory training group. Relationships between changes in speed of processing and peak variability and between peak variability and transition latency were significant.
| Processing Speed | Short-term Memory | Speech-in-Noise | Peak Variability | Transition Peak Latency | |
|---|---|---|---|---|---|
| Processing Speed | 1 | −0.160 | −0.070 | −0.499** | −0.126 |
| Short-term Memory | −0.160 | 1 | 0.110 | −0.035 | 0.045 |
| Speech-in-Noise | −0.070 | 0.110 | 1 | −0.160 | −0.225 |
| Peak Variability | −0.499** | −0.035 | −0.160 | 1 | 0.575** |
| Transition Peak Latency | −0.126 | 0.045 | −0.225 | 0.575** | 1 |
p < 0.01.
4. Discussion
The maintenance of the training benefits produced by commercial software programs is an important index of training success. Here we report auditory training effects on subcortical responses to speech and behavioural measures of speed of processing, speech-in-noise recognition, and short-term memory in older adults, some of which persisted for six months. We found that the most robust effects of the original training study, reductions in peak latencies in response to the CV transition of a speech syllable and interpeak variability, were maintained for six months after the completion of training. Interestingly, improvement in our behavioural measure of speed of processing also persisted for six months, while memory and speech-in-noise performance gains did not. See Table 4 for a summary of the initial training effects and their maintenance after six months.
Table 4.
Summary of training (pre to post) and maintenance effects (pre to post6) in the auditory training group with asterisks indicating significance levels for each effect. The neurophysiology effects that were maintained were the reduction in peak latencies to the CV transition presented in two-talker babble and the reduction in inter-peak variability. The improvement in speed of processing was the only behavioural effect that persisted.
| Training Effect | Pre to post | Pre to post-6 |
|---|---|---|
| Neurophysiology | ||
| Reduction in latency in quiet | ||
| Transition | * | |
| Steady state | ||
| Reduction in latency in noise | ||
| Transition | *** | * |
| Steady state | * | |
| Reduction in inter-peak variability | * | * |
| Reduction in quiet-to-noise timing shift | * | |
| Behavior | ||
| Speech-in-noise perception | ** | |
| Short-term memory | ** | |
| Speed of processing | ** | * |
p < 0.05,
p < 0.01,
p < 0.001.
4.1. Maintenance of changes in response peak timing
To add to previous reports of cortical auditory plasticity in humans (Du et al., 2013; Hayes, Warrier, Nicol, Zecker, & Kraus, 2003; Tremblay, Shahin, Picton, & Ross, 2009), mounting evidence now suggests that short-term training can modify subcortical auditory processing (Carcagno & Plack, 2011; Chandrasekaran, Kraus, & Wong, 2012; de Boer & Thornton, 2008; Song et al., 2012). One of these studies specifically examined the maintenance of these effects in young adults (Song et al., 2012). Although the current study is the first to examine maintenance of neural changes to short-term training initiated by older adults, the benefits of music lessons received during childhood can persist into older adulthood (White-Schwoch, Carr, Anderson, Strait, & Kraus, 2013). In the White-Schwoch et al. study, older adults with music training in childhood had earlier subcortical peak latencies in response to speech than those who had no musical training, with years of training correlating with peak latencies. The authors proposed that this training early in life may prime the auditory system to selectively respond to auditory inputs to better encode the acoustic complexity and the salient information of the signal. The results from the current study suggest that short-term training initiated later in life can instill changes that persist for up to six months.
The neural timing benefits (earlier peak timing) in the current study and that of White-Schwoch et al. (2013) served to offset one of the most pervasive aspects of age-related declines – an overall slowing in neural speed of processing and a decrease in synchronous responses. In our original study, we speculated that an increase in inhibitory neurotransmission in auditory midbrain occurred in response to the training, potentially due to training demands of attention to rapidly changing details of the CV transition. This conjecture was based in part on animal models of auditory plasticity that have demonstrated increased numbers of inhibitory gamma-aminobutyric acid (GABA) receptors in auditory cortex following auditory training (Guo et al., 2012) and that pharmacologic treatment with a GABAergic agent can improve gap detection performance (Gleich, Hamann, Klump, Kittel, & Strutz, 2003). GABA is necessary for the precise temporal coding of the fast-changing formants in consonant-vowel transitions (as reviewed in Caspary, Ling, Turner, & Hughes, 2008). Cholinergic input to auditory cortex from nucleus basalis in the basal forebrain is required to induce plasticity in auditory perception (Bakin & Weinberger, 1996; Leach, Nodal, Cordery, King, & Bajo, 2013; Puckett, Pandya, Moucha, Dai, & Kilgard, 2007). GABA neurons in the mesolimbic reward system (ventral tegmental area and nucleus accumbens) target cholinergic interneurons to modulate learning (Creed, Ntamati, & Tan, 2014). The training used in our study, with its intrinsic rewards from feedback on improved performance, may have resulted in changes in these neurotransmitter levels, which may in turn have contributed to a reduction in neural variability (Zinke et al., 2006). Due to the extensive series of descending corticofugal projections, we suggest that this GABAergic increase propagated from auditory cortex to midbrain leads to more precise (i.e., more synchronous and faster) temporal coding of fast-changing sounds, eventually creating long-lasting changes as reflected in our studies. Assuming that the initial changes in peak latencies arose from an increase in inhibitory neurotransmission, we speculate that these changes persisted to some extent for six months. In our original study, the greatest latency changes were found in response to the CV transition presented in noise, and these were the only latency changes that persisted for six months. These results are consistent with our neurotransmitter view, because inhibitory function is especially important for coding rapidly changing sounds (Caspary et al., 2008), as found in the CV transition.
4.2. Maintenance of changes in cognitive skills
Gains in speed of processing, but not in short-term memory, persisted for six months. Interestingly, reduction in peak variability related to improvements in processing speed over the course of the study, while changes in sentence recognition in noise and short-term memory did not relate to any neurophysiologic changes. The relationship between changes in processing speed and peak variability suggests that peak variability in neural responses to sensory inputs may be a factor in processing speed deficits in older adults.
The persistence of benefits for speed of processing but not for memory are consistent with findings of Borella et al. (2010), who showed that older adults maintained training enhancements for fluid intelligence and speed of processing but not for memory. In their study they trained verbal working memory using an adaptive procedure and then determined whether this training resulted in performance gains on the trained task (following 2 weeks of training), short-term transfer effects, and long-term maintenance (8 months) of gains. Salthouse (1996) proposed that speed of processing is a basic aspect of cognitive function that declines with age and underlies changes in specific cognitive skills such as working memory. Borella et al. postulated that the training causes a boost in overall cognitive processing (i.e. speed of processing) but that the change for specific skills, such as working memory, is not robust enough to resist erosion over time. In our training regimen, the reduction in inter-stimulus intervals in module 1 or compression of CV transitions in modules 2 to 6 (see Methods 2.2.1) may have necessitated an increase in processing speed that continued into everyday tasks and was therefore maintained for an additional six months. Of course, there are also opportunities to practice memory in everyday life. However, a higher practice threshold may be necessary to maintain improved memory skills, and this maintenance may require more initial training or explicit practice.
Aging may also affect retention of enhanced memory performance. Li et al. (2008) compared working memory training effects in younger versus older adults. Both age groups improved on the trained task and performance generalized to a more demanding working memory task. This performance improvement was maintained for three months in the young adults; the older adults showed a decline relative to post-training session, but their end performance at three months was still significantly better than at pre-test. Another study found maintenance in working memory gains for 18 months in both younger and older adults; however, in the older adults there was no transfer of learning to an untrained task (Dahlin, Nyberg, Backman, & Neely, 2008).
4.3. Implications for speech-in-noise perception
We were surprised that the improvement in SIN recognition did not hold, especially given the likelihood of having many opportunities to practice this skill in real-world environments after training had ceased. Older adults, both with normal hearing and with hearing loss, have deficits in auditory temporal processing that lead to impaired perception. For example, older adults require longer consonant transition durations to discriminate between words that differ only on this temporal cue (e.g., “beat” vs. wheat”) in isolated words (Gordon-Salant, Yeni-Komshian, Fitzgibbons, & Barrett, 2006) and sentences (Fitzgibbons, Gordon-Salant, & Friedman, 2006). Furthermore, older adults have more difficulty recognizing time-compressed speech materials presented in quiet (Gordon-Salant & Fitzgibbons, 2001) and at different SNRs (Jafari, Omidvar, & Jafarloo, 2013), suggesting that older adults have difficulty processing brief temporal cues that are characteristic of rapid speech. Therefore, given that the subcortical temporal processing gains in our study were maintained, we would have expected to see similar results for our perceptual measures. We suspect that factors other than enhanced neural timing were at least partly responsible for the initials gains in speech perception. The QuickSIN sentences used to assess SIN recognition are long – 8 to 11 words – and therefore impose strong demands on short-term memory and attention. While we believe that the both cognitive and sensory processing factors contribute to speech-in-noise performance (Anderson, White-Schwoch, Parbery-Clark, & Kraus, 2013a), the fact that improvement in QuickSIN scores did not persist despite the maintenance of improvements in subcortical peak timing and variability has led us to believe that the initial improvements in QuickSIN may have been driven in part by the cognitive components of the training. The training did not explicitly teach participants strategies for improving their ability to hear in noise. Perhaps, actual listening-in-noise exercises may be necessary to engender lasting changes in skills or strategies used while listening to speech in adverse listening environments. In our literature search, we found only a few reports of long-term maintenance of auditory training. The benefits of digits-in-noise training in older individuals with cochlear implants (ages 46 to 78) were maintained for one month after cessation of training and generalized to untrained tests of speech-in-noise performance, but no results were reported for a longer period of maintenance (Oba, Fu, & Galvin, 2011). Words-in-noise training produced significant improvement in older listeners (ages 65 to 75) that were maintained six months after the cessation of training, but the training gains did not generalize to recognition of the trained words in sentences (Burk, Humes, Amos, & Strauser, 2006). Listening and Communication Enhancement (LACE™, Neurotone, Redwood City, CA) is an example of a home-based computerized training program that explicitly trains listening in competing or background noise, in addition to other top-down strategies. Young adults who underwent LACE training showed maintenance effects lasting six months that generalized to two non-trained measures of sentence recognition in noise (Song et al., 2012). This study also showed a maintenance of enhanced subcortical responses to speech, with greater representation of the fundamental frequency in response to a speech syllable embedded in noise after 4 weeks of training with LACE, and then again at 6 months after the discontinuation of LACE training.
One explanation for the difference in maintenance outcomes between our study and the Song et al. (2012) study may be the study populations: Song et al. trained young adults whereas we trained older adults. Training-induced changes in young adults may be more robust and resistant to change than those in older adults. In fact, two studies that trained perception of time-compressed speech found that younger but not older adults retained training benefits (Barcroft et al., 2011; Peelle & Wingfield, 2005).
Younger adults may be more efficient in the engagement of the neural circuits necessary to learn a new skill compared to older adults, and this enhanced efficiency may account for the persistence of training benefits in younger adults. Park and Reuter-Lorenz (2009) introduced the term “scaffolding” to describe the engagement of complementary and redundant neural circuits in response to challenges that might be posed during a learning task or when sensory input is reduced. Although older adults use scaffolding as a compensatory mechanism, their scaffolding is less efficient than that in young adults; therefore, training-induced changes may not persist in the older population.
4.4. Ingredients for successful learning
Although memory exercises may be insufficient in themselves to effect lasting changes in SIN recognition, memory does play an important role in successful listening in noise in both younger and older adults (Pichora-Fuller et al., 1995). In fact, memory may be more important for older listeners than younger listeners, an idea that would be broadly consistent with the Cognitive Compensation Hypothesis, which suggests that cognitive processes, such as memory and attention, compensate for sensory losses in older adults (Wong, Ettlinger, Sheppard, Gunasekera, & Dhar, 2010). Even in young listeners, cognitive influences can compensate for degraded sensory input (Nahum, Nelken, & Ahissar, 2008).
In older adults, neuroimaging studies support the idea that cortical areas dedicated to executive function have greater activation during speech-in-noise listening tasks in older adults than in younger adults (Wong et al., 2010). Furthermore, a structural equation model of speech-in-noise perception has demonstrated a strong role for both cognitive factors and central auditory processing in understanding speech in noise, with nonsignificant contributions to the model from peripheral function (Anderson, White-Schwoch, Parbery-Clark, et al., 2013a). Therefore, cognitive training (e.g., memory, attention) may be an important component of SIN training, but the training dosage may need to be increased or booster sessions may be necessary to maintain learning. Older adults who received supplemental booster sessions in cognitive training on either episodic memory, reasoning, or speed of processing tasks had better maintenance of training gains in reasoning and speed of processing, but not in memory, 11 months later when compared to groups who had received similar training without the booster sessions (Ball, Berch, F., & et al., 2002). The training program we used did not include the option of booster sessions following completion of training, but we recognize that this is an important consideration in the selection or design of training programs for any age, particularly in older adults whose performance is less likely to be stable over time compared to that of younger adults. This is an important area of follow-up in future dosage studies.
4.5. Limitations
In this study we wished to establish an effect using a commercially available training program, but one of the limitations of selecting a pre-packaged program is its lack of flexibility. For example, although our primary interest was in the training effects on untrained tasks (rather than on program-trained perceptual tasks), it would have been beneficial to assess performance on these trained tasks after six months. Doing so would have allowed us to determine, among other things, if there is a relationship between behavioral performance on trained and untrained tasks and neurophysiologic measures. This information would be useful in guiding the development of future training programs and in tracking of trainee progress.
We used a visual measure of processing speed, the Visual Memory subtest of the Woodcock-Johnson III Tests of Cognitive Abilities, rather than an auditory measure; therefore, we cannot with certainty conclude these results generalize to the auditory domain. However, the fact that changes in a behavioral measure of visual processing speed correlate with changes in a neurophysiological measure of auditory processing suggests that the training may have had a domain general rather than a domain specific effect. It would be interesting to compare training effects on visual vs. auditory processing speed measures in a future study design.
Finally, as mentioned above, performance on the QuickSIN may be have been affected by short-term memory demands. For this reason, it would be beneficial to include speech recognition measures that are less affected by memory, such as the Bamford-Kowal-Bench Speech-in-Noise Test and the Words-in-Noise Test (Wilson, McArdle, & Smith, 2007), in future training studies designed to improved speech-in-noise performance.
4.6. Future directions
Future work should consider factors that are important for the maintenance of training. One such factor is compliance with the training regimen. Programs that are engaging may enhance compliance with training (Nacke, Nacke, & Lindley, 2009). Individuals are less likely to return to booster sessions when the task becomes less interesting. Recent studies have examined the use of engaging video games to improve executive function (Anguera et al., 2013) and to enhance learning capacity (i.e. learning to learn) (Bavelier, Green, Pouget, & Schrater, 2012), as they may improve compliance by providing a more entertaining and rewarding training system. We envision future applications of similar technology for improving speech perception in older adults.
Future work should also consider the role of hearing loss in training outcomes. We did not include any individual with more than a mild-moderate sensorineural hearing loss, and given the complex interactions between working memory, hearing loss, and the temporal coding of speech (Anderson, Parbery-Clark, White-Schwoch, Drehobl, & Kraus, 2013; Lin, Yaffe, Xia, & al., 2013; Rudner, Rönnberg, & Lunner, 2011), older adults with more severe hearing loss may have a different training and maintenance profile (Anderson, White-Schwoch, Choi, & Kraus, 2013). We also acknowledge that there may be individual differences in training outcomes independent of hearing status and future work should investigate factors that might contribute to success (or not) in older adults.
4.6. Conclusions
These findings demonstrate that short-term training-induced enhancements in auditory temporal precision persist for at least six months in older adults. These results are particularly important in light of the known pervasive declines in neural temporal precision in older adults (Anderson et al., 2012; Hughes et al., 2010; Lister et al., 2011). However, we did not find equivalent maintenance across all the training effects that we reported in Anderson, White-Schwoch, Parbery-Clark, et al. (2013b). Specifically, while most of the neural changes persisted (earlier peak timing, reduction in peak variability), speed of processing was the only behavioral measure that maintained improvement for six months. The improvements in sentence-in-noise recognition and short-term memory did not persist. The fact that we did not find maintenance across all domains suggests that training may not engender a domain general plasticity but instead may train a series of systems with different parameters and limits for changes. Broadly speaking, our results suggest that short-term training can instill changes in a number of cognitive and sensory functions that have different conditions for improvement and long-term maintenance and may suggest that diverse training regimens are most effective for lasting improvements.
Table 1.
Groups were matched on all demographic data. Means (S.D.s) are displayed for age, sex distributions, test-retest delay (weeks), pure-tone average (.5–4kHz; dB HL), click wave V latency (ms), IQ (standard score) and MOCA score. Means represent performance at the pre-test.
Group profiles
| Auditory Training N = 30 |
Active Control N = 32 |
|
|---|---|---|
|
| ||
| Age (years) | 62.3 (3.4) | 63.6 (4.1) |
|
| ||
| Females : Males | 16:14 | 18:14 |
|
| ||
| Test-retest delay in weeks: | ||
| post | 9.54 (1.21) | 9.9 (2.3) |
| post6 | 27.3 (3.6) | 27.4 (1.6) |
|
| ||
| Hearing (Pure-tone average .5–4kHz; dB HL) | 17.68 (9.8) | 18.2 (6.7) |
|
| ||
| Click Wave V Latency (ms) | 6.0 (0.3) | 6.0 (0.4) |
|
| ||
| IQ (WASI; standard score) | 119 (11) | 120 (13) |
Training-induced neural timing gains in older adults are maintained for 6 months.
Behavioral speed-of-processing improvement is also maintained.
Improvements in speech-in-noise recognition and memory did not persist.
Acknowledgments
The authors thank Sarah Drehobl, Rafael Escobedo, and Samantha O’Connell for their assistance with data collection. We thank Trent Nicol, Laurence Anderson, and two anonymous reviewers for their helpful reviews and suggestions for the manuscript. We also thank our participants for their willingness to return for multiple testing sessions. This work was supported by the National Institutes of Health (T32 DC009399 & R01 DC10016) and the Knowles Hearing Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson S, Parbery-Clark A, White-Schwoch T, Drehobl S, Kraus N. Effects of hearing loss on the subcortical representation of speech cues. The Journal of the Acoustical Society of America. 2013;133:3030–3038. doi: 10.1121/1.4799804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. The Journal of Neuroscience. 2012;32:14156–14164. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Auditory brainstem response to complex sounds predicts self-reported speech-in-noise performance. Journal of Speech, Language & Hearing Research. 2013;56:31–43. doi: 10.1044/1092-4388(2012/12-0043). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. The Journal of Neuroscience. 2010;30:4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Choi HJ, Kraus N. Training changes processing of speech cues in older adults with hearing loss. Frontiers in Systems Neuroscience. 2013:7. doi: 10.3389/fnsys.2013.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. A dynamic auditory-cognitive system supports speech-in-noise perception in older adults. Hearing Research. 2013a;300:18–32. doi: 10.1016/j.heares.2013.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, White-Schwoch T, Parbery-Clark A, Kraus N. Reversal of age-related neural timing delays with training. Proceedings of the National Academy of Sciences - USA. 2013b;110:4357–4362. doi: 10.1073/pnas.1213555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera J, Boccanfuso J, Rintoul J, Al-Hashimi O, Faraji F, Janowich J, Kong E, Larraburo Y, Rolle C, Johnston E, Gazzaley A. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proceedings of the National Academy of Sciences - USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CL, Ash IK. Impact of sensory acuity on auditory working memory span in young and older adults. Psychology and Aging. 2011;26:85–91. doi: 10.1037/a0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Berch DB, FHK, et al. Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association. 2002;288:2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcroft J, Sommers MS, Tye-Murray N, Mauzé E, Schroy C, Spehar B. Tailoring auditory training to patient needs with single and multiple talkers: Transfer-appropriate gains on a four-choice discrimination test. International Journal of Audiology. 2011;50:802–808. doi: 10.3109/14992027.2011.599868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Green CS, Pouget A, Schrater P. Brain plasticity through the life span: learning to learn and action video games. Annual Review of Neuroscience. 2012;35:391–416. doi: 10.1146/annurev-neuro-060909-152832. [DOI] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The influence of perceptual training on working memory in older adults. Plos ONE. 2010;5:e11537. doi: 10.1371/journal.pone.0011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella E, Carretti B, Riboldi F, De Beni R. Working memory training in older adults: Evidence of transfer and maintenance effects. Psychology and Aging. 2010;25:767. doi: 10.1037/a0020683. [DOI] [PubMed] [Google Scholar]
- Borella E, Carretti B, Zanoni G, Zavagnin M, De Beni R. Working memory training in old age: an examination of transfer and maintenance effects. Archives of Clinical Neuropsychology. 2013;28:331–347. doi: 10.1093/arclin/act020. [DOI] [PubMed] [Google Scholar]
- Burk MH, Humes LE, Amos NE, Strauser LE. Effect of training on word-recognition performance in noise for young normal-hearing and older hearing-impaired listeners. Ear & Hearing. 2006;27:263–278. doi: 10.1097/01.aud.0000215980.21158.a2. [DOI] [PubMed] [Google Scholar]
- Burkard R, Hecox KE. The effect of broadband noise on the human brain-stem auditory evoked response. III. Anatomic locus. The Journal of the Acoustical Society of America. 1987;81:1050. doi: 10.1121/1.394677. [DOI] [PubMed] [Google Scholar]
- Byrne D, Dillon H. The National Acoustic Laboratories’ (NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear & Hearing. 1986;7:257–265. doi: 10.1097/00003446-198608000-00007. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and Task-specific Age Effects on Brain Activity during Working Memory, Visual Attention and Episodic Retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Campbell T, Kerlin J, Bishop C, Miller L. Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography. Ear and Hearing. 2012;33:144–150. doi: 10.1097/AUD.0b013e3182280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carcagno S, Plack C. Subcortical plasticity following perceptual learning in a pitch discrimination task. Journal of the Assocation for Research in Otolaryngology. 2011;12:89–100. doi: 10.1007/s10162-010-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Ling L, Turner JG, Hughes LF. Inhibitory neurotransmission, plasticity and aging in the mammalian central auditory system. Journal of Experimental Biology. 2008;211:1781–1791. doi: 10.1242/jeb.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekaran B, Kraus N, Wong PCM. Human inferior colliculus activity relates to individual differences in spoken language learning. Journal of Neurophysiology. 2012;107:1325–1336. doi: 10.1152/jn.00923.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard C, Tremblay K. Aging degrades the neural encoding of simple and complex sounds. Journal of the American Academy of Audiology. 2013;24:590–599. doi: 10.3766/jaaa.24.7.7. [DOI] [PubMed] [Google Scholar]
- Coch D, Sanders LD, Neville HJ. An event-related potential study of selective auditory attention in children and adults. Journal of Cognitive Neuroscience. 2005;17:605–622. doi: 10.1162/0898929053467631. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. Hillsdale, N.J.; New York: Lawrence Erlbaum Associates; 1975. [Google Scholar]
- Creed MC, Ntamati NR, Tan KR. VTA GABA neurons modulate specific learning behaviours through the control of dopamine and cholinergic systems. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin E, Nyberg L, Backman L, Neely AS. Plasticity of executive functioning in young and older adults: immediate training gains, transfer, and long-term maintenance. Psychology and Aging. 2008;23:720. doi: 10.1037/a0014296. [DOI] [PubMed] [Google Scholar]
- de Boer J, Thornton ARD. Neural correlates of perceptual learning in the auditory brainstem: efferent activity predicts and reflects improvement at a speech-in-noise discrimination task. The Journal of Neuroscience. 2008;28:4929–4937. doi: 10.1523/JNEUROSCI.0902-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, He Y, Arnott SR, Ross B, Wu X, Li L, Alain C. Rapid Tuning of Auditory “What” and “Where” Pathways by Training. Cerebral Cortex. 2013:bht251. doi: 10.1093/cercor/bht251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahle M. Perceptual learning: specificity versus generalization. Current Opinion in Neurobiology. 2005;15:154–160. doi: 10.1016/j.conb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Gordon-Salant S, Friedman SA. Effects of age and sequence presentation rate on temporal order recognition. The Journal of the Acoustical Society of America. 2006;120:991–999. doi: 10.1121/1.2214463. [DOI] [PubMed] [Google Scholar]
- Gleich O, Hamann I, Klump GM, Kittel M, Strutz J. Boosting GABA improves impaired auditory temporal resolution in the gerbil. NeuroReport. 2003;14:1877–1880. doi: 10.1097/00001756-200310060-00024. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Fitzgibbons P. Sources of age-related recognition difficulty for time-compressed speech. Journal of Speech, Language, and Hearing Research. 2001;44:709–719. doi: 10.1044/1092-4388(2001/056). [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Yeni-Komshian GH, Fitzgibbons PJ, Barrett J. Age-related differences in identification and discrimination of temporal cues in speech segments. The Journal of the Acoustical Society of America. 2006;119:2455–2466. doi: 10.1121/1.2171527. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature Reviews Neuroscience. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Zhang J, Zhu X, Cai R, Zhou X, Sun X. Auditory discrimination training rescues developmentally degraded directional selectivity and restores mature expression of GABAA and AMPA receptor subunits in rat auditory cortex. Behavioural Brain Research. 2012;229:301–307. doi: 10.1016/j.bbr.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clinical Neurophysiology. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Hughes LF, Turner JG, Parrish JL, Caspary DM. Processing of broadband stimuli across A1 layers in young and aged rats. Hearing Research. 2010;264:79–85. doi: 10.1016/j.heares.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari Z, Omidvar S, Jafarloo F. Effects of ageing on speed and temporal resolution of speech stimuli in older adults. Medical Journal of The Islamic Republic of Iran (MJIRI) 2013;27:195–203. [PMC free article] [PubMed] [Google Scholar]
- Killion M, Niquette P, Gudmundsen G, Revit L, Banerjee S. Development of a quick speech-in-noise test for measuring signal-to-noise ratio loss in normal-hearing and hearing-impaired listeners. Journal of the Acoustical Society of America. 2004;116:2935–2405. doi: 10.1121/1.1784440. [DOI] [PubMed] [Google Scholar]
- Klatt D. Software for a cascade/parallel formant synthesizer. Journal of the Acoustical Society of America. 1980;67:971–995. [Google Scholar]
- Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nature Reviews Neuroscience. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- Krishnan A, Gandour JT, Bidelman GM. The effects of tone language experience on pitch processing in the brainstem. Journal of Neurolinguistics. 2010;23:81–95. doi: 10.1016/j.jneuroling.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach ND, Nodal FR, Cordery PM, King AJ, Bajo VM. Cortical Cholinergic Input Is Required for Normal Auditory Perception and Experience-Dependent Plasticity in Adult Ferrets. The Journal of Neuroscience. 2013;33:6659–6671. doi: 10.1523/JNEUROSCI.5039-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KZH, Lindenberger U. Relations between aging sensory/sensorimotor and cognitive functions. Neuroscience Biobehavioral Reviews. 2002;26:777–783. doi: 10.1016/s0149-7634(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Li SC, Schmiedek F, Huxhold O, Rocke C, Smith J, Lindenberger U. Working memory plasticity in old age: practice gain, transfer, and maintenance. Psychology and Aging. 2008;23:731. doi: 10.1037/a0014343. [DOI] [PubMed] [Google Scholar]
- Lin F, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. Journal of the American Medical Association Internal Medicine. 2013;173:293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ, Gonzalez VB. Auditory evoked response to gaps in noise: Older adults. International Journal of Audiology. 2011;50:211–225. doi: 10.3109/14992027.2010.526967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: scientific bases for a novel intervention. In: Aage RM, editor. Progress in brain research. Vol. 157. Elsevier; 2006. pp. 81–109. [DOI] [PubMed] [Google Scholar]
- Nacke LE, Nacke A, Lindley CA. Brain training for silver gamers: Effects of age and game form on effectiveness, efficiency, self-assessment, and gameplay experience. Cyberpsychology Behavior. 2009;12:493–499. doi: 10.1089/cpb.2009.0013. [DOI] [PubMed] [Google Scholar]
- Nahum M, Nelken I, Ahissar M. Low-level information and high-level perception: The case of speech in noise. PLoS biology. 2008;6:e126. doi: 10.1371/journal.pbio.0060126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oba SI, Fu QJ, Galvin JJI. Digit training in noise can improve cochlear implant users’ speech understanding in noise. Ear & Hearing. 2011;32:573–581. doi: 10.1097/AUD.0b013e31820fc821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annual Reviews of Psychology. 2009;60:173. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Bartlett EL. Age-related auditory deficits in temporal processing in F-344 rats. Neuroscience. 2011;192:619–630. doi: 10.1016/j.neuroscience.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Peelle JE, Wingfield A. Dissociations in perceptual learning revealed by adult age differences in adaptation to time-compressed speech. Journal of Experimental Psychology: Human Perception and Performance. 2005;31:1315. doi: 10.1037/0096-1523.31.6.1315. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller M, Schneider B, Daneman M. How young and old adults listen to and remember speech in noise. Journal of the Acoustical Society of America. 1995;97:593–608. doi: 10.1121/1.412282. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Levitt H. Speech comprehension training and auditory and cognitive processing in older adults. American Journal of Audiology. 2012;21:351. doi: 10.1044/1059-0889(2012/12-0025). [DOI] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. Journal of Neurophysiology. 2007;98:253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Rudner M, Rönnberg J, Lunner T. Working memory supports listening in noise for persons with hearing impairment. Journal of the American Academy of Audiology. 2011;22:156–167. doi: 10.3766/jaaa.22.3.4. [DOI] [PubMed] [Google Scholar]
- Sabin A, Clark C, Eddins D, Wright B. Different patterns of perceptual learning on spectral modulation detection between older hearing-impaired and younger normal-hearing adults. Journal of the Assocation for Research in Otolaryngology. 2013;14:283–294. doi: 10.1007/s10162-012-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T. Constraints on theories of cognitive aging. Psychonomic Bulletin & Review. 1996;3:287–299. doi: 10.3758/BF03210753. [DOI] [PubMed] [Google Scholar]
- Skoe E, Kraus N. Auditory brain stem response to complex sounds: A tutorial. Ear & Hearing. 2010;31:302–324. doi: 10.1097/AUD.0b013e3181cdb272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. Journal of the American Geriatrics Society. 2009;57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: Biological mechanisms. Cerebral Cortex. 2012;22:1180–1190. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay K, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clinical Neurophysiology. 2003;114:1332–1343. doi: 10.1016/s1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Shahin AJ, Picton T, Ross B. Auditory training alters the physiological detection of stimulus-specific cues in humans. Clinical Neurophysiology. 2009;120:128–135. doi: 10.1016/j.clinph.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O’Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. The Journal of Neuroscience. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Age-related changes in glycine receptor subunit composition and binding in dorsal cochlear nucleus. Neuroscience. 2009;160:227–239. doi: 10.1016/j.neuroscience.2009.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Schwoch T, Carr KW, Anderson S, Strait DL, Kraus N. Older Adults Benefit from Music Training Early in Life: Biological Evidence for Long-Term Training-Driven Plasticity. The Journal of Neuroscience. 2013;33:17667–17674. doi: 10.1523/JNEUROSCI.2560-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, McArdle RA, Smith SL. An evaluation of the BKB-SIN, HINT, QuickSIN, and WIN materials on listeners with normal hearing and listeners with hearing loss. Journal of Speech, Language & Hearing Research. 2007;50:844–856. doi: 10.1044/1092-4388(2007/059). [DOI] [PubMed] [Google Scholar]
- Wong PCM, Ettlinger M, Sheppard JP, Gunasekera GM, Dhar S. Neuroanatomical characteristics and speech perception in noise in older adults. Ear & Hearing. 2010;31:471–479. doi: 10.1097/AUD.0b013e3181d709c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PCM, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia. 2009;47:693–703. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Zhu J, Garcia E. The Wechsler Abbreviated Scale of Intelligence (WASI) New York: Psychological Corporation; 1999. [Google Scholar]
- Zinke W, Roberts M, Guo K, McDonald J, Robertson R, Thiele A. Cholinergic modulation of response properties and orientation tuning of neurons in primary visual cortex of anaesthetized Marmoset monkeys. European Journal of Neuroscience. 2006;24:314–328. doi: 10.1111/j.1460-9568.2006.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]