Abstract
We studied the influence of the rs1182 polymorphism of the TOR1A gene on the risk of dystonia spread in two representative cohorts of patients presenting with primary blepharospasm (BSP), one from Italy and the other from the United States of America. The relationship between rs1182 polymorphism and spread was estimated by Kaplan-Meier survival curves and Cox proportional hazard regression models adjusted by age and sex, age of BSP onset. In both series, patients carrying the T allele (G/T or T/T) in the rs1182 polymorphism were more likely to have dystonia spread as compared with the homozygous carriers of the common G allele. The comparable findings obtained in two independent cohorts support a genetic contribution to BSP spread.
Keywords: TOR1A, single-nucleotide polymorphisms, blepharospasm, primary adult-onset, dystonia, spread
A single mutation in TOR1A, the gene encoding torsin A protein, is responsible for most cases of early-onset generalized primary dystonia but has no significant role in primary late-onset dystonia.1 Recent case-control studies in Icelandic and Italian populations nevertheless raised the possibility that the 191G/T single nucleotide polymorphism (SNP) located at the 3_untranslated region of the TOR1A gene (SNP ID: rs1182) influences the risk of developing late-onset dystonia.2–4 However, these findings were not confirmed in other series from Germany or the United States of America.3,5
Previous studies did not take into account that late-onset dystonia may remain focal or spread to adjacent body regions,6,7 and left open the question whether normal TOR1A variants affect the susceptibility to spread. Because primary blepharospasm (BSP) have a higher risk of spread and spread faster (usually within the first 5 yr of history) than focal cervical dystonia (CD) or hand dystonia (FHD),2–4 we tested whether the rs1182 SNP affects the risk of spread in two independent Italian and U.S. cohorts of cases presenting with primary BSP.
Patients and Methods
Italian patients were consecutive outpatients periodically seen at the movement disorder clinics of the participating centres between 2002 and 2007. The majority of the United States of America patients were members of the Benign Essential Blepharospasm Research Foundation and were examined in nine major cities across the USA in a two-yr period. A smaller number of patients were seen in outpatient visits at the National Institutes of Health (NIH) in Bethesda, Maryland. Two-thirds of patients had already been included in our previous genetic studies.2,3 The study was approved by local ethical committees.
Inclusion criteria were focal BSP or BSP as part of a segmental/multifocal dystonia diagnosed according to standard criteria;1 BSP as the onset manifestation of dystonia; age at BSP onset >20 yr; and duration of disease >5 yr for patients with focal BSP. Exclusion criteria were features suggesting secondary or heredo-degenerative dystonia,1 Most patients were receiving botulinum toxin which seemed to have no effect on spread.8 No patient was exposed to neuroleptic drugs after BSP onset.
Spread of dystonia to oromandibular region -mouth, jaw and tongue-, neck, larynx, and limbs was assessed by standardized examination including triggering manoeuvres for dystonic movements or postures in apparently asymptomatic subjects. Dystonia was diagnosed when slow dystonic movements and definitely abnormal postures occurred at rest or were activated by specific tasks. Subtle signs like unusual tight hand gripping during writing (three cases) and shoulder elevation without significant limitation of shoulder movement (five cases) were not considered dystonic.9,10 No patient had hand tremor.11 Assessors were unaware of study hypothesis.
In the Italian series, information on age at BSP onset and date of spread (approximated to 1 yr) obtained at the initial clinical evaluation was supported by records from other physicians whenever available. In about half of cases, however, the date of spread was obtained from our own observation at follow-up visits. We, therefore evaluated the agreement of the diagnosis of dystonia at different body sites among the examiners from the participating centers by k statistics12 using viderecordings from 20 patients with late-onset dystonia, 10 patients with movement disorders other than dystonia, and 10 healthy controls. According to the Landis classification,13 substantial (k index between 0.6 and 0.8) to almost perfect (k > 0.8) interobserver agreement was obtained for the diagnosis of OMD (k = 0.71), CD (k = 0.82), laryngeal dystonia (k = 0.73) and FHD (k = 0.75).
In the United States of America series, information was obtained from a one time face-to-face examination performed by the same examiner (EP) who evaluated BSP patients for spread of dystonia as above reported. Diagnosis was confirmed by the senior investigator (MH). The dates of BSP onset and of dystonia spread were obtained from the patient's historical report and records from other physicians whenever available.
The rs1182 SNP was genotyped by real-time polymerase chain reaction and a site specific enzymatic cleavage (primers DYT1-F: 5_TGACAGTCATGATTGGCAGCCG-3_; and DYT1-R: 5_ATCTGAGCAGTCTCTCATAATG-3_) as reported.2,3
The relationship between rs1182 SNP and spread was estimated by Kaplan-Meier survival curves and multivariable Cox proportional hazard regression models assuming time to spread as the primary end-point.12,14 The STATA8 package computed survival curves and hazard ratio (HR), 95% confidence interval (CI) and p values. Significance was set at the 0.05 level. Statistical power was assessed according to Parmar and Marchin.12 Sensitivity of the T allele testing was the proportion of patients with BSP as part of a segmental/multifocal dystonia who also carried the T allele; specificity was the proportion of BSP patients who remained focal and did not carry the T allele.
Results
A total of 144 Italian patients and 257 USA patients (all Caucasians) met eligibility criteria. In both series, females predominated, BSP had its onset in the fifth to sixth decade and in most cases spread within the first 5 yr (Table 1, Fig. 1). Age at BSP onset and frequency of spread were significantly higher in the Italian series (Table 1). In both series, age of BSP onset was greater in the patients who spread (< 0.01) whereas duration of disease tended to be longer in those who did not (data not shown). BSP spread to one body site in 40 Italian and 38 United States of America patients and to a second body site in 12 Italian and 22 United States of America patients (P = 0.12). Dystonia spread more frequently to the oromandibular region, less frequently to neck, larynx and upper limb (Table 1). The genotype frequency for the rs1182 SNP was similar in Italian and United States of America patients (Table 1).
Table 1. Demographic and clinical features, and genotype distribution of the TOR1A single nucleotide polymorphism rs1182 in Italian and United States of America series.
| Italian series | USA series | P* | |
|---|---|---|---|
| Number of patients | 144 | 257 | |
| Sex (men/women) | 42/102 | 57/200 | 0.12 |
| Mean age (yr) ± SD. | 68.6 ± 10.2 | 66.2 ± 9.3 | 0.90 |
| Mean age (yr) of blepharospasm onset ± SD | 57.2 ± 7.9 | 52 ± 8.7 | <0.0001 |
| Mean years of follow up ± SD | 11.4 ± 5.7 | 14.5 ± 8.4 | <0.0001 |
| Number of patients (%) who experienced spread | 52 (36%) | 60 (23%) | 0.006 |
| Mean time to initial spread (years) ± SD | 2.8 ± 2.8 | 2.9 ± 3.8 | 0.6 |
| Number of patients who experienced spread ** to | |||
| Oromandibular region | 42 | 40 | 0.22 |
| Larynx | 4 | 10 | |
| Neck | 15 | 25 | |
| Upper limb | 4 | 7 | |
| rs 1182 - genotype distribution (%) | |||
| GG | 91 (63%) | 170 (66%) | 0.50 |
| GT | 41 (28%) | 73 (28%) | |
| TT | 12 (9%) | 14 (6%) |
P by by Student's t test and Chi-square test.
Blepharospasm spread to only one body site in 40 Italian and 38 United States of America patients, to a second body site in 12 Italian and 24 United States of America patients.
Fig. 1.
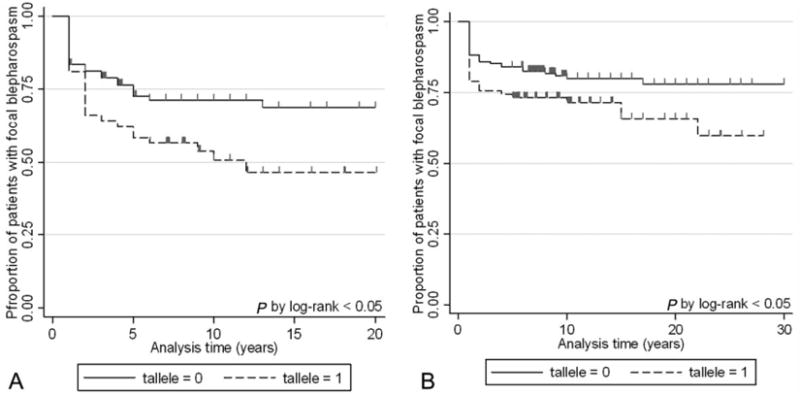
Kaplan–Meier survival analysis of spread of dystonia in the Italian (A) and USA (B) series according to the presence of the T allele in the TOR1A single nucleotide polymorphism rs1182. Censored observations are displayed by ticks. In the Italian series, number of at risk patients was 144 at time zero, 92 at 5 yr, 50 at 10 yr, 23 at 15 yr, and 12 at 20 yr. In the USA series, there were 257 patients at time zero, 209 at 5 yr, 132 at 10 yr, 84 at 15 yr, 49 at 20 yr, and 20 at 30 yr.
In both series, patients carrying the T allele (GT or TT) were significantly more likely to experience spread than those without (Fig. 1). Multivariable Cox analysis taking into account age, sex, and age at BSP onset (and referral center in the Italian series) confirmed a significantly higher risk of spread in patients carrying the T allele as compared with homozygous carriers of the common G allele (Italian series: adjusted HR, 1.9; 95%CI, 1.1–3.2; P = 0.03. USA series: adjusted HR, 2.1; 95%CI, 1.1–3.9; P = 0.02). Stratification by genotype yielded an increased risk of spread in GT patients (Italian series: adjusted HR, 1.8; 95%CI, 1.1–3.3; P = 0.04. USA series: adjusted HR, 2.2; 95%CI, 1.1–4.2; P = 0.02). whereas the TT group failed to reach statistical significance (Italian series: adjusted HR, 1.8; 95%CI 0.7–4.8; P = 0.25. USA series: adjusted HR, 1.7; 95%CI, 0.7–4.7; P = 0.50). The study had an estimated <80% chance of detecting a two-fold change in the risk of spread for the TT genotype with =0.05 (two-sided).
United States of America patients carrying the T allele yielded a higher risk of spread to either the oromandibular region (adjusted HR, 2.0; 95%CI, 1.1–3.9; P = 0.03) or extracranial sites (neck, larynx, and upper limb) (adjusted HR, 2.1; 95% CI, 1.1–4; P = 0.03). Non significant trends towards increased risk of spread were observed in the Italian series (Oromandibular region: adjusted HR, 1.7; 95%CI, 0.9–3.1; P = 0.11. Extracranial sites: adjusted HR, 2.0; 95%CI, 0.8–4.9; P = 0.14), but study power was <80%.
The T allele testing yielded 50% (26/52) sensitivity and 71% (65/92) specificity in the Italian series, 45% (27/60) sensitivity and 70% (137/197) specificity in the United States of America group.
Discussion
In our samples, the risk of spread was higher in patients carrying the T allele than in homozygous carriers of the common G allele. The association between T allele and spread was independent of possible confounders and, probably, of the site of spread. The comparable findings obtained in two independent cohorts add an extra validation to the association of the rs1182 polymorphism to dystonia spread in patients presenting with primary BSP. This finding also receives support from current knowledge. Although the effect of the rs1182 SNP upon the expression and functioning of torsin A is unknown and we cannot exclude that rs1182 might be the tagging SNP for a different causative variant in the haplotype block, dystonia associated with the TOR1A_GAG mutation has a high tendency to generalize within few years from onset.1,7
Our study may have limitations. This was not a population-based study, but recruiting criteria yielded case series resembling the general population of cases in both demographic and clinical features.1,6,7,15 The difference in the frequency of spread between Italian and United States of America populations probably reflected the different age at dystonia onset. In patients presenting with BSP, the higher the age at BSP onset, the higher was the tendency to spread.6,7,15 The satisfying levels of interobserver agreement on the diagnosis of dystonia at different body sites minimized an observer bias causing misclassification of spread events. Assessing self-reported age at dystonia onset and timing to spread in half of the Italian patients and in the majority of the United States of America patients might expose to recall bias. Nevertheless, we showed that age at dystonia appearance may be reliably determined from retrospective reports in primary late-onset dystonia.6 The sample size did not allow us to examine the influence of the rs1182 polymorphism on the risk of second spread, and to adequately investigate the effect of the TT genotype. We calculated15 that about 700 patients would be needed to detect a two- to three-fold change in the risk of spread for the TT genotype with 50.05 (two-sided), β > 80%, 30% frequency of spread events at 5 yr, and 10% frequency of TT genotype. A study of such size may be difficult to perform in reasonable time owing to BSP prevalence.16
In conclusion, this study provides new information suggesting that genetic factors may contribute to the spread of primary BSP. The low sensitivity of the T allele testing indicates that spread of dystonia is probably a multi-factorial event. The 70% specificity also indicates that excluding the T allele may help to identify BSP patients who are less likely to have spread.
Acknowledgments
This work was funded by the Comitato Promotore Telethon, Italy (Grant No. GGP05165); the Benign Essential Blepharospasm Research Foundation, Beaumont, TX, USA; and the Intramural Research Programs of the National Institute on Aging and National Institute of Neurological Disorders and Stroke, National Institutes of Health: Department of Health and Human Service(project number Z01 AG000957-05), Bethesda, MD, USA.
Footnotes
Potential conflict of interest: None reported.
Author Roles: Giovanni Defazio: Conception and organization of the research project; design and execution of the statistical analysis; writing of the first draft and revision of the manuscript; Mar Matarin: Organization and execution of the research project; review and critique of the manuscript; Elizabeth L Peckham: Organization and execution of the research project; review and critique of the manuscript; Davide Martino: Organization and execution of the research project; execution and revision of the statistical analysis; review and critique of the manuscript; Enza M Valente: Organization and execution of the research project; review and critique of the manuscript; Andrew Singleton: Organization and execution of the research project; review and critique of the manuscript; Anthony Crawley: Execution of the research project; Maria Stella Aniello: Execution of the research project; Francesco Brancati: Execution of the research project; Giovanni Abbruzzese: Execution of the research project; review and critique of the manuscript; Paolo Girlanda: Execution of the research project; review and critique of the manuscript; Paolo Livrea: Review and critique of the manuscript; Mark Hallett: Conception and organization of the research project; design, review and critique of the statistical analysis; review and critique of the manuscript; Alfredo Berardelli: Conception, organization and execution of the research project; review and critique of the manuscript.
References
- 1.Bressman SB. Dystonia genotypes, phenotypes, and classification. In: Fahn S, Hallett M, DeLong MR, editors. Dystonia 4, Adv neurol. Vol. 94. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 101–107. [PubMed] [Google Scholar]
- 2.Clarimon J, Asgeirsson H, Singleton A, et al. Torsin A haplotype predisposes to idiopathic dystonia. Ann Neurol. 2005;57:765–767. doi: 10.1002/ana.20485. [DOI] [PubMed] [Google Scholar]
- 3.Clarimon J, Brancati F, Peckham E, et al. Assessing the role of DRD5 and DYT1 in two different case-control series with primary blepharospasm. Mov Disord. 2007;22:162–166. doi: 10.1002/mds.21182. [DOI] [PubMed] [Google Scholar]
- 4.Kamm C, Asmus F, Mueller J, et al. Strong genetic evidence for association of TOR1A/TOR1B with idiopathic dystonia. Neurology. 2006;67:1857–1859. doi: 10.1212/01.wnl.0000244423.63406.17. [DOI] [PubMed] [Google Scholar]
- 5.Hague S, Klaffke S, Clarimon J, et al. Lack of association with TorsinA haplotype in German patients with sporadic dystonia. Neurology. 2006;66:951–952. doi: 10.1212/01.wnl.0000203344.43342.18. [DOI] [PubMed] [Google Scholar]
- 6.Abbruzzese G, Berardelli A, Girlanda P, et al. Long term assessment of the risk of spread in primary late-onset focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:392–396. doi: 10.1136/jnnp.2007.124594. [DOI] [PubMed] [Google Scholar]
- 7.Weiss EM, Hershey T, Karimi M, et al. Relative risk of spread of symptoms among the focal onset primary dystonias. Mov Disord. 2006;21:1175–1181. doi: 10.1002/mds.20919. [DOI] [PubMed] [Google Scholar]
- 8.Skogseid IM, Kerty E. The course of cervical dystonia and patient satisfaction with long term botulinum toxin A treatment. Eur J Neurol. 2005;12:163–170. doi: 10.1111/j.1468-1331.2004.01053.x. [DOI] [PubMed] [Google Scholar]
- 9.Bressman SB, Raymond D, Wendt K, et al. Diagnostic criteria for dystonia in DYT1 families. Neurology. 2002;59:1780–1782. doi: 10.1212/01.wnl.0000035630.12515.e0. [DOI] [PubMed] [Google Scholar]
- 10.Wright RA, Ahlskog JE. Focal shoulder-elevation dystonia. Mov Disord. 2000;15:709–713. doi: 10.1002/1531-8257(200007)15:4<709::aid-mds1017>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54(Suppl. 4):S2–S6. [PubMed] [Google Scholar]
- 12.Parmar KB, Machin D. Survival analysis: a practical approach. New York: John Wiley; 1995. [Google Scholar]
- 13.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 15.Defazio G, Berardelli A, Abbruzzese G, et al. Risk factors for spread of primary adult onset blepharospasm: a multicentre investigation of the Italian movement disorders study group. J Neurol Neurosurg Psychiatry. 1999;67:613–619. doi: 10.1136/jnnp.67.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Defazio G, Abbruzzese G, Livrea P, Berardelli A. Epidemiology of primary dystonia. Lancet Neurol. 2004;3:673–678. doi: 10.1016/S1474-4422(04)00907-X. [DOI] [PubMed] [Google Scholar]


