Abstract
Ultrahigh contrast fluorescence molecular imaging has long been pursued over the past few decades from basic sciences to clinics. Although new classes of near-infrared (NIR) molecular probes are emerging, the requirement of fluorophores with high quantum yield, high signal to noise (S/N) ratio, and being activatable to microenvironment changes can hardly be fulfilled. In this study, a new NIR dye embedded fluorogenic nanoprobe (fg-nanoprobe) was developed for ultrahigh contrast in vitro and in vivo imaging with negligible background interference. The achieved S/N ratio was found to be attributed to the synergistic effects of the cellular compartmental triggered fluorogenicity and pH tunable fluorescence on/off character. In addition, this constructed fluorogenic nanoprobe could be coupled with image processing method for super-resolution subdiffraction imaging. The developed fg-nanoprobe integrated photophysical merits of the synthesized NIR fluorophore and advantages of engineered nanoparticle for enhanced fluorescence molecular imaging. This probe may open another avenue for ultrahigh contrast fluorescence molecular imaging in the future.
Keywords: Fluorescence imaging, Subdiffraction imaging, Fluorogenic PLGA nanoparticle, BODIPY NIR dye, pH sensitive, Molecular binding
1. Introduction
With the advances of molecular imaging techniques and fluorescent reporter strategies, near-infrared (NIR) fluorescence imaging has evolved as one of the major focuses for non-invasive diagnosis and monitoring of diseases in the clinic [1] and in basic sciences to explore the fundamental biological events [2, 3]. For effective high contrast fluorescence imaging, the fluorophores with absorbance and emission in the NIR region (650–900 nm) have been investigated due to the increased optical transparency, reduced cell/tissue autofluorescence and low light scattering [4, 5]. This class of fluorophores enables rapid and wide expansion of their applications for in vitro and in vivo fluorescence imaging.
Although new classes of NIR molecular probes are emerging [6], the requirement of fluorophores with high quantum yield, high signal to noise (S/N) ratio and being activatable to microenvironment changes can hardly be fulfilled. It is highly desirable to develop the next generation NIR probes with optimal photophysical properties along with stimuli tunable fluorescence at the target sites to enhance S/N ratio for ultracontrast fluorescence imaging. Most of the conventional environment responsive fluorophores, however, can only be tuned by single stimulus, such as pH [7], or calcium [8]. Nevertheless, the inherent complexity of biological environment, such as culture media and animal body fluids, may still non-specifically activate the single stimulus responsive fluorophores, resulting in high background noise and low S/N ratios. Thus, the fluorescence emission of NIR fluorophores that can be simultaneously fine-tuned by multiple factors would significantly enhance S/N ratio for ultrahigh contrast fluorescence imaging. Additionally, it is well-known that lipophilic dyes are much likely to penetrate cell membrane for extensive whole cell staining and imaging. It has been reported, for instance, that PKH67, a lipophilic dye for cell membrane staining, could be used for long-term (3 weeks) cancer stem cell tracking in vivo [9]. Currently, these lipophilic dyes are only applied for in vitro cell labeling and imaging. The aqueous condition of extracellular environment limits their applications for real-time in vivo fluorescence imaging, even if some dyes do have high contrast and long fluorescence life time.
Fluorophores doped in engineered nanoparticles can result in enhanced photophysical properties and are being extensively investigated in nanomedicine and biotechnology [10]. Recent advances in engineered nanoparticles encapsulating fluorophores [11] or fluorescent semiconductors [12] have evidenced the advantages of fluorophore doped nanoparticles, especially those biodegradable nanoparticles, for biological applications. In addition, fluorophores doped in nanoparticles could prevent them from non-specific interaction with other molecules during blood circulation to the target sites, where the fluorophore could be released and function properly.
In this study, we reported a dual-factor triggered lipophilic distyryl boron dipyrromethene (BODIPY) based NIR probe (denoted as fgBODIPY, Scheme S1) doped in engineered biodegradable nanoparticles (termed as fg-nanoprobe) (Figure 1) with enhanced S/N ratio for ultrahigh contrast NIR fluorescence imaging and super-resolution subdiffraction imaging.
Figure 1.
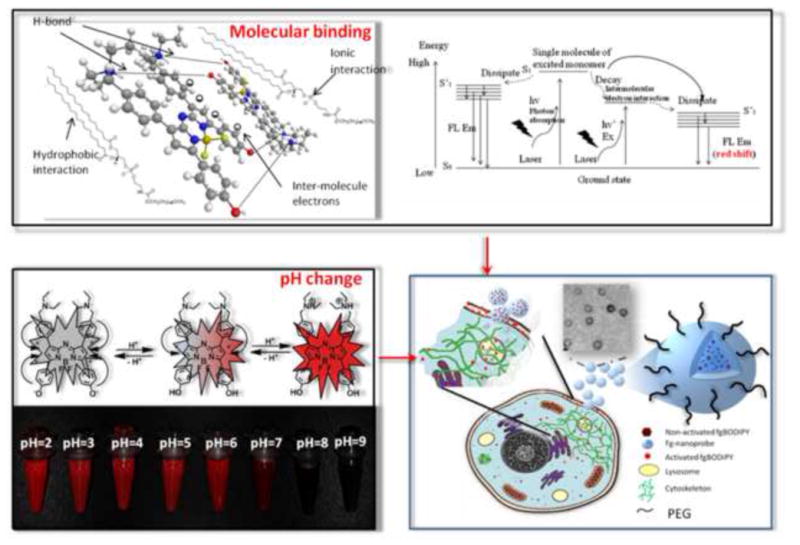
Illustration of the mechanism of dual-factor triggered fluorogenic nanoprobe (fg-nanoprobe) for cell associated fluorogenecity in NIR fluorescence imaging. (inset shows the TEM image of fg-nanoprobe. Scale = 100 nm).
2. Materials and methods
2.1 Chemicals and instruments
All chemicals used in this study were purchased from Sigma-Aldrich. Inc. unless otherwise indicated. Fluorescence of free or particle loaded dye was measured using Hitachi F-7000 Fluorescence Spectrophotometer (Tokyo, Japan). The UV-Vis spectra were recorded on a Genesys 10s UV-Vis spectrophotometer (Thermo Scientific, Swedesboro, NJ). Optical imaging acquirement and analysis were conducted on Maestro 2.10 in vivo imaging system (Cambridge Research & Instrumentation, Woburn, MA). Laser Scanning Confocal microscopy study was conducted on Olympus Fluoview FV10i confocal microscopy with 60x water objective lens. Subdiffraction imaging was conducted on Nikon N-STORM microscopy system.
2.2 Preparation and characterization of fg-nanoprobe
The nanoparticle was prepared by nanoprecipitation method according to our previously published approach [19]. Briefly, appropriate amount of the synthesized fgBODIPY dye was dissolved in 1 ml acetonitrile along with 5 mg PLGA (Resomer® 502H). 1 mg 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxy(polyethylene glycol)-2000] (DSPE-PEG2K) (Avanti polar lipid) was dissolved in 5 ml 4% ethanol aqueous solution, and aqueous solution was adjusted to pH 7.4 by diluted NaOH solution, then the solution was heated to 70°C. Ten min after heating the aqueous solution at 70°C, the acetonitrile solution was dropped into the heated solution under mild magnetic stirring, followed by vigorous vortexing for 5 min before magnetic stirring for another 4 h for nanoparticle self-assembly. The resulting nanoparticles were washed extensively with PBS (pH 7.4) by untrafiltration method (Amicon® Ultra, M.W.C.O. = 100 k) until there is no trace of UV/Vis absorption of the dye under spectrometer measurement. The concentrated fg-nanoprobe was kept in PBS (pH 7.4) in the refridgerator for further use. The size and distribution of the fg-nanoprobe was monitored by dynamic light scattering (Marvin Nano90, U.K.). The dried morphology of fg-nanoprobe was observed with a transmission electron microscope (TEM) (Philips/FEI CM200).
2.3 Cell culture
The U87G and MDA-MB-435 human cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA). U87MG cells were cultured in αMEM medium containing 10% (v/v) fetal bovine serum (GIBCO, Grand Island, NY) supplemented with penicillin (100 μg/mL), streptomycin (100 μg/mL), non-essential amino acids (100 μM) and sodium pyruvate (1 mM) at 37 °C with 5% CO2. MDA-MB-435 cells were cultured in L-15 medium supplemented with penicillin (100 μg/mL), streptomycin (100 μg/mL), non-essential amino acids (100 μM), 10 μg/ml bovine insulin, 10 μg/ml glutathione at 37 °C. Human umbilical vascular endothelial cells (HUVECs) were obtained from ATCC and cultured in pre-formulated HUVEC complete medium (Cell systems, Kirkland, WA) at 37 °C with 5% CO2.
2.4 Cell uptake and live cell imaging assays
Cells were seeded in Lab-Tek® chambers (Nalgene, Nunc Inc., Denmark) at a density of 2 × 104 per well, and allowed to attach for overnight. For HUVEC culture, the chamber was first coated with 0.2% gelatin at 37 °C for 2 h before culturing HUVEC. For fixed cell imaging, cells were incubated with 50 μM fg-nanoprobe over pre-determined time period, followed by 3 times PBS washing and fixation in 4% neutralized formalin solution for 10 min and in 0.1% Triton X-100 (TX-100) for 5 min. Then, Texas Red® conjugated phalloidin pre-diluted solution (0.5% BSA in PBS) was incubated with the cell monolayer for 30–40 min before washing 3 times with PBS, and mounted with Vectashiled mounting media with DAPI. For immunofluorescence staining, available cells were fixed by 4% neutralized formalin solution for 10 min, and permeabilized by 0.1% TX-100 for 10 min, followed by incubation with the primary antibody (anti-beta tubulin antibody, 1: 500, Cell Signaling, Inc.; anti-clathrin antibody, 1:300, Abcam), and corresponding fluorescent dye conjugated secondary antibodies. After extensive washing, the slides were mounted with Vectashield mounting media containing DAPI. For live cell imaging, cells were incubated with pre-determined concentrations of fg-nanoprobe, followed by incubation over pre-determined time period. Then, the live cell imaging was conducted without changing medium or washing steps. For lysosome/fg-nanoprobe co-localization study, the LysoTracker kit (Molecular Probes, Eugene, OR) was used according to the manufacturer’s instructions.
2.5 STORM based fgPAINT live cell imaging
Glass bottom cell culture dish (MatTek, Natick, MA) was pre-coated with 0.2% gelatin at 37 °C for 2 h before HUVECs were cultured (5 × 104 cells/dish). HUVECs were allowed to attach for overnight. The stock solution of fg-nanoprobe (500 μM) was serial diluted to achieve 1 nM final concentration. Then, the cultured HUVEC medium was changed with 1 nM fg-nanoprobe containing medium for the STORM imaging. We used Plan ApoVC100X 1.40 Oil objective lens, 647 nm (100 mW) as excitation laser with 660 nm long pass emission filter. The camera was set at 30 frames/sec. The imaging process was automatically terminated until 10,000 signals were obtained. Then, the contour image of the entire cell was re-constructed by software accompanying the STORM system.
Gaussian fitting was analyzed separately by MatLab (Mathworks, Natick, MA). Briefly, 100 fluorescent points were randomly selected in the cell to analyze the histogramic distribution. The average intensity with the fitted curve of Gaussian function is shown in Fig. S13. The full width at half maximum (FWHM) of the curve is ~ 35 nm, which can be considered as the spatial resolution of the system.
2.6 Animal model
Female athymic nude mice were supplied from Harlan (Indianapolis, IN) at 4 to 5 wk of age. U87MG tumor model was generated by subcutaneous injection of 5×106 cells in Matrigel into the right front flank of the nude mice at a volume of about 100 μL. The mice were used for optical imaging studies when the tumor volume reached about 200 mm3. Fg-nanoprobe was injected from tail vein in 100 μL PBS at 1 mM dye concentration under isoflurane anesthesia. All animal studies were conducted in accordance with the principles and procedures outlined in the Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Clinical Center, NIH.
2.7 Pharmacokinetic analysis of fg-nanoprobe in mouse
Three Balb/c mice were used in this study. Mice were intravenously administrated 1 mM fg-nanoprobe in 100 μl PBS solution. 10–15 μl blood was collected into heripinized tube at each predetermined time point through tail vail. 200 μl acetonitrile was added into blood, followed by vigorous vortex for 1 min to extract fgBODIPY encapsulated in fg-nanoprobe. HPLC was used to determine the fgBODIPY concentration in blood. The mobile phase: A: 0.1% TFA in H2O; B: 0.1% TFA in acetonitrile with gradient: 20% B for 5min, 20 −95% B in 20 min, and then 95–20% B in 5 min. The detection wavelength was set at 680nm.
2.8 Statistical analysis
Results were expressed as mean ± SD. Two-tailed paired and unpaired Student’s t tests were used to test differences within groups and between groups, respectively. P values < 0.05 were considered statistically significant.
3. Results and discussion
One merit of the fgBODIPY is the pH-switchable fluorogenic property. Its fluorescence is switched on at low pH value (pH < 5), whereas neutral condition (pH = 7), like extracellular environment, effectively keeps the fluorescence off. Nevertheless, the acidic environment (low pH value) in cytoplasm is restricted mainly in lysosome which most nanoscale materials temporally encounter upon cellular uptake. Hence, the simple single pH-dependent fluorogenecity could only transiently sustain for short-term cell highlighting. To further enhance the S/N ratio for ultrahigh constrast fluorescence imaging, the fgBODIPY additionally integrates dimer formation capacity upon binding with other biomolecules for fluorogenecity regardless of environmental pH values. This unique character makes the fgBODIPY’s fluorescence tunable by two indenpendent factors, pH value and molecular binding, which markedly enhance S/N ratio of fgBODIPY as a cell highlighter (Figure 1). Furthermore, the encapsulation of fgBODIPY into biodegradable polymer nanoparticles (fg-nanoprobe) provides additional protection from undesirable activation of fgBODIPY until the fg-nanoprobe is engulfed by targeted cells/tissues for fluorescence imaging. In addition, the lipophilic character of the released fgBODIPY results in extensive and efficient whole cell staining with good biocompatibility.
We chose the well-characterized BODIPY as the template, as it is a versatile NIR dye with tunable excitation and emission properties by chemical modification of the π-π conjugate systems [13]. The strong absorbance and high quantum yield at NIR region make BODIPY dyes attractive for biological applications [14,15]. For the molecular structure design, we manipulated the dye to improve photophysical properties, structure stability and stimuli sensitivity. Different from reported dialkylated aniline substituted BODIPY molecules [7], which showed strong highest occupied molecular orbital (HOMO) energy levels to cause electron transfer towards BODIPY fluorophore, our fgBODIPY molecule employed electron pairs across terminal tertiary amine and phenol alcohol as the electron bridge over the BODIPY core with balanced pKa for enhanced pH sensitivity. As a result, a dramatic change of fluorescence intensity took place when the pH value of solution was changed (Figure 2a–d). Moreover, the symmetrical structure of fgBODIPY made the dye highly sensitive to pH variation (0.2 unit) (Figure S1) and was very stable without detectable trace of degradation in solvent within 3 months.
Figure 2.

Fluorogenecity and stability of fgBODIPY and fg-nanoprobe: (a) Absorbance and Fluorescence spectra of fgBODIPY in 25mM HCl MeOH-H2O (1:1); (b) Concentration dependent fluorescence increase under acidic pH value (pH 5.5). Inset presents the linear regression of corresponding peak fluorescence values; (c) pH titration curve of fgBODIPY. Data were normalized to the highest fluorescence reading; (d) pH controlled fluorescence on/off cycles (off: pH 8; on: pH 5.5). (ex: 680nm; em:740nm); (e) Fluorogenecity of fgBODIPY dye in different surfactants (pH = 7); (f) Fg-nanoprobe incubated in different conditioned media for 6 h; (g) fg-nanoprobe size stability; and (h) PDI distribution over 6 h in PBS (pH = 7 or pH = 5).
Since the intrinsic lipophilic property of BODIPY makes it affinitive to hydrophobic structures, like lipid bilayer, we therefore tested the interaction between fgBODIPY and lipid molecules. Interestingly, we discovered that the fluorescence of fgBODIPY can also be triggered by some amphiphilic moleculesor or cell lysates in PBS buffer (pH = 7.4) (Figure 2e). This implies that the fluorescence emission of fgBODIPY can alternatively be fine-tuned independent of pH change. It was well known that the low solubility of BODIPY dyes will impair the fluorescence quantum yield due to the fluorophore self-aggregation in the aqueous environment [16]. Unfortunately, this explanation cannot account for the fluorogenic phenomenon of fgBODIPY at neutral pH, as it was inherently non-fluorescent until it was fully protonated in the aqueous medium. We further investigated the fluorogenicity of fgBODIPY in various kinds of surfactants (neutral, cationic, ionic and zwitterionic). We found that all surfactants tested could trigger the fluorescence emission at neutral pH under their concentrations over the respective critical micelle concentration (CMC), whereas different ionic strengths and charges of surfactants led to distinctive fluorogenic spectra (Figure 2e). We postulate that once the concentrations of the added surfactants reach a value close to or above the CMC, the formed micellar structures of surfactants could incorporate the dye into the lipophilic core of micelles (homomicelles) for dye solubilization. Meanwhile, the charges of surfactants also interact with fgBODIPY. Thus, the microenvironmental changes due to surfactant encapsulation and interaction [17] lead to altered pKa of fgBODIPY.
Since fgBODIPY is highly sensitive to pH variation (0.2 unit) (Figure 1 and Figure S1), the symmetrically designed fgBODIPY shows multi-stimuli responses in fluorogenicity. Moreover, we noted about 14 nm red shift in fluorescence emission upon incubation of fgBODIPY with cell lysates and phospholipids (DSPE-PEG2000), which distinguishes the phospholipid from other surfactants (Figure S2). As phospholipid is one of the major components in cell structures, it is highly possible that phospholipid and cell lysate share the same fluorogenic mechanism. We attribute the dramatic red shift emission to the intermolecular dimer formation of fgBODIPY after being trapped in the hydrophobic core of phospholipids, as the BODIPY structure is likely to form dimer at appropriate concentrations [18].
In this study, the lipophilic fgBODIPY dye was encapsulated in the biodegradable PLGA polymeric nanoparticle engineered via a modified nanoprecipitation method [19]. The preparation procedure was robust, and the resulting nanoparticle size was about 130 nm (Figure 2g) with fairly uniform size distribution (PDI < 0.2) (Figure 2h). The particles were stable at different pH values in 10% fetal bovine serum (FBS) culture medium at 37 °C over 6 h (Figure 2g-h). The engineered nanoparticles could protect fgBODIPY from protonation at pH 5 buffer (Figure S3). This guarantees that the encapsulated fgBODIPY molecules are not dissociated from the nanoparticles until being internalized by target cells. These above traits of fg-nanoprobe ensure its applications for ultrahigh contrast fluorescence imaging.
Next we investigated the cellular uptake behavior and fluorogenic mechanism of fg-nanoprobe in both cancer cells (MDA-MB-435, and U87MG) and primary cells (human umbilical vein endothelial cells (HUVEC)). In all cell lines, fg-nanoprobe presented time-dependent endocytosis, which was saturated after 24 h incubation (Figure S4-5) with good biocompatibility (Figure S6). Live cell imaging study (movie 1) showed similar time-dependent cellular uptake pattern. We then used time lapse confocal microscopy to study the step-by-step fluorogenic progress during endocytosis (Figure 3 and Figure S7). Results showed that fgBODIPY was not activated upon being immediate contact of fg-nanoprobe with cells, but it was gradually released from the biodegradable carrier into cell membrane bilayer, followed by fast diffusion into cytoplasm, where the dye molecules were accumulated and activated (Figure 3a–b). To clearly illustrate the subcellular localization of fg-nanoprobe via endocytosis and activation, we used lower probe concentration (50 nM) and normal cells (HUVEC) (Figure 3b). Results showed that released fgBODIPY dyes were activated and anchored randomly first on cell membrane due to the burst release effect of biodegradable nanoparticles arising from the microporous channels on the particle surfaces (Figure 3c). When the fg-nanoprobe was further trapped into the endo/lysosome compartments in cytosol (Figure S8), the acidic environment and associated lipids accelerated the release of fg-BODIPY, followed by fast protonation for fluorogenicity. At later time points, the released dyes would additionally accumulate in the membrane structured micellar-like compartments, such as clathrin (Figure 3d). Hence, the fluorogenic property of fg-nanoprobe during cellular uptake underwent series of activation processes from membrane lipid association, pH change to cytosol membrane compartments (Figure S9).
Figure 3.
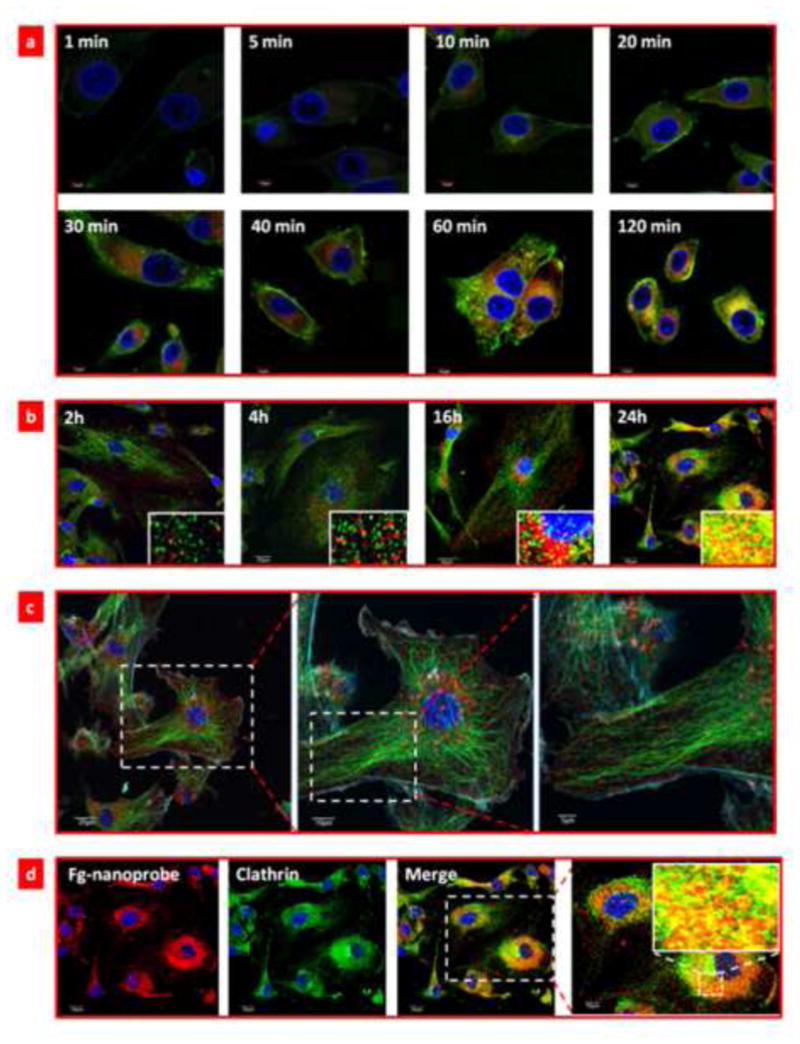
Fluorogenic progress of fg-nanoprobe during cellular uptake. (a) Time-lapse confocal microscopy (TLCM) study of the fluorogenic progress of fg-nanoprobe during endocytosis process. The nanoparticle is covalently conjugated with FITC for tracing the delivery progress. Blue: cell nucleus; green: FITC-labeled nanoparticle; red: activated fgBODIPY; (b) Time dependent HUVEC uptake of fg-nanoprobe and subcellular (clathrin) distribution analysis. Inset in each time point picture shows the colocalization of activated fg-nanoprobe with clathrin. Blue: nucleus; green: clathrin; orange/yellow: co-localization of activated fgBODIPY and clathrin; red: activated fgBODIPY from fg-nanoprobe; (c) Early time point (4 h) subcellular distribution of fg-nanoprobe in HUVEC cells. Blue: nucleus; cyan: actin; green: tubulin; red: fg-nanoparticle; (d) Late time point (24 h) subcellular distribution of fg-nanoprobe in HUVEC cells.
In light of this dual-factor triggered fluorogenecity feature of fg-nanoprobe, we applied this molecular probe for no-washing ultrahigh contrast live cell imaging with high S/N ~10 (Figure 4a). This indicates that the fg-nanoprobe is suitable for dynamic live cell labelling in the presence of extracellularly disspersed imaging probes to investigate cell biology events at the original growth condition of cells.
Figure 4.
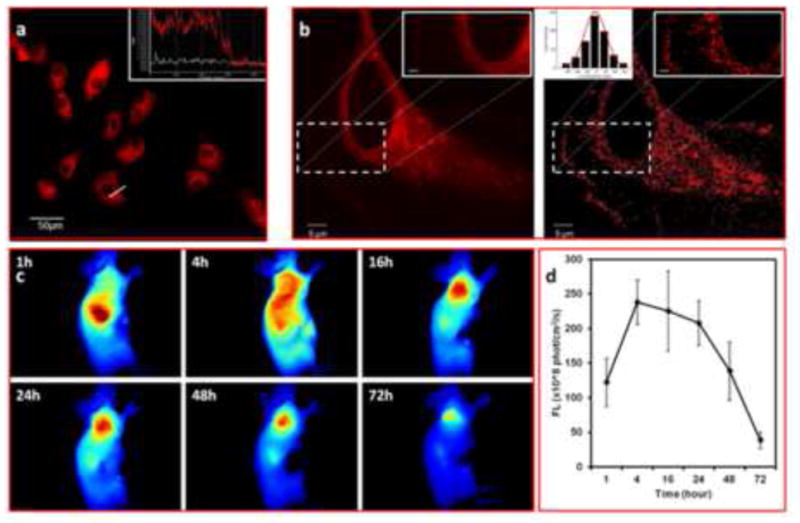
Ultrahigh contrast in vitro and in vivo imaging and subdiffraction imaging by fg-nanoprobe. (a) No washing live cell imaging of HUVEC in present of fg-nanoprobe in medium over 4 h. Inset: signal analysis of single live cell indicating significant fluorescence signal intensity contrast inside and outside of cell. The white line represents the DIC background, and the red line indicates the fluorescence signal intensity as highlighted by the crossing line. [c]fg-nanoprobe = 1μM; (b) Subdiffraction high resolution imaging of HUVEC incubated with 1 nM fg-nanoprobe in culture medium: (left) conventional confocal imaging and (right) synthetic subdiffraction imaging. Inset at upright corner scale bar = 0.5 μm; Inset at downright corner presents fgPAINT data. Gaussian fitting curve was generated by Matlab software to determine the full width at half maximum (FWHM), representing the resolution of fgPAINT (FWHM ~35nm); (c) In vivo tumor uptake of fg-nanoprobe over 72 h. The tumor area has high contrast at 16 h after intravenous injection; (d) Progressive fluorescence intensity change at tumor site.
In addition to the whole live cell ultrahigh contrast imagings, this dual-factor triggered fluorogenic fg-nanoprobe is also a good agent for subdiffraction imaging (figure 4b). Different from other super-resolution imaging methods, such as stochastic optical reconstruction microscopy (STORM) [20] or photoactivation localization microscopy (PALM) [21] which require special fluorophores to be photoswitchable upon laser triggering [22]. The fgBODIPY explores imaging results by using the diffusion and Brownian motion of very diluted fg-nanoprobe into the lipid microdomain on the membrane structures, followed by mathematic image processing [23, 24], as stated by points accumulation for imaging in nanoscale topography (PAINT) method [24]. In this study, we further extended the PAINT method by using this fg-nanoprobe (here termed fgPAINT) for live cell subdiffraction mapping (Figure 4b). Owing to the dual-factor triggered fluorogenic property, the fluorescence of fg-nanoprobe was negligible until it was triggered by both binding to cellular lipid structures and protonation in acidic environment of the lysosome. Figure 4b shows the improved imaging contrast quality of fgPAINT imaging (right) as compared with the image obtained by confocal microscopy (left). The concentration of fg-nanoprobe was tailored so that less than 5 fluorescent spots could be collected in a single frame scanning (30 frames/sec) with resolution about 35 nm Gaussian fitting (Figure 4b inset).
It is well-known that optical imaging often suffers from unwanted background inteference with low S/N ratio [25], which seriously comprises the imaging quality. Our fg-nanoprobe showed rapid clearance from the blood (Figure S10) and was almost in dark state in blood stream until it is taken up by the targeted cells. Figure 4d shows the prominent tumor highlighting effect of fg-nanoprobe upon intravenous adminstration. At 4 hour post-injection, the tumor area demonstratred significant signal enhancement. After 16–24 hours, the tumor was dominately distinguished over other tissues (Figure 4c-d, Figure S11). This probe combines the advantages of nanotechnology of prolonged circulation time in blood fluids for passive tumor targeting along with the improved photophysical properties of the synthetic fgBODIPY for cell triggered fluorogenicity. Therefore, this fg-nanoprobe is a good candidate for ultrahigh contrast fluorescence imaging.
Ther functional NIR nanoparticles, like the recently developed quantum dots [26–28] and upconversion nanocrystals [29–31], could combine imaging and therapy into a single delivery system. Similarily, our fg-nanoprobe could be further investigated by introuduction of therapeutic agent, as an theranostic agent, for biomedical application.
4. Conclusions
We have designed and synthesized a symmetrically structured fgBODIPY dye with sensitive pH adjustable and cell compartment triggered fluorogenic ability via bridge-mediated electron transfer. The triggered fluorogenicity by cell membrane or amphiphilic molecules has been illustrated to be caused by microenvironmental pKa change upon hydrogen bonding and static electrical interaction of fgBODIPY dye to the surrounding molecules. After loading the fgBODIPY into the engineered PLGA nanoparticle to form fg-nanoprobe, it presented superior properties for in vitro and in vivo ultrahigh contrast fluorescence imaging. The fg-nanoprobe can also be applied to subdiffraction imaging under specific conditions at resolution of 35 nm. Thus, the fg-nanoprobe meets the goal of developing next generation smart fluorescent probes for biomedical applications.
Supplementary Material
Acknowledgments
This work was supported, in part, by the National Basic Research Program of China (973 program, 2013CB733802), Henry M. Jackson Foundation and the Intramural Research Program (IRP), NIBIB/NIH, U.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitney MA, Crisp JL, Nguyen LT, Friedman B, Gross LA, Steinbach P, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29:352–6. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nat Biotechnol. 2005;23:313–20. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Huang P, Bhirde A, Jin A, Ma Y, Niu G, et al. A nanoscale graphene oxide-peptide biosensor for real-time specific biomarker detection on the cell surface. Chem Commun (Camb) 2012;48:9768–70. doi: 10.1039/c2cc31974h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiessler M, Hennrich U, Pipkorn R, Waldeck W, Cao L, Peter J, et al. Theranostic cRGD-bioshuttle constructs containing temozolomide-and Cy7 for NIR-imaging and therapy. Theranostics. 2011;1:381–94. doi: 10.7150/thno/v01p0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Li Z, Wang X, Liu F, Cheng Y, Zhang B, et al. In vivo cancer targeting and imaging-guided surgery with near infrared-emitting quantum dot bioconjugates. Theranostics. 2012;2:769–76. doi: 10.7150/thno.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayerhoffer U, Fimmel B, Wurthner F. Bright near-infrared fluorophores based on squaraines by unexpected halogen effects. Angew Chem Int Ed Engl. 2012;51:164–7. doi: 10.1002/anie.201107176. [DOI] [PubMed] [Google Scholar]
- 7.Urano Y, Asanuma D, Hama Y, Koyama Y, Barrett T, Kamiya M, et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat Med. 2009;15:104–9. doi: 10.1038/nm.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8. [PubMed] [Google Scholar]
- 9.Kusumbe AP, Bapat SA. Cancer stem cells and aneuploid populations within developing tumors are the major determinants of tumor dormancy. Cancer Res. 2009;69:9245–53. doi: 10.1158/0008-5472.CAN-09-2802. [DOI] [PubMed] [Google Scholar]
- 10.Stark WJ. Nanoparticles in biological systems. Angew Chem Int Ed Engl. 2011;50:1242–58. doi: 10.1002/anie.200906684. [DOI] [PubMed] [Google Scholar]
- 11.Muddana HS, Morgan TT, Adair JH, Butler PJ. Photophysics of Cy3-encapsulated calcium phosphate nanoparticles. Nano Lett. 2009;9:1559–66. doi: 10.1021/nl803658w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BY, Jiang W, Oreopoulos J, Yip CM, Rutka JT, Chan WC. Biodegradable quantum dot nanocomposites enable live cell labeling and imaging of cytoplasmic targets. Nano Lett. 2008;8:3887–92. doi: 10.1021/nl802311t. [DOI] [PubMed] [Google Scholar]
- 13.Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angew Chem Int Ed Engl. 2008;47:1184–201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- 14.Bozdemir OA, Guliyev R, Buyukcakir O, Selcuk S, Kolemen S, Gulseren G, et al. Selective manipulation of ICT and PET processes in styryl-bodipy derivatives: applications in molecular logic and fluorescence sensing of metal ions. J Am Chem Soc. 2010;132:8029–36. doi: 10.1021/ja1008163. [DOI] [PubMed] [Google Scholar]
- 15.Bura T, Retailleau P, Ziessel R. Efficient synthesis of panchromatic dyes for energy concentration. Angew Chem Int Ed Engl. 2010;49:6659–63. doi: 10.1002/anie.201003206. [DOI] [PubMed] [Google Scholar]
- 16.Palma A, Alvarez LA, Scholz D, Frimannsson DO, Grossi M, Quinn SJ, et al. Cellular uptake mediated off/on responsive near-infrared fluorescent nanoparticles. J Am Chem Soc. 2011;133:19618–21. doi: 10.1021/ja208086e. [DOI] [PubMed] [Google Scholar]
- 17.Garcia ME, Sanz-Medel A. Dye-surfactant interactions: a review. Talanta. 1986;33:255–64. doi: 10.1016/0039-9140(86)80060-1. [DOI] [PubMed] [Google Scholar]
- 18.Karolin J, Johansson LBA, Strandberg L, Ny T. Fluorescence and absorption spectroscopic properties of dipyrrometheneboron difluoride (bodipy) derivatives in liquids, lipid-membranes, and proteins. J Am Chem Soc. 1994;116:7801–6. [Google Scholar]
- 19.Wang Z, Ho PC. Self-assembled core-shell vascular-targeted nanocapsules for temporal antivasculature and anticancer activities. Small. 2010;6:2576–83. doi: 10.1002/smll.201001122. [DOI] [PubMed] [Google Scholar]
- 20.Rust MJ, Bates M, Zhuang X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM) Nat Methods. 2006;3:793–5. doi: 10.1038/nmeth929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- 22.Huang B, Babcock H, Zhuang X. Breaking the diffraction barrier: super-resolution imaging of cells. Cell. 2010;143:1047–58. doi: 10.1016/j.cell.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannone G, Hosy E, Levet F, Constals A, Schulze K, Sobolevsky AI, et al. Dynamic superresolution imaging of endogenous proteins on living cells at ultra-high density. Biophys J. 2010;99:1303–10. doi: 10.1016/j.bpj.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharonov A, Hochstrasser RM. Wide-field subdiffraction imaging by accumulated binding of diffusing probes. Proc Natl Acad Sci U S A. 2006;103:18911–6. doi: 10.1073/pnas.0609643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang P, Lin J, Wang X, Wang Z, Zhang C, He M, et al. Light-triggered theranostics based on photosensitizer-conjugated carbon dots for simultaneous enhanced-fluorescence imaging and photodynamic therapy. Adv Mater. 2012;24:5104–10. doi: 10.1002/adma.201200650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Lin M, Yan Y, Wang Z, Ho PC, Loh KP. CdSe/AsS core-shell quantum dots: preparation and two-photon fluorescence. J Am Chem Soc. 2009;131:11300–1. doi: 10.1021/ja904675a. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Loh KP, Wang Z, Yan Y, Zhong Y, Xu QH, et al. Fluorescent nanogel of arsenic sulfide nanoclusters. Angew Chem Int Ed Engl. 2009;48:6282–5. doi: 10.1002/anie.200900586. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Wang TH. Quantum dot enabled molecular sensing and diagnostics. Theranostics. 2012;2:631–54. doi: 10.7150/thno.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Bu W, Pan L, Shi J. NIR -triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica. Angew Chem Int Ed Engl. 2013;52:4375–9. doi: 10.1002/anie.201300183. [DOI] [PubMed] [Google Scholar]
- 30.Xing H, Bu W, Ren Q, Zheng X, Li M, Zhang S, et al. A NaYbF4: Tm3+nanoprobe for CT and NIR-to-NIR fluorescent bimodal imaging. Biomaterials. 2012;33:5384–93. doi: 10.1016/j.biomaterials.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Chen G, Ohulchanskyy TY, Swihart MT, Prasad PN. Facile synthesis and potential bioimaging applications of hybrid upconverting and plasmonic NaGdF4: Yb3+, Er3+/silica/gold nanoparticles. Theranostics. 2013;3:275–81. doi: 10.7150/thno.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


