Abstract
This analysis examined the relative contributions of sex, age, body mass index (BMI), and puberty (Tanner) stage on salivary melatonin amplitude. Sixty-nine children and adolescents (30 females; 9.6–17.8 years) were examined for Tanner stage. Serial salivary melatonin samples were collected in controlled conditions, from which these melatonin amplitude measures were derived: area under the curve (AUC) and maximum value (MAX). AUC declined with advancing Tanner stage. This melatonin decline was similar between boys and girls, but girls secreted more melatonin compared to boys. Tanner stage and sex explained AUC variability, but age and BMI did not; similar results emerged for MAX. These results indicate that puberty stage may either mediate the decline of melatonin, or the decrease in melatonin amplitude may be an indicator of pubertal progression. These findings also indicate that the melatonin decline during puberty is not entirely accounted for by body mass or by age.
Keywords: melatonin, adolescent, puberty, circadian, pineal, human
Melatonin, a hormone secreted by the pineal gland, fluctuates with a circadian rhythm such that circulating levels of the hormone are lowest during the daytime (light phase) and highest at night (dark phase). The amplitude of this endogenous melatonin rhythm varies among individuals (e.g., Burgess & Fogg, 2008) and across the lifespan (Waldhauser & Steger, 1986; Waldhauser, Weiszenbacher, Tatzer, Gisinger, Waldhauser, Schemper, & Frisch, 1988). Previous studies report a decline of nocturnal plasma melatonin (Attanasio, Borrelli, & Gupta, 1985; Salti, Galluzzi, Bindi, Perfetto, Tarquini, Halberg, & Cornelissen, 2000; Waldhauser, Weiszenbacher, Frisch, Zeitlhuber, Waldhauser, & Wurtman, 1984) in healthy children and adolescents with advancing puberty (Tanner) stage (Tanner, 1962). A similar melatonin decline emerged in female rhesus monkeys that were followed longitudinally across sexual maturation (Wilson & Gordon, 1989). Other studies, however, did not confirm (Cavallo, Richards, & Smith, 1992; Ehrenkranz, Tamarkin, Comite, Johnsonbaugh, Bybee, Loriaux, & Cutler, 1982) or contradicted this finding (Penny, 1982). These discrepant results may be explained by small sample sizes or methodological differences among studies, such as sampling frequency (single or multiple), bright or unreported ambient light levels, and uncontrolled sleep/wake schedules.
The functional significance, if any, of a decline in melatonin as it relates to the onset of puberty is debated (Cavallo, 1993) because other factors coincident with puberty stage may contribute to melatonin amplitude. For example, in one study, melatonin levels decreased as a function of age (Waldhauser et al., 1988), and when age was accounted for, the puberty-related decline of melatonin disappeared (Cavallo, 1992). Furthermore, Young and colleagues argued that the pineal gland secretes the same amount of melatonin across puberty, and the change in melatonin levels measured during this developmental period is accounted for by increasing body mass and associated diffusion of melatonin in larger body habitus (Young, Francis, Leone, Stovell, & Silman, 1988). This latter study, however, did not measure pubertal stage, and evidence from several studies does not support this finding (Cavallo & Dolan, 1996; Fideleff, Boquete, Fideleff, Albornoz, Lloret, Suarez, Esquifino, Honfi, & Cardinali, 2006; Salti et al., 2000). Sex is yet another factor that may contribute to melatonin amplitude during puberty. Studies of adults reported that women secrete more melatonin compared to men (Cain, Dennison, Zeitzer, Guzik, Khalsa, Santhi, Schoen, Czeisler, & Duffy, 2010; Wetterberg, Bratlid, von Knorring, Eberhard, & Yuwiler, 1999). Several studies of adults (Burgess & Fogg, 2008) and adolescents (Cavallo & Dolan, 1996; Cavallo et al., 1992; Griefahn, Brode, Blaszkewicz, & Remer, 2003; Salti et al., 2000), however, do not find these sex differences or find a difference accounted for by the youngster’s age (Penny, 1982).
A well-controlled assessment of pubertal adolescents with repeated nocturnal melatonin sampling that also accounts for previously described factors that may or may not contribute to a melatonin decline during puberty is needed to address inconsistent findings in the literature. Thus, the aims of the current analysis were (1) to examine whether or not melatonin amplitude changes across puberty stage when multiple salivary melatonin samples are taken in dim light conditions across an entire night after controlled light/dark exposure; and (2) to examine the relative contributions of sex, age, body mass index (BMI), and pubertal stage on salivary melatonin amplitude collected in constant conditions.
Materials and Methods
Participants
Data from 69 children and adolescents (30 females) ages 9.6 to 17.8 years (mean = 12.7, SD = 1.5 years) were included in this analysis. Parents reported their child’s race; 52 were identified as Caucasian, 6 as African American, 3 as Hispanic or Latino, 4 as Asian, 6 as multiracial, and 2 were another or unknown race. These youngsters participated in different laboratory studies using the same methodology, and some of these data have been previously reported (Acebo, Labyak, & Carskadon, 2003). Data were collected from 1996 to 2002 during June, July, and August at 42 degrees northern latitude. Participants were recruited to these studies using fliers at local schools, libraries, and other cooperating public establishments; letters sent to parents and students at home; newspaper advertisements; and word of mouth in the local community.
Participants reported no sleep problems, major illnesses, endocrine disorders, or psychopathology, and they were free from prescription and over-the-counter medications that may influence the sleep/wake cycle, alertness, or melatonin production. Body mass index (BMI), computed from height (cm) and weight (kg) at the time of the study, ranged from 14.7 to 32.6 (mean = 20.6, SD = 4.0).
Pubertal status was determined by consensus among two clinicians using the criteria of Tanner (Tanner, 1962), a staging system based on secondary sexual characteristics. Data are presented for pubic hair growth (Tanner, 1962). The distribution of Tanner stage in this sample included 15 Tanner 1 (pre pubertal; 7 girls), 15 Tanner 2 (3 girls), 10 Tanner 3 (5 girls), 17 Tanner 4 (9 girls), and 12 Tanner 5 (post pubertal; 6 girls). Of the 30 girls included in the sample, 16 had not begun to menstruate (N=7 Tanner 1; N=2 Tanner 2; N=4 Tanner 3; and N=3 Tanner 4); menarche status for one Tanner 3 girl was unavailable.
The E.P. Bradley Hospital or Lifespan Institutional Review Board approved each protocol. Each child’s parent gave informed consent, participants cosigned to indicate their assent to participate, and each child received monetary compensation for participating.
Procedures
The experimental protocol for this study has been described previously (Carskadon, Labyak, Acebo, & Seifer, 1999). Briefly, participants kept a fixed (2200h to 0800h) 10-hour sleep (dark) opportunity while living at home for at least 10 days. Compliance was confirmed with daily telephone messages, sleep logs, and wrist actigraphy. Participants slept on this fixed schedule for another night in the laboratory and then began a constant routine (CR) (Czeisler, Brown, Ronda, Kronauer, Richardson, & Freitag, 1985) procedure. During the CR, participants remained in individual bedrooms (light levels<20 lux) in a semi-recumbent (45 degrees) posture, were given frequent small meals, and were unaware of time of day. Thirty-eight saliva samples (2 mL) were collected during the CR at 30-minute intervals using Salivettes (Starstedt, Nümbrecht, Germany) beginning at 1750 and ending at 1220 the next day. Samples were later radioimmunoassayed (RIA) for melatonin concentration (pg/mL) using Alpco melatonin assay kits (Windham, NH, USA). Two amplitude measures were derived from these salivary melatonin samples: area under the curve (AUC), an integrated value computed by the trapezoid method using KaleidaGraph (Synergy Software, Reading PA), and maximum value (MAX).
Statistical Analysis
We computed a 2 (male vs. female) by 5 (Tanner Stages 1 – 5) analysis of variance to determine if melatonin AUC and MAX changed across Tanner stage and whether this change differed between boys and girls. Least Significant Difference (LSD) post-hoc comparisons identified the source of differences among Tanner Stages. We also computed separate multiple linear regression analyses to examine the relative contributions of Tanner stage, age, sex, and BMI on AUC and MAX. Sex was coded as a binary variable (1=male or 2=female). We report multiple R and R2 coefficients, as well as partial correlation coefficients. Results are described in terms of effect sizes (Cohen, 1988): “small” is equivalent to R=0.14, R2=0.02, and partial correlation=0.10; “medium” is equivalent to R=0.36, R2=0.13, and partial correlation=0.30; and “large” is equivalent to R=0.51, R2=0.26, and partial correlation=0.50.
Results
One participant (Tanner 1 male) manifested a melatonin secretory pattern exceeding the sample means by about 4 SD (MAX=116 pg/mL, AUC=728 pg/mL); therefore, his data were excluded from this analysis.
Figure 1A illustrates mean salivary melatonin levels at 30-minute intervals across the night for each Tanner stage. AUC decreased across Tanner Stage [F(4,59)=3.45, p=.013]; AUC of Tanner 1 participants (mean=275 pg/mL, SD=147) was larger compared to Tanner 4 (mean=189 pg/mL, SD=83; p=.032) and Tanner 5 (mean=145 pg/mL, SD=90; p=.004) participants; and AUC of Tanner 2 (mean=252 pg/mL, SD=95) participants was larger than Tanner 5 (p=.013). Figure 1B illustrates mean salivary melatonin levels and the associated error terms (SEM) for pre-pubertal (Tanner 1) and post-pubertal (Tanner 5) participants only, which shows the decline of salivary melatonin over the course of puberty more clearly. AUC of female (mean=240 pg/mL, SD=132) participants was larger compared to males (mean=198 pg/mL, SD=98) [F(1,58)=3.94, p=.052] (see Figure 1C). This main effect of sex may be driven by Tanner 1 and 5 participants since these groups descriptively show the largest mean differences between males and females (see Figure 1C). Post-hoc analyses, however, are under-powered to detect a statistical difference between Tanner 1 males and females [t(12) = 1.57, p = 0.14] and Tanner 5 males and females [t(10) = 1.70, p=0.12]. An interaction between Tanner stage and sex did not emerge [F(4,58)=3.94, p=.70]. Similar results (not shown) were found for melatonin amplitude as estimated by MAX.
Figure 1.
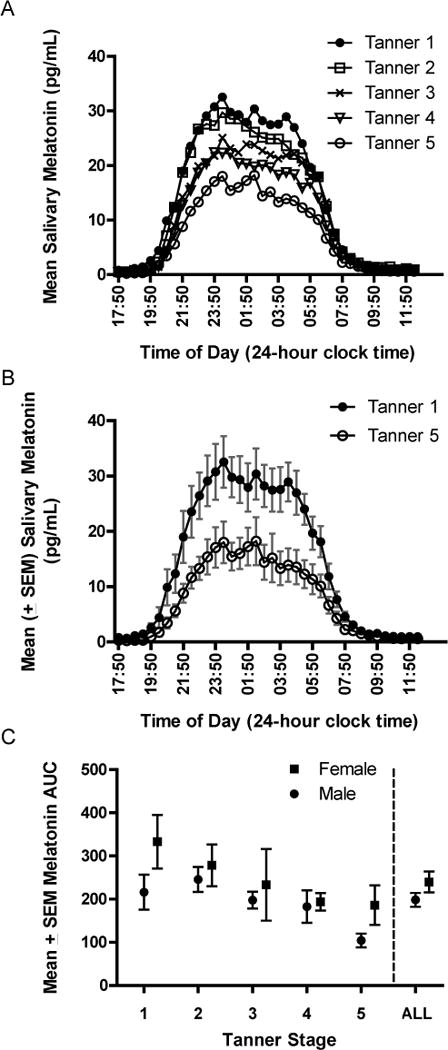
Mean salivary melatonin (y-axis) at each 30-minute sample time (x-axis) from 1750 on the first day to 1220 the following day is illustrated for each Tanner stage (A). Mean salivary melatonin data with associated standard error terms for Tanner 1 and Tanner 5 participants only highlight pre- and post-pubertal differences (B). Area Under the Curve (AUC, pg/mL), an estimate of salivary melatonin amplitude (y-axis), is shown separately for males (circles) and females (squares) at Tanner stages 1 through 5 and aggregated across all Tanner stages (C)
Results from multiple linear regression analyses and associated effect sizes are shown in Table 1. Tanner stage showed a negative association with both AUC and MAX when linear effects of sex, age, and BMI were removed: melatonin amplitude was greater in less mature participants. Tanner stage significantly contributed to the regression models predicting AUC (p<.001) and MAX (p=.010). The partial correlation coefficient for age was small, and age did not significantly contribute to the regression models predicting AUC (p=.068) or MAX (p=.149). Sex was associated with AUC and MAX when the linear effects of Tanner Stage, age, and BMI were removed; melatonin amplitude of females was larger than males. Sex significantly contributed to the regression models predicting AUC (p=.010) and MAX (p=.017). BMI did not contribute to the regression models predicting AUC (p=.845) or MAX (p=.671).
Table 1.
Regression analyses and associated partial correlations.
| Dependent Variable | Area Under the Curve (AUC) |
Melatonin Maximum (MAX) |
|---|---|---|
| Multiple R | 0.50** | 0.46** |
| Multiple R2 | 0.25** | 0.21** |
| Partial Correlations: | ||
| Tanner Stage | −0.43** | −0.38** |
| Sex | 0.32** | 0.30** |
| Age | 0.23* | 0.18* |
| BMI | −0.03 | −0.05 |
small effect size;
medium effect size;
large effect size.
To explore the sex difference that emerged in melatonin amplitude further, linear regression analyses were computed separately for boys and girls to examine whether Tanner stage, age, or BMI differentially contributed to melatonin amplitude between boys and girls. Separating the data by sex did not alter the results (see Table 2). Tanner stage continued to show a negative association with both AUC and MAX when linear effects of age and BMI were removed for both boys and girls. Tanner stage significantly contributed to the regression models predicting AUC in girls (p=.01) and boys (p=.02). Tanner stage also contributed to the regression models predicting MAX in girls (p=.02), and a trend emerged in boys (p=.06). Neither Age nor BMI significantly contributed to the regression models predicting AUC or MAX in boys or girls.
Table 2.
Regression analyses and associated partial correlations computed separately for boys (top) and girls (bottom).
| Dependent Variable | Area Under the Curve (AUC) |
Melatonin Maximum (MAX) |
|---|---|---|
| Boys | ||
|
| ||
| Multiple R | 0.47** | 0.43** |
| Multiple R2 | 0.22** | 0.18** |
| Partial Correlations: | ||
| Tanner Stage | −0.39** | −0.32** |
| Age | 0.22* | 0.15* |
| BMI | −0.07 | −0.12* |
| Girls | ||
|
| ||
| Multiple R | 0.49** | 0.45** |
| Multiple R2 | 0.24** | 0.20** |
| Partial Correlations: | ||
| Tanner Stage | −0.46** | −0.43** |
| Age | 0.24* | 0.23* |
| BMI | 0.02 | 0.02 |
small effect size;
medium effect size;
large effect size.
Discussion
In summary, the current analysis showed lower salivary melatonin amplitude across pubertal maturation; pre- and early-pubertal youngsters showed higher melatonin amplitude compared to their late- and post-pubertal peers. This melatonin decline was similar between boys and girls, but overall, girls secreted more melatonin compared to boys. Of the factors examined, Tanner stage and sex explained the salivary melatonin amplitude decline during this developmental period, but age and BMI did not.
Previous studies comparing melatonin amplitude of males and females reported inconsistent results. Our findings that adolescent females have more circulating melatonin compared to males is consistent with a recent study of adults by Cain and colleagues (Cain et al., 2010), who sampled plasma melatonin in constant conditions similar to the current study. Their study also matched men and women based on habitual sleep schedule and age, a procedure that was not utilized in studies where a sex difference was not found (Burgess & Fogg, 2008; Cavallo & Dolan, 1996; Cavallo et al., 1992; Griefahn et al., 2003; Salti et al., 2000). The current analysis did not match boys and girls, but with age range small and sleep timing fixed, a sex difference in melatonin amplitude similar to Cain and colleagues was found. The significance of females secreting more melatonin than males during adolescence and adulthood is unknown; however, we suggest that the sex difference in melatonin amplitude may contribute to sleep disorders (e.g., insomnia) where prevalence rates differ by sex.
This analysis confirmed a decline of melatonin amplitude across pubertal development. Mixed results among previous studies may have resulted from uncontrolled experimental conditions. Our data were collected under controlled conditions in a sample of healthy boys and girls spanning all 5 Tanner stages. We documented and controlled light/dark history, which can influence the duration of melatonin secretion (e.g., Aeschbach, Sher, Postolache, Matthews, Jackson, & Wehr, 2003), by controlling sleep/wake timing for at least 10 days before sampling salivary melatonin. Under this experimental control, our findings indicate that melatonin amplitude declines during puberty, although the cross-sectional design limits this conclusion (Kraemer, Yesavage, Taylor, & Kupfer, 2000).
Our results indicate that puberty stage predicts melatonin amplitude better than chronological age, consistent with the study of Salti and colleagues (Salti et al., 2000), who sampled plasma melatonin in a small group (n=16, Tanner 1–4) of boys and girls wherein melatonin amplitude measured by AUC showed a large decline as a function of puberty; this same pattern emerged as a function of age, but to a lesser degree. These data suggest that sexual maturation and the reactivation of the HPG axis may explain melatonin amplitude more precisely than age during this developmental period. Waldhauser and colleagues reported an inverse relationship between melatonin and LH secretion in children and young adults (Waldhauser et al., 1984), and suggested that melatonin may be a gonadotropin inhibitor. More recent data show that melatonin may act indirectly to inhibit GnRH secretion through a novel neurohormone called gonadotropin-inhibitory hormone (GnIH) (Tsutsui, 2009; Tsutsui, Saigoh, Ukena, Teranishi, Fujisawa, Kikuchi, Ishii, & Sharp, 2000; Ubuka, Bentley, Ukena, Wingfield, & Tsutsui, 2005), which further supports a role of melatonin in the reactivation of the HPG axis.
Our results are inconsistent, however, with those of Cavallo and colleagues (Cavallo, 1992), who found no association between plasma melatonin peak and puberty stage in 62 participants when age was covaried. Cavallo and colleagues noted that because puberty stage and age change together it is difficult to understand the independent contribution of each to melatonin amplitude changes. Pubertal onset disorders have been used to tease apart the biological processes of puberty and the coincident factor of age. Studies of patients with precocious puberty, for example, showed lower melatonin levels in patients compared to normal controls (Commentz & Helmke, 1995; Waldhauser, Boepple, Schemper, Mansfield, & Crowley, 1991). Other studies, however, did not replicate this finding (Cavallo, 1991; Ehrenkranz et al., 1982), and pituitary-gonadal suppression by a GnRH agonist does not alter (Berga, Jones, Kaufmann, & Yen, 1989) or decreases (Waldhauser et al., 1991) circulating melatonin levels in patients with precocious puberty. Many studies have also shown a decline of melatonin across the lifespan (e.g., Iguchi, Kato, & Ibayashi, 1982; Sack, Lewy, Erb, Vollmer, & Singer, 1986; Waldhauser et al., 1988; Zhao, Xie, Fu, Bogdan, & Touitou, 2002). Therefore, the decline of melatonin we observe in the current study occurs on the backdrop of a life-long age-related decline of melatonin. It remains unclear, however, whether the pronounced adolescent drop in melatonin, which is likely regulated by other processes or structural changes than in older adults, prompts the onset of puberty or processes particular to puberty contribute to a decline of melatonin.
Our results do not resolve the debate surrounding the functional significance of a melatonin decline during puberty, yet they indicate that stage of sexual maturation may mediate the decline of circulating melatonin or that a decrease in melatonin amplitude may be an indicator of pubertal progression. Our findings also show that the adolescent maturational decline of melatonin amplitude is not accounted for by larger body mass or by age.
Acknowledgments
This work was supported by two NIH grants (MH52415 and MH01358) awarded to M. Carskadon. We thank Susan Labyak, Ph.D. and Ron Seifer, Ph.D. for their assistance on experimental protocols and analyses, David Bushnell and Caroline Gredvig-Ardito for organizing demographic data, and Jennifer Maxwell for assistance in database management. We would also like to acknowledge the E.P. Bradley Hospital Sleep and Chronobiology laboratory’s technical staff and research assistants, William C. Dement summer research apprentices, and the participants and families who took part in these studies.
References
- Acebo C, Labyak SE, Carskadon MA. Dim light melatonin profiles during constant routines: amplitude and development. Sleep. 2003;26:A113–114. [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. Journal of Clinical Endocrinology and Metabolism. 2003;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Attanasio A, Borrelli P, Gupta D. Circadian rhythms in serum meltonin from infancy to adolescence. Journal of Clinical Endocrinology and Metabolism. 1985;61(2):388–390. doi: 10.1210/jcem-61-2-388. [DOI] [PubMed] [Google Scholar]
- Berga SL, Jones KL, Kaufmann S, Yen SS. Nocturnal melatonin levels are unaltered by ovarian suppression in girls with central precocious puberty. Fertil Steril. 1989;52(6):936–941. doi: 10.1016/s0015-0282(16)53155-0. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neuroscience Letters. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Cavallo A. Plasma melatonin rhythm in disorders of puberty: interactions of age and pubertal stages. Horm Res. 1991;36(1–2):16–21. doi: 10.1159/000182099. [DOI] [PubMed] [Google Scholar]
- Cavallo A. Plasma melatonin rhythm in normal puberty: interactions of age and pubertal stages. Neuroendocrinology. 1992;55(4):372–379. doi: 10.1159/000126147. [DOI] [PubMed] [Google Scholar]
- Cavallo A. Melatonin and human puberty: current perspectives. J Pineal Res. 1993;15(3):115–121. doi: 10.1111/j.1600-079x.1993.tb00517.x. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Dolan LM. 6-Hydroxymelatonin sulfate excretion in human puberty. J Pineal Res. 1996;21(4):225–230. doi: 10.1111/j.1600-079x.1996.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Cavallo A, Richards GE, Smith ER. Relation between nocturnal melatonin profile and hormonal markers of puberty in humans. Horm Res. 1992;37(4–5):185–189. doi: 10.1159/000182307. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, N.J.: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Commentz JC, Helmke K. Precocious puberty and decreased melatonin secretion due to a hypothalamic hamartoma. Horm Res. 1995;44(6):271–275. doi: 10.1159/000184640. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Brown EN, Ronda JM, Kronauer RE, Richardson GS, Freitag WO. A clinical method to assess the endogenous circadian phase (ECP) of the deep circadian oscillator in man. Sleep Research. 1985;14:295. [Google Scholar]
- Ehrenkranz JR, Tamarkin L, Comite F, Johnsonbaugh RE, Bybee DE, Loriaux DL, et al. Daily rhythm of plasma melatonin in normal and precocious puberty. J Clin Endocrinol Metab. 1982;55(2):307–310. doi: 10.1210/jcem-55-2-307. [DOI] [PubMed] [Google Scholar]
- Fideleff HL, Boquete H, Fideleff G, Albornoz L, Lloret SP, Suarez M, et al. Gender-related differences in urinary 6-sulfatoxymelatonin levels in obese pubertal individuals. J Pineal Res. 2006;40(3):214–218. doi: 10.1111/j.1600-079X.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Brode P, Blaszkewicz M, Remer T. Melatonin production during childhood and adolescence: a longitudinal study on the excretion of urinary 6-hydroxymelatonin sulfate. J Pineal Res. 2003;34(1):26–31. doi: 10.1034/j.1600-079x.2003.02931.x. [DOI] [PubMed] [Google Scholar]
- Iguchi H, Kato K, Ibayashi H. Age-dependent reduction in serum melatonin concentrations in healthy human subjects. Journal of Clinical Endocrinology and Metabolism. 1982;55:27–29. doi: 10.1210/jcem-55-1-27. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Yesavage JA, Taylor JL, Kupfer D. How can we learn about developmental processes from cross-sectional studies, or can we? Am J Psychiatry. 2000;157(2):163–171. doi: 10.1176/appi.ajp.157.2.163. [DOI] [PubMed] [Google Scholar]
- Penny R. Melatonin excretion in normal males and females: increase during puberty. Metabolism. 1982;31:816–823. doi: 10.1016/0026-0495(82)90081-6. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. Human melatonin production decreases with age. Journal of Pineal Research. 1986;3:379–388. doi: 10.1111/j.1600-079x.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Salti R, Galluzzi F, Bindi G, Perfetto F, Tarquini R, Halberg F, et al. Nocturnal melatonin patterns in children. Journal of Clinical Endocrinology and Metabolism. 2000;85:2137–2144. doi: 10.1210/jcem.85.6.6656. [DOI] [PubMed] [Google Scholar]
- Tanner J. Growth at Adolescence. Blackwell, Oxford: 1962. [Google Scholar]
- Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Prog Neurobiol. 2009;88(1):76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, et al. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun. 2000;275(2):661–667. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci U S A. 2005;102(8):3052–3057. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldhauser F, Boepple PA, Schemper M, Mansfield MJ, Crowley WF., Jr Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J Clin Endocrinol Metab. 1991;73(4):793–796. doi: 10.1210/jcem-73-4-793. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Steger H. Changes in melatonin secretion with age and pubescence. Journal of Neural Transmission, Suppl. 1986;21:183–197. [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1(8373):362–365. doi: 10.1016/s0140-6736(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Waldhauser F, Weiszenbacher G, Tatzer E, Gisinger B, Waldhauser M, Schemper M, et al. Alterations in nocturnal serum melatonin levels in humans with growth and aging. J Clin Endocrinol Metab. 1988;66(3):648–652. doi: 10.1210/jcem-66-3-648. [DOI] [PubMed] [Google Scholar]
- Wetterberg L, Bratlid T, von Knorring L, Eberhard G, Yuwiler A. A multinational study of the relationships betwwen nighttime urinary melatonin production, age, gender, body size, and latitude. European Archives of Psychiatry and Clinical Neuroscience. 1999;249:256–262. doi: 10.1007/s004060050095. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Gordon TP. Nocturnal changes in serum melatonin during female puberty in rhesus monkeys: a longitudinal study. J Endocrinol. 1989;121(3):553–562. doi: 10.1677/joe.0.1210553. [DOI] [PubMed] [Google Scholar]
- Young IM, Francis PL, Leone AM, Stovell P, Silman RE. Constant pineal output and increasing body mass account for declining melatonin levels during human growth and sexual maturation. J Pineal Res. 1988;5(1):71–85. doi: 10.1111/j.1600-079x.1988.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Zhao ZY, Xie Y, Fu YR, Bogdan A, Touitou Y. Aging and the circadian rhythm of melatonin: A cross-sectional study of Chinese subjects 30–110 yr of age. Chronobiology International. 2002;19:1171–1182. doi: 10.1081/cbi-120015958. [DOI] [PubMed] [Google Scholar]


