Abstract
The ability to visualize neural circuits in zebrafish in vivo is one of the most useful aspects of this model organism in neuroscience. To maintain the transparency of embryos, however, drugs, such as 1-pheyl-2-thiourea (PTU) must be added, or researchers can use mutants that do not develop pigment (e.g., the casper). The behavioral characteristics of such strains, however, have not been documented. Here, we tested adult zebrafish from the casper line, as well as wild-type (Tübingen, TU) and wild-types treated as embryos with PTU on three commonly used behavioral endpoints in neuroscience: novel tank test (similar to open-field in rodents), conditioned place preference for nicotine, and social cohesion (using a new method of cluster analysis). We found no differences between the casper and the TU, but the adult TU treated with PTU as embryos showed a marked increase in anxiety during the novel tank test. These data suggest that where possible, labs interested in analysis of developmental processes involved in adult phenotypes should avoid the use of PTU in favor of transparent mutants, such as casper.
Introduction
Recent years have seen a significant escalation in the use of adult zebrafish in behavioral neuroscience research.1–5 One of the many benefits of using zebrafish in neuroscience research is the relative ease with which high-resolution visualization of neural circuits can be facilitated with embryos at various developmental stages.6 At very early stages (0–48 hpf ), for example, embryos are completely transparent, and the development of melanophores can be further delayed with the application of 1-phenyl-2-thiourea (PTU).7,8 In addition, there are a number of mutants that have been developed, which do not develop melanophores or iridiophores, such as the casper, a double mutant of nacre (mutation of mitfa) and roy orbison (mutated allele unknown),9 so called because of its completely transparent nature, or the widely used nacre or albino mutants,10 both of which do not develop melanophores. This has facilitated high resolution imaging at even later developmental stages without the need for chemical manipulation.
Recent developments of transgenic fish harboring Genetically-Encoded Ca2+ Indicator (GECI)11–13 have facilitated in vivo study of neural activation. For example, Muto et al.11 recently demonstrated that zebrafish larvae expressing GCaMP in the optic tectum could be imaged performing food-search behavior in real time at the single-cell level of spatial resolution, highlighting the benefits of using zebrafish for research into neural circuits. With the development of completely transparent mutants, such as casper, the potential for scanning deeper in the zebrafish brain could soon be realized. However, while some efforts have been made to characterize the behavioral profiles of the albino,14 and nacre15 neither casper, nor adults raised as embryos in PTU have previously been characterized behaviorally. This is essential if these potentially invaluable mutants are to have any translational relevance as a model organism for behavioral studies.
PTU does not appear to affect dopamine expression levels in embryos,16 but it is known to alter endocrine function.17 The casper was developed for use primarily in cancer research, and little is known about functional neural systems that may be affected by the mutation. Here we examine the responses of both these groups against wild-type (TU) fish in three common behavioral endpoints in neuroscience: anxiety, conditioned place preference (CPP) (for nicotine) and social cohesiveness (shoaling). This phenotyping is essential for a number of reasons, but most notably perhaps in the light of recent demonstrations of poor reproducibility of behavioral phenotypes within mouse strains between different laboratories.18 Further, previous studies have already identified strain-related differences in anxiety in zebrafish, including the albino14 underscoring the necessity for this kind of research. In addition in this paper, we introduce a new method of measuring social cohesiveness using cluster analysis.19
Methods
Subjects
All fish used in this experiment were bred in-house. Embryos of casper9 and TU wild-type (w-t) were collected on the same day from large breeding tanks (100 L) and placed in separate petri-dishes (n~40/dish). Half of the TU w-t fish were treated with PTU for the first 5 dpf (see below). The casper mutant fish used in this experiment were generated from established casper breeders in our facility. All fish were reared in-house according to established protocols up to 4 months of age.6 All groups were age and sex matched before testing. During this time, fish from each treatment group were housed in groups of ~10 per tank (5 L). The shoaling assay was carried out first on groups of five fish from the same housing tank. Before the novel tank test, fish were housed in pairs (1 L tanks) for 2-weeks with a clear divider separating tank-mates to facilitate identification. For the novel tank test, previous research has indicated that this method of housing produces the most reliable results, and avoids ceiling effects during the test.20 Finally, before the CPP the fish were individually housed. All procedures were carried out under the Animals (Scientific Procedures) Act, 1986, and under local ethical guidelines (Queen Mary University of London).
PTU treatment
TU w-t + PTU embryos were treated with PTU according to standard protocols.6 Briefly, PTU (0.2 mM) was added to egg water 24 hpf, and replaced with fresh PTU every 24 h for the first 5dpf. After this, the embryos were returned to normal fish water and reared in the aquarium.
Apparatus and procedure
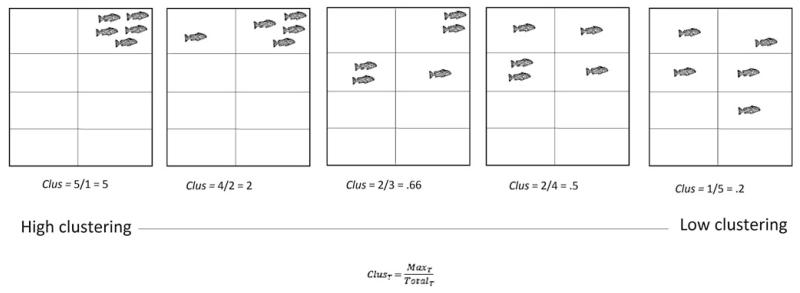
The cluster analysis (Fig. 1) was based on a technique developed for measuring general group cohesion in laying hens.19 Briefly, five fish were removed from larger groups and placed into a rectangular tank measuring (W × L × H) 35 × 50 × 15 cm. They were left to habituate for 5 min, after which they were filmed, from above, for a 10-min period. The data were analyzed according to strain (n = 3 TU w-t + PTU; n = 6 TU w-t; n = 9 casper).
FIG. 1. Cluster analysis.
Groups of n = 5 fish were observed in a rectangular tank (W × L × H: 35 × 50 × 15 cm) over a 10 min period. The tanks were split into eight equal segments (locations) for analysis. Cluster scores (Clus) were calculated for each time point (T) once every 30-s to ascertain shoaling by dividing the maximum number of fish in one location of the tank (MaxT) by the total number of segments occupied (TotalT).
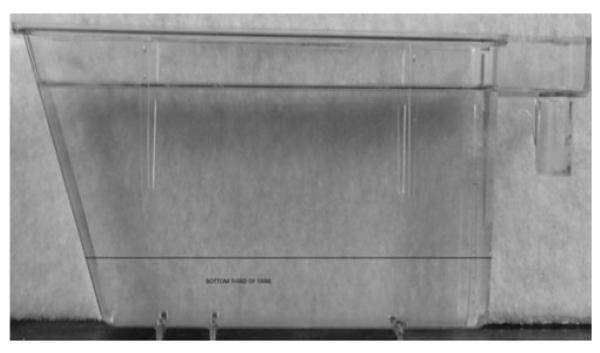
The novel tank test was carried out as previously described.20 Briefly, fish were housed in pairs for 2-weeks before tank diving. They were then transported to the testing room, and left to habituate for 1 h. The tank diving task was carried out in 1.5 L trapezoid tanks (W × L [Top] × L [Bottom] × H) 7.1 × 27.9 × 22.5 × 15.2 cm, filled with aquarium treated water from the main aquarium supply. Fish were tested individually by placing them into the novel tank (Fig. 2) for 5 min. During this time, the amount of time spent in the bottom third of the tank was recorded, as was their movement (distance travelled & velocity) and analyzed by strain (n = 11 TU w-t + PTU; n = 27 TU w-t; n = 23 casper. Data were collected automatically using Ethovision (Noldus Technologies).
FIG. 2. Tank used in novel tank test.
Fish were placed in the novel tank for 5-min and the duration (s) spent at the bottom of the tank was recorded via an automated video tracking system (Ethovision).
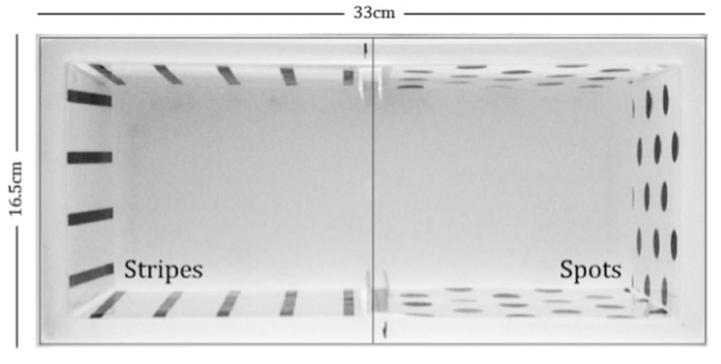
CPP was measured in a tank measuring (W × L × H) 16.5 × 33 × 15 cm which contained 3 L of water. On the walls of the tanks were placed the visual stimuli (see Fig. 3) that were either black spots or black vertical lines. All fish were first placed in the conditioning tanks, with no nicotine added, for a period of 20 min. This was repeated on the second day, during which baseline preference for spots or stripes was recorded during the last 5-min of the session. After this, the fish were conditioned to their least-preferred stimulus, depending on their baseline preference. Two days of conditioning were carried out, after which (Day 3) we conducted a probe trial. During conditioning trials, all fish were initially exposed to the nondrug side for a period of 20 min. A barrier in the tank prevented access to the drug side. The barrier was then lifted to allow the fish to move to the drug side of the tank. On entry, the barrier was replaced and nicotine (5 μM; nicotine hemi-sulphate, Sigma) was added to the tank water (n.b., nicotine solution was added to both ends of the tank to ensure equal dispersion of the solution through the water) until the bath concentration of nicotine reached 5 μM. This procedure was repeated on the following day. After conditioning, all fish were tested in a single probe trial, during which they were placed in a tank with the barrier lifted such that both sides could be accessed freely for 5-min. Time spent in the proximity to the drug-paired stimulus was recorded and analyzed according to strain (n = 9 TU w-t + PTU; n = 10 TU w-t; n = 12 casper). Data were collected automatically using Ethovision (Noldus Technologies).
FIG. 3. Tank used to measure conditioned place preference (CPP).
After assessment of baseline preference for spots or stripes, fish were conditioned for 2 days to their least-preferred stimulus with 5 μM nicotine solution. Preference was then reassessed in a drug-free probe trial.
Data analysis
Cluster analysis data were analyzed using random intercept linear mixed effects model (LMM; package lme4 for R21), with strain (3-levels: TU w-t, TU w-t + PTU, casper) as the fixed effect and time as a covariate. Group ID was taken as the unit of replication (groups of n = 5) and this was added as a random effect, as was housing tank. The response was cluster score. To calculate these, videos were reviewed by a single observer, and cluster scores (Clus) were ascertained once every 30-s (instantaneous sampling) according to the following equation: ClusT = MaxT/TotalT, where Max represented the maximum number of fish in one location (see Fig. 1), and Total the total number of locations occupied at any given time point (T). Locations were equal sizes and were demarcated before video analysis. This method generated cluster scores that fell in the range of 0.2:5, where lower scores suggested that the fish were more dispersed, and higher scores, more clustered (Fig. 1). Novel tank test data were analyzed with an LMM, with bottom duration (s) as the response, strain (3-levels: TU w-t, TU w-t + PTU, casper) and time (5-levels: min 1- min 5) as fixed factors, and distance travelled as a covariate. Random effects were fish ID and housing tank. CPP data were analyzed again using a random intercept LMM, with strain (3-levels: TU w-t, TU w-t + PTU, casper) and time (2-levels: baseline, postconditioning) as fixed factors, fish ID as a random effect (fish were individually housed), and preference for conditioned cue (s) as the response. Finally, for all LMMs, to facilitate Type-III null-hypothesis testing, F-statistics were estimated using the Kenward-Roger (K-R) approximation for denominator degrees of freedom.22 All data were analyzed in R (version 2.15.0) for Macintosh (www.r-project.org).
Results
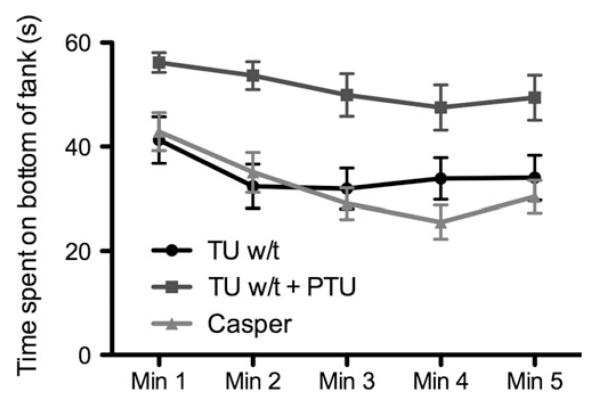
Data from the novel tank test are displayed in Figure 4. The response of the TU w-t and casper mutants were fairly similar, but the TU w-t + PTU group appeared to spend more time on the bottom of the tank during testing. This was confirmed by fitting a random intercept (fish ID as random effect) linear mixed-effects model (LMM) with time (added as a factor with 5-levels) and strain (three levels) as fixed effects, distance travelled (s) as a covariate and time spent on the bottom of the tank as the response variable. There was a main effect of time, F (4, 231) = 6.91, p < 0.001, with time on the bottom decreasing after min 1 (Min 1 . Min 2, p = 0.02; Min 1 vs. Min 3, p < 0.01; Min 1 vs. Min 4, p < 0.01; Min 1 vs. Min 5, p < 0.01). There was also a main effect of strain, F (2, 58) = 6.37, p < 0.01. TU w-t + PTU spent significantly more time on the bottom of the tank than TU w-t ( p < 0.01) and casper ( p < 0.01), but there was no significant difference between TU w-t and casper ( p = 0.96). There was no significant time × strain interaction, F < 1, suggesting that the pattern of tank exploration during the test did not differ, only the overall time on the bottom.
FIG. 4. Novel tank test.
Time (s) spent in the bottom third of a novel tank over a 5-min exposure for TU w/t, TU w/t treated with PTU (TU w/t + PTU) and casper mutants. Error bars represent standard error.
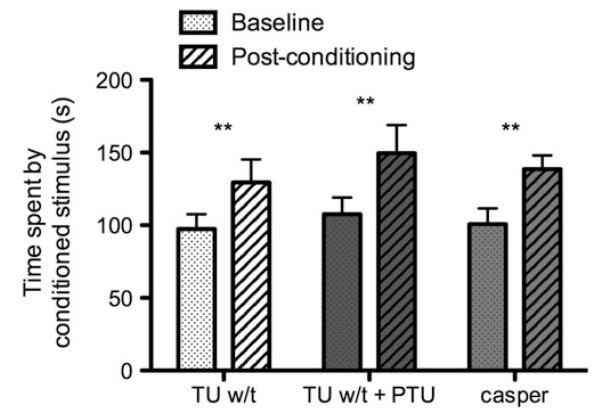
Figure 5 displays the preference of TU w-t, TU w-t treated with PTU (TU w-t + PTU) and casper mutants for drug-paired CS (spots or stripes, depending on initial preference) at baseline, and after 2 days of conditioning with 5 μM nicotine. All groups showed an increase in preference for the drug-paired cue. This was confirmed with a random intercept LMM, with strain (3-levels) and condition (2-levels) as fixed effects, and time spent in proximity to the conditioned cue (s) as the response. There was a significant effect of condition, F (1, 34) = 13.21, p < 0.001, with all strains showing a significant increase in preference for the drug-paired side. There was no effect of strain, nor was there a time × strain interaction (Fs < 1).
FIG. 5. Conditioned place preference for nicotine.
Time spent in the proximity of the conditioned cue before and after conditioning with 5 μM nicotine for 2 days for TU w/t, TU w/t treated with PTU (TU w/t + PTU) and casper mutants. Error bars represent standard error. **p < 0.01.
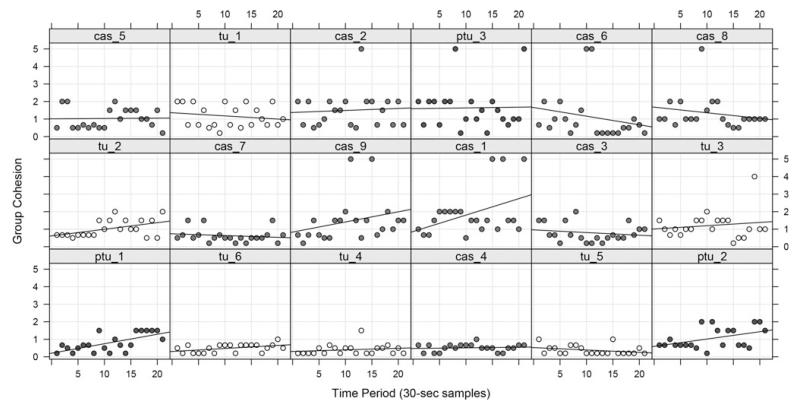
Figure 6 displays the cluster scores for each individual shoal of TU w-t, TU w-t treated with PTU (TU w-t + PTU) and casper mutant during each of the time points, ordered by intercept value. There were no clear patterns of change during the 10-min period, with the slopes of the regression lines looking randomly distributed across individual shoals. This was confirmed with a random intercept LMM with time as a covariate, clustering as the response, and individual group ID (the unit of replication) as a random effect, F (1, 359) = 2.9, p = 0.1 (time). There was also no difference between the scores of the three groups (TU w-t: 0.78 ± 0.18; TU w-t + PTU: 1.17 ± 0.25; casper: 1.14 ± 0.14), LMM = F (2, 15) = 1.45, p = 0.3 (strain), nor a time × strain interaction (F < 1).
FIG. 6. Cluster analysis of zebrafish shoals over 10-min.
Individual group dynamics for shoals from casper, TU w/t, and TU w/t + PTU, ordered by intercept (bottom-left–top-right panels). Group cohesion (clustering) was measured every 30-s (instantaneous sampling) for a 10-min undisturbed period. Lines represent first order least-squares regression (see text for details).
Discussion
The aim of this study was to characterize the adult behavioral phenotypes of casper mutant zebrafish and TU wild-type adults, which had been exposed as embryos to PTU in three commonly used behavioral endpoints for zebrafish researchers: anxiety (novel tank test), drug preference (CPP), and shoaling (cluster analysis). We found no evidence for a difference between the casper mutants and the wild-type fish in any of the three tests. Previously albino mutants, which have a genetic suppression of melanophores, but not both melanophores and iridiophores like casper, were observed to show a reduction in exploration of the tank during the novel tank test, suggesting increased stress reactivity.14 Although the comparison group in that study was the wild-type AB strain, whereas here we used the wild-type TU, this does suggest that, while albino might not be behaviorally equivalent to wild-type, no such problems are apparent with the casper.
We did, however, observe strong differences in novel tank diving responses of the PTU-treated embryos. These observations suggest that using PTU to visualize embryos should be approached with caution, in particular if researchers are interested in phenotypes relevant to stress and anxiety. PTU has previously been shown to affect development of embryos beyond the mere suppression of melanocytes. For example, there is evidence that it disrupts thyroid function,23 but also increases concentrations of peptides, such as follicle stimulating hormone and luteinizing hormone,17 suggesting that developmental exposure to PTU may disrupt organization of the endocrine system. This would explain why the PTU-treated adults showed differences in stress responsivity in the novel tank test. There were no differences found in tyrosine hydroxylase expression in embryos treated with PTU when compared with nacre mutants,16 and this would correspond to our findings that the PTU treated embryos showed no differences in the CPP assay. We also found that PTU did not appear to affect shoaling behavior, suggesting that PTU-treated embryos may be suitable for assays designed to examine social behavior. However, the interaction between stress-reactivity and social behavior is complex,24,25 and we would suggest caution in interpretation of data until more is understood about the molecular effects of PTU in relation to neural circuits relevant to social behavior.
Finally, in this study we have introduced a new method for quantifying shoaling behavior that may be used to complement existing methods (e.g., nearest neighbor). There are several benefits to using this method; for example, it is relatively simple and can be carried out quickly by a single observer. However, there are some potential limitations. This method lacks the detailed spatial resolution that is available from nearest-neighbor analyses.26 This method is also currently limited by the fact that it is analyzed in 2D, but using 3D tracking of fish may be a fruitful way forward in this procedure.
As we have found no evidence to suggest that casper mutants show different responding in three tasks commonly used in behavioral neuroscience, this suggests that it may be preferable to PTU to use this strain in work where researchers wish to look at developmental and adult phenotypes from the same cohort. In the light of emerging evidence of the capabilities of adult zebrafish to show complex cognitive processing, such as attention and impulse control,27 and behavioral flexibility,28,29 it may be possible to visualize neural circuits in adult zebrafish in vivo using the casper strain. This offers an unprecedented opportunity to visualize these circuits at the single-cell level of resolution.
Acknowledgment
All experimental work, as well as the salaries of M.O.P. and M.E.M., was funded by a research grant from the National Center for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) G1000053.
Footnotes
Disclosure Statement: No competing financial interests exist.
References
- 1.Parker MO, Brennan CH. Zebrafish (Danio rerio) models of substance abuse: Harnessing the capabilities. Behaviour. 2012;149:10–12. [Google Scholar]
- 2.Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioral genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaser RE, Chadwick L, McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 5.Kyzar E, Zapolsky I, Green J, Gaikwad S, Pham M, Collins C, et al. The Zebrafish Neurophenome Database (ZND): a dynamic open-access resource for zebrafish neurophenotypic data. Zebrafish. 2012;9:8–14. doi: 10.1089/zeb.2011.0725. [DOI] [PubMed] [Google Scholar]
- 6.Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) M. Westerfield; Eugene, OR: 1993. [Google Scholar]
- 7.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson J, von Hofsten J, Olsson PE. Generating transparent zebrafish: a refined method to improve detection of gene expression during embryonic development. Mar Biotechnol. 2001;3:522–527. doi: 10.1007/s1012601-0053-4. [DOI] [PubMed] [Google Scholar]
- 9.White RM, Sessa A, Burke C, Bowman T, LeBlanc J, Ceol C, et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell. 2008;2:183–189. doi: 10.1016/j.stem.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lister JA, Robertson CP, Lepage T, Johnson SL, Raible DW. Nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate. Development. 1999;126:3757–3767. doi: 10.1242/dev.126.17.3757. [DOI] [PubMed] [Google Scholar]
- 11.Muto A, Ohkura M, Kotani T, Higashijima S-i, Nakai J, Kawakami K. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc Natl Acad Sci. 2011;108:5425–5430. doi: 10.1073/pnas.1000887108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muto A, Ohkura M, Abe G, Nakai J, Kawakami K. Real-time visualization of neuronal activity during perception. Curr Biol. 2013;23:307–311. doi: 10.1016/j.cub.2012.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Muto A, Kawakami K. Imaging functional neural circuits in zebrafish with a new GCaMP and the Gal4FF-UAS system. Commun Integr Biol. 2011;4:566–568. doi: 10.4161/cib.4.5.15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Malley DM, Sankrithi NS, Borla MA, Parker S, Banden S, Gahtan E, et al. Optical physiology and locomotor behaviors of wild-type and nacre zebrafish. Methods Cell Biol. 2004;76:261–284. doi: 10.1016/s0091-679x(04)76013-6. [DOI] [PubMed] [Google Scholar]
- 16.Holzschuh J, Ryu S, Aberger F, Driever W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech Dev. 2001;101:237–243. doi: 10.1016/s0925-4773(01)00287-8. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Zhang X, Deng J, Hecker M, Al-Khedhairy A, Giesy JP, et al. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ Sci Technol. 2010;45:769–775. doi: 10.1021/es102659p. [DOI] [PubMed] [Google Scholar]
- 18.Richter SH, Garner JP, Würbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nat Methods. 2009;6:257–261. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- 19.Collins LM, Asher L, Pfeiffer DU, Browne WJ, Nicol CJ. Clustering and synchrony in laying hens: The effect of environmental resources on social dynamics. Appl Anim Behav Sci. 2011;129:43–53. [Google Scholar]
- 20.Parker MO, Millington ME, Combe FJ, Brennan CH. Housing conditions differentially affect physiological and behavioral stress responses of zebrafish, as well as the response to anxiolytics. PloS One. 2012;7:e34992. doi: 10.1371/journal.pone.0034992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. JML. 2008;59:390–412. [Google Scholar]
- 22.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997:983–997. [PubMed] [Google Scholar]
- 23.Elsalini OA, Rohr KB. Phenylthiourea disrupts thyroid function in developing zebrafish. Dev Genes Evol. 2003;212:593–598. doi: 10.1007/s00427-002-0279-3. [DOI] [PubMed] [Google Scholar]
- 24.Kagan J. Temperamental contributions to social behavior. Am Psychol. 1989;44:668. [Google Scholar]
- 25.von Dawans B, Fischbacher U, Kirschbaum C, Fehr E, Heinrichs M. The social dimension of stress reactivity acute stress increases prosocial behavior in humans. Psychol Sci. 2012;23:651–660. doi: 10.1177/0956797611431576. [DOI] [PubMed] [Google Scholar]
- 26.Miller N, Gerlai R. Quantification of shoaling behavior in zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Parker MO, Millington ME, Combe FJ, Brennan CH. Development and implementation of a three-choice serial reaction time task for zebrafish (Danio rerio) Behav Brain Res. 2012;227:73–80. doi: 10.1016/j.bbr.2011.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker MO, Gaviria J, Haigh A, Millington ME, Brown VJ, Combe FJ, et al. Discrimination reversal and attentional sets in zebrafish (Danio rerio) Behav Brain Res. 2012;232:264–268. doi: 10.1016/j.bbr.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira R. Social plasticity in fish: integrating mechanisms and function. J Fish Biol. 2012;81:2127–2150. doi: 10.1111/j.1095-8649.2012.03477.x. [DOI] [PubMed] [Google Scholar]