Abstract
Nonalcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM) frequently coexist due to shared risk factors. Their rising prevalence parallels the growing epidemic of obesity and insulin resistance (IR). In patients with T2DM and biopsy-proven NAFLD, a significantly higher prevalence of nonalcoholic steatohepatitis (NASH) (63–87%), any fibrosis (22–60%), and advanced fibrosis (4–9%) is noted. Possible risk factors for more advanced liver disease include concomitant metabolic syndrome with three or more components, visceral obesity, older age, increased duration of diabetes, and family history of diabetes. Liver biopsy is strongly suggested in these patients. Cardiovascular disease (CVD) and malignancy are the leading causes of death in this population, but a growing body of evidence shows liver-related mortality as an important cause of death, including an increased rate of hepatocellular carcinoma (HCC) in diabetes. The presence of NAFLD in T2DM is also associated with increased overall mortality. We aim with this review to summarize the results from studies investigating NAFLD in T2DM and to outline the factors that predict more advanced liver histology as well as the impact of these hepatic changes on CVD, overall and liver-related mortality.
Keywords: NAFLD, NASH, Fibrosis, Diabetes, Insulin resistance, Obesity, Metabolic syndrome, Mortality, CVD
1. Introduction
For the last two decades, NAFLD and its more advanced form NASH have been increasingly recognized as major contributing factors for morbidity and mortality in patients with T2DM (Adams et al., 2010; Younossi, Gramlich, Matteoni, Boparai, & McCullough, 2004). The rising prevalence of NAFLD parallels the growing epidemic of obesity and related IR, T2DM and metabolic syndrome (MS). Recent analysis of National Health and Nutrition Examination Survey (NHANES) data from three periods between 1999 and 2008 revealed a steadily increasing prevalence of NAFLD (5.51% [1988–1994], 9.84% [1999–2004] 11.01% [2005–2008]), T2DM (5.55%, 7.88%, 9.11%), and obesity (35.18%, 48.16%, 51.43%), respectively (Younossi et al., 2011). NAFLD and T2DM frequently coexist and share common risk factors, in particular visceral obesity. Visceral fat accumulation significantly affects free fatty acid and glucose metabolism (Gastaldelli et al., 2007). This leads to liver fat deposition, hepatic inflammation, development of NASH and eventually fibrosis. NAFLD and NASH promote hepatic IR and increase the risk for incident T2DM or worsen the existing one (Byrne, 2012). T2DM, on the other hand, is a strong contributing factor for more advanced liver fibrosis (Angulo, Keach, Batts, & Lindor, 1999). This pathogenic mechanism links T2DM and NASH in an inextricable relationship.
The estimated prevalence of NAFLD (both simple steatosis and NASH) in T2DM is as high as 75% (Angulo, 2002; Medina, Fernández- Salazar, García-Buey, & Moreno-Otero, 2004) in comparison to 20–30% in the general population (Bellentani, Scaglioni, Marino, & Bedogni, 2010). Most of these data come from population-based studies in which the diagnosis of NAFLD and NASH were based on imaging studies and elevated liver enzymes. Despite several recently developed non-invasive scoring systems for the diagnosis and prognosis of NAFLD, liver biopsy remains the gold standard for assessment of necroinflammatory changes, cellular injury and degree of fibrosis in NASH. Changes in liver histology remain not only the best diagnostic tool but also the most sensitive and specific method for determining prognosis (Angulo & Lindor, 2002). This specifically applies to diabetic patients with NAFLD who were noted to have more advanced liver fibrosis, increased overall and liver-related mortality (Younossi et al., 2004) as well as increased risk of CVD independently of classical risk factors (Targher et al., 2005; Targher et al., 2007).
In the light of the above evidence, in this review, we aim to evaluate the likelihood of NASH among diabetics and factors that are predictive of NASH and advanced fibrosis in this population. We will also review specific histolologic changes in obese patients with T2DM and NASH as well as other histological alterations in diabetics that should be differentiated from NAFLD. Finally, we will assess the impact of liver histology in diabetics on cardiovascular, overall and liver-related mortality.
A PubMed search was undertaken using words such as “diabetes”, “nonalcoholic fatty liver disease”, “nonalcoholic steatohepatitis”, “liver histology”, “fibrosis”, “insulin resistance” and “obesity”. We reviewed one hundred and fifty original articles, reviews and meta-analyses in English and Spanish published from January 1990 to present. Only papers on adult NAFLD were used.
2. NAFLD in T2DM
2.1. Clinical predictors of NAFLD in T2DM
Most of the studies evaluating NAFLD in T2DM have relied exclusively on ultrasonography or elevated liver enzymes for diagnosis. This is mainly due to the high prevalence of NAFLD as well as the perceived risks and difficulty obtaining liver biopsies in a large population. However, non-invasive methods cannot reliably assess the degree of liver disease. NAFLD represents a spectrum of liver diseases ranging from isolated steatosis to NASH, the progressive form of NAFLD. NAFLD patients with isolated steatosis have macrovesicular fat accumulation exceeding 5% in the absence of excessive alcohol consumption or other known liver disease (Kleiner et al., 2005; Neuschwander-Tetri & Caldwell, 2002; Sanyal, 2011a). NASH, on the other hand, encompasses steatosis with lobular inflammation and ballooning degeneration with or without a varying degree of fibrosis that is centered on the hepatic venulae. It is generally accepted that patients with NASH, but not those with isolated (or also referred to as simple steatosis), can progress to cirrhosis (Kleiner et al., 2005; Sanyal, 2011a). For this reason, monitoring and reversal of NASH should improve liver-related outcomes, but additional studies are needed to show improvement in long-term outcomes with treatment of NASH. Examples of simple steatosis and NASH are shown in Figs. 1 and 2, respectively.
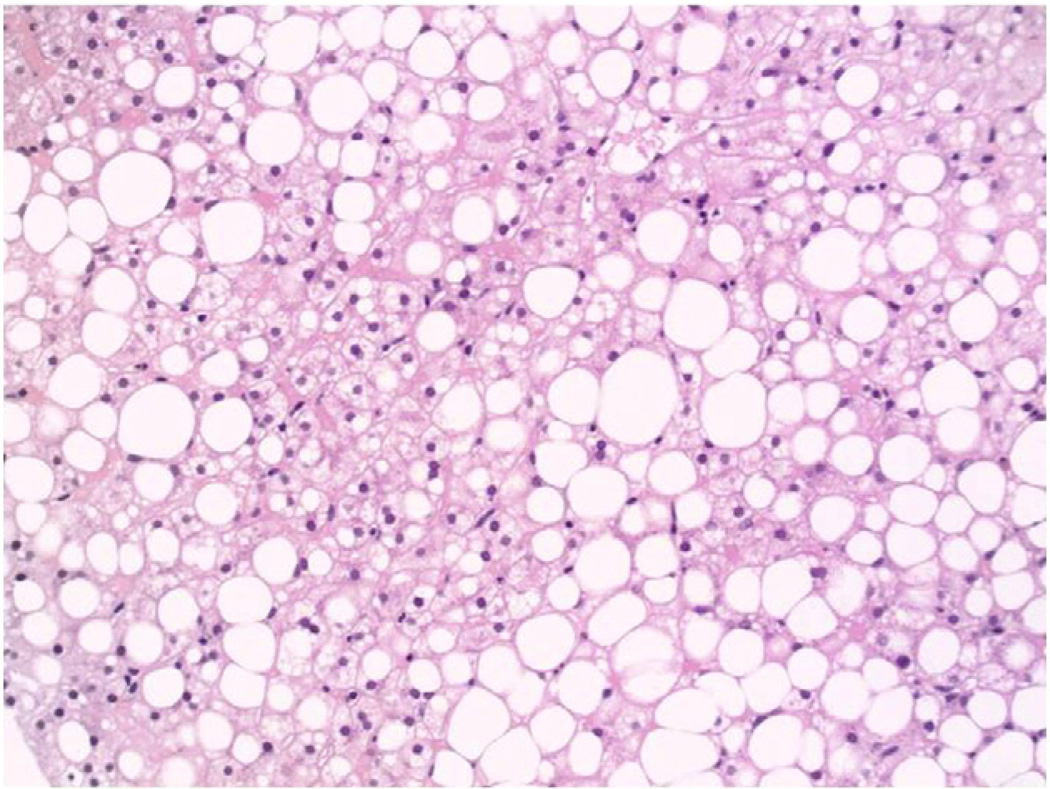
Fig. 1.
(20×, H&E): Liver biopsy illustrating severe steatosis, without significant inflammation or hepatocellular ballooning. Much of the fat is found in large cytoplasmic vacuoles, termed macrovesicular steatosis.
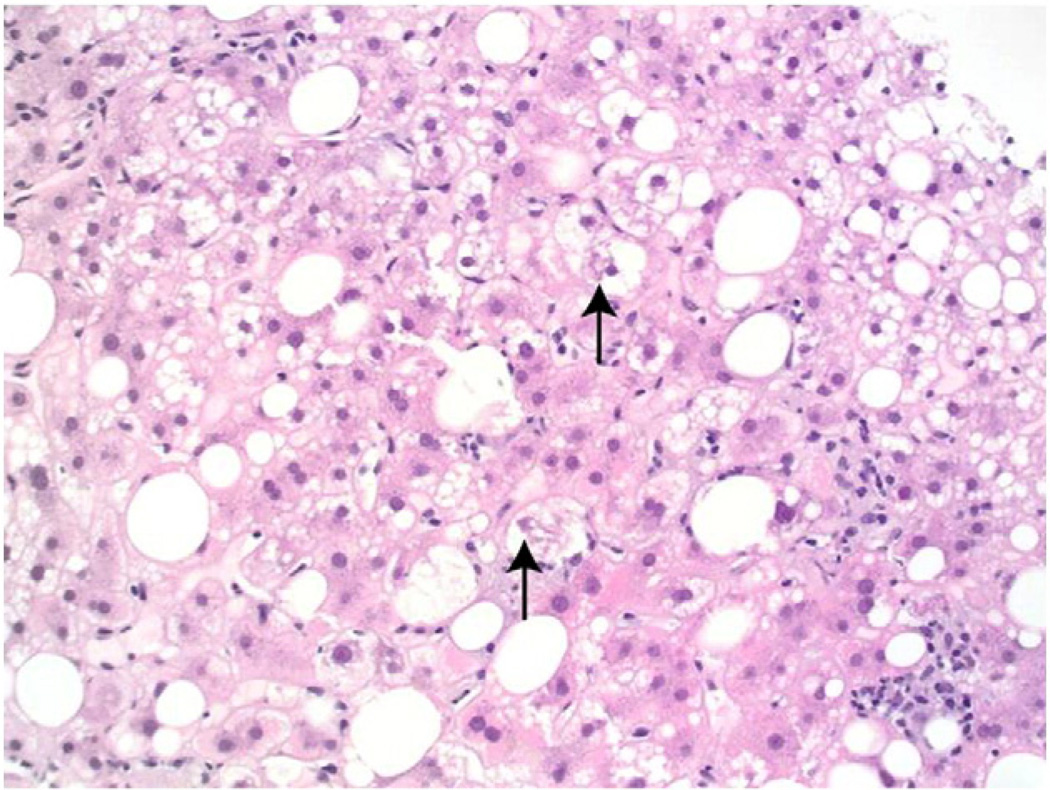
Fig. 2.
(20×, H&E): Liver biopsy showing steatosis, as well as inflammation and hepatocellular ballooning, diagnostic of steatohepatitis. The arrows indicate Mallory- Denk bodies within ballooned hepatocytes. Two microgranulomas are also observed on the middle and lower right.
Histopathological scoring systems have been proposed by Matteoni et al. for NAFLD (Matteoni et al., 1999) and by Brunt et al. for NASH (Brunt, Janney, Di Bisceglie, Neuschwander-Tetri, & Bacon, 1999). In 2005, Kleiner et al. introduced the NAFLD activity score (NAS) for use in clinical trials in NASH. It semiquantitatively measures steatosis (graded 0–3), lobular inflammation (0–3), and hepatocellular ballooning (0–2) with NAS ranging from 0 to 8. A NAFLD activity score≥5 correlates well with a diagnosis of NASH (Kleiner et al., 2005). After reviewing large clinical and histological data, Brunt et al. recently cautioned that a numerical value alone is not sufficient for definitive diagnosis of steatohepatitis and NAS remains a more subjective assessment of liver biopsy by an experienced pathologist (Brunt et al., 2011).
2.2. Prevalence of NAFLD in T2DM
The higher prevalence of NAFLD and NASH in T2DM has been demonstrated in a recent prospective study of 328 asymptomatic patients, 16.5% of whom had T2DM (Williams et al., 2011). Patients initially underwent an abdominal ultrasound and if steatosis was present, a liver biopsy was performed. The prevalence of NAFLD and NASH in diabetics vs. the entire cohort was 74% vs. 46% and 22% vs. 12%, respectively. Increased ALT and insulin levels were independent predictors of NASH in the entire cohort and patients with significant fibrosis (stage 2–4) were more insulin-resistant than those with mild fibrosis (Williams et al., 2011). These results illustrate not only the crucial role of T2DM on liver histology, but also a possible independent relationship between IR and NASH fibrosis in non-diabetic patients. Another recent study assessing the prevalence of NAFLD in T2DM included 939 older subjects (age 61–76 years) who were participants in the Edinburg Type 2 Diabetes Study. After exclusion of all other possible causes for liver disease, they found a 43% prevalence of NAFLD based on the finding of grade 3 steatosis on ultrasonography (Williamson et al., 2011). This may be an underestimate due to a low sensitivity of ultrasound in the diagnosis of NAFLD, especially when liver steatosis is less than 30% (Saadeh et al., 2002). Liver biopsy, however, remains the gold standard for identifying the degree of liver changes.
Several studies have assessed liver histology in diabetics and correlated this with risk factors (Amarapurkar et al., 2006; Gupte et al., 2004; Leite et al., 2011; Prashanth et al., 2009). We will review their findings and will discuss possible predictive factors.
All 4 studies included only patients with T2DM (Amarapurkar et al., 2006; Gupte et al., 2004; Leite et al., 2011; Prashanth et al., 2009). Subjects were initially screened with ultrasound and offered a liver biopsy if steatosis was found and/or if elevated liver enzymes were noted. The main goal of the studies was to investigate the prevalence of NAFLD in diabetes and to identify independent predictors of NASH and advanced fibrosis in this population. Baseline characteristics of the patients and the most important findings are shown in Table 1. The prevalence of NASH was 63–87%, any fibrosis was found in 22–60%, with stage 3 fibrosis or cirrhosis in 3–9%. Elevated liver enzymes were detected only in 13–31%. Alanine aminotransferase (ALT) was independently associated with NASH in the study by Leite et al. (p=0.044) (Leite et al., 2011). Prashanth al. found that in NASH patients ALT and alkaline phosphatase (ALP) were significantly higher, although within normal limits, in comparison to those with normal biopsy (Prashanth et al., 2009). However, in the study by Gupte et al. there was no significant correlation between liver enzymes and NASH or fibrosis (Gupte et al., 2004). Only gamma-glutamyl transpeptidase (GGT) correlated with degree of fibrosis (p=0.002) (Leite et al., 2011).
Table 1.
Patient characteristics and liver histology in diabetic patients undergoing liver biopsy.
| Study | N Patients | Mean Age |
Female (%) |
BMI (kg/m2) |
DM Duration (years) |
HTN (%) |
HL (%) |
↑LFTs (%) |
GGT (%) |
NASH (%) |
Fibrosis (%) |
Cirrhosis (%) |
Predictors for NASH |
Predictors for fibrosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leite et al., 2011 Brazil | 92 | 55.6 | 64 | 31.4 | 7.8 | 88 | 86 | AST 14 ALT 16 |
13 | 78 | 34–60 | 3 | ↑Tg, ↓HDL, ↑ALT | ↑GGT for any fibrosis + age, male sex for advanced |
| Prashanth et al., 2009 India | 83 | 54.3 | 66 | 26.4 | 8.2 | 60 | 84 | 63 | 37.3 | 4 (stage 3 and 4) | ↑ALT | |||
| Amarapurkar et al., 2006 India | 36 | 50.8 | 47 | 24.9 | 80.6 | 30.5 | ↑AST,↑ALT, ↑AST/ALT | |||||||
| Gupte et al., 2004 India | 32 | 63 | 31 | 87 | 21.8, adv.9.3 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DM, diabetes mellitus; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; HL, hyperlipidemia; HTN, hypertension; LFTs, liver function tests; NASH, nonalcoholic steatohepatitis; Tg, triglycerides.
Advanced fibrosis refers to histological fibrosis stages 3 and 4.
The significance of liver enzymes in NAFLD was assessed by an Italian study which evaluated 458 liver biopsies of subjects with and without elevated ALT and presumed NAFLD (Fracanzani et al., 2008). The cutoff point for ALT was 40 U/L, but in the sensitivity analysis the authors used the upper limit of normal suggested by Prati et al. for ALT of 19 U/L for women and 30 U/L for men (Prati et al., 2002). Fifty nine percent of those with normal ALT had NASH (Fracanzani et al., 2008). The prevalence of fibrosis was similar in both groups. In patients with increased liver enzymes, diabetes was the strongest independent predictor of fibrosis≥2(OR 3.2, p=0.0001) while in those with normal ALT, homeostatic model assessment of insulin resistance (HOMA-IR) index was the only independent variable associated with fibrosis≥2 (OR 1.97, p=0.02) (Fracanzani et al., 2008). A similar discordance between ALT and fibrosis was seen in an earlier study where 51 patients with normal ALT underwent liver biopsies for unexplained hepatomegaly or as potential living donors (Mofrad et al., 2003). Diabetes was the only independent risk factor for advanced fibrosis in this group (OR 2.3, p<0.01). Based on these data, we propose that in patients with T2DM higher ALT increases the odds of NASH on biopsy, but normal ALT does not exclude NASH or fibrosis and additional risk factors should be sought.
2.3. Role of metabolic syndrome and its components
The presence of MS has been associated with a higher risk of NASH (OR 3.2, p=0.026) and more advanced fibrosis (OR 3.5, p=0.032) in non-diabetic patients (Marchesini et al., 2003). In diabetics, the prevalence of NASH rose from 54% to 83% if metabolic syndrome components increased from 3 to 5 (Prashanth et al., 2009). In addition, MS was present in all patients with advanced fibrosis (stage 3 or 4) (Prashanth et al., 2009). Hypertriglyceridemia and low HDL-cholesterol, the most typical metabolic pattern of dyslipidemia, are frequently encountered in T2DM and in more than 50% of patients with NAFLD (Smith & Adams, 2011). Leite et al. found that high triglycerides (TG) and low HDL-cholesterol independently correlate with NASH, but MS was not analyzed separately in this study (Leite et al., 2011). The underlying pathogenic mechanism of this correlation most likely includes hyperinsulinemia which leads to increased TG formation and decreased release of TG in the form of very low-density lipoprotein (VLDL)(Medina et al., 2004). Additionally, NASH patients were found to have elevated levels of both fasting and postprandial TG, which is associated not only with increased liver fat, but also with enhanced atherosclerotic risk (Cassader et al., 2001). This finding was confirmed in another study which attributed the reduced TG clearance to genetic variants in the allele of apolipoprotein C3 (APOC3) and linked this to NAFLD in lean individuals (Petersen et al., 2010).
Another important component of MS is visceral obesity. Prashanth et al. found that 66% of patients with truncal obesity had NASH and 40% had fibrosis, compared to 40% and 21%, respectively, in those without truncal obesity (Prashanth et al., 2009).The pivotal role of visceral obesity is illustrated in the study of Kelley et al. where patients with T2DM who met computer tomography (CT) criteria for NAFLD were compared to lean and obese non-diabetic subjects with NAFLD (Kelley, McKolanis, Hegazi, Kuller, & Kalhan, 2003). In the diabetic cohort, a decreased liver to spleen ratio for CT attenuation, as a marker for NAFLD, most strongly correlated to visceral adipose tissue (VAT) (r=−0.64, p<0.0001). There was no correlation with overall fat mass and subcutaneous adiposity (Kelley et al., 2003). These data suggest that visceral obesity correlates well with hepatic steatosis among patients with T2DM.
2.4. Role of age
Advanced age appears to be an important risk factor for NASH and advanced fibrosis in patients with NAFLD (Angulo et al., 1999). Studies are emerging and these data would help refine the management of patients with NAFLD with and without existing diabetes (Noureddin et al., 2011).
2.5. Role of family history of diabetes
Family history of diabetes should be routinely obtained in patients with NAFLD. Chitturi et al. showed that high prevalence (39%) of positive family history of diabetes mellitus (DM) was associated with histologically proven NASH in non-diabetics (Chitturi et al., 2002). A very recent large study on 1069 patients confirmed this finding and demonstrated that in non-diabetics, family history of diabetes increased the risk of NASH and any fibrosis with an adjusted-odds ratio (OR) of 1.51, p=0.04 and OR 1.49, p=0.04, respectively. Furthermore, it reinforced the strong association between DM and risk of NASH (age-sex-BMI-adjusted OR of 2.48, p=0.01), any fibrosis (OR 2.94, p<0.01) and advanced fibrosis (OR 6.03, p<0.0001) (Loomba et al., 2012). The study highlights the importance of family history of diabetes in stratifying patients with NAFLD for advanced liver histology, specifically in non-diabetics, although in those with T2DM it can be also useful.
2.6. Practical recommendations: When to consider liver biopsy in diabetes?
Table 2 summarizes the possible risk factors for NAFLD in diabetic and prediabetic patients. Overall, in patients with T2DM there are no definite predictors of NASH and fibrosis. The presence of MS with greater number of components, visceral obesity, hypertriglyceridemia, higher aspartate aminotransferase (AST) to ALT ratio (Williams & Hoofnagle, 1988), lower platelet count (suggesting portal hypertension and seen in advanced fibrosis) (Bashour, Teran, & Mullen, 2000), older age, and diabetes duration might be predictive for more advanced liver disease. Liver biopsy should be considered in these patients. Some of the outlined above risk factors are components of the NAFLD Fibrosis Score (a noninvasive score, consisting of age, BMI, impaired fasting glucose/diabetes, AST/ALT ratio, platelet count and albumin), which can predict a presence or absence of advanced fibrosis in NAFLD (Angulo et al., 2007).
Table 2.
Risk factors for NASH in prediabetics and diabetic patients with NAFLD: Who to biopsy?
| Risk factors | |
|---|---|
| Diabetic patients at high risk of NASH and/or advanced fibrosis | Older age |
| Concomitant metabolic syndrome, especially 3 or more features | |
| Visceral adiposity | |
| Longer duration of diabetes | |
| Family history of diabetes | |
| High GGT | |
| Higher AST/ALT ratio | |
| Prediabetics at increased risk for NAFLD | Older age |
| Family history of diabetes | |
| High GGT | |
| Higher AST/ALT ratio |
2.6.1. Prevalence of NASH, cirrhosis and hepatocellular carcinoma (HCC) in diabetics and non-diabetics
Few studies have compared the degree of inflammation and fibrosis between diabetics and non-diabetics. Most of them focused on NASH in the stage of cirrhosis and HCC. Younossi et al. retrospectively evaluated132 patients with histologically proven NAFLD, of which 33% had DM. Cirrhosis occurred in 25% of diabetics with NAFLD vs. 10% of non-diabetics with NAFLD (p=0.04) (Younossi et al., 2004).When diabetics were compared to non-diabetics, both overall mortality (56.8% vs. 27.3%, p=0.001) and liver-related mortality (18.2 vs. 2.3%, p=0.02) were higher in diabetic group and remained statistically significant even after adjustment for potential confounders. DM was also the single most important predictor for severe NASH and fibrosis in a Chinese study with 42 patients (Wong et al., 2004). Furthermore, the authors noted that in patients without DM, higher fasting glucose correlated with more severe histology (Wong et al., 2004). In a large prospective cohort study conducted among 173,643 veterans with T2DM and 650,620 without diabetes, T2DM doubled the risk of chronic nonalcoholic liver disease and HCC (El-Serag, Tran, & Everhart, 2004). Diabetes carried the highest risk among patients with longer than 10 years of follow-up. However, this study has several limitations: First, it was based on computerized national database of the Department of Veterans Affairs (VA) and hence 98% of the patients were men. Additionally, the duration of diabetes remains disputable as it was accepted based on index hospitalization with this diagnosis, and finally, although the authors excluded history of prior liver diseases and left out in their analysis all subjects with incident hepatitis B, hepatitis C or alcoholic liver disease during the follow-up period, there was no screening for these diseases. Despite these limitations, similar findings were observed in another population-based case-control study which found that T2DM was associated with an almost threefold increase in the risk of HCC after adjusting for other major risk factors, including hepatitis C, hepatitis B, alcohol, and hemochromatosis (OR 2.87, CI 2.49–3.30) (Davila, Morgan, Shaib, McGlynn, & El-Serag, 2005). Furthermore, there is a growing number of case series and reports describing HCC associated with NASH in non-cirrhotic livers in the setting of T2DM and other metabolic components (Ertle et al., 2011; Guzman et al., 2008; Takuma & Nouso, 2010). This raises the important question of the need for regular surveillance in this population.
2.6.2. The role of diabetes in NASH and fibrosis in morbidly obese patients
Several studies have evaluated liver biopsies in obese patients performed at the time of bariatric surgery. We will review seven observational studies which included 14–28% prevalence of diabetics in their cohorts (Beymer et al., 2003; Boza et al., 2005; Dixon, Bhathal, & O'Brien, 2001; Gholam, Flancbaum, Machan, Charney, & Kotler, 2007; Lima, Mourão, Diniz, & Leite, 2005; Ong et al., 2005; Spaulding, Trainer, & Janiec, 2003). All the studies controlled for other etiologies of liver disease, including alcohol use, and only Dixon et al. included nine patients with significant alcohol intake. Baseline characteristics of the patients and the most important findings of the studies are demonstrated in Table 3. In summary, this population consisted predominantly of females (62–83%), mean age 39–42, and mean BMI ranging between 42–59 kg/m2. The overall prevalence of NAFLD was 85–99% and of NASH was 24–58%. The broad range in NASH prevalence can probably be explained by ethnic and geographical differences between patients in the studies, use of different scoring systems and interobserver variability. Any fibrosis was noted in 22–49%, advanced (bridging) fibrosis was reported separately in three studies and ranged between 9% and 12% (Beymer et al., 2003; Dixon et al., 2001; Ong et al., 2005). Only one or two patients per study group (0.9–2%) were found to have cirrhosis with no symptoms. In all seven studies NASH was more prevalent in patients with T2DM in comparison to non-diabetics. In three studies (Beymer et al., 2003; Dixon et al., 2001; Gholam et al., 2007) fibrosis was more prevalent in diabetics vs. non-diabetics, and Ong et al. found more advanced fibrosis in T2DM (Ong et al., 2005). Insulin resistance, quantified by HOMA-IR index was predictive of both NASH and fibrosis in one study (Gholam et al., 2007). Dixon et al. also found that IR, assessed by both HOMA and quantitative insulin sensitivity check index (QUICKI), was a predictor of NASH, and particularly of zone 3 steatosis, inflammation and fibrosis. However, elevated C-peptide alone was a predictor of advanced fibrosis (stage 3 and 4), thus suggesting the more important role of hyperinsulinemia in this process (Dixon et al., 2001). Further evidence of this is noted in a recent study of 173 biopsy-proven NAFLD patients without a history of DM who underwent an oral glucose tolerance test (OGTT) showing that postprandial hyperinsulinemia at 120 minutes was associated with advanced fibrosis (Kimura et al., 2011). In order to better predict the threshold for HOMA-IR, Boza et al. constructed receiver operating characteristic curves demonstrating that patients with HOMA-IR>5.8 and AST>31 IU/L had an increased risk of NASH (50% vs. 7.8% if values were below this threshold). When T2DM was included in this model, the risk of NASH rose to 75% (Boza et al., 2005). Gholam et al. also developed two mathematical models related to this. The first used AST and diagnosis of diabetes to predict the presence of NASH. A second model used ALT and hemoglobin A1C to predict fibrosis (Gholam et al., 2007). Interestingly, BMI was not a predictive factor in any of the seven studies, neither was age, but the latter finding could be explained with the relatively young age of the patients in all groups. Similar to the studies involving only diabetic patients (Amarapurkar et al., 2006; Gupte et al., 2004; Leite et al., 2011; Prashanth et al., 2009), the studies with obese patients had a low prevalence of elevated transaminases (12–18% for AST and 23–30% for ALT) (Boza et al., 2005; Dixon et al., 2001; Gholam et al., 2007). However, if elevated, AST and/or ALT were independent predictors of NASH (Boza et al., 2005; Spaulding et al., 2003) or of both NASH and fibrosis (Dixon et al., 2001; Gholam et al., 2007).
Table 3.
Characteristics of obese patients undergoing liver biopsy during bariatric study.
| Study | N patients | Age | Female (%) |
BMI (kg/m2) |
T2DM (%) |
↑LFTs (%) |
HTN (%) |
DLP (%) | NAFLD (%) |
NASH (%) | Fibrosis % (total/ advanced) |
Cirrhosis (%) |
Independent predictors for NASH |
Independent predictors for fibrosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Dixon et al., 2001 Australia |
105 | 41 ± 11 | 78 | 47 ± 7 | 18 | AST 18 ALT 30 |
38 | 96 | 25 | 25/9 | 1 | HOMA-IR, ↑ALT, HTN | ↑ALT, HTN, ↑ C-peptide | |
|
Beymer et al., 2003 USA |
48 | 42 ± 10 | 69 | 59.9 ± 12 | 19 | ↑Tg in 23 | 85 | 33 | 35/12 | none | DM (OR 128, CI 5.2–3137) | Severe fibrosis greater in DM than in non-DM (OR 75, CI 7.5–1247) |
||
|
Spaulding et al., 2003 USA |
48 | 84 | 51 ± 20 | 16 | 18 | 47 | 61 | 90 | 56 | 49/NA | 2 | DM (p=0.029), abnormal LFTs (p=0.029) |
||
|
Ong et al., 2005 USA |
212 | 42 ± 10 | 80 | 48 ± 9 | 24 | 45 | 30 | 93 | 24 | 23.5/7 | 0.9 | DM, male gender, ↑ALT | Waist/hip ratio, AST, focal necrosis | |
|
Lima et al., 2005 Brazil |
112 | 40 ± 10 | 74 | 49 ± 8 | 28 | 68 | 40 | 99 | 58 (type 3 and 4 NAFLD) |
21/14 | none | Type 4 NAFLD correlated with T2DM (p=0.018) |
||
|
Boza et al., 2005 Chile |
127 | 40 ± 11 | 62 | 42 ± 6 | 14 | 41 | 63 | 26 | 22/1.6 | 1.6 | HOMA-IR>5.8, AST>31 | |||
|
Gholam et al., 2007 USA |
97 | 39 ± 10 | 83 | 55 ± 12 | 24 | 26 AST 12 ALT 23 |
43 | ↑Tg in 35 ↓HDL in 59 |
89 | 36 | 25/NA | none | DM, AST | ALT, HbA1C |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; DLP, dyslipidemia; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; HTN, hypertension; LFTs, liver function tests; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; T2DM, type 2 diabetes mellitus; Tg, triglycerides.
Age, mean ± standard deviation.
Independent predictors for NASH/fibrosis refer to variables that are independently associated with a diagnosis of NASH/fibrosis.
Advanced fibrosis refers to histological fibrosis stages 3 and 4.
Type 3 and type 4 NAFLD refers to the NAFLD classification system proposed by Matteoni et al.: type 1 (steatosis), type 2 (steatosis and lobular inflammation), Type 3 (steatosis, lobular inflammation and hepatocellular ballooning), type 4 (similar to type 3+ fibrosis and/or Mallory's hyaline) (Matteoni et al., 1999).
An interesting histological finding in the study of Lima et al. was that the presence of glycogenated nuclei correlated with steatosis, lobular inflammation and perisinusoidal fibrosis but not with diabetes (Lima et al., 2005). In contrast, a previous study showed that glycogenated nuclei are significantly more prevalent in obese patients with DM than in those without DM, a fact that suggests an accelerated senescence of the liver in patients who have diabetes and NAFLD (Silverman et al., 1990).
2.7. Recommendations for liver biopsy in patients undergoing bariatric surgery
Patients with NAFLD who are undergoing bariatric surgery should be considered for a liver biopsy during the surgery. Patients with diabetes and metabolic syndrome in particular are at increased risk of NASH and advanced fibrosis, therefore a liver biopsy is strongly suggested. Other variables to consider include lower platelet count and higher AST/ALT ratio.
3. Special clinicopathologic entities in diabetics
3.1. Glycogenichepatopathy (GH)
The term GH was initially introduced by Torbenson et al. based on a review of 14 cases of poorly controlled type 1 diabetes mellitus (DM), hepatomegaly and varying degrees of elevated transaminases. Liver biopsies characteristically showed enlarged and pale hepatocytes with excessive glycogen accumulation demonstrated on periodic acid- Schiff stain as well as glycogenated nuclei and giant mitochondria. Liver architecture was preserved with no or minimal steatosis, inflammation and fibrosis (Torbenson et al., 2006) (Fig. 3). GH has been reported to recur in parallel with diabetic decompensation (van den Brand, Elving, Drenth, & van Krieken, 2009) and resolves when diabetes is controlled (Hudacko, Manoukian, Schneider, & Fyfe, 2008), with repeat liver biopsy showing normal liver histology (Torbenson et al., 2006).
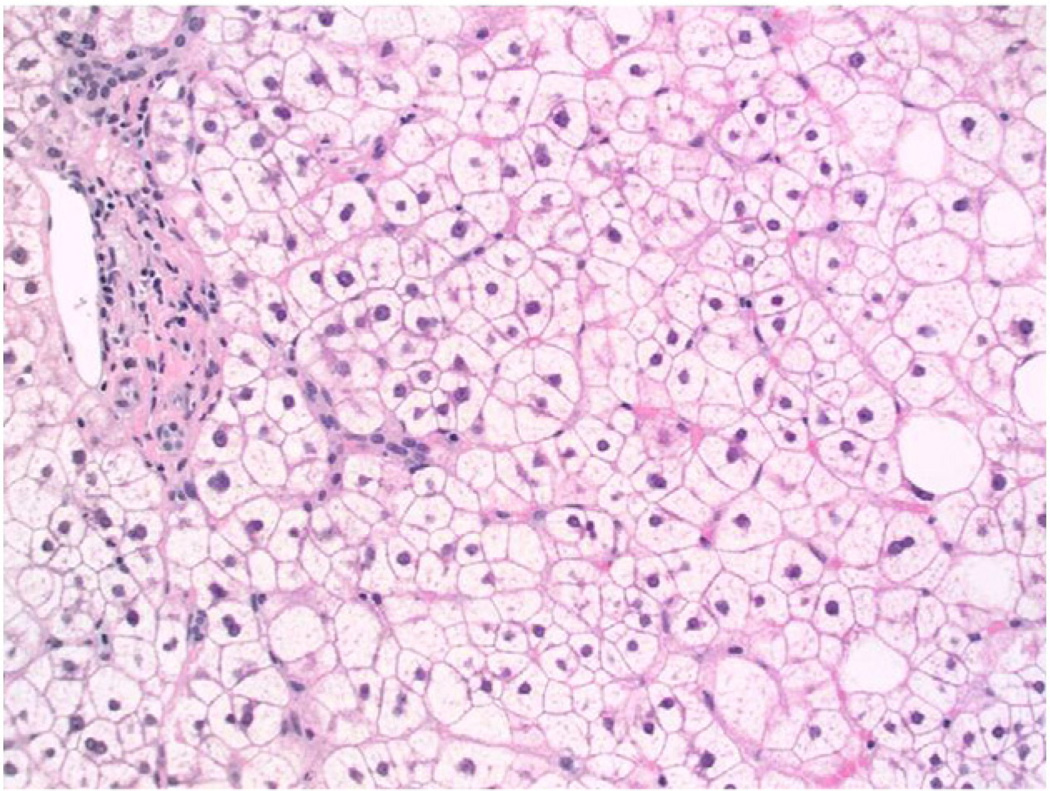
Fig. 3.
(20×, H&E): Liver biopsy showing glycogenic hepatopathy. The hepatocytes are enlarged and pale, but without steatosis. For comparison, several hepatocytes with macrovesicular steatosis are seen on the right aspect of the image.
3.2. Diabetic hepatosclerosis (DH)
DH is an uncommon pattern of liver disease seen only in patients with longstanding severe type 1 or 2 DM and multiorgan microangiopathy with end-organ damage. Harrison and colleagues reviewed 12 cases and described dense perisinusoidal fibrosis with immunohistochemical staining positive for laminin and collagen IV without co-existent NAFLD on biopsies. In addition to capillarization of sinusoids, histology was also notable for perivenular fibrosis and hepatic artery thickening (Harrison, Brunt, Goodman, & Di Bisceglie, 2006). Two more recent autopsy studies examined DH and found only one case in 57 diabetic patients and 12% in 159 cases (Hudacko, Sciancalepore, & Fyfe, 2009; Chen & Brunt, 2009). In all reports, patients with DH had concomitant severe diabetic nephropathy but no other definite risk factors have been identified (Harrison et al., 2006; Chen & Brunt, 2009; Hudacko et al., 2009). The exact pathogenic mechanism and clinical significance of DH have not been elucidated yet.
3.3. Hepatitis C virus infection (HCV), glucose metabolism and steatosis
Currently there is convincing evidence that HCV alters glucose metabolism by inducing hepatic IR even at early stages of infection. IR is related to severity of liver steatosis, as it can progress to overt T2DM and trigger metabolic derangements (Bugianesi, Salamone, & Negro, 2012). The prevalence of steatosis in HCV ranges between 20 and 30% (Sanyal, 2011b), but in patients with HCV genotype 3 it has been reported to be as high as 77% (Adinolfi et al., 2001). Only in HCV genotype 3, the severity of steatosis correlates with viremia level (Adinolfi et al., 2001) and reduces significantly with antiviral treatment (Poynard et al., 2003). However, in patients with other HCV genotypes, NAFLD is strongly associated with metabolic syndrome and its components (Sanyal et al., 2003). Diabetes is an independent predictor of advanced fibrosis in HCV patients (Sanyal et al., 2003) and IR-induced inflammatory cascade is considered an important contributor in the pathogenesis of HCC in this population (Sanyal, Yoon, & Lencioni, 2010). Insulin resistance, T2DM, steatosis, and the resultant accelerated fibrosis in HCV lead to a reduced antiviral response (Bugianesi, Salamone, & Negro, 2012). The exception to this is steatosis in HCV genotype 3, which is not associated with fibrosis, suggesting a different pathogenic mechanism (Bugianesi et al., 2006). Thus, it is important to exclude HCV infection in all patients with T2DM and NAFLD, and if present, comorbidities must be managed promptly.
4. Role of liver histology in DM in predicting CVD and liver-related mortality
Cardiovascular disease is the leading cause of mortality in type 2 diabetic patients (Morrish, Wang, Stevens, Fuller, & Keen, 2001). The question of whether NAFLD in T2DM is an independent risk factor for CVD or just a coexisting feature is still controversial. Targher et al. carried out a prospective nested case-control study including participants from the Valpolicella Heart Diabetes Study. They assessed incident CVD over 5 years of follow-up and concluded that NAFLD was an independent predictor of CVD in diabetics (OR 1.84, p<0.001). This association remained significant, although attenuated, even after adjustment for MS (Targher et al., 2005). Another study from the same authors included 2,839 diabetics with a 69.5% prevalence of NAFLD. When compared to those without fatty liver, patients with NAFLD had higher rates of coronary heart disease (26.6% vs. 18.3%), cerebrovascular disease (20% vs. 13.3%) and peripheral vascular disease (15.4% vs. 10%). In addition, NAFLD again was an independent predictor of CVD after adjusting for classical risk factors and MS (Targher et al., 2007). These studies, however, used ultrasonography for diagnosis of NAFLD. There is insufficient evidence for the independent role of necroinflammatory changes and advanced liver fibrosis as risk factors for CVD in diabetics. When patients with NAFLD were followed for 14 years in one study, the leading cause of death in biopsy-proven NASH was CVD. However, the authors did not have data for T2DM at the onset of the study and direct comparison between CVD in NASH and non- NASH patients was not performed (Ekstedt et al., 2006). The suggested pathogenic mechanisms by which NAFLD affects CVD are oxidative stress, subclinical inflammation, impaired fibrinolytic activity, deranged cytokines profile, increased small dense LDL, reduced large HDL and altered cardiac metabolism (Targher et al., 2005; Smith & Adams, 2011). A recent study of uncomplicated type 2 diabetics without CVD aimed to assess early myocardial abnormalities in this population and found that patients with high liver triglyceride content have decreased myocardial perfusion, glucose uptake, and high-energy phosphate metabolism in comparison to those with low liver triglyceride content (Rijzewijk et al., 2010). This suggests the possible independent role of fatty liver as a risk factor for CVD.
Conversely, a recent large population-based study based on NHANES III data did not find any association of NAFLD (defined by hepatic steatosis on ultrasound with or without elevated ALT), with CVD and overall mortality over a median follow-up of 14.5 years (Lazo et al., 2011). However, a prior study also based on NHANES III data but with a smaller cohort and NAFLD defined as elevated liver enzymes with lower cut-off points showed that NAFLD was an independent predictor of CVD and overall mortality only in the 45–54 year-old group (Dunn et al., 2008). Furthermore, a recent retrospective study compared patients with histologically proven NASH to a non-NASH fatty liver cohort. There was no difference in the prevalence of CVD between the two groups, but patients with diabetes had a higher prevalence of CVD than non-diabetics (13% vs. 3%, p<0.001) (Domanski, Park, & Harrison, 2012). However, no further analysis on diabetics with and without NASH was performed. The results of all these studies are difficult to compare due to different criteria used to define NAFLD and heterogeneity of endpoints included in CVD diagnosis. Further prospective studies are needed to clarify the role of NAFLD and severity of hepatic histology in CVD in diabetics.
Several population-based studies have demonstrated that faster progression of liver fibrosis in diabetics with NAFLD considerably affects mortality. In the Verona Diabetes Study of 7,148 type 2 diabetics, more than a twofold increased risk of dying from cirrhosis was found after 5 years of follow-up (standardized mortality ratio, SR=2.5) in comparison to the general population (Trompetta, Spiazzi, Zoppini, & Muggeo, 2005). In addition, a higher risk of mortality from HCC was noted after 10 years. The latter finding was even more significant in patients with concomitant obesity. Although the study did not adjust for alcohol consumption and hepatitis, the changes in mortality were attributed mostly to NASH (Trompetta, Spiazzi, Zoppini, & Muggeo, 2005). In another study based on residents of Olmsted County, Minnesota, 337 diabetics were followed for a mean of 10.5 years and 34% developed NAFLD diagnosed by ultrasound or liver biopsy (Adams et al., 2010). The presence of NAFLD was associated with a 2.4-fold higher risk of overall mortality. This was partly attributed to liver-related deaths which accounted for 19% of deaths in patients with NAFLD and DM (Adams et al., 2010). In the Verona Diabetes Study, CVD was the leading cause of mortality (40%) (de Marco et al., 1999), while in the Olmsted County study, the most common cause of mortality was malignancy (33%), followed by ischemic heart disease (19%) and liver-related complications (19%) (Adams et al., 2010).
5. Conclusions
In summary, diabetes increases the likelihood of NASH and advanced fibrosis on biopsy among patients with NAFLD. It is likely that the increased risk of cirrhosis and HCC among diabetics is related to the presence of NASH and advanced fibrosis on liver histology. We speculate that liver histology has a prognostic value among patients with diabetes and future studies are needed to examine whether diabetics with NASH and fibrosis are the ones who are at higher risk of death from liver disease and HCC as compared to diabetics with mild NAFLD and no evidence of NASH or fibrosis on liver biopsy. Further studies are warranted to assess the role of non-invasive biomarkers in predicting risk of future liver-related complications in patients with diabetes.
6. Future research considerations
A clearly defined study of NAFLD patients with diabetes is needed to develop a clinical model of predicting NASH and fibrosis in these patients. This would significantly aid in efficient resource allocation and recommending liver biopsy in those who are at higher risk of progression. Further studies are needed to better understand the mechanisms underlying why diabetics have more advanced liver histology and a greater risk of cirrhosis, HCC and liver mortality. It also remains to be shown whether reversing NASH would result in an improvement in the risk of cirrhosis, HCC and liver mortality in patients with diabetes. Furthermore, whether treating NASH would also improve CVD outcomes is also an important research priority in the field.
Footnotes
Conflict of interest: No conflicts of interest exist.
References
- Adams LA, Harmsen S, St Sauver JL, Charatcharienwitthaya P, Enders FB, Therneau T, et al. Nonalcoholic fatty liver disease increases risk of death among patients with diabetes: a community based cohort study. The American Journal of Gastroenterology. 2010;105:1567–1573. doi: 10.1038/ajg.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- Amarapurkar DN, Amarapurkar AD, Patel ND, Agal S, Baigal R, Gupte P, Pramanik S. Annals of Hepatology. 2006;5:30–33. [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. The New England Journal of Medicine. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- Angulo P, Keach JC, Batts KP, Lindor KP. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- Angulo P, Lindor KD. Non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology. 2002;17(Suppl S):186–190. doi: 10.1046/j.1440-1746.17.s1.10.x. [DOI] [PubMed] [Google Scholar]
- Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. The American Journal of Gastroenterology. 2000;95:2936–2939. doi: 10.1111/j.1572-0241.2000.02325.x. [DOI] [PubMed] [Google Scholar]
- Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Digestive Diseases. 2010;28:155–161. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Archives of Surgery. 2003;138:1240–1244. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- Boza C, Riquelme A, Ibañez L, Duarte I, Norero E, Viviani P, et al. Predictors of nonalcoholic steatohepatitis (NASH) in obese patients undergoing gastric bypass. Obesity Surgery. 2005;15:1148–1153. doi: 10.1381/0960892055002347. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. The American Journal of Gastroenterology. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA NASH Clinical Research Network (CRN) Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugianesi E, Marchesini G, Gentilcore E, Cua IH, Vanni E, Rizzetto M, George J. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44:1648–1655. doi: 10.1002/hep.21429. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Salamone F, Negro F. The interaction of metabolic factors with HCV infection: does it matter? Journal of Hepatology. 2012;56(Suppl. 1):S56–S65. doi: 10.1016/S0168-8278(12)60007-5. [DOI] [PubMed] [Google Scholar]
- Byrne CD. Dorothy Hodgkin Lecture 2012* Non-alcoholic fatty liver disease, insulin resistance and ectopic fat: a new problem in diabetes management. Diabetic Medicine. 2012;29:1098–1107. doi: 10.1111/j.1464-5491.2012.03732.x. [DOI] [PubMed] [Google Scholar]
- Cassader M, Gambino R, Musso G, Depetris N, Mecca F, Cavallo-Perin P, et al. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Lipids. 2001;36:1117–1124. doi: 10.1007/s11745-001-0822-5. [DOI] [PubMed] [Google Scholar]
- Chen G, Brunt EM. Diabetic hepatosclerosis: a 10-year autopsy series. Liver International. 2009;29:1044–1050. doi: 10.1111/j.1478-3231.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population-based case control study. Gut. 2005;54:533–539. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco R, Locatelli F, Zoppini G, Verlato G, Bonora E, Muggeo M. Cause-specific mortality in type 2 diabetes. The Verona Diabetes Study. Diabetes care. 1999;22:756–761. doi: 10.2337/diacare.22.5.756. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- Domanski JP, Park SJ, Harrison SA. Cardiovascular disease and nonalcoholic fatty liver disease: does histologic severity matter? Journal of Clinical Gastroenterology. 2012;46:427–430. doi: 10.1097/MCG.0b013e31822fb3f7. [DOI] [PubMed] [Google Scholar]
- Dunn W, Xu R, Wingard DL, Rogers C, Angulo P, Younosssi ZM, Schwimmer JB. Suspected nonalcoholic fatty liver disease and mortality risk in a population-based cohort study. The American Journal of Gastroenterology. 2008;103:2263–2271. doi: 10.1111/j.1572-0241.2008.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Ertle J, Dechene A, Sowa JP, Penndorf V, Herzer K, Kaiser G, et al. Nonalcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. International Journal of Cancer. 2011;128:2436–2443. doi: 10.1002/ijc.25797. [DOI] [PubMed] [Google Scholar]
- Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. Nonalcoholic fatty liver disease in severely obese subjects. The American Journal of Gastroenterology. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- Gupte P, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. Journal of Gastroenterology and Hepatology. 2004;19:854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- Guzman G, Brunt EM, Petrovic LM, Chejfec G, Layden TJ, Cotler SJ. Does nonalcoholic fatty liver disease predispose patients to hepatocellular carcinoma in the absence of cirrhosis? Archives of Pathology & Laboratory Medicine. 2008;132:1761–1766. doi: 10.5858/132.11.1761. [DOI] [PubMed] [Google Scholar]
- Harrison SA, Brunt EM, Goodman ZD, Di Bisceglie AM. Diabetic hepatosclerosis: diabetic microangioapthy of the liver. Archives of Pathology & Laboratory Medicine. 2006;130:27–32. doi: 10.5858/2006-130-27-DHDMOT. [DOI] [PubMed] [Google Scholar]
- Hudacko RM, Manoukian AV, Schneider SH, Fyfe B. Clinical resolution of glycogenic hepatopathy fallowing improved glycemic control. Journal of Diabetes and its Complications. 2008;22:320–330. doi: 10.1016/j.jdiacomp.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Hudacko RM, Sciancalepore JP, Fyfe BS. Diabetic microangiopathy in the liver: an autopsy study of incidence and association with other diabetic complications. American Journal of Clinical Pathology. 2009;132:494–499. doi: 10.1309/AJCPQBFF42ZZXXRQ. [DOI] [PubMed] [Google Scholar]
- Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. American Journal of Physiology, Endocrinology and Metabolism. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Hyogo H, Ishitobi T, Nabeshima Y, Arihiro K, Chayama K. Postprandial insulin secretion pattern is associated with histological severity in non-alcoholic fatty liver disease patients without prior known diabetes mellitus. Journal of Gastroenterology and Hepatology. 2011;26:517–522. doi: 10.1111/j.1440-1746.2010.06567.x. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, Clark JM. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. British Medical Journal. 2011;343:d6891. doi: 10.1136/bmj.d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite NC, Villela-Nogueira CA, Pannain VL, Bottino AC, Rezende GF, Cardoso CR, Salles GF. Histopathological stages of nonalcoholic fatty liver disease in type 2 diabetes: prevalences and correlated factors. Liver International. 2011;31:700–706. doi: 10.1111/j.1478-3231.2011.02482.x. [DOI] [PubMed] [Google Scholar]
- Lima ML, Mourão SC, Diniz MT, Leite VH. Hepatic histopathology of patients with morbid obesity submitted to gastric bypass. Obesity Surgery. 2005;15:661–669. doi: 10.1381/0960892053923888. [DOI] [PubMed] [Google Scholar]
- Loomba R, Abraham M, Unalp A, Wilson L, Lavine J, Doo E, Bass NM NASH Clinical Research Network. Association between diabetes, family history of diabetes and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver disease, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Medina J, Fernández-Salazar LI, García-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl. 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- Noureddin M, Vaughn IA, Neuschwander-Tetri BA, Sanyal AJ, McCullough AJ, Merriman RB, et al. Clinical and histological determinants of nonalcoholic steatohepatitis (NASH) and advanced fibrosis in the elderly [abstract] Hepatology. 2011;54(Suppl.):1118A. doi: 10.1002/hep.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong JP, Elariny H, Collantes R, Younoszai A, Chandhoke V, Reines HD, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obesity Surgery. 2005;15:310–315. doi: 10.1381/0960892053576820. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, et al. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. The New England Journal of Medicine. 2010;362:1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynard T, Ratziu V, McHutchinson J, Manns M, Goodman Z, Zeuzem S, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38:75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Journal of the Association of Physicians of India. 2009;57:205–210. [PubMed] [Google Scholar]
- Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vacchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Annals of Internal Medicine. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Rijzewijk LJ, Jonker JT, van der Meer RW, Lubberink M, de Jong HW, Romijn JA, et al. Effects of hepatic triglyceride content on myocardial metabolism in type 2 diabetes. Journal of the American College of Cardiology. 2010;56:225–233. doi: 10.1016/j.jacc.2010.02.049. [DOI] [PubMed] [Google Scholar]
- Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ. NASH: A global health problem. Hepatology Research. 2011a;41:670–674. doi: 10.1111/j.1872-034X.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ. Role of insulin resistance and hepatic steatosis in the progression of fibrosis and response to treatment in hepatitis C. Liver International. 2011b;31(Suppl1):23–28. doi: 10.1111/j.1478-3231.2010.02397.x. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Stravitz RT, Mills AS. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. The American Journal of Gastroenterology. 2003;98:2064–2071. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- Silverman JF, O'Brien KF, Long S, Leggett N, Khazanie PG, Pories WJ, et al. Liver pathology in morbidly obese patients with and without diabetes. The American Journal of Gastroenterology. 1990;85:1349–1355. [PubMed] [Google Scholar]
- Smith BW, Adams LA. Non-alcoholic fatty liver disease. Critical Reviews in Clinical Laboratory Sciences. 2011;48:97–113. doi: 10.3109/10408363.2011.596521. [DOI] [PubMed] [Google Scholar]
- Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obesity Surgery. 2003;13:347–349. doi: 10.1381/096089203765887633. [DOI] [PubMed] [Google Scholar]
- Takuma Y, Nouso K. Nonalcoholic steatohepatitis-associated hepatocellular carcinoma: our case series and literature review. World Journal of Gastroenterology. 2010;16:1436–1441. doi: 10.3748/wjg.v16.i12.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Rodella S, Tessari R, Zenari L, Lippi G, Arcaro G. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- Torbenson M, Chen YY, Brunt E, Cummings OW, Gottfried M, Jakate S, et al. Glycogenic hepatopathy: an underrecognized hepatic complication of diabetes mellitus. The American Journal of Surgical Pathology. 2006;30:508–513. doi: 10.1097/00000478-200604000-00012. [DOI] [PubMed] [Google Scholar]
- Trompetta M, Spiazzi G, Zoppini G, Muggeo M. Review article: type 2 diabetes and chronic liver disease in the Verona diabetes study. Alimentary Pharmacology and Therapeutics. 2005;22(Suppl 2):24–27. doi: 10.1111/j.1365-2036.2005.02590.x. [DOI] [PubMed] [Google Scholar]
- van den Brand M, Elving LD, Drenth JP, van Krieken JH. Glycogenic hepatopathy: a rare cause of elevated serum transaminases in diabetes mellitus. The Netherlands Journal of Medicine. 2009;67:394–396. [PubMed] [Google Scholar]
- Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis.Relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Williamson RM, Price JF, Glancy S, Perry E, Nee LD, Hayes PC, et al. Prevalence of and risk factors for hepatic steatosis and nonalcoholic fatty liver disease in people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes Care. 2011;34:1139–1144. doi: 10.2337/dc10-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Chan HL, Hui AY, Chan KF, Liew CT, Chan FK, Sung JJ. Clinical and histological features of non-alcoholic fatty liver disease in Hong Kong Chinese. Alimentary Pharmacology and Therapeutics. 2004;20:45–49. doi: 10.1111/j.1365-2036.2004.02012.x. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Gramlich T, Matteoni CA, Boparai N, McCullough AJ. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clinical Gastroenterology and Hepatology. 2004;2:262–265. doi: 10.1016/s1542-3565(04)00014-x. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clinical Gastroenterology and Hepatology. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]