Abstract
Deficits in impulse control are related to a number of psychiatric diagnoses, including attention deficit hyperactivity disorder, addiction, and pathological gambling. Despite increases in our knowledge about the underlying neurochemical and neuroanatomical correlates, understanding of the molecular and cellular mechanisms is less well established. Understanding these mechanisms is essential in order to move towards individualized treatment programs and increase efficacy of interventions. Zebrafish are a very useful vertebrate model for exploring molecular processes underlying disease owing to their small size and genetic tractability. Their utility in terms of behavioral neuroscience, however, hinges on the validation and publication of reliable assays with adequate translational relevance. Here, we report an initial pharmacological validation of a fully automated zebrafish version of the commonly used five-choice serial reaction time task using a variable interval pre-stimulus interval. We found that atomoxetine reduced anticipatory responses (0.6 mg/kg), whereas a high-dose (4 mg/kg) methylphenidate increased anticipatory responses and the number of trials completed in a session. On the basis of these results, we argue that similar neurochemical processes in fish as in mammals may control impulsivity, as operationally defined by anticipatory responses on a continuous performance task such as this, making zebrafish potentially a good model for exploring the molecular basis of impulse control disorders and for first-round drug screening.
Keywords: Five-choice serial reaction time task, Zebrafish, Impulsivity, Addiction, ADHD, Atomoxetine, Methylphenidate
Introduction
Impulsivity, as operationally defined in terms of anticipatory responding on a continuous performance task, has been linked to a number of psychiatric diagnoses, including attention deficit hyperactivity disorder (ADHD) (Urcelay and Dalley 2012; Winstanley et al. 2006), substance abuse (Dalley et al. 2011; Everitt et al. 2008; Hosking and Winstanley 2011), and pathological gambling (Alessi and Petry 2003). Despite a recent increase in our understanding of the neurochemical and neuroanatomical correlates of impulsivity (Caprioli et al. 2013; Dalley and Roiser 2012), the underlying cellular processes are somewhat less clear.
Zebrafish provide an excellent model for studying the molecular basis of human disease owing to their prolific breeding, low maintenance costs, and genetic tractability (Guo 2004; Parker and Brennan 2012; Parker et al. 2013a). We previously demonstrated that adult zebrafish perform well in terms of their general response characteristics (accuracy, anticipatory responding, and omissions) on a three-choice (Parker et al. 2012a) and later a five-choice version (Parker et al. 2013b) of the commonly used five-choice serial reaction time task (5-CSRTT) (Carli et al. 1983; Robbins 2002) for rodents. Impulsivity, as operationalized by the rate of anticipatory responding on the task, is a strong predictor for compulsive drug seeking (Belin et al. 2008) and relapse following withdrawal from drugs (Economidou et al. 2009). Understanding the cellular and molecular basis of impulsivity may help us to develop individualized treatment for recovering addicts but also potentially to design early interventions for at-risk individuals.
In the present paper, we carried out an initial pharmacological validation of the 5-CSRTT in adult zebrafish using drugs that have previously been shown to affect rodents’ performance on the task with well-defined and frequently replicated results. Methylphenidate is a dopamine (DA) and noradrenaline reuptake inhibitor and has long been used to treat the symptoms of ADHD (Barkley 1997), but its effects on anticipatory responding in the 5-CSRTT are less clear with some studies showing increases in anticipatory response, and some decreases, at various doses (Bizarro et al. 2004; Navarra et al. 2008). Atomoxetine (Tomoxetine hydrochloride, LY 139603) is a selective noradrenaline reuptake inhibitor, which has also been successfully used in the treatment symptoms of ADHD (Michelson et al. 2001). Atomoxetine has shown high efficacy in reducing anticipatory responding on the 5-CSRTT in rodents (Economidou et al. 2011; Economidou et al. 2012; Fernando et al. 2012; Robinson et al. 2008). We incubated adult zebrafish in different doses of each of the drugs prior to probing anticipatory response rates on the 5-CSRTT using variable interval (VI) pre-stimulus intervals (PSI).
Method
Subjects
Nineteen adult, mixed-sex, wild-type (TU strain) zebrafish were bred in our aquarium facility at Queen Mary University of London (QMUL) and reared up to 4 months of age according to established protocols (Westerfield 1993). At 4 months, the fish were moved into our behavioral testing facility and pair-housed (26–28 °C, 160 lx ambient lighting; 14/10 h light/dark cycle) for 1 week prior to commencing the experiment. They remained pair-housed throughout the experimental period. Throughout the experiment, all fish were fed live brine shrimp and flake food at weekends and brine shrimp liquidized with bloodworm during testing (see below) supplemented with commercial dried flake food in the evening after testing. All procedures were carried out in accordance with the Animals (Scientific Procedures) Act of 1986 and local ethical guidelines.
Apparatus
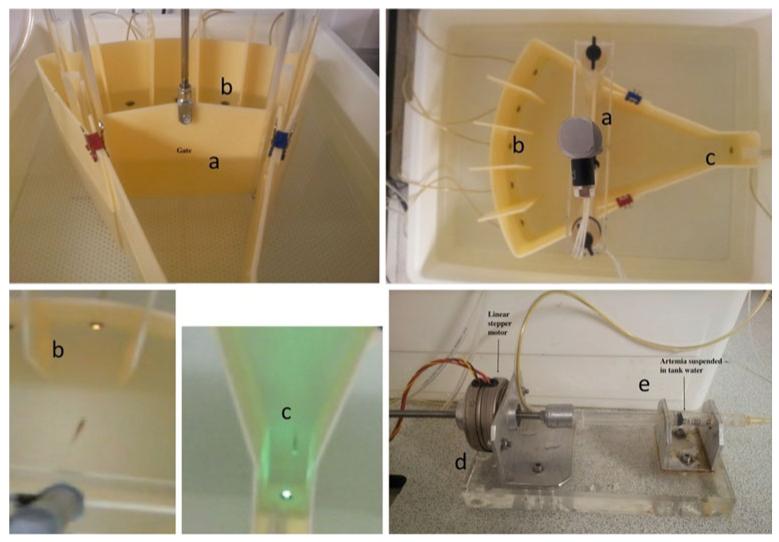
The fish were trained in a custom-built testing arena (Fig. 1) manufactured in-house at QMUL (Parker et al. 2013b). Briefly, the entire length of the testing unit was 36 cm, split into two halves by the gate (21 cm from food area to gate and 15 cm from gate to stimulus areas). The gate is used in order to signal the start and end of trials and to ensure that all of the fish start each trial from the same vantage point. In the rodent version of the task, the box is smaller in comparison to the size of the animal. We have attempted to use a smaller box in previous implementations of this task, but the fish do not perform well if confined to small spaces. The external tank (W×L×H: 42×49×15 cm) was purchased commercially (Ikea, UK). The base was constructed from 10 mm clear cast acrylic and drilled to fix two uprights to support the gate mechanism. The testing unit was constructed from opaque acrylic, and a 96-channel i-o card drove the actuators (National Instruments, Austin, TX). The apparatus were controlled via a program written in LabView (National Instruments, Austin, TX) that also collected the data during training sessions. The gate was operated via a pneumatic cylinder (RS Components, UK). The movements of the fish in the tank, and hence the actuation of the hardware, was performed by a custom-written (Python) camera-based fish detection system. The cameras were located above the tanks (Windows LifeCam HD). Food delivery was controlled by a linear stepper motor (RS Components, UK), calibrated to deliver ~10 μl liquidized bloodworm/brine shrimp mixture via a syringe and a length of 1 mm catheter tubing. The stimuli at the stimulus end of the tank comprised five super-bright yellow LEDs (RS components, UK), and the stimulus in the magazine area comprised a single super-bright green LED.
Fig. 1. Testing environment used to train zebrafish on 5-CSRTT.
(a)Gate mechanism, controlled by pneumatic piston. The gate raised to reveal the stimulus area containing the stimulus apertures (b) and the food delivery area containing the food magazine (c). The correct stimulus aperture (b) was signaled by illuminating a super-bright yellow LED, and food availability was signaled in the food magazine (c) by illuminating a super-bright green LED. Food, liquidized bloodworm and brine shrimp, was delivered via a 2-ml plastic syringe (e), driven by a linear stepper motor (d), all mounted on an acylic base. Image detection was carried out using custom software (Python) and an HD webcam from above the tanks. (Figure reproduced, with permission, from Parker et al. 2013b)
Atomoxetine (Tomoxetine hydrochloride, Tocris Bioscience, Bristol, UK; 0.5 (0.15 mg/kg), 1 (0.3 mg/kg), and 2 μM (0.6 mg/kg)) and methylphenidate (threo-methylphenidate hydrochloride, Tocris Bioscience, Bristol, UK; 5 (1.3 mg/kg), 10 (2.6 mg/kg), and 15 μM (4 mg/kg)) were dissolved in aquarium-treated water and administered to each fish at three different doses via incubation in the drug solution for 30 min prior to testing. The incubation tank was a 1-l transparent acrylic tank, identical to the fishes’ housing tanks, located adjacent to the testing tanks.
Procedure
Prior to training, all fish were acclimated to the behavioral testing room for 1 week (week 0). All testing sessions lasted for 30 min and were carried out Monday–Friday. The time of day that the fish were tested was staggered to avoid potential diurnal performance confounds, but the tank in which each fish was tested remained the same for every session. In the first week of pre-training (week 1), the fish were habituated to the testing tanks. During this time, all of the lights remained illuminated and the gate was raised. Food was delivered intermittently according to a 1-min fixed time schedule following entry to the food magazine. In the second week of pre-training (week 2), the fish were “magazine trained.” During this phase, the gate was closed and the fish was isolated in the food delivery end of the tank. The magazine light was illuminated for up to 30 s (1-min inter-trial interval (ITI)) or until the fish entered the food magazine. Correct entries (i.e., entry during the stimulus exposure) were reinforced in a discrete trial manner (see above). Entries during the ITI were neither reinforced nor punished. In the third and final week of pre-training (week 3), the fish were trained to approach the stimulus lights at the far end of the tank. At the start of a session, the fish was isolated in the food delivery area of the tank, with the magazine light illuminated. Entry to the magazine started the session. After an ITI of 20 s, the gate was raised to reveal the stimulus apertures. All LEDs were illuminated contiguously for 1 min. During this time, entry to any of the stimulus apertures was conditionally reinforced by illumination of the magazine light. As the fish swam past the gate, it was lowered, and entry to the food magazine was reinforced. The following trial began after a 20-s ITI. Late entries were not reinforced or punished, but the fish was isolated in the food delivery area following re-entry for a 20-s ITI. The fish were then trained on the 5-CSRTT. The general procedure was as in week 3, but only one stimulus light was illuminated at any one time, and we introduced a PSI, which represented the delay between the gate being raised and the stimulus being illuminated.
Training was split into three distinct phases. The criterion for moving from each phase to the next was that the fish performed ≥20 trials in each session for a minimum of three consecutive days. In the first phase (weeks 4–5), the stimulus duration was 30 s, and the PSI was 1 s (FI schedule). In the second phase (weeks 6–9), the stimulus duration remained at 30 s, but the pre-stimulus interval changed to a 5-s VI schedule. The third phase (weeks 10–15) incorporated the drug trials. Atomoxetine was administered at 0.5, 1, and 2 μM and methylphenidate at 5, 10, and 15 μM. These dose ranges were based on previous work with rodents (Bizarro et al. 2004; Economidou et al. 2011; Fernando et al. 2012; Milstein et al. 2010; Navarra et al. 2008; Robinson et al. 2008) and with zebrafish (Lange et al. 2012). During each drug treatment week, the treatment schedule was as follows: Monday, baseline; Tuesday, drug; Wednesday–Thursday, baseline; and Friday, drug. This allowed for a minimum of 2 days of washout between drug treatments. Each fish received all doses of both drugs twice during the course of the experiment, with each fish receiving the same drug twice in the same week. The order in which the drugs and doses were given was counterbalanced between fish to avoid any possibility of order effects. Performance parameters were calculated as thus:
Finally, data were analyzed using general or generalized linear mixed effects models (LME), fit by restricted maximum likelihood with drug as a fixed effect with seven levels (Baseline: methylphenidate, 5, 10, and 15 μM; atomoxetine, 0.5, 1, and 2 μM) and fish ID (random intercept) and day as scalar random effects, followed by pairwise comparisons (least significant difference). We used two drug days for each fish specifically as the fishes’ performance on the task is far more variable than that of rodents. Thus, each drug was given twice in the same week and we employed a mixed effects model to deal with any issues of inter-class correlations and pseudoreplication.
Fixed effects were evaluated initially with compound symmetry assumed, and subsequently with diagonal, first-order autoregressive or unstructured covariance structures. The best fitting model was ascertained by comparisons of Akaike’s information criterion. Denominator degrees of freedom were estimated according to the Satterthwaite approximation. Data were analyzed in IBM® SPSS® Statistics (Version 21 for Macintosh). All test statistics were evaluated with respect to an α-level of 0.05. All descriptive statistics are reported as mean±standard error.
Results
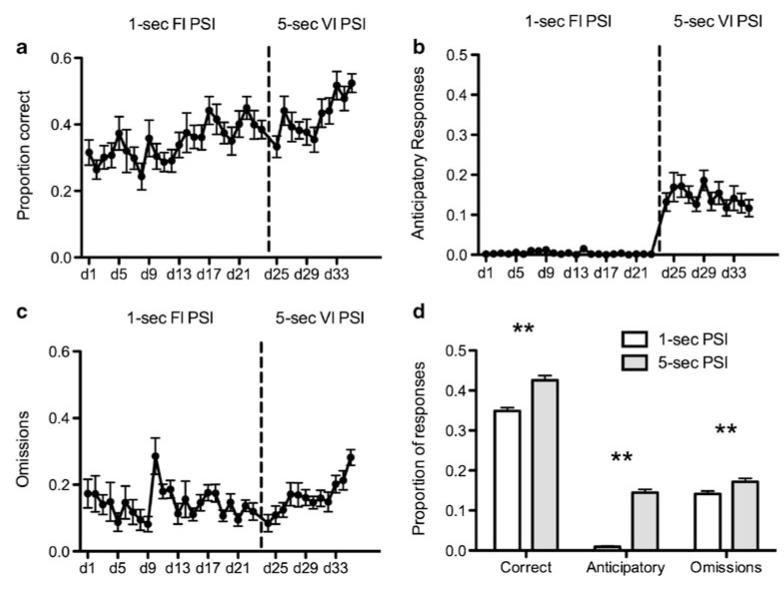
Figure 2 displays the learning curves during the first (1-s FI PSI) and second (5-s VI PSI) learning phases. As is clear, all fish increased their response accuracy during the course of the training, and this continued after the introduction of the 5-s VI PSI. This was confirmed with a general LME comparing the first and second phase of learning (1-s FI PSI vs 5-s VI PSI; F1,696=34.38, p<0.001). There was also a significant increase in anticipatory responses (F1,724=588.01, p<0.001 and omissions (F1, 725=7.54, p<0.01), upon introduction of the 5-s VI PSI. Finally, with respect to approach latency, there was no significant difference between the first and second phases of the experiment (F1,439=2.79, p=0.1; phase 1=3.6±1.42 s vs. phase 2=3.42±1.11 s) nor was there a difference for return latency (F1,335=1.23, p=0.27; phase 1=12.61±1.34 s vs. phase 2=12.15±0.93 s).
Fig. 2. Training data from 1-s FI PSI and 5-s VI PSI of 5-CSRTT.
Criterion for moving from phase 1 to 2 was ≥20 trials per session for three consecutive sessions. a Correct responses increased steadily thoughout training and significantly increased between phases 1 and 2. b Anticipatory responses increased on initiation of the 5-s VI PSI. c Omission errors increased significantly in phase 2. d Summary of data in each training phase. Error bars represent SEM. Note: **p<0.01, post-hoc pairwise comparisons
Stability of baseline
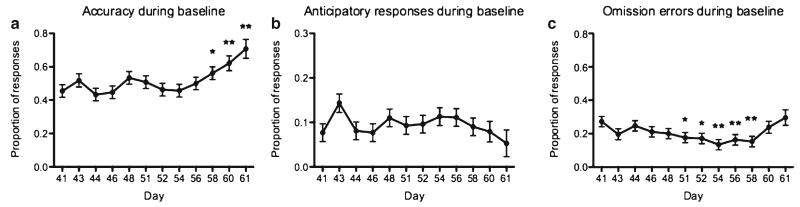
Drug testing did not commence until the fish were performing ≥20 trials in a session. Prior to drug testing, we examined performance over the final five sessions of pre-testing to ensure stability. A LME with day as the fixed effect revealed that accuracy had stabilized prior to drug testing commencing (F4,83=1.95, p=0.11) as had anticipatory responding (F<1). Omission errors, however, were not stable (F4,83=4.97, p<0.01). Stability during the baseline days of drug training was confirmed for anticipatory responding (F11,181=1.14, p=0.33). However, accuracy (F11,181=3.38, p<0.01) and omission errors (F11,179=2.46, p<0.01) were variable during baseline (see Fig. 3).
Fig. 3. Performance stability during baseline sessions of drug trials.
There was variability in accuracy (a), with accuracy increasing significantly in the last 3 days of baseline (days 58–61). There was also variability in omission errors (c), with omission error decreasing during days 51–58 of the drug delivery period, but re-stabilizing thereafter. Anticipatory response rate (b) was stable throughout the drug period. Error bars represent SEM. Note: Differs from day 41; *p<0.05; **p<0.01, post-hoc pairwise comparisons
Training
Figure 4 displays the number of trials, accuracy, anticipatory responding, omissions, correct latency, and return latency during the drug phase. There was a significant effect of drug treatment on total number of trials completed (generalized LME with Poisson distribution; F6, 378=2.32, p<0.05). Post-hoc pairwise comparisons confirmed a dose-dependent change for methylphenidate treatment. There was a significant increase in trials between baseline and 15 μM methylphenidate (p=0.041), but no differences between baseline and 10 (p=0.14) or 5 μM (p=0.18), nor between methylphenidate doses (p>0.65). There was no difference between baseline and atomoxetine at any of the doses (p>0.22) nor between atomoxetine doses (p>0.89).
Fig. 4. Dose-related effects of atomoxetine and methylphenidate on performance parameters of zebrafish in 5-CSRTT.
a Total trials in a session increased significantly (compared with baseline) following 15 μM methylphenidate but not at any other dose of either drug; b accuracy (proportion of correct responses) was not affected by either drug; c proportion of anticipatory responses was reduced (relative to baseline) following exposure to 2 μM atomoxetine and increased following exposure to 15 μM methylphenidate; d proportion of omission errors was not affected by either drug; e approach latency and freturn latency to collect food were also unaffected by either drug. Error bars represent SEM. Note: Differs significantly from baseline; *p<0.05; **p<0.01, post-hoc pairwise comparisons
Drug treatment had no significant effect on proportion of correct responses during sessions (F<1). There was a significant main effect of drug treatment on anticipatory responses (F6, 363=2.64, p<0.05). Pairwise comparison revealed that atomoxetine had a dose-dependent effect. Specifically, 2 μM atomoxetine reduced anticipatory responses relative to baseline (p<0.01) but neither 1 nor 0.5 μM atomoxetine had any effect (p>0.23). There was no difference at 2, 1, or 0.5 μM atomoxetine (p>0.13). Methylphenidate also affected anticipatory responding, increasing it relative to baseline at 15 μM (p<0.5). There were no differences at 5 or 10 μM compared with baseline (p>0.25). The fish also performed significantly more anticipatory responses at 15 μM methylphenidate than at 10 μM (p<0.05), but no difference between 15 and 5 μM (p=0.2). There was no effect of drug treatment on omissions (F6,361=1.68, p=0.12) or approach latency (F<1) or return latency (F6,109=1.86, p=0.09).
Discussion
The aim of the present study was to carry out an initial pharmacological validation of a fully automated version of the 5-CSRTT for studying impulse control in zebrafish. We previously demonstrated that a low dose of amphetamine (0.025 mg/kg) reduced anticipatory responding relative to saline injection on a three-choice version of this task (Parker et al. 2012a). Here, we show that atomoxetine reduced anticipatory responding in a dose-dependent manner (2 μM (0.6 mg/kg)) and methylphenidate increased anticipatory responding at higher doses (15 μM (4 mg/kg)). Methylphenidate also increased the number of trials completed during training sessions, suggesting increased general activity levels following exposure to higher doses of this drug. Neither compound had an effect on performance accuracy or omissions, nor any aspect of response latency at the doses tested here. However, performance of zebrafish was variable during baseline in terms of omission errors and to a lesser extent, accuracy, suggesting that the present manifestation of this task may not be suitable for addressing attentional performance. Our data show that in fish, selective increases in noradrenergic activity increase the ability to withhold a response on this task representing similar patterns to those observed in rats (Robinson et al. 2008) and human patients with ADHD (Chamberlain et al. 2007). This suggests some degree of conservation of the neurobiological underpinnings of the ability to withhold a response across species.
We also observed a higher proportion of anticipatory responses following incubation in the maximum dose of methylphenidate (15 μM) and intensification of general activity at all doses, with the latter as evidenced by the significant increase in completed trials in a session. The increase in anticipatory responding and the increase in general activity levels are similar to those observed in rats following comparably high doses of methylphenidate (5 mg/kg) (Navarra et al. 2008) and amphetamine (Cole and Robbins 1987; 1989). Methylphenidate blocks both the norepinephrine and DA transporter, thus causing a general increase in catecholamine neurotransmission (Bymaster et al. 2002). The fact that methylphenidate did not reduce anticipatory responding in the fish at the lower doses used here may suggest that the doses used here may not have been appropriate for this species. This hypothesis is partially supported by the fact that in a previous study, we found that a very low dose of amphetamine (0.025 mg/kg), a similar catecholaminergic transporter blocker, reduced anticipatory responding relative to saline injection (Parker et al. 2012a). However, we based the doses here on previous work with larval zebrafish (Lange et al. 2012) as well as effective doses used in mammalian models (Bizarro et al. 2004). In addition, the effect of methylphenidate on anticipatory responses on the 5-CSRTT are highly variable, with some studies finding increases (Milstein et al. 2010; Navarra et al. 2008), some no effect (Fernando et al. 2012) and some decreases (Bizarro et al. 2004) even at comparable doses to one another (2.5-10 mg/kg).
Zebrafish share a large degree of homology with mammals with respect to catecholaminergic and monoaminergic neurotransmitter systems (Parker et al. 2013a). Functional homologues for midbrain regions related to impulsivity are present in zebrafish, such as the caudal raphe complex (Rink and Wullimann 2002), from which serotoninergic (5-HT) neurons project to the dorsal pallium (fish) and pre-frontal regions (mammals). It is also clear that both DA and 5-HT projections from the pallium to thalamic regions are very similar to those seen in mammals (Guo et al. 1999; Holzschuh et al. 2001; Rink and Guo 2004; Rink and Wullimann 2001; 2002). Of relevance to this study, zebrafish have strikingly similar projection patterns of catecholaminergic neurons; for example, norepinephrine neural projections from the locus coeruleus to the subpallium in zebrafish and to the cortex in mammals (Holzschuh et al. 2001; Korf et al. 1973; Ma 1997; Tay et al. 2011). The currently accepted hypothesis is that the route of action of both atomoxetine and methylphenidate is via the reduction of locus coeruleus activity (Pliszka et al. 1996). In addition, atomoxetine (1 μM) and methylphenidate (10 μM) rescued the hyperactive/motor-impulsive phenotype observed in a putative ADHD model using morpholino oligonucleotide-treated zebrafish larvae with a transient loss of function in the latrophilin 3 gene (Lange et al. 2012). This, in conjunction with our findings that adult zebrafish respond similarly to atomoxetine in terms of anticipatory responding on a 5-CSRTT to mammalian models and humans, suggest that this species may represent a useful model system for examining the cellular and molecular basis of psychiatric disorder linked to impulse control and for first-round drug screening.
There are a number of performance, task-related, and methodological differences between fish and mammals on this task that should be addressed here. First, the proportion of correct responses is lower in fish (~60 % at asymptote) than rodents (~80–90 % at asymptote) and the response and return latencies are much longer in fish (~5 s in fish vs. ~1 s in rodents). In addition, stability of baseline responding in terms of accuracy and omission errors appears to be difficult to attain in fish. It may be that further refinement of the procedure will improve this in the future, or it may reflect specific differences in task performance between the species. For example, fish may become satiated faster than rodents owing to their size and the amount of food deliverable in each trial. If this were the case, we may expect the rate of omission errors to be correlated with accuracy, which we did not observe when all baseline sessions were considered. However, in the final three baseline sessions, where accuracy increases (see Fig. 3a), omissions increased in a similar manner consistent with the satiety hypothesis. Previously, we found omission and accuracy to be correlated (Parker et al. 2012a). Alternatively, it may be that fish do not stay on-task in the same way as rodents, meaning that they may not be capable of sustaining attention for prolonged periods. This would result in lower reliability for accuracy and omission errors but will not necessarily affect premature responding as this aspect of performance would be related to trial-specific motivation to approach the stimulus aperture.
There is some evidence that fish have differences in cognitive capacity; for example a number of studies in the 1960s suggested that fish did not form attentional sets (Behrend et al. 1965; Bitterman 1965; Bitterman and Mackintosh 1969). However, this has since been shown to have been the result of poorly defined task parameters (Parker et al. 2012b; Woodward et al. 1971). Second, the duration of the stimuli are shorter in the rodent version (~0.5 s) than in fish (30 s) (Bari et al. 2008). We are unable to test fish at shorter stimulus durations, in particular because zebrafish will become very stressed and not perform if confined to small areas. As such, our testing tank is far larger in size relative to the size of the fish than the rodent assay. Therefore we are not claiming that this task will be suitable for measuring aspects of attention in the fish under the current protocol, but we hope that in the future, this might be incorporated into the assay. Finally, in our design we incorporate a start gate in the apparatus. In the classical design of the 5-CSRTT, the animal is required to perform a nose-poke the magazine and turn around to start a trial. In our version, the fish has to return to the start area in order to drop the gate, and subsequently re-start the task. In this sense, both versions rely on the animal performing an observing response in order to gain access to the task stimuli. We have found that what appear to be pre-potent responses in the fish can be induced by a variable interval pre-stimulus delay. Furthermore we can, to some extent, control this with a noradrenaline transporter blocker (atomoxetine), in a similar manner to that consistently observed in rodents. We would argue therefore that this study represents a useful starting point for future research.
Zebrafish offer a valuable model for studying the genetics and molecular basis of psychiatric disease in general (Guo 2004). There are numerous ethical and practical difficulties relating to GWAS and CNV studies in humans, including an inability to test cause/effect relations. This has led to the extensive use of animal models, often examining phenotypes retrospectively using reverse-genetic procedures such as knock out/knock down of candidate genes in murine models. Forward genetic screening procedures that use mutagenesis to introduce random variation into the genome complement these studies and can uncover novel alleles and pathways contributing to specific disease phenotypes (Muto et al. 2005). Mutagenesis studies in rodents have been limited by both ethical and practical considerations, not least of which is the small number of offspring in each generation (rodents have 5–10 offspring per pairing in comparison to the 200–300 obtained from fish) and because levels of chemical mutagens required to induce the high density of mutations per genome seen in zebrafish (1/300 kb) are not tolerated by rodents. By contrast, mutagenesis screening in zebrafish has been used to great effect to uncover genetic modifiers of developmental processes (Amsterdam et al. 1999; Amsterdam et al. 2004; Darland and Dowling 2001; Golling et al. 2002). The data we have described here allow for behavioural screening in adult zebrafish to identify genetic modifiers of impulse control.
In summary, we have demonstrated that wild-type adult zebrafish show reduced anticipatory responding on the 5-CSRTT with a comparable dose of atomoxetine (2 μM) to those observed in mammals. Taken with previous data from our lab (Parker et al. 2012a) and from larval models of ADHD (Lange et al. 2012), this highly tractable and useful system, zebrafish, is emerging as a potentially useful model for studying the cellular basis of impulsivity and for first-round drug screening.
Acknowledgments
This research was funded by project grant G1000053 from the National Center for the Replacement, Reduction and Refinement of animals in research (NC3Rs; UK) and by the Medical Research Council (MRC; UK). CHB is a Royal Society (UK) Industrial Research Fellow. We acknowledge the contributions of Dennis Ife, Jun Ma and Chris Straw of the School of Engineering and Materials Science at Queen Mary University of London for building and engineering the automated testing arena, and Dr Fabrizio Smeraldi (Electronic Engineering and Computer Science) and Mahesh Pancholi (School of Biological and Chemical Sciences) for writing the visual tracking programming. We also thank the two anonymous reviewers for their helpful and constructive comments on earlier versions of this manuscript.
References
- Alessi S, Petry N. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Burgess S, Golling G, Chen W, Sun Z, Townsend K, Farrington S, Haldi M, Hopkins N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13:2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101:12792–12797. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3:759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Behrend ER, Domesick VB, Bitterman M. Habit reversal in the fish. J Comp Physiol Psychol. 1965;60:407. doi: 10.1037/h0022566. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman M. Phyletic differences in learning. Am Psychol. 1965;20:396. doi: 10.1037/h0022328. [DOI] [PubMed] [Google Scholar]
- Bitterman M, Mackintosh N. Habit reversal and probability learning: rats, birds, and fish. Animal Discrimination Learning. 1969:163–185. [Google Scholar]
- Bizarro L, Patel S, Murtagh C, Stolerman I. Differential effects of psychomotor stimulants on attentional performance in rats: nicotine, amphetamine, caffeine and methylphenidate. Behav Pharmacol. 2004;15:195–206. [PubMed] [Google Scholar]
- Bymaster F, Katner J, Nelson D, Hemrick-Luecke S, Threlkeld P, Heiligenstein J, Morin S, Gehlert D, Perry K. Atomoxetine increases extracellular levels of norepinephrine and dopamine in pre-frontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, Voon V, Carpenter TA, Everitt BJ, Robbins TW. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Robbins T, Evenden J, Everitt B. Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Del Campo N, Dowson J, Müller U, Clark L, Robbins TW, Sahakian BJ. Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry. 2007;62:977–984. doi: 10.1016/j.biopsych.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Cole B, Robbins T. Amphetamine impairs the discrimination performance of rats with dorsal bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology. 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Cole B, Robbins T. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012 doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Dalley J, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top–down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Darland T, Dowling JE. Behavioral screening for cocaine sensitivity in mutagenized zebrafish. Proc Natl Acad Sci U S A. 2001;98:11691–11696. doi: 10.1073/pnas.191380698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Economidou D, Dalley JW, Everitt BJ. Selective norepinephrine reuptake inhibition by atomoxetine prevents cue-induced heroin and cocaine seeking. Biol Psychiatry. 2011;69:266–274. doi: 10.1016/j.biopsych.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Economidou D, Theobald DE, Robbins TW, Everitt BJ, Dalley JW. Norepinephrine and Dopamine Modulate Impulsivity on the Five-Choice Serial Reaction Time Task Through Opponent Actions in the Shell and Core Sub-Regions of the Nucleus Accumbens. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Phil Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, Caprioli D, Moreno M, Hipolito L, Aspinall AT, Robbins TW, Dalley JW. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology. 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–140. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Guo S, Wilson SW, Cooke S, Chitnis AB, Driever W, Rosenthal A. Mutations in the zebrafish unmask shared regulatory pathways controlling the development of catecholaminergic neurons. Dev Biol. 1999;208:473–487. doi: 10.1006/dbio.1999.9204. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- Hosking J, Winstanley C. Impulsivity as a mediating mechanism between early-life adversity and addiction: theoretical comment on Lovic et al. Behav Neurosci. 2011;125:681–686. doi: 10.1037/a0024612. [DOI] [PubMed] [Google Scholar]
- Korf J, Aghajanian G, Roth R. Increased turnover of norepinephrine in the rat cerebral cortex during stress: role of the locus coeruleus. Neuropharmacology. 1973;12:933–938. doi: 10.1016/0028-3908(73)90024-5. [DOI] [PubMed] [Google Scholar]
- Lange M, Norton W, Coolen M, Chaminade M, Merker S, Proft F, Schmitt A, Vernier P, Lesch K, Bally-Cuif L. The ADHD-susceptibility gene lphn3. 1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol Psychiatry. 2012;17:946–954. doi: 10.1038/mp.2012.29. [DOI] [PubMed] [Google Scholar]
- Ma PM. Catecholaminergic systems in the zebrafish. III. Organization and projection pattern of medullary dopaminergic and noradrenergic neurons. J Comp Neurol. 1997;381:411–427. [PubMed] [Google Scholar]
- Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose–response study. Pediatrics. 2001;108:e83–e83. doi: 10.1542/peds.108.5.e83. [DOI] [PubMed] [Google Scholar]
- Milstein JA, Dalley JW, Robbins TW. Methylphenidate-induced impulsivity: pharmacological antagonism by beta-adrenoreceptor blockade. J Psychopharmacol. 2010;24:309–321. doi: 10.1177/0269881108098146. [DOI] [PubMed] [Google Scholar]
- Muto A, Orger MB, Wehman AM, Smear MC, Kay JN, Page-McCaw PS, Gahtan E, Xiao T, Nevin LM, Gosse NJ. Forward genetic analysis of visual behavior in zebrafish. PLoS Genet. 2005;1:e66. doi: 10.1371/journal.pgen.0010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarra R, Graf R, Huang Y, Logue S, Comery T, Hughes Z, Day M. Effects of atomoxetine and methylphenidate on attention and impulsivity in the 5-choice serial reaction time test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:34–41. doi: 10.1016/j.pnpbp.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Parker M, Brennan C. Zebrafish (Danio rerio) models of substance abuse: harnessing the capabilities. Behaviour. 2012;149:10–12. [Google Scholar]
- Parker M, Millington M, Combe F, Brennan C. Development and implementation of a three-choice serial reaction time task for zebrafish (Danio rerio) Behav Brain Res. 2012a;227:73–80. doi: 10.1016/j.bbr.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MO, Gaviria J, Haigh A, Millington ME, Brown VJ, Combe FJ, Brennan CH. Discrimination reversal and attentional sets in zebrafish (Danio rerio) Behav Brain Res. 2012b;232:264–268. doi: 10.1016/j.bbr.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M, Brock A, Walton R, Brennan C. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front Neural Circ. 2013a;7:63. doi: 10.3389/fncir.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M, Ife D, Ma J, Pancholi M, Smeraldi F, Straw C, Brennan C. Development and automation of a test of impulse control in zebrafish. Front Syst Neurosci. 2013b;7:65. doi: 10.3389/fnsys.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka S, McCracken J, Maas J. Catecholamines in attention-deficit hyperactivity disorder: current perspectives. J Am Acad Child Adolesc Psychiatry. 1996;35:264–272. doi: 10.1097/00004583-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Rink E, Guo S. The too few mutant selectively affects subgroups of monoaminergic neurons in the zebrafish forebrain. Neuroscience. 2004;127:147–154. doi: 10.1016/j.neuroscience.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum) Brain Res. 2001;889:316–330. doi: 10.1016/s0006-8993(00)03174-7. [DOI] [PubMed] [Google Scholar]
- Rink E, Wullimann MF. Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res Dev Brain Res. 2002;137:89–100. doi: 10.1016/s0165-3806(02)00354-1. [DOI] [PubMed] [Google Scholar]
- Robbins T. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, Dalley JW, Robbins TW. Similar effects of the selective noradrena-line reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Tay T, Ronneberger O, Ryu S, Nitschke R, Driever W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat Commun. 2011;2:171. doi: 10.1038/ncomms1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay G, Dalley J. Linking ADHD, impulsivity, and drug abuse: a neuropsychological perspective. Curr Top Behav Neurosci. 2012;9:173–197. doi: 10.1007/7854_2011_119. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) M. Westerfield; Eugene, OR: 1993. [Google Scholar]
- Winstanley C, Eagle D, Robbins T. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward WT, Schoel WM, Bitterman ME. Reversal learning with singly presented stimuli in pigeons and goldfish. J Comp physiol Psychol. 1971;76:460–467. doi: 10.1037/h0031402. [DOI] [PubMed] [Google Scholar]