Abstract
Purpose
Differences in clinical outcomes between primary and secondary bladder carcinoma is situ (CIS) are still unclear. We sought to compare the clinical outcomes of primary versus secondary CIS and to identify predictive factors.
Materials and Methods
A retrospective analysis of 476 patients with high grade cTis (221 primary and 255 secondary CIS) from 1990 to 2008 in a high-volume cancer center after transurethral resection (TUR) and intravesical bacillus Calmette-Guérin (BCG) therapy. Our endpoints were time to progression to invasive disease (≥cT1) or radical cystectomy (RC) before progression, and progression to muscle-invasive disease (≥cT2) or RC before progression. Cox proportional hazards regression models were used.
Results
Patients with primary CIS responded significantly more within 6 months of BCG therapy than secondary CIS (65% vs 39%; p<0.001). The 5-year cumulative incidence of progression to ≥cT1 was 43% (95% CI, 36%–51%) and 32% (95% CI, 27%–39%) in the primary and secondary CIS groups, respectively; progression to ≥cT2 was 17% (95% CI, 12%–23%) and 8% (95% CI, 5%–13%), respectively. In multivariable analyses, primary CIS was significantly more likely to progress to ≥cT1 or RC (HR: 1.38; 95% CI, 1.05–1.81; p=0.020), and to ≥cT2 or RC (HR: 1.72; 95% CI, 1.27–2.33; p=0.001). We found no significance for age, gender, or response to BCG therapy as predictors of outcome. The median follow-up time was 5.1 years.
Conclusions
Patients presenting with primary CIS have a worse outcome compared to those with secondary CIS, suggesting a need to differentiate these two entities in the treatment decision process.
Keywords: Bladder, Bladder Neoplasms, Carcinoma in situ, BCG
Introduction
Since 1952, when Melicow first described the importance of bladder carcinoma in situ (CIS) in the recurrence and progression rates of urothelial bladder carcinoma (UBC), the understanding of this disease has evolved greatly, allowing improvements in patient care.1, 2 The pathologic finding of CIS implies a worse prognosis in non-muscle-invasive UBC patients, despite a widely variable outcome in the long term.3 Although the clinical and biological impact of CIS continues to be controversial, it has been suggested that CIS represents a distinct entity.4 More recently, authors have begun to distinguish between primary CIS (isolated CIS with no prior or concomitant papillary tumors—de novo CIS) and secondary CIS (diagnosed concomitantly to or after a papillary tumor).3, 5, 6
However, it still remains unclear whether primary or secondary CIS represents a worse prognosis.7 Moreover, the distinction between primary and secondary CIS has not yet been shown to be clinically relevant or associated with particular oncologic outcomes after receiving intravesical bacillus Calmette-Guérin (BCG) therapy. Although many authors have addressed the issue of the clinical significance of primary or secondary CIS, studies have shown that conclusions have been drawn from cohorts with a small number of patients from each of these categories or inadequate patient selection in mixed stages, not allowing a thorough understanding of the natural history of this disease.3, 8–11
In this context, we sought to compare the clinical outcomes of a large cohort of patients presenting with primary or secondary CIS at a tertiary referral cancer center.
Patients and Methods
A retrospective analysis of our institutional database was performed with the approval of the institutional review board. The diagnosis of CIS was based on urine cytology, cystoscopy with biopsy or transurethral resection (TUR), bimanual examination, as well as pathologic evaluation by a dedicated genito-urinary pathologist at MSKCC. We excluded patients whose pathology slides had been unavailable for review. Patients were followed every 3 months with urine cytology and cystoscopy. Random biopsies and repeat TUR were performed in all suspicious cases. Positive cytology was considered as a recommendation for random biopsies and upper tract imaging, even when cystoscopy was not suspicious. A negative cytology was acceptable, since all cases required random biopsies and pathologic confirmation of CIS. BCG therapy consisted of an induction course of 6 weekly intravesical instillations.
The study comprised a consecutive cohort of 476 patients diagnosed with primary or secondary CIS from 1990 to 2008 (221 primary CIS and 255 secondary CIS). Primary CIS was defined as an isolated high-grade cTis on the first transurethral resection (TUR) without any prior or concomitant papillary tumor, and secondary CIS as high grade cTis diagnosed concomitantly to or after a prior papillary cTa tumor. Patients with CIS concomitant to ≥cT1 were not included.
For the analysis of response to BCG therapy, patients who progressed before receiving BCG therapy (48) or were missing the date they received BCG therapy (36) were excluded, leaving 392 patients for analysis (182 primary CIS and 210 secondary CIS).
The diagnosis was based on the TNM system of the International Union Against Cancer and graded according to the World Health Organization/International Society of Urological Pathology (WHO/ISUP) 1998 grading system of urothelial neoplasms of the urinary bladder.12 The medical records were reviewed for clinical information related to patient characteristics.
Statistical Methods
In order to compare the clinical outcomes of primary versus secondary CIS, we analyzed the time to separate endpoints: progression to invasive disease, defined as cT1 or higher (≥cT1); and progression to muscle-invasive disease, defined as cT2 or higher (≥cT2). Because radical cystectomy (RC) is an adverse outcome that may be related to disease severity, we considered the earlier of either RC or progression as a single endpoint in our analyses. As many patients underwent RC before progression to invasive disease, we plotted the risk of progression using the cumulative incidence function in the presence of a competing risk.
We created separate multivariable Cox regression models for each of the endpoints, as follows: (a) progression to ≥cT1 or RC before progression; and (b) progression to ≥cT2 or RC before progression. We used as predictors: CIS presentation (primary vs secondary), age, gender, and response to intravesical BCG therapy. We defined ‘responders’ as those whose disease did not recur within 6 months of receiving BCG therapy, and ‘non-responders’ as those whose disease recurred within 6 months of BCG therapy.
All analyses were conducted using SPSS 16.0 (SPSS Inc, Chicago, IL) and R (R Foundation for Statistical Computing, http://www.R-project.org) with the cmprsk package.
Results
A total of 476 patients received BCG therapy after presenting with CIS. The majority of patients were male (n=389; 82%), white (n=446; 94%), and current or former smokers (n=341; 72%)(Table 1). Gross hematuria was more frequently diagnosed in the secondary CIS group (51% vs 31%), while voiding symptoms (irritative or obstructive), were more commonly reported by the patients diagnosed with primary CIS (29% vs 10%; p<0.001). Overall, the median follow-up time was 5.1 years (IQR:2.5, 8.2).
Table 1.
Clinical characteristics of patients who received BCG therapy for primary or secondary CIS. All values are median (interquartile range) or frequency (proportion).
| All Patients | Initial CIS Presentation | |||
|---|---|---|---|---|
|
| ||||
| N=476 | Primary n=221 |
Secondary n=255 |
P value | |
| Age at first diagnosis of bladder cancer (years) | 66.7 (13.1) | 68.6 (11.8) | 65.2 (14.6) | 0.002*** |
| Male | 389 (81.7%) | 185 (83.7%) | 204 (80.0%) | 0.342**** |
| White | 446 (93.7%) | 210 (95.5%) | 236 (92.9%) | 0.329**** |
| Smoking history | 0.027**** | |||
| None | 111 (23.3%) | 63 (28.5%) | 48 (18.8%) | |
| Former | 282 (59.2%) | 125 (56.6%) | 157 (61.6%) | |
| Current | 59 (12.4%) | 22 (10.0%) | 37 (14.5%) | |
| Unknown | 24 (5.0%) | 11 (5.0%) | 13 (5.1%) | |
| Initial symptoms | <0.001**** | |||
| Asymptomatic* | 101 (21.2%) | 48 (21.7%) | 53 (20.8%) | |
| Gross hematuria | 198 (41.6%) | 69 (31.2%) | 129 (50.6%) | |
| Voiding symptoms (irritative or obstructive)** | 88 (18.5%) | 63 (28.5%) | 25 (9.8%) | |
| Unknown | 89 (18.7%) | 41 (18.6%) | 48 (18.8%) | |
Asymptomatic: includes incidental finding and microhematuria
Other voiding symptoms: irritative, obstructive
Mann-Whitney U-test
Chi-square tests
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ
Sixty-five percent of the patients in the primary CIS group and 39% in the secondary CIS group responded to BCG therapy within 6 months (p<0.001) (Table 2). In total, 179 patients progressed to invasive disease and 57 patients progressed to muscle-invasive UBC. The median time to progression to ≥cT1/RC was 3.4 years (IQR: 2.4, 4.5) and 5.8 years (IQR: 3.7, 7.8), and to ≥cT2/RC was 5.2 years (IQR: 3.2, 7.4) and 9.9 years (IQR: 5, 12.7) in the primary and secondary CIS groups, respectively. The median follow-up for patients in the primary CIS group was 3.4 years (IQR: 2.4, 4.5) and in the secondary CIS group was 5.7 years (IQR: 3.7, 7.8). Median follow-up for patients who did not experience disease progression was 3.9 years (IQR: 1.2, 6.7). Direct progression from CIS to cT2 occurred in 27 patients in the primary CIS group and in 17 patients in the secondary CIS group. Overall, 173 patients underwent RC (92 primary CIS and 81 secondary CIS). RC before progression was performed in 132 patients (67 primary CIS vs 66 secondary CIS). The pathology stage at RC did not differ between the two groups (p=0.26). In the primary CIS group, 31 patients (34.5%) had ≥pT2 at RC, while in the secondary CIS group, 23 patients (29.2%) had ≥pT2 at RC. Disease specific-survival at 10 years was 82% and 96% in the primary and secondary CIS groups, respectively.
Table 2.
Clinical outcomes of patients who received BCG therapy for primary or secondary CIS
| All Patients | Initial CIS | ||
|---|---|---|---|
|
| |||
| N=476 | Primary n=221 |
Secondary n=255 |
|
| Intravesical BCG therapy* | |||
| Responders | 243 (51%) | 144 (65%) | 99 (39%) |
| Non-responders | 233 (49%) | 77 (35%) | 156 (61%) |
| Intravesical chemotherapy | 79 (17%) | 33 (15%) | 46 (18%) |
| Recurrence | 367 | 182 | 185 |
| Progression to ≥T1 | 179 | 93 | 86 |
| Progression to ≥T2 | 57 | 37 | 20 |
| Direct progression to T2 | 44 | 27 | 17 |
| Radical cystectomy | |||
| RC for all stages | 173 | 92 | 81 |
| RC before progression | 132 | 67 | 66 |
| RC for CIS | 57 | 27 | 30 |
| RC for T1 | 75 | 40 | 36 |
| Distant metastasis | 51 | 28 | 23 |
| Second primary urothelial carcinoma | |||
| Upper tract | 32 | 10 | 22 |
| Urethral | 25 | 8 | 17 |
| Death | 95 | 50 | 45 |
| Bladder cancer | 33 | 18 | 15 |
| Upper tract urothelial carcinoma | 6 | 0 | 6 |
| Other causes | 28 | 11 | 17 |
| Unknown | 28 | 21 | 7 |
p<0.001
BCG = bacillus Calmette-Guérin; CIS = carcinoma in situ; RC = radical cystectomy
In multivariable analyses, primary CIS had a significantly higher risk of progression to ≥cT1/RC (HR: 1.38; 95% CI, 1.05–1.81; p=0.020), or to ≥cT2/RC (HR: 1.72; 95% CI, 1.27–2.33; p=0.001) compared to secondary CIS (Table 3). Age, gender and response to BCG therapy were not significantly associated with disease progression or RC.
Table 3.
Multivariable analyses of progression to invasive disease (≥cT1) and to muscle-invasive disease (≥cT2), adjusting for RC before progression in 392 patients
| Variables | Progression to invasive disease (≥cT1) or radical cystectomy* | Progression to muscle- invasive disease (≥cT2) or radical cystectomy* | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Primary vs secondary CIS | 1.37 (1.05–1.81) | 0.020 | 1.72 (1.27–2.33) | 0.001 |
| Age | 1.01 (0.99–1.02) | 0.178 | 1.01 (0.99–1.02) | 0.568 |
| Gender | 1.18 (0.86–1.63) | 0.300 | 1.15 (0.80–1.65) | 0.455 |
| Response to BCG | 1.12 (0.85–1.46) | 0.421 | 1.03 (0.76–1.39) | 0.865 |
(84 patients were excluded due to progression before BCG therapy or missing data)
RC before progression to invasive disease
RC = radical cystectomy; CIS = carcinoma in situ; BCG = bacillus Calmette-Guérin
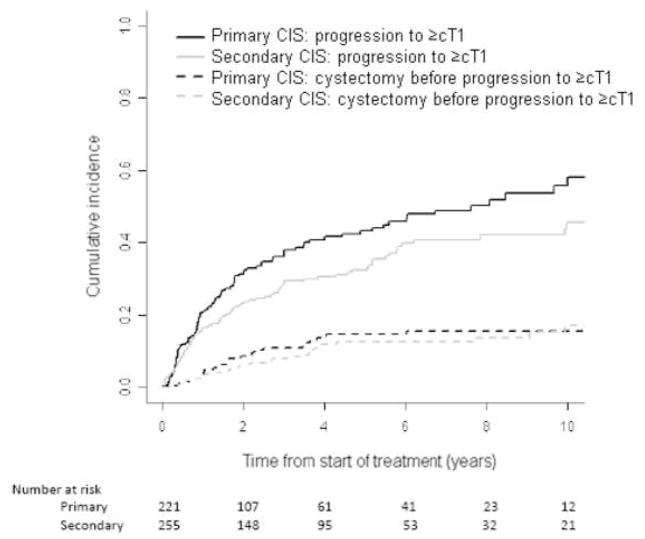
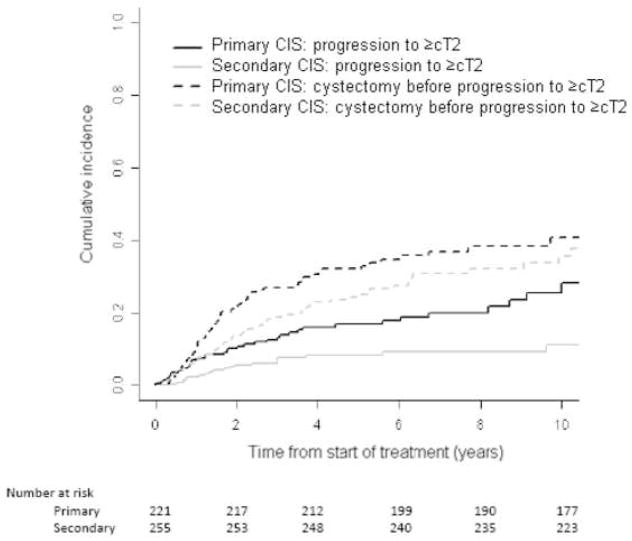
Figure 1 shows a higher cumulative incidence of progression to ≥cT1 in patients with primary CIS. RC before progression to ≥cT1 was similar in the two groups. In Figure 2, progression to ≥cT2 is shown to be consistently higher in the primary CIS group, despite the higher incidence of RC before progression to ≥cT2 in this group. The competing risk of RC before progression was greater than the cumulative incidence of progression to ≥cT2 in both groups. This inversion is related to the higher recommendation of RC before progression for cT1 than for CIS.
Figure 1.
Cumulative incidence of progression to invasive disease (≥cT1; solid lines) or radical cystectomy before progression (dashed lines) in primary or secondary CIS
Figure 2.
Cumulative incidence of progression to muscle-invasive disease (≥cT2; solid lines) or radical cystectomy before progression (dashed lines) in primary or secondary CIS
The 5-year cumulative incidence of progression to ≥cT1 was 43% (95% CI, 36%–51%) in the primary CIS group and 32% (95% CI, 27%–39%) in the secondary CIS group. In patients with primary CIS, RC for <cT1 was 15% (95% CI, 10%–21%) and for <cT2 was 32% (95% CI, 26%–39%) at 5 years. In patients with secondary CIS, RC for <cT1 was 12% (95% CI, 7%–19%) and for <cT2 was 25% (95% CI, 20%–32%) at 5 years. The 5-year cumulative incidence of progression to ≥cT2 was 17% (95% CI, 12%–23%) in the primary CIS group and 8% (95% CI, 5%–13%) in the secondary CIS group.
Discussion
In this large cohort of patients with primary or secondary CIS, we compared the oncologic outcomes of patients after intravesical BCG failure and identified evidence that primary CIS represents a higher risk for progression than secondary CIS. We found that primary CIS was significantly associated with higher progression to invasive disease or RC and muscle-invasive disease or RC.
The mechanism of progression from CIS to a life-threatening disease may be related to the pattern of invasion. Historical studies have introduced the concept of divergent routes for flat and papillary tumors, and presumably, these patterns behave differently.2, 13, 14 Therefore, if we consider secondary CIS an initiation for the papillary type of tumor—that is, if two patterns of invasion actually coexist—then distinct clinical outcomes might be expected from the different patterns of invasion. Recent evidence showed genomic alterations related to CIS carcinogenesis that further distinguishes flat and papillary lesions by giving support to the presence of two separate biological pathways in bladder tumors. Zieger et al found a series of chromosomal instability characterized by gains of chromosomes (5p, 6p22.3, 10p15.1) and losses of heterozygosity (5q, 13q13-q14) associated with CIS lesions, while FGFR3 mutations were associated with papillary tumors.6
Despite not clearly defining the impact of CIS on oncologic outcomes, results from several previous studies have identified the presence of CIS as a poor prognostic factor because it is associated with increasing both the risk of progression to muscle-invasive disease and the risk of death from bladder cancer.3, 15–17 However, very few authors aimed at comparing the outcomes of primary versus secondary CIS patients and consequently could not determine whether the use of this classification holds any importance to the management of this disease.8, 18
Previously, no study has been able to clearly demonstrate significant differences in outcomes between primary and secondary CIS in a homogenous cohort of non-muscle-invasive UBC. Most have suggested similar rates of disease progression or have shown confounding results by favoring primary CIS as a better biological behavior, mostly due to small samples or mixed stage groups.
A landmark study by Orozco et al compared patients with primary CIS versus patients with CIS associated with UBC at any level of invasion, of which more than 50% had muscle-invasive disease at initial diagnosis.19 Not surprisingly, the mixed secondary CIS group had a worse outcome, leading to a misleading conclusion that primary CIS may be a less aggressive disease. More recently, however, Cheng et al found in a small cohort that patients with primary CIS had a lower progression-free survival at 15 years than secondary CIS, although not statistically significant (54% vs 65%; p=0.34).20 Gofrit et al studied 104 patients with CIS (primary and secondary combined), showing no significant difference between pure and concomitant CIS.8 Takenaka et al published findings based on small number of patients, also showing no difference between type of CIS. Although reporting a sample of 185 patients with CIS, the analysis included primary, secondary, and concomitant disease in a same risk-group.21
In our study, the higher cumulative incidence of progression to invasive and to muscle-invasive disease at 5 years, adjusting for the competing risk of RC before progression, underscores the aggressiveness of primary CIS. If we would consider at risk patients with primary CIS who underwent RC before progression to cT2, the 5-year cumulative incidence of progression to muscle-invasive UBC could be as high as 49% in the absence of RC before progression. Moreover, with RC being recommended more frequently in the primary CIS group with the goal of avoiding progression, one could expect a reduced incidence of progression in this group, contrary to our findings in this cohort. Therefore, our results support recommending surgery in presumably higher-risk patients and even suggest that more frequent recommendations for surgical treatment could benefit patients with primary CIS.
Radical cystectomy before progression is an acceptable recommendation in patients with non-muscle-invasive UBC after BCG therapy failure.22–24 In our study, the relatively high incidence of RC before progression to muscle-invasive UBC demonstrates a trend towards considering these patients at high risk. Moreover, similar rates of RC before progression to ≥cT1 in both CIS groups and a higher RC rate before progression to ≥cT2 in the primary CIS group suggest that the poorer outcome in the primary CIS group is reliable because it is not biased by the surgical treatment that could preclude this group from progressing in an even higher rate. However, several other factors may influence the timing of RC, which could also have affected the results.
Herr has shown that aging was significantly associated with recurrence in a large cohort of 805 patients with non-muscle-invasive disease (Ta or T1 with concomitant CIS in 78% of patients).25 In the present study, age was not associated with progression to muscle-invasive disease or RC before progression. This may possibly be related to a higher cumulative incidence of RC before progression in the younger population in the primary CIS group.
Although response to BCG therapy was significantly higher in the primary CIS group than in the secondary (65% vs 39%; p<0.001), we found that it did not impact on progression rates when controlled for age, gender, and CIS group. This may due to the higher rate of RC before progression in the primary CIS group. Recent studies have also shown no significant association of response to BCG with outcome.8 Andius et al, however, found that the first cystoscopy had a predictive value for progression, BCG failure, and death in a cohort of 173 patients, but no difference between type of CIS.9
There are several limitations to our study. It is retrospective in nature. Patients treated at our tertiary referral hospital may differ from bladder cancer patients treated at community centers. And also, the cohort covered a 19-year period, during which a trend towards earlier RC occurred. Although the indication of earlier RC has only recently gained more support, this treatment option had been part of our recommendation during this time interval, which may have prevented a greater impact on our series.22
Conclusion
Our results suggest that primary CIS represents a more aggressive tumor than secondary CIS and that these two types of tumor are distinct. Although primary CIS patients respond better to BCG therapy, we found no association with progression. Although RC before progression was recommended more frequently in the primary CIS group, it was not enough to reduce the progression rates close to the secondary CIS group. Even though additional data is required to validate these findings, the distinction between these two entities may influence the clinical management of this disease, on decision-making for RC before progression, and on prognostic models.
Acknowledgments
Supported by: The Sidney Kimmel Center for Prostate and Urologic Cancers. Dr. Chade is a research fellow supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil). Dr. Shariat is a research fellow in urologic oncology supported by NIH T32-CA82088.
Abbreviations
- CIS
carcinoma is situ
- TUR
transurethral resection
- RC
radical cystectomy
- UBC
urothelial bladder carcinoma
- BCG
bacillus Calmette-Guérin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melicow MM. Histological study of vesical urothelium intervening between gross neoplasms in total cystectomy. J Urol. 1952;68:261. doi: 10.1016/S0022-5347(17)68193-X. [DOI] [PubMed] [Google Scholar]
- 2.Utz DC, Farrow GM. Carcinoma in situ of the urinary tract. Urol Clin North Am. 1984;11:735. [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden A, Witjes JA, et al. High grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90. doi: 10.1016/j.urology.2005.06.135. [DOI] [PubMed] [Google Scholar]
- 4.Weinstein RS, Miller AW, 3rd, Pauli BU. Carcinoma in situ: comments on the pathobiology of a paradox. Urol Clin North Am. 1980;7:523. [PubMed] [Google Scholar]
- 5.Nese N, Gupta R, Bui MH, et al. Carcinoma in situ of the urinary bladder: review of clinicopathologic characteristics with an emphasis on aspects related to molecular diagnostic techniques and prognosis. J Natl Compr Canc Netw. 2009;7:48. doi: 10.6004/jnccn.2009.0004. [DOI] [PubMed] [Google Scholar]
- 6.Zieger K, Marcussen N, Borre M, et al. Consistent genomic alterations in carcinoma in situ of the urinary bladder confirm the presence of two major pathways in bladder cancer development. Int J Cancer. 2009;125:2095. doi: 10.1002/ijc.24619. [DOI] [PubMed] [Google Scholar]
- 7.Hudson MA, Herr HW. Carcinoma in situ of the bladder. J Urol. 1995;153:564. doi: 10.1097/00005392-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Gofrit ON, Pode D, Pizov G, et al. The natural history of bladder carcinoma in situ after initial response to bacillus Calmette Guerin immunotherapy. Urol Oncol. 2009;27:258. doi: 10.1016/j.urolonc.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Andius P, Damm O, Holmang S. Prognostic factors in patients with carcinoma in situ treated with intravesical bacille Calmette Guerin. Scand J Urol Nephrol. 2004;38:285. doi: 10.1080/00365590410028692. [DOI] [PubMed] [Google Scholar]
- 10.Witjes JA. Bladder carcinoma in situ in 2003: state of the art. Eur Urol. 2004;45:142. doi: 10.1016/j.eururo.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Sylvester RJ. Natural history, recurrence, and progression in superficial bladder cancer. Scientific World Journal. 2006;6:2617. doi: 10.1100/tsw.2006.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuter VE, Epstein JI, Amin MB, et al. The “WHO/ISUP Consensus Classification of Urothelial (Transitional Cell) Neoplasms”: continued discussion. Hum Pathol. 1999;30:879. doi: 10.1016/s0046-8177(99)90153-3. [DOI] [PubMed] [Google Scholar]
- 13.Utz DC, Farrow GM, Rife CC, et al. Carcinoma in situ of the bladder. Cancer. 1980;45:1842. [PubMed] [Google Scholar]
- 14.Farrow GM, Utz DC, Rife CC, et al. Clinical observations on sixty nine cases of in situ carcinoma of the urinary bladder. Cancer Res. 1977;37:2794. [PubMed] [Google Scholar]
- 15.Lamm DL. Carcinoma in situ. Urol Clin North Am. 1992;19:499. [PubMed] [Google Scholar]
- 16.Althausen AF, Prout GR, Jr, Daly JJ. Non invasive papillary carcinoma of the bladder associated with carcinoma in situ. J Urol. 1976;116:575. doi: 10.1016/s0022-5347(17)58916-8. [DOI] [PubMed] [Google Scholar]
- 17.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Prout GR, Jr, Griffin PP, Daly JJ, et al. Carcinoma in situ of the urinary bladder with and without associated vesical neoplasms. Cancer. 1983;52:524. doi: 10.1002/1097-0142(19830801)52:3<524::aid-cncr2820520324>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Orozco RE, Martin AA, Murphy WM. Carcinoma in situ of the urinary bladder. Clues to host involvement in human carcinogenesis. Cancer. 1994;74:115. doi: 10.1002/1097-0142(19940701)74:1<115::aid-cncr2820740120>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, Cheville JC, Neumann RM, et al. Survival of patients with carcinoma in situ of the urinary bladder. Cancer. 1999;85:2469. [PubMed] [Google Scholar]
- 21.Takenaka A, Yamada Y, Miyake H, et al. Clinical outcomes of bacillus Calmette Guerin instillation therapy for carcinoma in situ of urinary bladder. Int J Urol. 2008;15:309. doi: 10.1111/j.1442-2042.2008.02012.x. [DOI] [PubMed] [Google Scholar]
- 22.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296. [PubMed] [Google Scholar]
- 23.Denzinger S, Fritsche HM, Otto W, et al. Early versus deferred cystectomy for initial high risk pT1G3 urothelial carcinoma of the bladder: do risk factors define feasibility of bladder sparing approach? Eur Urol. 2008;53:146. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Herr HW. Age and outcome of superficial bladder cancer treated with bacille Calmette Guerin therapy. Urology. 2007;70:65. doi: 10.1016/j.urology.2007.03.024. [DOI] [PubMed] [Google Scholar]