Abstract
The Hippo signaling pathway is an emerging growth control and tumor suppressor pathway that regulates cell proliferation and stem cell functions. Defects in Hippo signaling and hyperactivation of its downstream effectors YAP and TAZ contribute to the development of cancer, suggesting that pharmacological inhibition of YAP and TAZ activity may be an effective anticancer strategy. Conversely, YAP and TAZ can also play beneficial roles in stimulating tissue repair and regeneration following injury, therefore activation of YAP and TAZ may be useful in these contexts. Recently, a complex network of intracellular and extracellular signaling pathways that modulate YAP and TAZ activities have been identified. Here we review the regulation of the Hippo signaling pathway, its functions in normal homeostasis and disease, and recent progress in the identification of small molecule pathway modulators.
The Hippo signaling pathway is an emerging growth control pathway that is conserved throughout the animal kingdom. Growing interest in the Hippo pathway is fueled by studies that demonstrate its fundamental role in organ growth control, stem cell function, regeneration, and tumor suppression 1,2,3,4,5,6. Indeed, the Hippo pathway is deregulated with high frequency in many diverse cancers suggesting that altered Hippo signaling is tightly linked to tumor initiation and/or progression 7. Hence, there is much excitement and speculation about targeting the Hippo pathway to treat a wide variety of human malignancies 8, 9.
The Hippo pathway’s main function is to negatively regulate the activity of YAP and TAZ, two homologous transcriptional co-activators that are the main downstream mediators of the Hippo pathway 10. When activated, YAP and TAZ promote cell proliferation, inhibit cell death, and are hyperactivated in many human malignancies 11,12,13, 14,7,15,16–20,21. Therapeutic intervention for cancer would thus aim at reducing or inhibiting the oncogenic function of YAP and/or TAZ. However, to date few small-molecule inhibitors have been discovered that target the Hippo pathway and a prevalent view is that most Hippo pathway signaling components are not conventional drug targets. Indeed, YAP and TAZ are transcriptional co-activators with no known catalytic activity. Moreover, no known upstream regulators that specifically promote YAP and TAZ activity have enzymatic activity 7,6. Thus, inhibiting the function of YAP and TAZ may require targeting protein-protein interactions. A further complication is that YAP and TAZ are required for tissue repair and regeneration in some contexts 22–27,28,29, raising questions as to whether systemic and chronic manipulation of Hippo signaling might have potential deleterious side effects on normal tissue function and homeostasis. However, transient activation of YAP and TAZ may help to promote tissue repair and regeneration in the context of injury 28,29. These two faces of the Hippo pathway suggest that identification and proper application of small molecular modulators of Hippo signaling may provide exciting new approaches for cancer therapy and in regenerative medicine.
Overview of the Hippo pathway
The Hippo pathway relays signals from the plasma membrane into the nucleus where it regulates the expression of a battery of target genes controlling diverse cellular processes such as proliferation, survival, and differentiation 1,2,3,4,5,6. In this regard, Hippo signaling is similar to other well-known signal transduction pathways such as the EGF, TGFβ or WNT pathways. However, in contrast to these other pathways, the Hippo pathway does not appear to have dedicated extracellular signaling peptides and receptors, but rather is regulated by a network of upstream components and mechanisms, many of which are also involved in regulating cell adhesion and cell polarity 3, 6, 30. Nevertheless, the Hippo pathway bears considerable resemblance to other canonical signal transduction pathways in that many upstream regulators feed into the core of the pathway that is comprised of two serine/threonine kinases, known as the Hippo and Warts kinases in Drosophila 31–37 and the MST1/2 (Mammalian Sterile 20-like 1 and 2) and LATS1/2 (Large tumor suppressor 1/2) kinases in humans 37–40. These kinases and their essential roles in growth control were first discovered in Drosophila and function together in a novel signaling pathway, termed “the Hippo pathway” after one of its founding members 31–35. Since the initial discovery of Hippo and Warts, many additional components of the Hippo pathway have been identified and a complex signaling network that integrates multiple upstream inputs from the plasma membrane into the nucleus has emerged (Figures 1 and 2). In this review we will largely refer to Hippo signaling components using the mammalian nomenclature and the Drosophila components are listed in Table 1.
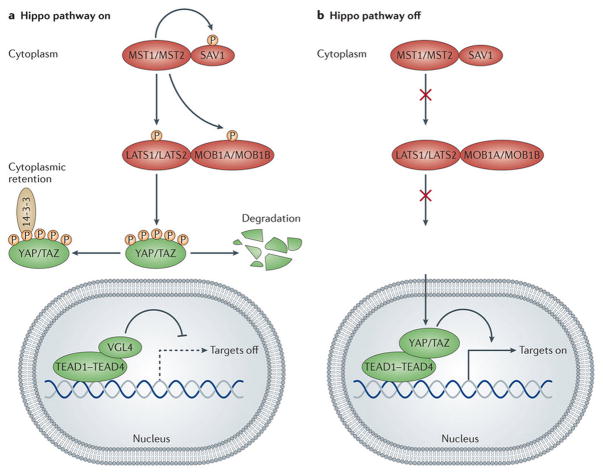
Figure 1. The core of the Hippo pathway and its mode of action.
Schematics of the core pathway components and how they interact. (A) When the Hippo pathway is ON, MST1/2 phosphorylate SAV1 and together they phosphorylate and activate MOB1A/B and LATS1/2, which then phosphorylate YAP and TAZ. Phosphorylated YAP and TAZ are sequestered in the cytoplasm by the 14-3-3 phosphopeptide binding proteins and shunted for proteasomal degradation. As a result, the TEAD transcription factors associate with VGL4 and suppress target gene expression. (B) When the Hippo pathway is OFF, the MST1/2 and LATS1/2 kinases are inactive, YAP and TAZ are not phosphorylated and accumulate in the nucleus where they displace VGL4 and complex with TEADs. YAP and TAZ are transcriptional co-activators and in complex with TEADs promote the expression of target genes.
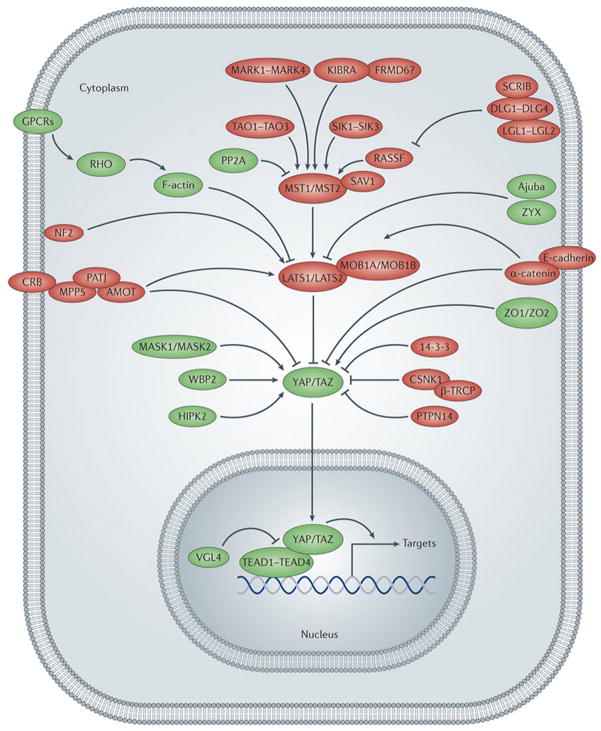
Figure 2. The Hippo pathway network.
Outline of a cell with nucleus and the Hippo pathway network. Hippo pathway components are shown in green when they promote YAP/TAZ activity or in red when they inhibit YAP/TAZ activity. Pointed and blunt arrowheads indicate activating and inhibitory interactions, respectively. Abbreviations: α-CAT (α-Catenin), AJUB (Ajuba), AMOT (Angiomotin), β-TRCP (β-transducing repeat containing protein), CK1 (Casein Kinase 1), CRB (Crumbs), E-CAD (E-cadherin), EX (Expanded), GPCR (G-protein coupled receptor), HIPK (Homeodomain interacting protein kinase), KIBRA (Kidney brain), LATS (Large tumor suppressor), LGL (Lethal giant larvae), MASK (Multiple ankyrin single KH), MER (Merlin), MOB (Mps one binder), MST (Mammalian sterile 20 like), PALS (Protein Associated with Lin-7), PATJ (Pals1-associated tight junction protein), PP2A (Protein phosphatase 2A), PTPN14 (Protein tyrosine phosphatase non-receptor type 14), RASSF (Ras associated factor), SAV (Salvador), SCRIB (Scribble), SIK (Salt inducible kinase), TAO (Thousand and one amino acid protein), TAZ (transcriptional coactivator with PDZ-binding motif), TEAD (TEA domain protein), VGL4 (Vestigial-like 4), WBP2 (WW domain binding protein 2), YAP (Yes associated protein), ZO (Zonula occludens), ZYX (Zyxin).
Table 1.
List of Hippo pathway members and their molecular function.
| Human proteins | D. melanogaster protein | Protein function | Domain composition |
|---|---|---|---|
| Core components | |||
| MST1, MST2 | Hpo | Serine/threonine kinase, STE20 family | Kinase domain, SARAH domain |
| SAV1 (also known as WW45) | Sav | Adaptor protein | FERM domain-binding motif, two WW domains, SARAH domain |
| LATS1, LATS2 | Wts | Serine/threonine kinase, NDR family | Kinase domain |
| MOB1A, MOB1B | Mats | Cofactor | MOB domain |
| YAP, TAZ | Yki | Transcriptional co-activator | Two WW domains, 14-3-3 binding motif, TEAD-binding motif, PDZ-binding motif |
| TEAD1–TEAD4 | Sd | Transcription factor | TEAD DNA-binding domain, vestigial binding domain |
| Pathway modulators | |||
| CRB1–CRB3 | Crb | Transmembrane receptor | EGF domains, four laminin AG domains, transmembrane domain |
| PATJ, MUPP1 | Patj | Adaptor protein | Ribosomal protein L27, eight PDZ domains |
| MPP5 (also known as PALS1) | Sdt | Adaptor protein | Ribosomal protein L27, PDZ domain, SH3 domain, GUK domain |
| AMOT, AMOTL1, AMOTL2 | - | Adaptor protein | Coiled coil domain, PDZ binding motif |
| NF2 | Mer | Adaptor protein | FERM domain |
| KIBRA | Kibra | Adaptor protein | Two WW domains, C2 domain |
| FRMD6 (also known as EX1) | Ex | Adaptor protein | FERM domain |
| TAO1–TAO3 | Tao | Serine/threonine kinase | Kinase domain |
| MARK1–MARK4 | Par-1 | Serine/threonine kinase | Kinase domain |
| E-cadherin | E-cadherin | Transmembrane receptor | Five cadherin domains, transmembrane domain |
| α-catenin | α-catenin | Adaptor protein | VH1–VH3 domains |
| Ajuba, LIMD1, WTIP | Jub | Adaptor protein | Three LIM domains |
| ZYX, LPP, TRIP6 | Zyx | Adaptor protein | Three LIM domains |
| RASSF1-RASSF6 | Rassf | Adaptor protein | RAS association domain, SARAH domain |
| PP2A | STRIPAK-PP2A complex (dSTRIPAK) | Phosphatase | Phosphatase domain |
| SCRIB | Scrib | Adaptor protein | 16 LRR domains, 4 PDZ domains |
| LGL1, LGL2 | Lgl | Adaptor protein | Four WD40 domains |
| DLG1–DLG4 | Dlg | Adaptor protein | Three PDZ domains, SH3 domain, GUK domain |
| PTPN14 | Pez | Phosphatase | FERM domain, phosphatase domain |
| CSNK1 | Dco | Serine/threonine kinase | Kinase domain |
| β-TRCP | Slimb | SCF-type E3 ubiquitin ligase | F-box domain, β-TRCP domain, WD40 domain |
| HIPK | Hipk | Serine/threonine kinase | Kinase domain |
| MASK1, MASK2 | Mask | Adaptor protein | Two ankyrin domains, KH domain |
| WBP2 | Wbp2 | Cofactor | GRAM domain |
| VGL4 | Tgi | Cofactor | Two tondu domains |
AMOT, angiomotin; AMOTL, angiomotin-like protein; β-TRCP, β-transducin repeat-containing E3 ubiquitin protein ligase; CRB1, Crumbs homolog 1; CSNK1, casein kinase 1;D. melanogaster, Drosophila melanogaster; Dco, discs overgrown; Dlg, discs large; EGF, epidermal growth factor; Ex, Expanded; FERM, protein 4.1, ezrin, radixin and moesin; GUK, guanylyl kinase; HIPK, homeodomain-interacting protein kinase; Hpo, Hippo; KIBRA, kidney and brain protein; LATS1, large tumour suppressor homolog 1; Lgl, lethal giant larvae; LIMD1, LIM domain-containing protein 1; LPP, lipoma-preferred partner; LRR, leucine-rich repeat; MARK, MAP/ microtubule affinity-regulating kinase; MASK, multiple ankyrin repeats single KH domain-containing protein; Mats, Mob as tumour suppressor; Mer, Merlin; MOB1A, MOB kinase activator 1A; MPP5, membrane protein, palmitoylated 5; MST, mammalian STE20-like protein kinase; MUPP1, multiple PDZ domain protein 1; NDR, nuclear DEF2-related; NF2, neurofibromin 2; PATJ, PALS1-associated tight junction protein; PP2A, protein phosphatase 2A; PTPN14, protein tyrosine phosphatase, non-receptor type 14; RASSF, RAS association domain-containing family protein; SARAH, Sav–Rassf-Hpo domain; SAV1, Salvador homolog 1; SCF, SKP1–cullin-1–F-box complex; SCRIB, Scribble; Sd, Scalloped; Sdt, Stardust; SH3, SRC homology 3; Slimb, supernumerary limbs; dSTRIPAK, Drosophila striatin-interacting phosphatase and kinase; TAO, thousand and one amino acid protein, TAZ, transcriptional co-activator with PDZ-binding motif; TEAD, TEA domain-containing sequence-specific transcription factor; Tgi, tondu domain-containing growth inhibitor; TRIP6, thyroid hormone receptor interactor 6; VGL4, vestigial-like protein 4; VH1, vinculin head 1; WBP2, WW domain-binding protein 2; WTIP Wilms’tumour 1 interacting protein; Wts, Warts; YAP, Yes-associated protein; Yki, Yorkie; ZYX, Zyxin.
Hippo pathway signaling
The core of the Hippo pathway comprises a highly conserved signaling module that functions similarly in mammals and in Drosophila and contains the MST1/2 and LATS1/2 serine/threonine kinases 31–37 and their respective scaffolding proteins SAV1 (Salvador) 41–43 and MOB1A/B 41–43, the transcriptional co-activators YAP and TAZ 44, and the TEA-domain containing sequence specific transcription factors TEAD1-4 (Figure 1) 45–49. YAP and TAZ are transcriptional co-activators that are not able to bind to DNA themselves, but they form complexes with TEADs 45–49 and other transcription factors such as SMADs 50–52, TBX5 53, 54, RUNX1/2 55 and p73 56 to regulate gene expression. The Hippo pathway is considered to be in the active state when the MST and LATS kinases are active (Figure 1A): The MST kinases in complex with SAV1 phosphorylate and activate the LATS kinases and MOB1 co-factors 34, 57–59,60, which in turn phosphorylate their downstream targets YAP and TAZ 11, 21, 44, 61–64. Phosphorylation of YAP and TAZ results in their nuclear export, cytoplasmic retention, and βTRCP dependent degradation by the proteasome 11, 21, 61, 62, 65–68. Instead of binding YAP and TAZ, the TEAD factors then form complexes with VGL4, which repress target gene expression 69, 70. Thus, when the Hippo pathway is ON, YAP and TAZ activity is inhibited and YAP/TAZ driven gene expression is suppressed. Conversely, when the Hippo pathway is OFF, YAP and TAZ accumulate in the nucleus where they drive gene expression in complex with TEAD and other transcription factors (Figure 1B) 45–49, 71–75. Thus, the Hippo pathway acts primarily by inhibiting the nuclear functions of YAP and TAZ.
Regulation of activity
A key question concerning the Hippo pathway relates to the signals and mechanisms that regulate its activity and ultimately that of YAP and TAZ. To date over 20 regulators have been identified that intersect with the core of the Hippo pathway at different levels (Figure 2) 1,2,3,4,5,6, 30. In mammals, at least four interconnected upstream branches regulate the Hippo pathway. These inputs are (1) the Crumbs complex, (2) regulators that act upstream of the MST kinases, (3) the Actin cytoskeleton, and (4) the adherens junction. Each of these inputs is described below.
The Crumbs (Crb) complex contains CRB proteins that are transmembrane proteins localizing to apical junctions which are required to specify the apical plasma membrane domain 76. CRB proteins have short intracellular domains with protein docking sites that assemble multi-protein complexes that function in cell polarity and also regulate the Hippo pathway 77–81. Most prominently for its regulation of the Hippo pathway in mammals, the CRB complex recruits members of the Angiomotin (AMOT) family of adaptor proteins that directly bind Hippo pathway components. Initial reports showed that AMOT inhibits YAP nuclear localization by directly binding to YAP and by activating LATS kinases to promote YAP phosphorylation and nuclear exclusion 77,82–87. In contrast to these studies, however, a recent report showed that AMOT is required for YAP dependent overgrowth of Nf2 mutant mouse livers, that AMOT promotes nuclear localization of YAP and that it forms a functional complex with YAP and TEADs on target gene DNA 88. AMOT may thus have growth promoting and growth-suppressing functions depending on cellular and molecular context, however, reasons for these seemingly paradoxical results is not known and requires further understanding of the function of AMOT.
A second branch of the Hippo pathway consists of kinases and other regulators that modulate the activity of the MST kinases. These include the TAO kinases and the cell polarity kinase PAR-1 that directly phosphorylate MST kinases and regulate their activity 89, 90,91. In addition, MSTs are regulated by the adaptor protein KIBRA, and in Drosophila also by the adaptor Expanded (Ex) 92–95.
A third branch of the Hippo pathway is defined by an as yet poorly understood signaling mechanism that is mediated by the actin cytoskeleton. Here, the mechanical properties of the extracellular matrix and cell-matrix attachment regulate YAP/TAZ localization and activity, in a process that requires F-actin and that is also present in Drosophila 96–100. In addition, G-protein coupled receptors (GPCRs) that relay signals from soluble extracellular cues such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) regulate YAP and TAZ activity through Rho GTPases that likely affect YAP/TAZ via modulating the actin cytoskeleton 101,102,103. Thus, pathways that regulate the structure of the actin cytoskeleton, for example by activating Rho signaling, affect the Hippo pathway. However, while it is generally appreciated that F-actin intersects the pathway downstream of the MST kinases, the exact mechanism is not known and may involve LATS kinase dependent and independent regulation of YAP and TAZ 96–100,104.
A fourth branch of Hippo pathway inputs emanates from the adherens junction. Engagement of E-Cadherin (E-Cad) at adherens junctions suppresses YAP nuclear localization and activity through regulating MST activity 105. Additionally, the E-cadherin associated protein α-catenin regulates YAP directly by sequestering YAP/14-3-3 complexes in the cytoplasm 106, 107. Furthermore, the junction associated Ajuba protein family members directly inhibit LATS kinase activity 108. In Drosophila, the related Zyxin protein also regulates Warts levels 109. Whether these adherens junction associated regulators of the Hippo pathway act independently of each other or whether they function coordinately to regulate Hippo signaling is not yet known. In addition, crosstalk between the different regulatory branches most likely exists. For example, CRB and adherens junctions regulate the structure of the actin cytoskeleton, and polarity proteins and junction proteins regulate each other.
In addition to these four major branches of upstream regulators of Hippo signaling, there are several other proteins that modulate the activity of the pathway, most of which have conserved functions in Drosophila (Figure 2). These include proteins that directly interact with or affect YAP and TAZ (WBP2 110,111,112, MASK1/2 113,114, ZO1/2 115, 116, HIPK2 117,118, 14-3-3, PTPN14 119–123, CK1 and βTRCP 67, 68) or that interact with the upstream kinase complexes (RASSF 124–126, PP2A 127, Salt-inducible kinases (SIKs) 128, Scribble (SCRIB), and the Scribble associated proteins Discs large (Dlg) and Lethal giant larvae (Lgl) (so far shown in Drosophila 80, 129–133)). Scrib, Dlg and Lgl form a module that regulates cell polarity and establishes the basolateral domain, indicating that the Hippo pathway is independently modulated by several junctional complexes. The Drosophila Hippo pathway is also regulated by a signaling axis from the atypical cadherin Fat that regulates the levels and activity of Warts 2,3, 30. However, whether Fat homologs are involved in vertebrate Hippo pathway regulation is not clear 134.
Tissue-specific pathway regulation
While the net effect of deregulated YAP/TAZ activity in many tissues is similar, the control of YAP and TAZ activity appears to be under different regulatory mechanisms in different tissues. For example, while the MST kinases are essential to inhibit YAP and TAZ in the liver 135–138, intestine 139, pancreas 140, 141, and heart 142, they appear to be dispensable in the skin 106. Presumably, in the skin, other negative regulatory mechanisms predominate, and sequestration via α-catenin and AMOT have been suggested to circumvent the need for phosphorylation by LATS kinases 106, 107. Further evidence that there is cell-type specificity in Hippo pathway regulation is the observations that the MST kinases are largely dispensable in mouse embryo fibroblasts (MEFs) 135,137, while the LATS kinases are essential to inhibit YAP and TAZ activities 143. Tissue specific requirements for upstream components is also observed in Drosophila, where Fat signaling is dominant in imaginal discs but has no significant effect in the ovarian follicle cell epithelium 144,145, 146,147,148,149,150. Likewise, Mer has minor effects in imaginal discs but is a major regulator of the Hippo pathway in follicle cells 151,145,152,153,144,154,155.
The Hippo pathway in growth control and cancer
The dramatic overgrowth phenotypes caused by loss of Hippo pathway activity in Drosophila first led to the idea that the pathway may be important in the control of organ growth and act as a tumor suppressor in vertebrates. Indeed, many studies have now shown that loss of Hippo signaling or hyperactivation of YAP and TAZ promote growth and cell pluripotency depending on tissue context. Thus, loss of pathway activity in mouse models causes overgrowth of various organs such as the liver and heart and can lead to the development of cancer in the liver, skin, and intestine. Below, we highlight the phenotypes associated with loss of pathway activity in genetically engineered animal models, discuss cellular events that are controlled by YAP/TAZ, and summarize the evidence for hyperactive YAP and TAZ in human cancer.
Hippo signaling in growth control
A predominant function of the Hippo pathway is in regulating progenitor cell proliferation and organ size. In Drosophila, loss of function of the Hpo or Wts kinases, or overexpression of Yorkie (Yki), the Drosophila homolog of YAP and TAZ during development, result in severely overgrown imaginal discs leading to dramatic overgrowth of the corresponding adult structures 31–37, 44. These overgrowths arise because mutant cells have two defects. First, they have defects in regulating cell proliferation; they proliferate faster than wild-type cells and continue to proliferate when wild-type cells stop proliferating after tissues have reached their proper size. Second, mutant cells have defects in regulating apoptosis; they are resistant to apoptotic signals that are employed to eliminate extra cells. The combination of these defects then results in production of excess cells that cannot be eliminated, leading to increased organ size. Similarly in mice, YAP overexpression or loss of MST or LATS kinase activities increases liver and heart size by increasing cell number 11, 135–138, 142, 156, 157. However, the relationship between Hippo signaling and organ size is not absolute. In some tissues such as the skin and intestine, overexpression or activation of YAP causes an enlargement of the stem cell compartment in part due to a block in differentiation, but does not lead to an overall increase in organ size. 12, 106, 158. This may be due to the cellular composition of these tissues in that they continuously undergo a stem cell driven renewal program. Thus, the general conclusion from genetic studies of the Hippo pathway in mice and flies is that YAP and Yki drive cell proliferation and tissue growth. However, they may not drive growth in every tissue and they also have functions apart from regulating tissue growth, although non-growth related functions appear to be minor and related to cellular differentiation 1, 159, 160.
Several direct downstream target genes of the Hippo pathway have been identified and include genes such as cyclins, growth factors, and inhibitors of apoptosis that are involved in cell proliferation, cell survival and stem cell functions among others. In Drosophila, a combination of ChIPseq and RNAseq for Yki and the TEAD protein Scalloped (Sd) showed that Yki/Sd regulate a majority of genes expressed in imaginal discs, making them more akin to general transcriptional activators 161. At present, however, how YAP/TAZ and Yki drive cell proliferation and tissue growth is incompletely understood and likely involves the regulation of many direct target genes, some of which affect growth and survival directly while others affect these processes indirectly by global regulation of metabolism and other cellular processes 21, 161, 162,163.
Hippo signaling in cancer
The dramatic effects of YAP overexpression and hyperactivation on organ size and progenitor cell pools demonstrate a potent growth promoting activity. Indeed, there is considerable evidence that abnormal Hippo signaling is associated with a wide variety of human cancers 7. Elevated levels and nuclear localization of YAP and in some cases TAZ has been reported in a majority of solid cancers, suggesting widespread deregulation of Hippo signaling in human neoplasia 7,11,12, 15,16–20,21. Examples of abnormally elevated YAP levels and nuclear localization in human cancers include cancers in the liver, lung, breast, skin, colon and ovary 7,11,12, 15,16–20,21. In the liver, skin and colon, mouse models showed that attenuation of Hippo signaling or overexpression of YAP is sufficient to promote tumor formation 11, 12, 15, 106, 135–138. The exact mechanisms involved in transformation of normal cells to malignant tumor cells by deregulated YAP and TAZ are not known, but likely involve enhanced cell proliferation and survival coupled with acquisition of additional cancer cell phenotypes such as cancer stem cell character, epithelial to mesenchymal transition (EMT), drug resistance, and inhibition of senescence as these are all activities promoted by YAP/TAZ that are abnormally regulated in tumor cells.
The association of Hippo signaling with stem cell properties has recently been extended to cancer stem cells. In breast cancer, TAZ has been shown to be a potent stimulator of cancer stem cells in vitro 132. Overexpression of TAZ increases the ability of MFC10A cells to form mammospheres, an indication that TAZ enhances the proportion of MCF10A cells that have stem cell properties. Conversely, knockdown of endogenous TAZ inhibits mammosphere formation. TAZ is overexpressed in approximately 85% of high-grade human breast cancer with evidence for gene amplification in 15–20% of these cases 132. Taken together, these results suggest that activation of TAZ is a major event associated with breast cancer initiation and/or progression. In contrast, YAP does not appear to be effective in promoting cancer stem cell properties in the report of Cordenonsi et al., however, other studies support a role for YAP in promoting cancer-like properties in non-transformed mammary epithelial cells 15. Given that YAP and TAZ promote pluripotency in other stem cell contexts it is likely that their hyperactivation also contributes to cancer stem cell expansion, survival and self-renewal in other malignancies.
In addition to their self-renewal capacity, cancer stem cells are associated with epithelial-mesenchymal transition (EMT) a feature commonly observed in high-grade tumors and metastasis. Overexpression of YAP and/or TAZ can lead to acquisition of a mesenchymal phenotype in mammary epithelial cells suggesting Hippo signaling may play important roles in suppression of EMT 15, 61, 132. Indeed, loss of cell polarity determinants such as SCRIB leads to reduced Hippo pathway activity, activation of YAP/TAZ, EMT and cancer stem cell expansion 132. Thus, YAP/TAZ activation by loss of cell polarity may engage a positive feed-forward loop in which YAP/TAZ promotes EMT and loss of cell polarity, which then further activates YAP and TAZ. Importantly, hyperactivation of TAZ has been associated with EMT and high-grade tumors in human breast cancer and in glioma 132, 164.
Cancer stem cells are commonly resistant to chemotherapeutic agents and several recent studies indicate that hyperactivation of YAP and/or TAZ contributes to this resistance 15, 165–167. A feature of Hippo pathway inactivation and YAP/TAZ hyperactivation is increased cell survival mediated by suppression of apoptosis 11, 15, 41, 42. Several mechanisms contribute to this suppression, including transcriptional upregulation of pro-survival factors such Bcl2 family members 168. Additionally, it has been reported that the YAP/TAZ targets CTGF and Cyr61 inhibit apoptosis in liver cells 169, 170 and are responsible for taxol resistance in breast cancer cells 167. Other mechanisms likely contribute to YAP/TAZ-mediated drug resistance, including their ability to promote EMT and stem cell properties171, both of which are associated with increased resistance to chemotherapy in a variety of tumors and cell lines172.
In summary, deregulated YAP and TAZ activity promotes multiple cancer cell phenotypes beyond simply driving cell proliferation, suggesting that targeting YAP and TAZ may inhibit growth of cancer cells at many levels.
Mechanisms of Hippo pathway deregulation in cancer
YAP/TAZ protein levels and nuclear localization are elevated in many human cancers and YAP/TAZ drive acquisition of several important cancer cell phenotypes. However, the mechanism of Hippo pathway de-regulation and YAP/TAZ activation in human cancers is not well understood. This knowledge gap is important because development of Hippo pathway based therapies would benefit from insight into molecular mechanisms that are altered and responsible for elevated YAP/TAZ activity in cancer. Few germline or somatic mutations in Hippo pathway components have been discovered in targeted and whole-genome sequencing efforts to date suggesting that these may be generally uncommon events 7. One exception to this observation is the Neurofibromatosis type 2 (NF2) locus. NF2 encodes the MERLIN tumor suppressor protein that regulates the Hippo pathway by modulating the activity of the LATS kinases by promoting their localization to the plasma membrane 92, 173,174. NF2 is mutated with high frequency in neurofibromatosis, a condition that is characterized by malignant peripheral nerve sheath tumors, and it is thus a bona fide tumor suppressor gene 175, 176. Loss of MERLIN as well as the Hippo pathway components LATS2 or SAV1 is also frequently seen in malignant mesothelioma 177.
Why NF2 and LATS2 mutations are common in neurofibromatosis and mesothelioma but uncommon in other human cancers, despite widespread deregulation of YAP/TAZ is not understood. Additionally, activating mutations in YAP or TAZ have not been reported in human cancers 7, 178, despite the ability of such mutations to drive tumor formation in mouse models and to confer tumor properties onto non-tumorigenic human cell lines 11,12, 15. One possibility is that in human cancers, mutant YAP/TAZ may not confer a growth advantage because mutant YAP/TAZ may still be kept in check by mechanisms that act independently of LATS, for example by actin mediated regulation of YAP and TAZ 104, or because they also initiate as yet unknown growth inhibitory mechanisms. In support of the latter hypothesis, YAP and TAZ are found to be genomically amplified in several human cancers including oral cancers, intracranial ependymomas, hepatocellular carcinomas, gliomas and mammary tumors 179,180,181,19,16,15. The frequency of amplification is variable, with high occurrence in oral cancers and ependymomas and relatively low frequencies observed in hepatocellular carcinomas and in breast cancer. In contrast, approximately 50% of human liver cancers display elevated YAP levels 20, and TAZ overexpression is observed in over 80% of breast cancers 18,132. These findings imply that amplification is a common mechanism in some human cancers, but other mechanisms predominate in other cancers. The exact nature of these other mechanisms is currently not known, but may include promoter methylation and epigenetic silencing of the MST and LATS kinases 182–184,185 or expression and/or upregulation of proteins that control Yap transcription 186, 187,188, 189 or stability 188.
Hence, current evidence suggests that multiple mechanisms contribute to the deregulation of YAP and TAZ in human cancers, including promoter hypermethylation, mutation, and amplification. Importantly, while specific defects that deregulate the Hippo pathway in many cancers are not known, the core components are largely unaffected by irreversible mutations or genetic aberrations 7. Hence, at least in principle, these observations provide the opportunity for pharmaceutical interventions to reactivate or restore Hippo pathway function, thereby inhibiting oncogenic YAP and TAZ activities.
Approaches and prospects for Hippo-based therapies in cancer
Given the association of elevated and hyperactive YAP and TAZ with many cancers, direct or indirect attenuation of YAP and/or TAZ represents a rational and novel targeted approach for treatment and prevention of human malignancies. In this section, we discuss recent preclinical findings that support efforts directed towards the development of new Hippo pathway-based targeted therapies to prevent and treat malignancies.
Several lines of evidence from mouse and in vitro models show that reducing YAP activity is effective in limiting growth of tumor cells and preventing tumor formation. First, liver-specific deletion of Yap in the context of simultaneous NF2 deletion results in complete suppression of liver tumor formation induced by NF2 loss 173. Importantly, reducing, but not eliminating Yap gene dosage to 50% results in a similar suppression, indicating that partial inhibition of YAP is effective in preventing tumor formation in this model 173. In addition, expression of a dominant negative version of TEAD2 that lacks the DNA binding domain but that can still bind to YAP abolished hepatomegaly and the development of liver cancer in a NF2 mutant mouse model 190. A dosage effect for YAP was also seen in a mouse model of colon cancer where the Mst1 and Mst2 kinases were conditionally deleted in the intestinal epithelium with villin-cre 139. Villin-cre;Mst1/2 mice develop colonic adenomas by three months of age, associated with disruption of the normal colonic tissue architecture due to hyperactivation of YAP. Both of these phenotypes were effectively suppressed by removal of a single Yap allele 139. Hence these two studies show that reducing YAP dosage is effective in preventing tumor formation in situations predisposed to activation of YAP. Thus, therapeutics that target YAP may not need to fully block YAP activity for efficacy, thereby reducing the potential for negative side effects.
While these results are encouraging, whether reducing YAP dosage after tumor formation will cause a reduction in tumor burden has not yet been established. In addition, these studies activate YAP by mechanisms that are not documented in the corresponding human cancers. Hence, it will be important to test whether reducing YAP or TAZ activities in relevant genetically engineered mouse models that more closely recapitulate human cancer is effective. Also of concern is that a complete understanding of the long-term consequences of YAP and/or TAZ inhibition on normal and cancerous tissues is lacking. Potential for unexpected consequences of YAP inhibition is suggested by the finding that deletion of YAP in the mouse intestine can lead to WNT hypersensitivity with subsequent enhanced injury-induced stem cell expansion and hyperplasia 191. This effect was attributed to YAP-dependent cytoplasmic sequestration of the WNT signaling component DVL, a function of YAP that is independent of the transcriptional activity of YAP-TEAD. The growth promoting effect of YAP deletion may be due to more intestinal differentiation, resulting in gain of Paneth cells that define the stem cell niche and are a major source for WNT ligands 192. Thus, complete inhibition of YAP may have unintended consequences and result in increased colorectal tumor growth. Desirable effects may thus be best achieved by aiming to reduce the transcriptional activity of the YAP-TEAD complex, for example by disrupting the YAP-TEAD interaction or by promoting cytoplasmic localization of YAP, rather than completely removing YAP. Indeed, expression of a dominant negative TEAD2 does not cause overt liver abnormalities 22. Hence, available evidence supports the notion that selective inhibition of YAP/TEAD function may have therapeutic efficacy with minimal side effects.
Additional evidence that reducing YAP activity is effective in suppressing tumor growth comes from studies using cancer cell assays 193,186, 194–196. Results obtained with human cancer cell lines demonstrated the efficacy of reducing YAP dosage (by siRNA or shRNA depletion) in slowing or arresting the growth of tumor cells in vitro or in xenograft assays 193,186, 194–196. Other properties of cancer cells are also affected by YAP knockdown, including making cells more sensitive to chemotherapeutic reagents122, inhibition of cancer stem cell formation132, and reduction of metastatic potential 194, 197, suggesting that targeting YAP may be effective in modulating a number of cancer cell properties that are required for their expansion, survival and spread to distant tissues.
Notably, a recent study extends the potential for targeting YAP in human cancer to cancers that do not exhibit elevated levels of YAP 54. Genome-wide screens for shRNA induced synthetic lethality in a large panel of human cancer cells revealed that many tumor cell lines with activated WNT signaling are particularly sensitive to knock-down of YAP 54. In this case, YAP forms a transcriptional complex with β-catenin (the nuclear effector of canonical WNT signaling) and TBX5 (a sequence-specific DNA binding protein) that is necessary for cell survival 54. Interfering with complex assembly inhibits tumor cell survival and growth as effectively as knock down of YAP itself suggesting that the β-catenin/YAP/TBX5 complex is driving survival rather than the YAP-TEAD complex that is presumably also present in these cells. In these cancer cell lines YAP is constitutively nuclear and is not regulated by cell density indicating a non-canonical mode of YAP regulation. Indeed, LATS independent phosphorylation of YAP by the SRC family kinase YES1 is required for YAP-β-catenin-TBX5 complex formation and oncogenic properties 54. Thus, this study highlights that sensitivity to YAP inhibition may not always correlate with the levels or activity of YAP, and that TEAD-independent interactions of YAP may be important in some cancer contexts.
In summary, these studies imply that inhibiting the activity of YAP and TAZ may be effective in treating a variety of cancers, that complete inhibition is not required to show therapeutic efficacy, and that the sensitivity of a cancer cell to YAP/TAZ inhibition may not always correlate with the expression levels of YAP/TAZ.
The Hippo pathway in tissue repair and regeneration
Tissue repair and regeneration often involves stem cell activation and progenitor cell expansion and an emerging picture is that YAP and TAZ regulate the balance between stem, progenitor and differentiated cells. In general, enhanced YAP or TAZ activity is associated with stem and progenitor cell expansion coupled with inhibition of differentiation, whereas attenuation of YAP and TAZ activity tends to have the opposite effect. Examples include stem and progenitor cells of the liver, intestine, pancreas, heart, skin, and CNS 12, 106, 142, 156, 158,28, 157, 198. At present, there is little mechanistic insight into how YAP and TAZ regulate stem cell properties and inhibit differentiation. However, a recent study by Lian et al. suggests that in murine embryonic stem cells YAP directly binds to the promotors of genes that enhance pluripotency, suggesting that YAP may be a key component of the core pluripotency machinery 199. Whether this result can be generalized to tissue-specific stem cells in vivo remains to be determined.
In situations where progenitor cell expansion has been observed in vivo by YAP activation, it is not clear whether YAP functions to inhibit differentiation and expand stem cell pools or acts by reprogramming differentiated cells to more primitive and stem cell-like progenitor states. Indeed, in the case of murine embryonic stem cells and induced pluripotent cells, YAP appears to be capable of doing both 199. Enhanced YAP activity inhibits embryonic stem cell differentiation whereas inhibition of YAP leads to loss of pluripotency. Similarly, in induced pluripotent cell derivation from murine fibroblasts, YAP activity increases during the reprogramming process and negative regulators of YAP, such as the LATS kinases act as barriers to reprogramming 200. The situation in human induced pluripotent stem cells and embryonic stem cells is similar to what is observed in the mouse in that LATS kinases function as negative regulators of reprogramming 201. However, unlike in the murine situation, this effect appears to be mediated by TAZ 51. In human embryonic stem cells, TAZ is important for maintenance of the pluripotent state, while YAP is dispensable for this activity. Differences between mouse and human systems are not fully understood, but likely derive from distinct signaling pathways that regulate pluripotency and reprogramming in murine versus human cells. Taken together these results suggest that YAP and likely TAZ promote reprogramming of differentiated cells as well as expansion of stem and progenitor cell populations, both features that are important in applications to regenerative medicine.
An evolutionarily conserved essential function for YAP in tissue regeneration has been described through genetic studies of intestinal epithelial re-growth following injury. Elevated YAP expression is seen in mice treated with dextran sodium sulfate (DSS), a chemical that results in injury and inflammation of the large intestine and initiates a regenerative response 22. Mice that lack YAP in the colonic epithelium do not show overt defects in intestinal homeostasis but are unable to efficiently undergo a regenerative response upon DSS treatment 22, consistent with a role for Hippo signaling in repressing latent regenerative responses that involve stem cell activation. Another study, however, found that conditional elimination of YAP in the intestine promoted regeneration after irradiation and caused hypersensitivity to WNT ligands 191. The reasons behind the different outcomes in the Li et al. and the Cai et al. studies are unclear at the moment but may be due to differences in experimental set-up and time point of analysis. In any case, these two studies suggest that YAP has growth promoting as well as growth suppressing functions, potentially because it directly promotes progenitor cell expansion but also suppresses Paneth cell differentiation, which promotes stem cells by producing WNT ligands. The Drosophila intestine also harbors stem cells that are triggered to proliferate in response to tissue damage induced by feeding toxins or pathogens. The Drosophila YAP homolog Yki is required for this response 26,25,27,24, but unlike in mammals, Yki is activated in differentiated enterocytes which drives the expression of cytokines that then signal to stem cells and promote their proliferation 24. Thus, although Yki may not be directly involved in controlling intestinal stem cell proliferation in Drosophila, it is activated upon tissue damage. Thus, in the intestine, regulation of the Hippo pathway and activation of YAP is involved in driving regeneration of damaged tissue.
A similar situation may be operating in the liver, where loss of Hippo signaling results in expansion of oval cells 136, 202 a facultative progenitor cell population that contributes to liver repair following hepatocyte injury. In addition, YAP is required for neonatal heart regeneration in mouse 28,29. Notably, forced overexpression of an activated, S127A phospho-site mutant form of YAP in the adult mouse heart promoted heart regeneration after myocardial infarction 28. Thus, therapeutic elevation of YAP activity might prove beneficial for restoration of gut, heart, liver, and potentially other tissues following injury.
An important effect of experimental elevation of YAP activity with regard to regeneration is the promotion of cell cycle entry of non-dividing cells. This effect is seen in both the liver 11, 12 and heart 28, 142, 156,29. These are tissues that are composed of mostly quiescent cells with cellular turnover rates estimated to be on the order of one year for human hepatocytes 203 and once in a lifetime for human cardiomyocytes 204. While both cell types are normally quiescent, hepatocytes can be readily induced to re-enter the cell cycle and progress through cytokinesis following injury whereas adult cardiomyocytes do not efficiently undergo cell cycle re-entry and rarely, if at all, undergo cytokinesis. Because of this, the adult liver can regenerate while the heart cannot. Potentially relevant to these observations are the findings that, in mouse mutants of core Hippo pathway components, including Sav1, Mst1/2, there is unscheduled hepatocyte proliferation in adults 135, 202, 205, 206 and enhanced embryonic cardiomyocyte proliferation 142 Moreover, overexpression of YAP in adult murine cardiocytes induces cell cycle entry and cell division 156,28,29. Hence, transient elevation of YAP activity might prove to be useful in the repair of tissues that do not normally undergo regeneration such as the heart and to augment the proliferative capability of damaged tissues that normally regenerate, such as the liver. Altogether, while sustained activation of YAP or TAZ has clear oncogenic potential in a variety of tissues, transient up-regulation of YAP or TAZ activity by pharmacologic intervention might be useful in situations that require mobilization of stem and progenitor cell populations or even the reprogramming of adult differentiated cells.
Therapeutically targeting the Hippo pathway
The studies described above suggest that manipulation of the Hippo signaling pathway might be beneficial in cancer prevention and treatment as well as to expand stem cell populations for use in regenerative medicine. Targeting the Hippo pathway as an anticancer therapeutic strategy would aim to suppress YAP and TAZ activity, while targeting the Hippo pathway to facilitate regeneration and reprogramming of adult cells and tissues, would aim at elevating YAP and TAZ activity. However, although evidence is mounting that pharmacologic manipulation of Hippo signaling and YAP/TAZ activity would be beneficial, there is a need to develop effective means to manipulate Hippo signaling and YAP/TAZ activity in both a sustained and transient manner. In addition, tissue-specific control of YAP/TAZ may become important if chronic and/or long-term whole-body inhibition of YAP and/or TAZ results in deleterious side effects. This may be achieved by targeting individual branches of upstream regulators. While there is interest and potential in targeting genes and pathways that are downstream of Hippo/YAP/TAZ such as the Axl receptor kinase 207, connective tissue growth factor (CTGF, 208), EGFR signaling 209, 210, and others (reviewed in 211), below we focus on strategies to target Hippo signaling itself by manipulating pathway components.
Kinases
Decades of targeted drug development efforts suggest that kinases and other proteins with enzymatic activity are attractive targets for small-molecule therapeutics 212. Within the core of the Hippo signaling pathway are two pairs of kinases, MST1/2 and LATS1/2 that restrain YAP and TAZ activity. Small-molecule inhibitors of these kinases would thus be predicted to upregulate YAP and TAZ function, which might prove beneficial in regenerative medicine applications such as ex vivo stem and progenitor cell expansion and in vivo tissue repair. A small-molecule inhibitor for MST1, 9E1, has been developed 213. This inhibitor inhibits MST activity in vitro and in cultured Hela cells as measured by Histone H2B phosphorylation in response to apoptosis, although effects on YAP/TAZ activity have not yet been reported. 9E1 shows significant although not complete selectivity for MST1 over other kinases and it provides a starting point for the development of more selective MST1 inhibitors. In addition, rational strategies to target kinases are generally effective in identifying lead compounds that can be further developed and refined by traditional medicinal chemical approaches 214, 215. On the other hand, targeting the MST or LATS kinases for anticancer therapy is more challenging since small molecule agonists would be most desirable for which there are few options for rational design. Alternatively, the tyrosine kinase YES1 or the Homeodomain interacting kinase 2 (HIPK2) may be targeted for cancer therapy in some contexts as these kinases promote YAP activity in some assays: YES1 is required for cell survival and the activation of YAP in β-catenin active cancer cell lines 54 and HIPK2 promotes YAP activity in in vitro transcriptional assays 117. While there are no reported inhibitors of HIPK2, there is evidence that YES1 inhibition can selectively target cancer cell survival and growth. The YES1 kinase phosphorylates YAP thereby promoting the formation of a YAP/β-catenin/TBX5 transcriptional complex that is essential for the proliferation of β-catenin dependent colon cancer cell lines 54. Notably, the broad range tyrosine kinase inhibitor dasatinib, which inhibits the YES1 kinase, was effective in inhibiting growth of these β-catenin dependent cell lines in vitro and to inhibit tumor formation in xenographs by interfering with YAP/β-catenin/TBX5 complex assembly independent of Hippo pathway modulation 54. Thus, Inhibition of YES1 may be effective in a subset of β-catenin and YAP dependent cancers.
YAP/TAZ-TEAD complex
The most attractive anti-cancer targets in the Hippo pathway are YAP and TAZ, as they are the key downstream mediators of the pathway. However, pharmacological inhibition of YAP and TAZ is challenging as these proteins have no known catalytic activity and function by engaging domains that facilitate protein-protein interactions with the upstream kinases LATS1/2, the 14-3-3 phosphopeptide binding proteins, the AMOT and ZO1/2 polarity proteins, and the TEAD transcription factors. In many cases, the oncogenic properties of YAP and TAZ depend on their interaction with the TEAD proteins 194,71. Genetic disruption of this interaction by mutating amino acid residues critical for YAP-TEAD or TAZ-TEAD complex formation abolishes the transforming ability of YAP and TAZ in vitro 48, and in the case of YAP, in vivo, at least in a mouse liver cancer model 190. Since the co-crystal structure of the YAP/TEAD complex has been recently determined 216–218, it is possible that this detailed structural information can be leveraged to rationally design small-molecule inhibitors of YAP/TEAD by targeting residues that line the YAP/TEAD polypeptide interaction interface.
MASK/WBP2/Ajuba proteins
Additional components of the Hippo pathway that could be envisioned for targeted anti-cancer therapy are proteins that are required for YAP/TAZ activity such as the MASK proteins 114,113 and WBP2 110, 111, that directly interact with YAP/TAZ, and Ajuba family proteins, which stimulate LATS activity and thus inhibit YAP/TAZ indirectly 108. However, all of these are scaffold or regulatory proteins without enzymatic activity, which would require targeting a protein-protein interface. Nevertheless, because YAP and TAZ are regulated by multiple inputs, which often show tissue specific requirements, these and other proteins may provide means to inhibit YAP/TAZ in specific tissues, thereby aiding in the development of inhibitors that show organ and/or disease type specificity.
EGFR-PI3K pathway
Recently, small-molecule modulators of the EGFR-PI3K pathway have been shown to affect Hippo signaling 219. Mitogenic growth factors contained in serum as well as EGF treatment stimulated nuclear localization of YAP by inhibiting YAP phosphorylation and promoting nuclear localization 219, 220. Based on this observation, Fan et. al. screened small-molecule inhibitors of kinases and phosphatases that act downstream of EGF signaling and found that PI3K and PDK1 inhibitors, but not AKT inhibitors, were effective in blocking EGF-induced and lysophosphatidic acid (LPA)-induced YAP nuclear localization 219. Mechanistically, PDK1 physically associates with the core Hippo pathway kinase complex and this complex dissociates in response to EGF signaling thereby resulting in YAP activation 219. Thus, PI3K and PDK1 inhibitors may be effective in reducing YAP activity in cells with an intact Hippo signaling pathway that have elevated EGFR and/or reduced PTEN function.
GPCR signaling
Three recent reports link G-protein coupled receptors (GPCRs) to Hippo pathway regulation 101,102,103. LPA, sphingosine-1-phosphate (S1P), and thrombin were identified as novel signals that signal through Ga12/13 coupled GPCRs to stimulate YAP nuclear localization and activity 101,102,103. Importantly, representatives of many different types of GPCR subgroups affect YAP, demonstrating a general function of GPCRs in YAP regulation 101. GPCRs that act through Ga12/13, Gaq/11, or Gai/o stimulate YAP, while Gas coupled receptors have the opposite effect 101. GPCRs regulate YAP via Rho GTPases and the actin cytoskeleton to inhibit LATS kinases independently of MST kinases to cause dephosphorylation and nuclear accumulation of YAP 101, 102,103. Agonists of Gas-coupled receptors such as epinephrine, glucagon and the dopamine receptor agonist dihydrexidine result in enhanced YAP phosphorylation and inactivation 101. Importantly, GPCR agonists such as epinephrine also affect YAP phosphorylation in vivo causing an increase of YAP phosphorylation in the heart of injected mice 101. These results therefore indicate that YAP activity can be modulated with drugs that modulate GPCR signaling, although which agonists or antagonists will be effective in therapeutic applications remains to be determined.
F-actin
The identification of F-actin as an important regulator of YAP/TAZ localization and activity opens new opportunities for small molecule modulation of Hippo signaling. The F-actin destabilizers cytochalasin D and latrunculin A and B, as well as treatment with the non-muscle myosin II inhibitor blebbistatin, the myosin light chain kinase inhibitor ML-7, the Rho inhibitor botulinum toxin C3, and the Rho kinase inhibitor Y27632 all cause nuclear export of YAP/TAZ 96,98,100,99. These results suggest that drugs that target F-actin and its modulators may also be effective in modulating YAP/TAZ activity in vivo. This approach is complicated in that the actin cytoskeleton is not a Hippo pathway specific signal transduction component but required for many basic cellular functions. Nevertheless, non-lethal levels of actin modulators may enhance the effects of other drugs that target the Hippo pathway by different mechanisms.
High-throughput screening approaches
An attractive approach to identify small-molecule inhibitors and activators of Hippo signaling is through application of cell based high throughput screening. Indeed, a screen of approximately 3300 FDA approved drugs for inhibitors of the transcriptional activity of YAP identified 71 hits including several porphyrin compounds 190. One of them, verteporfin (VP), currently used as a photosensitizer in therapy for macular degeneration, was effective in vivo in delaying tumor progression in a NF2-depleted mouse liver model and to suppress liver overgrowth caused by overexpression of YAP using a regimen that involved repeated drug administration during the development of the cancer phenotype 190. VP was found to bind to YAP in vitro and to inhibit the interaction of YAP with TEAD 190. Although these data are exciting, future studies will be needed to determine if VP is effective in other in vitro and in vivo cancer models and also whether it is effective in the treatment of established cancers. In addition, the affinity of VP for YAP is relatively low (at the micromolar level) and higher affinity derivatives may be required.
In another cell based screen of 48 drugs for inhibitors of nuclear localization of YAP, Bao et. al. identified dobutamine, a G-protein coupled β-adrenergic receptor agonist used clinically to treat acute heart failure, as being effective in preventing nuclear accumulation of YAP and YAP-mediated transcriptional activation in osteoblastoma and HEK293 cells 221. Dobutamine treatment induced cytoplasmic translocation of YAP and phosphorylation of the main LATS phosphorylation site serine 127. Phosphorylation of this site was required for the effect of dobutamine, although it appeared to be phosphorylated by a kinase other than LATS 221. Dobutamine-mediated activation of β-adrenergic receptor likely acts through a pathway that involves Rho GTPases and F-actin, similar to other GPCRs 101,102,103.
Open questions and future challenges
The Hippo pathway, and in particular the YAP/TAZ-TEAD complex, is an emerging anti-cancer target and mounting evidence from mouse models as well as tissue culture assays indicates that targeting the Hippo pathway is effective in preventing disease and counteracting cellular mechanisms that promote oncogenic transformation. Although less appreciated, there is also considerable potential for application of small molecules that activate the YAP/TAZ-TEAD complex transiently for stem cell expansion and tissue repair following injury.
Work over the past decade has identified a complex network of Hippo pathway regulatory components and established robust assays by which pathway activity can be measured. These results now provide a rich landscape to identify small-molecule modulators of the Hippo pathway and a number of pharmacological modulators of Hippo signaling have already been discovered. The challenges are now to determine whether these drugs will be therapeutically useful, which combinations will be the most effective, and in what disease contexts they should be applied. Reflecting the complex regulation of the Hippo signaling pathway, these small molecules affect a variety of components and branches of the pathway. However, many of these do not specifically target Hippo pathway components and therefore another challenge is to develop novel modulators that specifically target the pathway and in particular the activity of the YAP-TEAD complex. Since many of the pathway components are adaptor proteins that may be difficult to target, it will be important to discover additional components of the Hippo pathway, especially ones that directly affect YAP/TAZ, which may lead to the identification of better drug targets. For example, the recent finding that SET7-dependent lysine monomethylation of YAP is important for cytoplasmic retention 222 as well as p300/CBP-mediated acetylation and SIRT1-mediated deacetylation of YAP 223, 224 suggests that identification and selective inhibition of YAP protein demethylases could be a novel approach to modulate YAP activity. Another challenge is to decipher how YAP/TAZ are deregulated in cancer as this will be critical in deciding which components in the pathway should be targeted.
In principle, targeting the Hippo pathway for regenerative medical applications may be more easily accomplished as the core of the pathway contains two kinases. It will thus be interesting to develop and test inhibitors of the MST and LATS kinases such as the 9E1 compound for transient stimulation of growth and regeneration of tissues following injury and in applications where stem/progenitor cell expansion is desired.
Although most attention has focused on small-molecule manipulation of YAP/TAZ activity that acts by controlling YAP/TAZ sub-cellular localization or their ability to complex with TEADs, there are other avenues for pharmacological manipulation of YAP/TAZ that warrant further investigation. For example, YAP/TAZ stability is controlled by phosphorylation-induced protein degradation 67, 68 and small molecules that enhance YAP/TAZ turnover would be expected to reduce nuclear transcriptional activities of these proteins. Finally, not all components of the Hippo pathway are required in all tissues and the pathway has tissue specific regulatory mechanisms. Targeting such tissue specific regulators provides opportunities to manipulate the pathway in specific cells, which may help in reducing toxicity and increasing therapeutic value.
In conclusion, while studies aimed at targeting the Hippo pathway for therapeutic uses are still in their infancy, promising preclinical genetic and pharmacological results have already been documented in the literature. As these studies mature, we anticipate that the full potential of harnessing the Hippo pathway in human disease prevention and treatment will be realized.
Figure 3. Hippo mutant phenotypes in flies and mice.

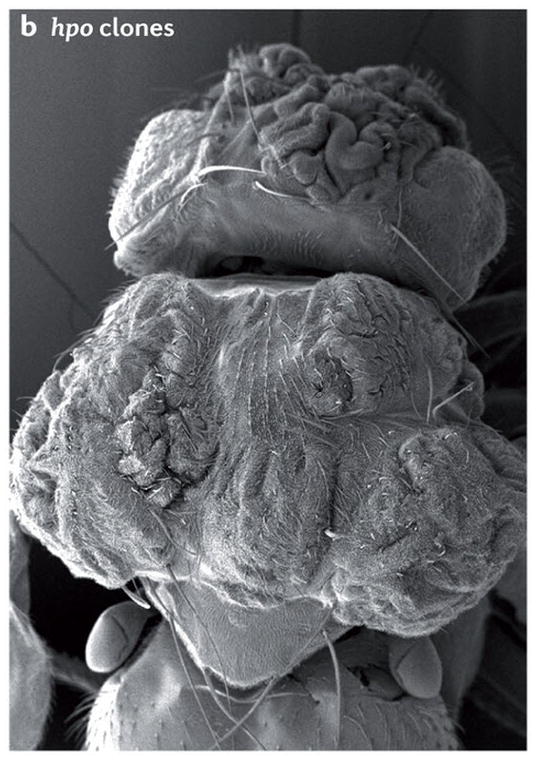
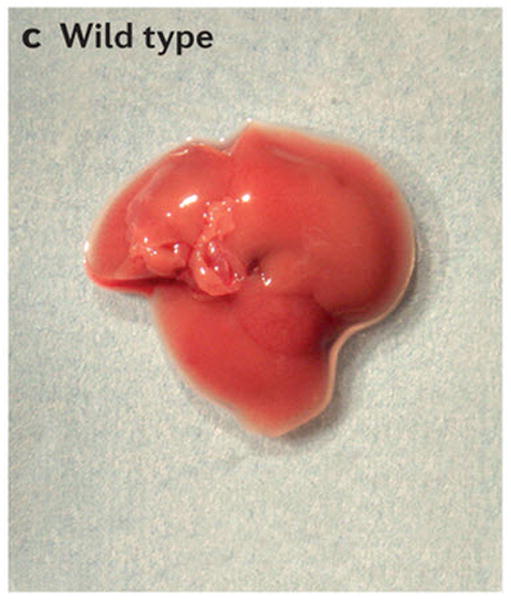
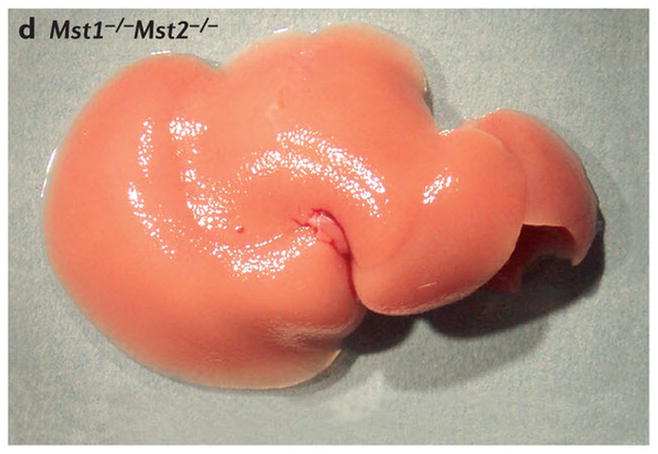
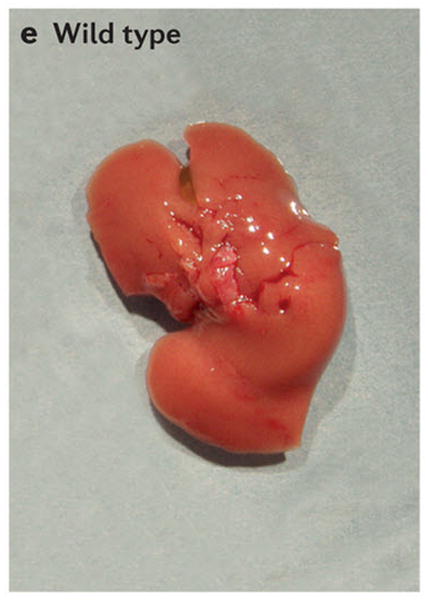
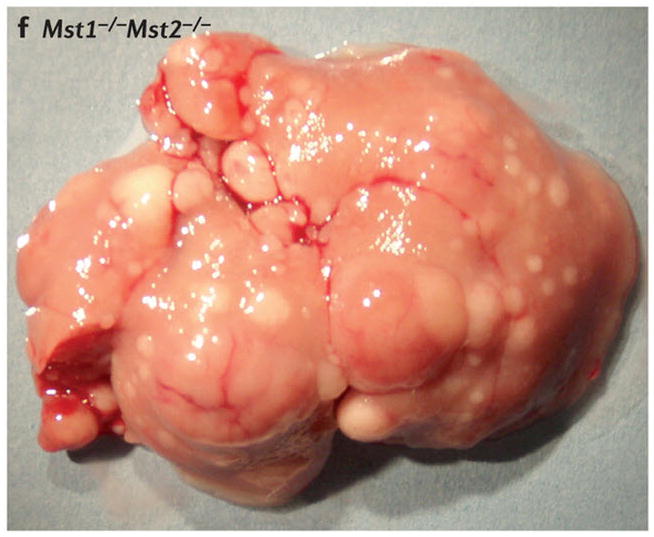
Scanning electron micrographs of (A) a wild-type fly and (B) a fly with patches (clones) of cells homozygous mutant for the hippo kinase. The hippo mutant tissues exhibit overgrowth of the adult cuticle. (C) A mouse liver at two months of age from a wild-type animal and (D) a liver at two months of age from a mouse mutant in which the two hippo homologs Mst1 and Mst2 have been conditionally deleted in the developing liver. (E) Normal mouse liver at 6 months and (F) a Mst1/2 double mutant liver at 6 months which is not only overgrown but also developed foci of hepatocellular carcinoma (HCC).
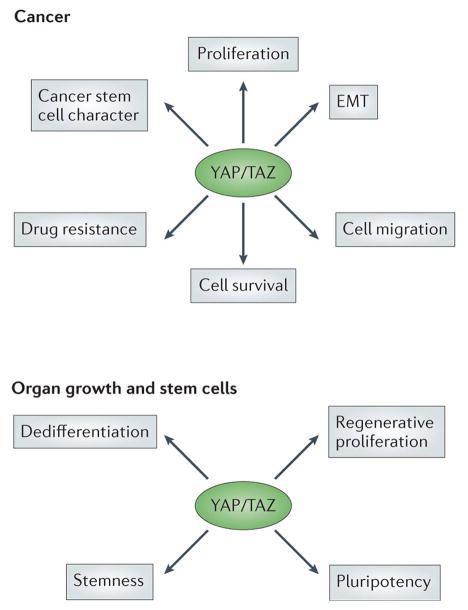
Figure 4. Cellular functions of YAP/TAZ and TEAD.
YAP and TAZ regulate several cellular properties that are important for the development of cancer and the regulation of stem cell behavior and regeneration. Some of these, such as the promotion of stemness and proliferation are important for cancer development and in regeneration, while others such as the regulation of EMT may be important only for the development of cancer. However, the function of YAP and TAZ in reprogramming mature and differentiated cells during regenerative behavior may be exploited during the development of cancer and help drive EMT and other phenotypes.
Table 2.
Mode of action of current small-molecule modulators of the Hippo pathway.
| Compounds | Targets | Effects | Refs |
|---|---|---|---|
| Verteporfin | YAP | Inhibits YAP-TEAD interaction and transcriptional activity in vitro; suppresses hepatomegaly and hepatocellular carcinoma caused by YAP overexpression or Nf2 deletion in mouse livers | 194 |
| 9E1 | MST1 | Inhibits MST1 kinase activity in vitro and in Hela cells; has significant but incomplete selectivity and also inhibits GSK3β and PIM1 | 213 |
| LPA, S1P, thrombin | LPA-, S1P-and thrombin receptors (GPCRs) | Signal through the Gα proteins G12/13 to activate RHO and actin, which inhibits LATS kinase activity, thereby causing the dephosphorylation of YAP and TAZ. This promotes the stability and nuclear accumulation of YAP and TAZ, resulting in enhanced target gene expression, cell proliferation and cell migration in different cell lines | 102–104 |
| Epinephrine, glucagon, dihydrexidine (agonist for dopamine receptors) | GPCRs | These molecules signal through GPCRs that signal through the Gα protein Gs, cAMP, PKA, RHO and actin to activate LATS, which results in the phosphorylation of YAP and inhibition of its function in cultured cells. Injection of epinephrine into mice results in enhanced phosphorylation of YAP in the heart — the physiological target of epinephrine | 102,221 |
| Dobutamine | β-adrenergic receptor agonist | Causes YAP Ser127 phosphorylation, cytoplasmic accumulation and suppression of YAP-TEAD transcriptional activity in vitro | 222 |
| Dasatinib | Tyrosine kinase inhibitor | Suppresses proliferation of β-catenin-active cell lines in vitro; this suppression depends on the inhibition of YES1 and resulting inactivation of the YAP–β-catenin–TBX5 complex. Inhibits the growth of Apc-null colon organoids and suppresses intestinal hyperplasia of Axin1-mutant zebrafish | 55 |
| Latrunculin A, latrunculin B, cytochalasin D | F-actin | All of these actomyosin cytoskeletal drugs inhibit YAP nuclear localization as well as YAP and TEAD activity in various cell lines | 97,99–101 |
| Blebbistatin | Non-muscle myosin | Inhibits YAP nuclear localization as well as YAP and TEAD activity in various cell lines | – |
| ML7 | MLCK | Inhibits YAP nuclear localization as well as YAP and TEAD activity in various cell lines | – |
| Botulinum toxin C3 | RHO | Inhibits YAP nuclear localization as well as YAP and TEAD activity in various cell lines | – |
| Y27632 | RHO kinase | Inhibits YAP nuclear localization as well as YAP and TEAD activity in various cell lines | – |
Apc, adenomatous polyposis coli; Axin1, gene encoding axis inhibition protein 1; cAMP, cyclic AMP; GPCR, G protein-coupled receptor; GSK3β glycogen synthase kinase 3β; LATS, large tumour suppressor homolog 1; LPA, lysophosphatidic acid; MLCK, myosin Light chain kinase; MST1, mammalian STE20-like protein kinase 1; NF2, neurofibromin 2; PKA, protein kinase A; S1P, sphingosine-1-phosphate; TAZ, transcriptional co-activator with PDZ-binding motif; TBX5, T-box transcription factor 5; TEAD, TEA domain-containing sequence-specific transcription factor; YAP, Yes-associated protein.
References
- 1.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Dev Dyn. 2012;241:3–15. doi: 10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem J. 2011;436:213–24. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- 4.Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–46. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–71. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 8.Stanger BZ. Quit your YAPing: a new target for cancer therapy. Genes & development. 2012;26:1263–7. doi: 10.1101/gad.196501.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu AM, Xu Z, Luk JM. An update on targeting Hippo-YAP signaling in liver cancer. Expert opinion on therapeutic targets. 2012;16:243–7. doi: 10.1517/14728222.2012.662958. [DOI] [PubMed] [Google Scholar]
- 10.Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–93. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, et al. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci. 2010 doi: 10.1111/j.1349-7006.2010.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, et al. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011;30:2181–6. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 15.Overholtzer M, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zender L, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–9. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan SW, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–8. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez LA, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–41. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu MZ, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–8. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grusche FA, Degoutin JL, Richardson HE, Harvey KF. The Salvador/Warts/Hippo pathway controls regenerative tissue growth in Drosophila melanogaster. Dev Biol. 2011;350:255–66. doi: 10.1016/j.ydbio.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–7. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw RL, et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–58. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–45. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren F, et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–9. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xin M, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heallen T, et al. Hippo signaling impedes postnatal cardiomyocyte regeneration. Development. 2013 doi: 10.1242/dev.102798. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder MC, Halder G. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol. 2012;23:803–11. doi: 10.1016/j.semcdb.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–20. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 32.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–67. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 33.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–7. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 35.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–9. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–46. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 37.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–63. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 38.Creasy CL, Chernoff J. Cloning and characterization of a member of the MST subfamily of Ste20-like kinases. Gene. 1995;167:303–6. doi: 10.1016/0378-1119(95)00653-2. [DOI] [PubMed] [Google Scholar]
- 39.Creasy CL, Chernoff J. Cloning and characterization of a human protein kinase with homology to Ste20. J Biol Chem. 1995;270:21695–700. doi: 10.1074/jbc.270.37.21695. [DOI] [PubMed] [Google Scholar]
- 40.Taylor LK, Wang HC, Erikson RL. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc Natl Acad Sci U S A. 1996;93:10099–104. doi: 10.1073/pnas.93.19.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapon N, et al. salvador Promotes Both Cell Cycle Exit and Apoptosis in Drosophila and Is Mutated in Human Cancer Cell Lines. Cell. 2002;110:467. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 42.Kango-Singh M, et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–30. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- 43.Lai ZC, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–85. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 44.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Goulev Y, et al. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–41. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 46.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–98. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–87. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229–41. doi: 10.1101/gad.888601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alarcon C, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–69. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varelas X, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 52.Ferrigno O, et al. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–84. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- 53.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt-Oram syndrome. Proc Natl Acad Sci U S A. 2005;102:18034–9. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenbluh J, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–62. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strano S, et al. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–73. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- 57.Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–76. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- 58.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–21. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chan EH, et al. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005 doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 60.Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. Embo J. 2007;26:1772–81. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lei QY, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–36. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–8. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 64.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J Biol Chem. 2008;283:27534–46. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 65.Kanai F, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14–3–3 and PDZ domain proteins. EMBO J. 2000;19:6778–91. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren F, Zhang L, Jiang J. Hippo signaling regulates Yorkie nuclear localization and activity through 14–3–3 dependent and independent mechanisms. Dev Biol. 2009;337:303–12. doi: 10.1016/j.ydbio.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu CY, et al. The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFbeta-TrCP E3 ligase. J Biol Chem. 2010 doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koontz LM, et al. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo T, et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013 doi: 10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–69. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- 72.Chan SW, et al. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347–58. doi: 10.1074/jbc.M901568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peng HW, Slattery M, Mann RS. Transcription factor choice in the Hippo signaling pathway: homothorax and yorkie regulation of the microRNA bantam in the progenitor domain of the Drosophila eye imaginal disc. Genes Dev. 2009;23:2307–19. doi: 10.1101/gad.1820009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, et al. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem. 2009;284:13355–62. doi: 10.1074/jbc.M900843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oh H, Irvine KD. Cooperative regulation of growth by Yorkie and Mad through bantam. Dev Cell. 2011;20:109–22. doi: 10.1016/j.devcel.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bazellieres E, Assemat E, Arsanto JP, Le Bivic A, Massey-Harroche D. Crumbs proteins in epithelial morphogenesis. Front Biosci. 2009;14:2149–69. doi: 10.2741/3368. [DOI] [PubMed] [Google Scholar]
- 77.Varelas X, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–44. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 78.Chen CL, et al. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–5. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein expanded. Curr Biol. 2010;20:582–90. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grzeschik NA, Parsons LM, Allott ML, Harvey KF, Richardson HE. Lgl, aPKC, and Crumbs regulate the Salvador/Warts/Hippo pathway through two distinct mechanisms. Curr Biol. 2010;20:573–81. doi: 10.1016/j.cub.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 81.Ling C, et al. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chan SW, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J Biol Chem. 2011;286:7018–26. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirate Y, et al. Polarity-Dependent Distribution of Angiomotin Localizes Hippo Signaling in Preimplantation Embryos. Curr Biol. 2013 doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–70. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paramasivam M, Sarkeshik A, Yates JR, 3rd, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22:3725–33. doi: 10.1091/mbc.E11-04-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yi C, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–40. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi C, et al. The p130 Isoform of Angiomotin Is Required for Yap-Mediated Hepatic Epithelial Cell Proliferation and Tumorigenesis. Sci Signal. 2013;6:ra77. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21:888–95. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 91.Huang HL, et al. Par-1 regulates tissue growth by influencing hippo phosphorylation status and hippo-salvador association. PLoS Biol. 2013;11:e1001620. doi: 10.1371/journal.pbio.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamaratoglu F, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- 93.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–8. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yu J, et al. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–99. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–16. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 96.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–35. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernandez BG, et al. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–46. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 98.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 99.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–14. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 100.Zhao B, et al. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miller E, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–62. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 103.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–43. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aragona M, et al. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–59. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 105.Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A. 2011;108:11930–5. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schlegelmilch K, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Silvis MR, et al. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Das Thakur M, et al. Ajuba LIM Proteins Are Negative Regulators of the Hippo Signaling Pathway. Curr Biol. 2010 doi: 10.1016/j.cub.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rauskolb C, Pan G, Reddy BV, Oh H, Irvine KD. Zyxin links fat signaling to the hippo pathway. PLoS Biol. 2011;9:e1000624. doi: 10.1371/journal.pbio.1000624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang X, Milton CC, Poon CL, Hong W, Harvey KF. Wbp2 cooperates with Yorkie to drive tissue growth downstream of the Salvador-Warts-Hippo pathway. Cell Death Differ. 2011;18:1346–55. doi: 10.1038/cdd.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan SW, et al. WW domain-mediated interaction with Wbp2 is important for the oncogenic property of TAZ. Oncogene. 2011;30:600–10. doi: 10.1038/onc.2010.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen HI, et al. Characterization of the WW domain of human yes-associated protein and its polyproline-containing ligands. J Biol Chem. 1997;272:17070–7. doi: 10.1074/jbc.272.27.17070. [DOI] [PubMed] [Google Scholar]
- 113.Sidor CM, Brain R, Thompson BJ. Mask proteins are cofactors of Yorkie/YAP in the Hippo pathway. Curr Biol. 2013;23:223–8. doi: 10.1016/j.cub.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 114.Sansores-Garcia L, et al. Mask is required for the activity of the Hippo pathway effector Yki/YAP. Curr Biol. 2013;23:229–35. doi: 10.1016/j.cub.2012.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Remue E, et al. TAZ interacts with zonula occludens-1 and -2 proteins in a PDZ-1 dependent manner. FEBS Lett. 2010;584:4175–80. doi: 10.1016/j.febslet.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 116.Oka T, et al. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461–72. doi: 10.1042/BJ20100870. [DOI] [PubMed] [Google Scholar]
- 117.Poon CL, Zhang X, Lin JI, Manning SA, Harvey KF. Homeodomain-interacting protein kinase regulates Hippo pathway-dependent tissue growth. Curr Biol. 2012;22:1587–94. doi: 10.1016/j.cub.2012.06.075. [DOI] [PubMed] [Google Scholar]
- 118.Chen J, Verheyen EM. Homeodomain-interacting protein kinase regulates Yorkie activity to promote tissue growth. Curr Biol. 2012;22:1582–6. doi: 10.1016/j.cub.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 119.Liu X, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32:1266–73. doi: 10.1038/onc.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang W, et al. PTPN14 is required for the density-dependent control of YAP1. Genes Dev. 2012;26:1959–71. doi: 10.1101/gad.192955.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Michaloglou C, et al. The tyrosine phosphatase PTPN14 is a negative regulator of YAP activity. PLoS One. 2013;8:e61916. doi: 10.1371/journal.pone.0061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang JM, et al. YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene. 2013;32:2220–9. doi: 10.1038/onc.2012.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poernbacher I, Baumgartner R, Marada SK, Edwards K, Stocker H. Drosophila Pez acts in Hippo signaling to restrict intestinal stem cell proliferation. Curr Biol. 2012;22:389–96. doi: 10.1016/j.cub.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 124.Polesello C, Huelsmann S, Brown NH, Tapon N. The Drosophila RASSF homolog antagonizes the hippo pathway. Curr Biol. 2006;16:2459–65. doi: 10.1016/j.cub.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–62. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ikeda M, et al. Hippo pathway-dependent and -independent roles of RASSF6. Sci Signal. 2009;2:ra59. doi: 10.1126/scisignal.2000300. [DOI] [PubMed] [Google Scholar]
- 127.Ribeiro PS, et al. Combined functional genomic and proteomic approaches identify a PP2A complex as a negative regulator of Hippo signaling. Mol Cell. 2010;39:521–34. doi: 10.1016/j.molcel.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 128.Wehr MC, et al. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat Cell Biol. 2013;15:61–71. doi: 10.1038/ncb2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen CL, Schroeder MC, Kango-Singh M, Tao C, Halder G. Tumor suppression by cell competition through regulation of the Hippo pathway. Proc Natl Acad Sci U S A. 2012;109:484–9. doi: 10.1073/pnas.1113882109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Menendez J, Perez-Garijo A, Calleja M, Morata G. A tumor-suppressing mechanism in Drosophila involving cell competition and the Hippo pathway. Proc Natl Acad Sci U S A. 2010;107:14651–6. doi: 10.1073/pnas.1009376107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun G, Irvine KD. Regulation of Hippo signaling by Jun kinase signaling during compensatory cell proliferation and regeneration, and in neoplastic tumors. Dev Biol. 2011;350:139–51. doi: 10.1016/j.ydbio.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cordenonsi M, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–72. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 133.Zhao M, Szafranski P, Hall CA, Goode S. Basolateral junctions utilize warts signaling to control epithelial-mesenchymal transition and proliferation crucial for migration and invasion of Drosophila ovarian epithelial cells. Genetics. 2008;178:1947–71. doi: 10.1534/genetics.108.086983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bossuyt W, et al. An evolutionary shift in the regulation of the Hippo pathway between mice and flies. Oncogene. 2013 doi: 10.1038/onc.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–6. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou D, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE. Hippo signaling regulates pancreas development through inactivation of Yap. Molecular and cellular biology. 2012;32:5116–28. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gao T, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–53. 1553, e1. doi: 10.1053/j.gastro.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yabuta N, et al. N-terminal truncation of Lats1 causes abnormal cell growth control and chromosomal instability. Journal of cell science. 2013;126:508–20. doi: 10.1242/jcs.113431. [DOI] [PubMed] [Google Scholar]
- 144.Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr Biol. 2007;17:1864–70. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- 145.Meignin C, Alvarez-Garcia I, Davis I, Palacios IM. The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr Biol. 2007;17:1871–8. doi: 10.1016/j.cub.2007.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–10. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 147.Bryant PJ, Huettner B, Held LI, Jr, Ryerse J, Szidonya J. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev Biol. 1988;129:541–54. doi: 10.1016/0012-1606(88)90399-5. [DOI] [PubMed] [Google Scholar]
- 148.Cho E, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–50. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 149.Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–9. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 150.Willecke M, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 151.MacDougall N, et al. Merlin, the Drosophila homologue of neurofibromatosis-2, is specifically required in posterior follicle cells for axis formation in the oocyte. Development. 2001;128:665–73. doi: 10.1242/dev.128.5.665. [DOI] [PubMed] [Google Scholar]
- 152.Milton CC, Zhang X, Albanese NO, Harvey KF. Differential requirement of Salvador-Warts-Hippo pathway members for organ size control in Drosophila melanogaster. Development. 2010;137:735–43. doi: 10.1242/dev.042309. [DOI] [PubMed] [Google Scholar]
- 153.Pellock BJ, Buff E, White K, Hariharan IK. The Drosophila tumor suppressors Expanded and Merlin differentially regulate cell cycle exit, apoptosis, and Wingless signaling. Dev Biol. 2007;304:102–15. doi: 10.1016/j.ydbio.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yu J, Poulton J, Huang YC, Deng WM. The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS ONE. 2008;3:e1761. doi: 10.1371/journal.pone.0001761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG. The Neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development. 2000;127:1315–1324. doi: 10.1242/dev.127.6.1315. [DOI] [PubMed] [Google Scholar]
- 156.von Gise A, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–9. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–5. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mikeladze-Dvali T, et al. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–87. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 160.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 161.Oh H, et al. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 2013;3:309–18. doi: 10.1016/j.celrep.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nagaraj R, et al. Control of mitochondrial structure and function by the Yorkie/YAP oncogenic pathway. Genes Dev. 2012;26:2027–37. doi: 10.1101/gad.183061.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kaneko KJ, Depamphilis ML. TEAD4 establishes the energy homeostasis essential for blastocoel formation. Development. 2013;140:3680–90. doi: 10.1242/dev.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bhat KP, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes Dev. 2011;25:2594–609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Imanaka Y, et al. MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J Hum Genet. 2011;56:270–6. doi: 10.1038/jhg.2011.1. [DOI] [PubMed] [Google Scholar]
- 166.Kawahara M, et al. Kpm/Lats2 is linked to chemosensitivity of leukemic cells through the stabilization of p73. Blood. 2008;112:3856–66. doi: 10.1182/blood-2007-09-111773. [DOI] [PubMed] [Google Scholar]
- 167.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–38. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 168.Huo X, et al. Overexpression of Yes-associated protein confers doxorubicin resistance in hepatocellullar carcinoma. Oncol Rep. 2013;29:840–6. doi: 10.3892/or.2012.2176. [DOI] [PubMed] [Google Scholar]
- 169.Urtasun R, et al. Connective tissue growth factor autocriny in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology. 2011;54:2149–58. doi: 10.1002/hep.24587. [DOI] [PubMed] [Google Scholar]
- 170.Juric V, Chen CC, Lau LF. Fas-mediated apoptosis is regulated by the extracellular matrix protein CCN1 (CYR61) in vitro and in vivo. Molecular and cellular biology. 2009;29:3266–79. doi: 10.1128/MCB.00064-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Piccolo S, Cordenonsi M, Dupont S. Molecular Pathways: YAP and TAZ Take Center Stage in Organ Growth and Tumorigenesis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013 doi: 10.1158/1078-0432.CCR-12-3172. [DOI] [PubMed] [Google Scholar]
- 172.Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Seminars in cancer biology. 2012;22:396–403. doi: 10.1016/j.semcancer.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang N, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yin F, et al. Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell. 2013;154:1342–55. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Asthagiri AR, et al. Neurofibromatosis type 2. Lancet. 2009;373:1974–86. doi: 10.1016/S0140-6736(09)60259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131:606–15. doi: 10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- 177.Murakami H, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–83. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- 178.Hall CA, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–25. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baldwin C, Garnis C, Zhang L, Rosin MP, Lam WL. Multiple microalterations detected at high frequency in oral cancer. Cancer Res. 2005;65:7561–7. doi: 10.1158/0008-5472.CAN-05-1513. [DOI] [PubMed] [Google Scholar]
- 180.Snijders AM, et al. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene. 2005;24:4232–42. doi: 10.1038/sj.onc.1208601. [DOI] [PubMed] [Google Scholar]
- 181.Modena P, et al. Identification of tumor-specific molecular signatures in intracranial ependymoma and association with clinical characteristics. J Clin Oncol. 2006;24:5223–33. doi: 10.1200/JCO.2006.06.3701. [DOI] [PubMed] [Google Scholar]
- 182.Jiang Z, et al. Promoter hypermethylation-mediated down-regulation of LATS1 and LATS2 in human astrocytoma. Neurosci Res. 2006;56:450–8. doi: 10.1016/j.neures.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 183.Takahashi Y, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–5. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 184.Seidel C, et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog. 2007;46:865–71. doi: 10.1002/mc.20317. [DOI] [PubMed] [Google Scholar]
- 185.Li H, et al. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver international : official journal of the International Association for the Study of the Liver. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang T, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–9. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 187.Wu H, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell reports. 2013;3:1663–77. doi: 10.1016/j.celrep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang J, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell. 2013;51:211–25. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Konsavage WM, Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730–9. doi: 10.1074/jbc.M111.327767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu-Chittenden Y, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Barry ER, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–10. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li VS, Clevers H. Intestinal regeneration: YAP-tumor suppressor and oncoprotein? Curr Biol. 2013;23:R110–2. doi: 10.1016/j.cub.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 193.Diep CH, et al. Down-regulation of Yes Associated Protein 1 expression reduces cell proliferation and clonogenicity of pancreatic cancer cells. PloS one. 2012;7:e32783. doi: 10.1371/journal.pone.0032783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lamar JM, et al. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci U S A. 2012;109:E2441–50. doi: 10.1073/pnas.1212021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhou Z, Zhu JS, Xu ZP, Zhang Q. Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits growth and induces apoptosis in the SGC7901 gastric cancer cell line. Molecular medicine reports. 2011;4:1075–82. doi: 10.3892/mmr.2011.543. [DOI] [PubMed] [Google Scholar]
- 196.Wang X, Su L, Ou Q. Yes-associated protein promotes tumour development in luminal epithelial derived breast cancer. European journal of cancer. 2012;48:1227–34. doi: 10.1016/j.ejca.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 197.Chen D, et al. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nature medicine. 2012;18:1511–7. doi: 10.1038/nm.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lavado A, et al. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development. 2013 doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–18. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Qin H, et al. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Hum Mol Genet. 2012;21:2054–67. doi: 10.1093/hmg/dds023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Qin H, et al. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Human molecular genetics. 2012;21:2054–67. doi: 10.1093/hmg/dds023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Fellous TG, et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–63. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- 204.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1431–6. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xu MZ, et al. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229–40. doi: 10.1038/onc.2010.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Neesse A, et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12325–30. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang J, et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444–50. doi: 10.1038/ncb1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Yang N, et al. TAZ induces growth factor-independent proliferation through activation of EGFR ligand amphiregulin. Cell cycle. 2012;11:2922–30. doi: 10.4161/cc.21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu AM, Xu MZ, Chen J, Poon RT, Luk JM. Targeting YAP and Hippo signaling pathway in liver cancer. Expert opinion on therapeutic targets. 2010;14:855–68. doi: 10.1517/14728222.2010.499361. [DOI] [PubMed] [Google Scholar]
- 212.Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nature reviews Drug discovery. 2002;1:309–15. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 213.Anand R, et al. Toward the development of a potent and selective organoruthenium mammalian sterile 20 kinase inhibitor. Journal of medicinal chemistry. 2009;52:1602–11. doi: 10.1021/jm8005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ghose AK, Herbertz T, Pippin DA, Salvino JM, Mallamo JP. Knowledge based prediction of ligand binding modes and rational inhibitor design for kinase drug discovery. Journal of medicinal chemistry. 2008;51:5149–71. doi: 10.1021/jm800475y. [DOI] [PubMed] [Google Scholar]
- 215.Norman RA, Toader D, Ferguson AD. Structural approaches to obtain kinase selectivity. Trends in pharmacological sciences. 2012;33:273–8. doi: 10.1016/j.tips.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 216.Chen L, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Li Z, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24:235–40. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tian W, Yu J, Tomchick DR, Pan D, Luo X. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci U S A. 2010;107:7293–8. doi: 10.1073/pnas.1000293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–96. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 221.Bao Y, et al. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- 222.Oudhoff MJ, et al. Control of the Hippo Pathway by Set7-Dependent Methylation of Yap. Developmental cell. 2013 doi: 10.1016/j.devcel.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 223.Hata S, et al. A novel acetylation cycle of transcription co-activator Yes-associated protein that is downstream of Hippo pathway is triggered in response to SN2 alkylating agents. The Journal of biological chemistry. 2012;287:22089–98. doi: 10.1074/jbc.M111.334714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mao B, et al. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2013 doi: 10.1038/onc.2013.88. [DOI] [PubMed] [Google Scholar]