Abstract
Single nucleotide polymorphisms (SNPs) occurring in noncoding sequences have largely been ignored in genome-wide association studies (GWAS). Yet, amounting evidence suggests that many noncoding SNPs especially those that are in the vicinity of protein coding genes play important roles in shaping chromatin structure and regulate gene expression and, as such, are implicated in a wide variety of diseases. One of such regulatory SNPs (rSNPs) is the E-cadherin (CDH1) promoter −160C/A SNP (rs16260) which is known to affect E-cadherin promoter transcription by displacing transcription factor binding and has been extensively scrutinized for its association with several diseases especially malignancies. Findings from studying this SNP highlight important clinical relevance of rSNPs and justify their inclusion in future GWAS to identify novel disease causing SNPs.
1. Introduction
Genetic variation contributes to virtually every human disease, conferring susceptibility or resistance or influencing interaction with environmental factors [1]. The most common type of human genetic variation is single nucleotide polymorphism (SNP), where two alternative bases occur at appreciable frequency (>1%) in the human population [2]. As of NCBI dbSNP Build 141 (http://www.ncbi.nlm.nih.gov/SNP/), there are about 43 million validated SNPs in human genome occurring about once in every 72 basepairs (bp). While much focus has been given to SNPs in coding sequences in genome-wide association studies (GWAS), the role of noncoding SNPs, which count more than coding SNPs, is much less studied. Many such noncoding SNPs that reside in the noncoding sequences (e.g., promoters, enhancers, and 3′ termini) surrounding protein coding genes have been shown to have profound effects on the expression of neighboring genes and can cause disease phenotypes [3, 4] and are thus called regulatory SNPs (rSNPs) [5, 6].
In 2000, when we were mapping DNA methylation in the CpG island region of the E-cadherin promoter in cancer samples using the bisulfite genomic sequencing technique [7], we accidently identified a novel C/A polymorphic site at the −160 location of the E-cadherin promoter within the mapped region. Further molecular characterization revealed that the two alleles confer the E-cadherin promoter different transcriptional activities. Since then, this SNP (reference SNP accession rs16260) has been extensively scrutinized for its association with different types of cancer and several noncancerous diseases (Table 1) by worldwide groups including our own [8, 9]. In this review, we summarize data accumulated in the past 13 years on the association of the E-cadherin −160C/A SNP with human conditions and highlight the important function of rSNPs as a risk factor for diseases. Nevertheless, this review is not intended to serve as a meta-analysis, many of which have already been published [10–13].
Table 1.
Association of E-cadherin −160C/A SNP and diseases.
| Disease | Case/control | Ethnicity | Positive association | No association |
|---|---|---|---|---|
| Gastric cancer | 505/246 | Italian | [44] | |
| 239/343 | Chinese | [59] | ||
| 387/392 | Chinese | [69] | ||
| 107/134 | Chinese | [57] | ||
| 201/196 | Chinese | [70] | ||
| 245/950 | European | [71] | ||
| 39/78 | Mexican | [72] | ||
| 153/303 | Japanese | [73] | ||
| 106/90 | Japanese | [46] | ||
| 53/70 | Italian | [74] | ||
| 192/170 | Omani | [75] | ||
| 412/408 | Italian | [76] | ||
| 572/589 | Chinese | [68] | ||
| 206/261 | Chinese | [77] | ||
| 292/146 | Korean | [78] | ||
| 433/466 | Canadian, German, Portuguese | [79] | ||
|
| ||||
| Prostate cancer | 82/188 | Dutch | [52] | |
| 1036/669 | Swedish | [80] | ||
| 183/168 | Slovenian | [81] | ||
| 200/159 | Japanese | [82] | ||
| 236/209 | Japanese | [83] | ||
| 801/1636 | Swedish | [84] | ||
| 86/126 | Caucasian | [9] | ||
| 49/117 | African American | |||
| 219/102 | European American | [85] | ||
| 119/112 | African American | [85] | ||
| 89/123 | Jamaicans | |||
| 98/0 | Caucasian | [86] | ||
| 219/219 | Japanese | [54] | ||
|
| ||||
| Bladder/urothelial cancer | 50/50 | Chinese | [8] | |
| 180/100 | Chinese | [53] | ||
| 314/314 | Japanese | [54] | ||
| 197/344 | Dutch | [55] | ||
| 302/0 | Caucasian, African American, Hispanic | [56] | ||
|
| ||||
| Colorectal cancer | 194/220 | German | [87] | |
| 5679/5412 | British | [88] | ||
| 130/130 | Brazilian | [58] | ||
| 505/246 | Italian | [44] | ||
| 334/171 | British | [89] | ||
|
| ||||
| Pancreatic cancer | Chinese | [90] | ||
|
| ||||
| Nasopharyngeal cancer | 302/140 | Tunisian | [60] | |
|
| ||||
| Ovarian cancer | 207/256 | Chinese | [91] | |
|
| ||||
| Renal cancer | 526/514 | Polish | [92] | |
|
| ||||
| Lung cancer | 95/85 | Chinese | [93] | |
|
| ||||
| Hepatocellular carcinoma | 131/347 | Chinese | [94] | |
| 93/0 | Chinese | [95] | ||
|
| ||||
| Benign diseases | ||||
|
| ||||
| Endometriosis | 505/246 | Italian | [44] | |
| 715/370 | Indian | [47] | ||
| 511/498 | Japanese | [61] | ||
| 152/189 | Chinese | [96] | ||
|
| ||||
| Nonsyndromic orofacial clefts (NSOC) | 140/107 | Chinese | [97] | |
|
| ||||
| Asthma | 299/383 | Asian | [63] | |
|
| ||||
| Nephrolithiasis | 127/152 | Chinese | [64] | |
|
| ||||
| PI-IBS | 228/581 | Caucasians | [65] | |
2. Regulatory Variants and Gene Expression
Unlike coding SNPs that either cause a change in amino acid sequences or do nothing, rSNPs may have an effect on the level of transcription of neighboring genes. Multiple mechanisms can be attributed to such effect including affecting binding affinity of protein transcription factor or altering promoter methylation [14]. It is also likely that rSNPs affect sequence specific binding of nonprotein transcriptional factor such as noncoding RNA. In this regard, it has recently been shown that miRNAs and long noncoding RNAs (ncRNAs) can regulate gene transcription or chromatin structure in a sequence-dependent fashion [15]. Some rSNPs have such a profound effect on gene transcription so as to create a new transcriptional promoter which directly contributes to the etiology of α-thalassemia, a genetic disease [16].
Normal variation in gene expression is common among individuals and can be attributed to genetic factors [17]. However, the underlying molecular mechanisms have remained unclear until recently when several genome-wide studies highlight the importance of regulatory variants in affecting gene expression by altering transcription factor binding and chromatin structure [18–21]. Epigenetic code has been known to underlie critical biological processes ranging from development, differentiation, and disease. However the fundamental question that remains unanswered is how epigenetic code per se is established and regulated [22]. After all, genetics still underlie epigenetic mechanisms of gene regulation. By combinatorial analysis of gene expression data and binding profiles of NFκB and RNA polymerase II (RNAP II), Kasowski et al. found extensive contribution of genetic variation to variation in TF binding, many of which can affect gene expression and are thus functional [18]. Similarly, McDaniell et al. found that individual-specific and allele-specific variation in chromatin structure and transcription factor binding can be transmitted from parents to children as a result of genetic variation [19]. Very recently, Kasowski et al. and Kilpinen et al. further showed that the mechanism underlying chromatin variation resulting from genetic variability is mainly through disrupting TF binding [20, 21].
3. The Function of E-Cadherin Gene
Epithelia are essential and abundant tissues in most eukaryotic organs, and over 90% of the malignant human tumors are derived from epithelia [23]. Development of malignant tumors is in part characterized by the ability of tumor cells to overcome cell-cell adhesion and to invade surrounding tissues [24]. E-cadherin, one of the classic cadherins, playing a major role in the establishment and maintenance of intercellular adhesion, cell polarity, and tissue architecture [25], has been implicated in carcinogenesis because it is frequently lost or downregulated in human epithelial cancers including prostate, breast, bladder, pancreas, stomach, and colon tumors [26–30]. Compelling evidence also indicates that E-cadherin is a potent tumor invasion suppressor [24, 31] by inhibiting epithelial to mesenchymal transition (EMT) [32].
The molecular mechanisms underlying the loss of E-cadherin expression in carcinomas are not fully understood. Somatic mutations in the E-cadherin gene have been identified in diffuse gastric carcinomas [33] and lobular breast carcinomas [34] and in a small proportion of gynecologic cancers [35]. However, in the majority of cancers, where E-cadherin expression is downregulated, the molecular mechanisms underlying this defect are still poorly understood. A major mechanism leading to the decrease in E-cadherin expression seems to result from a decrease in transcription [24, 36, 37], since mutations within the E-cadherin coding sequence have been reported as rare in breast, gastric, and gynecological cancers [34]. Additionally, inactivation of E-cadherin has been associated with hypermethylation of CpG islands within the proximal promoter region of the E-cadherin gene in a number of human cancers [7, 38, 39].
Dysfunction of E-cadherin has also been associated with a number of nonmalignant diseases such as ulcerative and Crohn's colitis, Langerhans' cell histiocytosis, endometriosis, and autosomal dominant polycystic kidney disease [40, 41].
4. E-Cadherin −160C/A SNP Affects E-Cadherin Transcriptional Activity
The E-cadherin −160C/A SNP is located at the −160 location relative to the transcription start site (TSS) of E-cadherin. Cloning the two alleles into the upstream of a promoterless luciferase reporter gene revealed that the A allele decreases transcriptional activity by 68% compared with the C allele in a reporter gene analysis, suggesting that the A allele may reduce E-cadherin expression in vivo [42]. This finding is supported by other studies that reported similar reduced transcriptional activity from the A allele [43, 44]. Based on footprinting and gel shift assays, the −160 site is probably bound by two protein complexes and the two alleles have very different binding affinity for nuclear proteins with the C allele bound by more proteins than the A allele as revealed by gel shift assay. Footprinting assay confirmed that only the C allele is protected from DNase digestion at the polymorphic site. The protected region contains a 7-nucleotide sequence which may be the binding site for unknown transcription factors that are required for achieving higher transcriptional activity (Figure 1).
Figure 1.
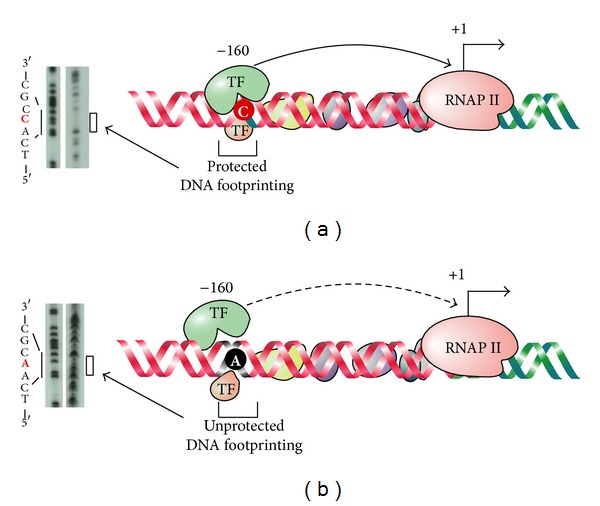
rSNPs regulate gene transcription by affecting transcription factor (TF) binding. rSNPs in regulatory sequences such as gene promoters may affect gene expression at the transcriptional level and this regulation is mainly realized through affecting transcription factor binding. In the example of −160C/A SNP in E-cadherin promoter, the −160 location is the binding site of putative TFs. The C allele of this site allows for binding of the TFs, as evidenced by a protected footprint on DNA footprinting assay, leading to active transcription of E-cadherin gene (a), whereas the A allele prevents the TFs from binding likely due to steric hindrance, resulting in the loss of footprint of the TFs and attenuated transcription (b) [42].
By bioinformatics analyses using the TFSEARCH and TESS databases, Borges Bdo et al. identified putative binding sites at the −160 location for RAR-β, ER-α, AP-1, StuAp, and CF-1. When the −160 C is changed to A, the binding site for CF-1 is eliminated and a putative de novo binding site is created for two transcription factors: RC2 and MCBF [45].
The decreased transcriptional activity from the A allele may be explained as the result of structure differences between the A and the C alleles, which hinders the access of DNA by transcription factors. However, the change of a cytosine to an adenosine in the DNA structure does not abandon the binding completely (Figure 1).
By analyzing E-cadherin protein expression in tissue samples, Kuraoka et al. showed that samples with C/C genotype have higher E-cadherin protein expression than C/A genotype [46], despite the fact that CC genotype is associated with higher risk of gastric cancer [46]. Similarly, expression of E-cadherin protein as assessed by immunohistochemistry and western blotting is lower in endometrium tissues of endometriosis patients carrying the A allele [47]. There is, so far, no enough evidence to indicate that the two alleles have an impact on E-cadherin expression in vivo. Further studies are needed to verify whether this SNP has an impact on E-cadherin expression in vivo.
5. E-Cadherin rSNP and DNA Methylation
It has been reported that SNPs can alter CpG methylation [48–50], representing one of the mechanisms that link genetic alternations to epigenetic changes. This view is corroborated by a recent genome-wide DNA methylation mapping study in which differentially methylated regions (DMRs) are found to contain enriched SNPs associated with cell-type related diseases revealed by GWAS [14]. Although the exact mechanism is unknown, differential protein/transcription factor binding can presumably contribute to the differential methylation profiles between different alleles, especially when a SNP occurs within a CpG site. In this regard, Borges Bdo et al. correlated −160C/A alleles with DNA methylation status in Brazilian gastric cancer patients and found that the −160A allele is positively associated with hypermethylation at the E-cadherin promoter and also with increased risk of developing gastric cancer [45]. However, in another study of Japanese gastric patients, the C/C genotype was found to be associated with higher risk of gastric cancer and higher E-cadherin expression but not associated with E-cadherin promoter hypermethylation [46]. This discrepancy might have arisen from disease stages/grades and the ages of the patients since those variables are known to be determinants of promoter hypermethylation [51].
6. E-Cadherin −160C/A SNP Genotype Frequency in General Populations
Based on data from the 1000 genome phase I population consisting of 1094 worldwide individuals, the global minor allele frequency (MAF) for A in the −160C/A SNP is 0.2323. The frequency varies considerably among ethnic groups with the lowest A allele frequency of 5.3% found in an Asian population (Coriell Cell Repositories, 38 chromosome counts) and the second lowest of 10.2% found in a population of African ancestry in southwest USA (98 chromosome counts). The highest A allele frequencies of 32.6% and 31.0% are found, respectively, in the Hispanics (46 chromosome counts) and Centre d'Etude du Polymorphisme Humain (CEPH) pedigrees [UTAH (93%), French (4%), and Venezuelan (3%)] (chromosome counts 184).
7. E-Cadherin −160C/A SNP and Cancer
The association of −160C/A SNP with various types of cancer has been extensively studied. As of April, 2014, there are at least 49 case-control studies examining the association of this SNP with gastric, prostate, bladder, breast, colorectal, nasopharyngeal, endometrial, pancreatic, cervical, lung, oral, liver, thyroid, and ovarian cancer and lymphoma (Table 1). At least 15 meta-analysis studies have been published with the most recent one summarizing 47 cancer-related case-control studies [10]. Results from these studies reveal that −160 SNP is a cancer type specific and also ethnicity specific risk factor.
7.1. E-Cadherin −160C/A SNP and Urological Cancer of the Prostate and the Bladder
The first-ever study associating −160C/A SNP with cancer risk was published in 2002 [52]. The authors genotyped 82 patients with localized prostate cancer including 57 with sporadic prostate cancer and 25 with hereditary prostate cancer and 188 controls from a Dutch population and found that carriers of the A had a 3.6-fold increased risk for prostate cancer compared to C-only carriers. Interestingly, heterozygous (CA) genotypes had an almost 4-fold increased risk of prostate cancer compared to CC genotype whereas homozygous (AA) had only a 1.7-fold increased risk. In addition, the A allele and AA/CA genotypes render less risk for hereditary prostate cancer than for sporadic prostate cancer. This first study was then followed by 9 others examining a total of 3,570 cases and 3,304 controls as summarized in the meta-analysis by Wang et al. [10]. These studies have found that the A allele is associated with higher risk for prostate cancer in the Europeans (OR = 1.56; 95% CI = 1.16–2.08) and Asians (OR = 1.10; 95% CI = 0.86–1.41), but not in black and white Americans [10].
Three case-control studies have observed that the A allele of E-cadherin C/A SNP confers higher risk for bladder cancer in the Chinese [53], Japanese [54], and Dutch [55] and is associated with invasive cancer [53]. Of particular note is a clinical outcome study following 302 patients with superficial bladder cancer after transurethral resection of the tumors for a median follow-up of 27.65 months [56]. Among 274 Caucasians in the cohort, 50% developed recurrence during the follow-up period. Compared to patients with CC genotype, patients carrying at least one A allele had a 32% reduction in recurrence risk (adjusted HR 0.68; 95% CI 0.48–0.96).
7.2. E-Cadherin −160C/A SNP and Gastrointestinal Tract Cancer
E-cadherin −160C/A SNP has been studied most intensively in gastric cancer resulting in at least 15 case-control and 6 meta-analysis studies. Findings from these studies suggest that −160C/A SNP is an ethnical dependent risk factor for gastric cancer. Interestingly, in Asian population, this SNP may be reversely associated with gastric cancer risk with the A allele possessing a protective effect on developing gastric cancer [46]. However, a recent study directly sequencing 167 gastric cancer (107 diffuse and 60 intestinal) cases and 134 controls in a Chinese population found that the −160 A allele was significantly higher in diffuse gastric cancer cases (OR 1.75, 95% CI, 1.014–3.022) [57].
7.3. E-Cadherin − 160C/A SNP and Cancer Metastasis
In a Brazilian study, the AA genotype is associated with a higher risk of metastatic disease at diagnosis (OR 3.43; 95% CI 1.27–9.27; P = 0.023) [58]. In a Japanese population of 106 gastric cancer cases, which had a higher CC genotype frequency compared to controls, patients positive for lymph node metastasis had a further higher CC genotype frequency than those without metastasis (OR 2.86; 95% CI 1.28–6.36; P = 0.01) [46]. The CC genotype in cases is significantly associated with poorly differentiated adenocarcinoma, deep invasion, and lymph node metastasis [46]. However, other studies could not identify an association of −160C/A SNP with lymphatic metastasis in esophageal squamous cell carcinoma, gastric cardia adenocarcinoma, [59] and nasopharyngeal cancer [60].
8. E-Cadherin −160C/A SNP and Noncancerous Diseases
While most studies on the −160C/A SNP focused on cancer, a few have examined its association with noncancerous diseases including orofacial clefts, asthma, urolithiasis, endometriosis, and infection. Song et al. genotyped 140 nonsyndromic orofacial clefts (NSOC) cases and 107 healthy individuals from a Chinese Han population. Although there is lack of association between this SNP and overall risk of NSOC, when all cleft cases were stratified into four groups (i.e., cleft lip with or without cleft palate, cleft lip only, cleft lip with cleft palate, and cleft palate only), the −160C/A SNP overall genotype frequencies in cleft palate only (CPO) groups were significantly different from those in the controls (P = 0.004) and AA genotype significantly increased the risk of CPO by 5.90-fold (OR 6.90; 95% CI 1.47–32.40), suggesting that E-cadherin activity may contribute to etiology of CPO.
Govatati et al. [47] studied the association of −160 SNP with endometriosis in Indian women (715 cases and 500 controls) and found that the −160A/A frequencies are higher in cases than in control (P < 0.0019). In another case-control study performed in Japanese women (520 cases and 520 healthy controls), no such association, however, was found [61].
It is known that levels of E-cadherin can affect airway remodeling which is a feature of chronic asthma and is characterized by an increased turnover of cells and extracellular matrix [62]. Very recently, Wang et al. studied the effects of environmental tobacco smoke (ETS) and E-cadherin −160C/A SNP on the risk of developing childhood asthma in 299 asthmatic children and 383 healthy controls. They found that EST exposure to more than 5 cigarettes/day and the presence of CDH1 AA/CA genotypes had a significantly increased risk for childhood asthma (OR 1.53; 95% CI 1.08–2.17), suggesting a role of gene and environment interactions in asthma risk [63].
In a hospital-based case-control study of 127 nephrolithiasis patients and 152 controls, Tan et al. genotyped the −160C/A SNP and found that CA/AA genotypes are associated with a significantly decreased risk of nephrolithiasis (OR = 0.53; 95% CI = 0.32–0.87), compared with the CC genotype. The association is even greater among subgroups of BMI > 24 kg/m2 (OR = 0.38; 95% CI = 0.17–0.85), age ≤ 57 years (OR = 0.47; 95% CI = 0.24–0.93), and men (OR = 0.56; 95% CI = 0.29–0.99) [64].
Genetic variation is known to affect susceptibility to infection. In an effort to examine genetic risk factors for postinfectious irritable bowel syndrome (PI-IBS), Villani et al. genotyped 71 functional variants including −160C/A SNP which, among the other 2, is an independent risk factor for developing PI-IBS [65]. Since E-cadherin is a transmembrane glycoprotein which forms the tight junctions with apical junctional complex which provides intestinal barrier function, decreased E-cadherin expression may contribute to PI-IBS symptoms by increasing intestinal permeability.
9. Concluding Remarks
Results from recent genome-wide sequencing analysis highlight the importance of rSNPs in modulating neighboring gene expression by affecting transcription factor binding and chromatin structure [20, 21]. Intensive studies in the past decade on the E-cadherin −160C/A rSNP have revealed that this rSNP can modify the risk of a number of diseases, especially gastric, prostate, and bladder cancer. In certain tumor types and ethnical groups, however, there are inconsistent results regarding the effect of the A allele on disease risk. It is possible that other nearby rSNPs in haplotype with −160C/A could mask the effect of the latter. In this regard, additional SNPs in the E-cadherin promoter have been reported such as the −347G/GA which could also modify promoter transcriptional activity and disease risk [66–68]. Future GWAS studies that include the −160 rSNPs as well as others in E-cadherin promoter are needed to further clarify the functional role of E-cadherin −160C/A SNP in diseases.
Conflict of Interests
No competing financial interests exist.
References
- 1.Collins FS, Guyer MS, Charkravarti A. Variations on a theme: cataloging human DNA sequence variation. Science. 1997;278(5343):1580–1581. doi: 10.1126/science.278.5343.1580. [DOI] [PubMed] [Google Scholar]
- 2.Wang DG, Fan JB, Siao CJ, et al. Large-scale identification, mapping, and genotyping of single-nucleotide polymorphisms in the human genome. Science. 1998;280(5366):1077–1082. doi: 10.1126/science.280.5366.1077. [DOI] [PubMed] [Google Scholar]
- 3.Schaub MA, Boyle AP, Kundaje A, Batzoglou S, Snyder M. Linking disease associations with regulatory information in the human genome. Genome Research. 2012;22(9):1748–1759. doi: 10.1101/gr.136127.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molineris I, Schiavone D, Rosa F, Matullo G, Poli V, Provero P. Identification of functional cis-regulatory polymorphisms in the human genome. Human Mutation. 2013;34(5):735–742. doi: 10.1002/humu.22299. [DOI] [PubMed] [Google Scholar]
- 5.Knight JC, Keating BJ, Rockett KA, Kwiatkowski DP. In vivo characterization of regulatory polymorphisms by allele-specific quantification of RNA polymerase loading. Nature Genetics. 2003;33(4):469–475. doi: 10.1038/ng1124. [DOI] [PubMed] [Google Scholar]
- 6.Hudson TJ. Wanted: regulatory SNPs. Nature Genetics. 2003;33:439–440. doi: 10.1038/ng0403-439. [DOI] [PubMed] [Google Scholar]
- 7.Li LC, Zhao H, Nakajima K. Methylation of the E-cadherin gene promoter correlates with progression of prostate cancer. Journal of Urology. 2001;166(2):705–709. [PubMed] [Google Scholar]
- 8.Zhang X, Ma X, Zhu QG, Li LC, Chen Z, Ye ZQ. Association between a C/A single nucleotide polymorphism of the E-cadherin gene promoter and transitional cell carcinoma of the bladder. The Journal of Urology. 2003;170(4, part 1):1379–1382. doi: 10.1097/01.ju.0000084297.43710.e9. [DOI] [PubMed] [Google Scholar]
- 9.Pookot D, Li LC, Tabatabai ZL, Tanaka Y, Greene KL, Dahiya R. The E-cadherin-160 C/A polymorphism and prostate cancer risk in white and black American men. The Journal of Urology. 2006;2006(2):793–796. doi: 10.1016/j.juro.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Wang G, Lu C, Feng B, Kang J. Contribution of the -160C/A polymorphism in the E-cadherin promoter to cancer risk: a meta-analysis of 47 case-control studies. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0040219.e40219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geng P, Chen Y, Ou J, Yin X, Sa R, Liang H. The E-cadherin (CDH1)-C160A polymorphism and colorectal cancer susceptibility: a meta-analysis. DNA and Cell Biology. 2012;2012(6):1070–1077. doi: 10.1089/dna.2011.1380. [DOI] [PubMed] [Google Scholar]
- 12.Cui Y, Xue H, Lin B, Ni P, Fang JY. A meta-analysis of CDH1 C-160A genetic polymorphism and gastric cancer risk. DNA and Cell Biology. 2011;30(11):937–945. doi: 10.1089/dna.2011.1257. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Kong CZ, Zhang Z, Yang CM, Li J. Role of CDH1 promoter polymorphism and DNA methylation in bladder carcinogenesis: a meta-analysis. DNA and Cell Biology. 2014;33(4):205–216. doi: 10.1089/dna.2013.2100. [DOI] [PubMed] [Google Scholar]
- 14.Ziller MJ, Gu H, Muller F, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang V, Place RF, Portnoy V, et al. Upregulation of Cyclin B1 by miRNA and its implications in cancer. Nucleic Acids Research. 2012;40(4):1695–1707. doi: 10.1093/nar/gkr934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Gobbi M, Viprakasit V, Hughes JR, et al. A regulatory SNP causes a human genetic disease by creating a new transcriptional promoter. Science. 2006;312(5777):1215–1217. doi: 10.1126/science.1126431. [DOI] [PubMed] [Google Scholar]
- 17.Cheung VG, Spielman RS. Genetics of human gene expression: mapping DNA variants that influence gene expression. Nature Reviews Genetics. 2009;10(9):595–604. doi: 10.1038/nrg2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasowski M, Grubert F, Heffelfinger C, et al. Variation in transcription factor binding among humans. Science. 2010;328(5975):232–235. doi: 10.1126/science.1183621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDaniell R, Lee BK, Song L. Heritable individual-specific and allele-specific chromatin signatures in humans. Science. 2010;328(5975):235–239. doi: 10.1126/science.1184655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasowski M, Kyriazopoulou-Panagiotopoulou S, Grubert F, et al. Extensive variation in chromatin states across humans. Science. 2013;342(6159):750–752. doi: 10.1126/science.1242510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilpinen H, Waszak SM, Gschwind AR, et al. Coordinated effects of sequence variation on DNA binding, chromatin structure, and transcription. Science. 2013;342(6159):744–747. doi: 10.1126/science.1242463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LC. Chromatin remodeling by the small RNA machinery in mammalian cells. Epigenetics. 2014;9(1):45–52. doi: 10.4161/epi.26830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 24.Frixen UH, Behrens J, Sachs M, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. The Journal of Cell Biology. 1991;113(1):173–185. doi: 10.1083/jcb.113.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. The Journal of Cell Biology. 1994;127(1):235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Umbas R, Schalken JA, Aalders TW, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Research. 1992;52(18):5104–5109. [PubMed] [Google Scholar]
- 27.Gamallo C, Palacios J, Suarez A, et al. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. American Journal of Pathology. 1993;142(4):987–993. [PMC free article] [PubMed] [Google Scholar]
- 28.Pignatelli M, Ansari TW, Gunter P. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. The Journal of Pathology. 1994;174(4):243–248. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 29.Mayer B, Johnson JP, Leitl F, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Research. 1993;53(7):1690–1695. [PubMed] [Google Scholar]
- 30.Kinsella AR, Lepts GC, Hill CL, Jones M. Reduced E-cadherin expression correlates with increased invasiveness in colorectal carcinoma cell lines. Clinical & Experimental Metastasis. 1994;12(4):335–342. doi: 10.1007/BF01753841. [DOI] [PubMed] [Google Scholar]
- 31.Efstathiou JA, Liu D, Wheeler JM, et al. Mutated epithelial cadherin is associated with increased tumorigenicity and loss of adhesion and of responsiveness to the motogenic trefoil factor 2 in colon carcinoma cells. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(5):2316–2321. doi: 10.1073/pnas.96.5.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gall TM, Frampton AE. Gene of the month: E-cadherin (CDH1) Journal of Clinical Pathology. 2013;66(11):928–932. doi: 10.1136/jclinpath-2013-201768. [DOI] [PubMed] [Google Scholar]
- 33.Becker KF, Atkinson MJ, Becker KF, Atkinson MJ, Reich U, et al. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Research. 1994;54(14):3845–3852. [PubMed] [Google Scholar]
- 34.Berx G, Cleton-Jansen AM, Nollet F, et al. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. The EMBO Journal. 1995;14(24):6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risinger JI, Berchuck A, Kohler MF, Boyd J. Mutations of the E-cadherin gene in human gynecologic cancers. Nature Genetics. 1994;7(1):98–102. doi: 10.1038/ng0594-98. [DOI] [PubMed] [Google Scholar]
- 36.Dorudi S, Sheffield JP, Poulsom R, Northover JM, Hart IR. E-cadherin expression in colorectal cancer. An immunocytochemical and in situ hybridization study. The American Journal of Pathology. 1993;142(4):981–986. [PMC free article] [PubMed] [Google Scholar]
- 37.Brabant G, Hoang-Vu C, Cetin Y, et al. E-cadherin: a differentiation marker in thyroid malignancies. Cancer Research. 1993;53(20):4987–4993. [PubMed] [Google Scholar]
- 38.Graff JR, Herman JG, Lapidus RG, et al. E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Research. 1995;55(22):5195–5199. [PubMed] [Google Scholar]
- 39.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(18):9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankowski JA, Bedford FK, Boulton RA, et al. Alterations in classical cadherins associated with progression in ulcerative and Crohn's colitis. Laboratory Investigation. 1998;78(9):1155–1167. [PubMed] [Google Scholar]
- 41.Gaetje R, Kotzian S, Herrmann G, Baumann R, Starzinski-Powitz A. Nonmalignant epithelial cells, potentially invasive in human endometriosis, lack the tumor suppressor molecule E-cadherin. The American Journal of Pathology. 1997;150(2):461–467. [PMC free article] [PubMed] [Google Scholar]
- 42.Li LC, Chui RM, Sasaki M. A single nucleotide polymorphism in the E-cadherin gene promoter alters transcriptional activities. Cancer Research. 2000;60(4):873–876. [PubMed] [Google Scholar]
- 43.Nakamura A, Shimazaki T, Kaneko K, et al. Characterization of DNA polymorphisms in the E-cadherin gene (CDH1) promoter region. Mutation Research. 2002;502(1-2):19–24. doi: 10.1016/s0027-5107(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 44.Cattaneo F, Venesio T, Molatore S. Functional analysis and case-control study of -160C/A polymorphism in the E-cadherin gene promoter: association with cancer risk. Anticancer Research. 2006;26(6B):4627–4632. [PubMed] [Google Scholar]
- 45.Borges Bdo N, Santos Eda S, Bastos CE, et al. Promoter polymorphisms and methylation of E-cadherin (CDH1) and KIT in gastric cancer patients from northern Brazil. Anticancer Research. 2010;30(6):2225–2233. [PubMed] [Google Scholar]
- 46.Kuraoka K, Oue N, Yokozaki H, et al. Correlation of a single nucleotide polymorphism in the E-cadherin gene promoter with tumorigenesis and progression of gastric carcinoma in Japan. International Journal of Oncology. 2003;23(2):421–427. [PubMed] [Google Scholar]
- 47.Govatati S, Tangudu NK, Deenadayal M, Chakravarty B, Shivaji S, Bhanoori M. Association of E-cadherin single nucleotide polymorphisms with the increased risk of endometriosis in Indian women. Molecular Human Reproduction. 2012;18(5):280–287. doi: 10.1093/molehr/gar079. [DOI] [PubMed] [Google Scholar]
- 48.Dayeh TA, Olsson AH, Volkov P, Almgren P, Rönn T, Ling C. Identification of CpG-SNPs associated with type 2 diabetes and differential DNA methylation in human pancreatic islets. Diabetologia. 2013;56(5):1036–1046. doi: 10.1007/s00125-012-2815-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savio AJ, Lemire M, Mrkonjic M, et al. MLH1 region polymorphisms show a significant association with CpG island shore methylation in a large cohort of healthy individuals. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0051531.e51531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kloth M, Goering W, Ribarska T, Arsov C, Sorensen KD, Schulz WA. The SNP rs6441224 influences transcriptional activity and prognostically relevant hypermethylation of RARRES1 in prostate cancer. International Journal of Cancer. 2012;131(6):E897–E904. doi: 10.1002/ijc.27628. [DOI] [PubMed] [Google Scholar]
- 51.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. Journal of the National Cancer Institute. 2005;97(2):103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 52.Verhage BA, van Houwelingen K, Ruijter TE, Kiemeney LA, Schalken JA. Single-nucleotide polymorphism in the E-cadherin gene promoter modifies the risk of prostate cancer. International Journal of Cancer. 2002;100(6):683–685. doi: 10.1002/ijc.10541. [DOI] [PubMed] [Google Scholar]
- 53.Ma X, Xu H, Zheng T, et al. DNA polymorphisms in exon 1 and promoter of the CDH1 gene and relevant risk of transitional cell carcinoma of the urinary bladder. British Journal of Urology. 2008;102(5):633–636. doi: 10.1111/j.1464-410X.2008.07634.x. [DOI] [PubMed] [Google Scholar]
- 54.Tsukino H, Kuroda Y, Nakao H, et al. E-cadherin gene polymorphism and risk of urothelial cancer. Cancer Letters. 2003;195(1):53–58. doi: 10.1016/s0304-3835(03)00130-7. [DOI] [PubMed] [Google Scholar]
- 55.Kiemeney LA, van Houwelingen KP, Bogaerts M, et al. Polymorphisms in the E-cadherin (CDH1) gene promoter and the risk of bladder cancer. European Journal of Cancer. 2006;42(18):3219–3227. doi: 10.1016/j.ejca.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 56.Lin J, Dinney CP, Grossman HB, et al. E-cadherin promoter polymorphism (C-160A) and risk of recurrence in patients with superficial bladder cancer. Clinical Genetics. 2006;70(3):240–245. doi: 10.1111/j.1399-0004.2006.00666.x. [DOI] [PubMed] [Google Scholar]
- 57.Chu CM, Chen CJ, Chan DC, et al. CDH1 polymorphisms and haplotypes in sporadic diffuse and intestinal gastric cancer: a case-control study based on direct sequencing analysis. World Journal of Surgical Oncology. 2014;12, article 80 doi: 10.1186/1477-7819-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Lima JM, de Souza LG, da Silva ID, Forones NM. E-cadherin and metalloproteinase-1 and -7 polymorphisms in colorectal cancer. The International Journal of Biological Markers. 2009;24(2):99–106. doi: 10.1177/172460080902400206. [DOI] [PubMed] [Google Scholar]
- 59.Zhang XF, Wang YM, Ge H, et al. Association of CDH1 single nucleotide polymorphisms with susceptibility to esophageal squamous cell carcinomas and gastric cardia carcinomas. Diseases of the Esophagus. 2008;21(1):21–29. doi: 10.1111/j.1442-2050.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 60.Ben Nasr H, Hamrita B, Batbout M. A single nucleotide polymorphism in the E-cadherin gene promoter—160 C/A is associated with risk of nasopharyngeal cancer. Clinica Chimica Acta. 2010;411(17-18):1253–1257. doi: 10.1016/j.cca.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida K, Yoshihara K, Adachi S, et al. Possible involvement of the E-cadherin gene in genetic susceptibility to endometriosis. Human Reproduction. 2012;27(6):1685–1689. doi: 10.1093/humrep/des080. [DOI] [PubMed] [Google Scholar]
- 62.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Current Opinion in Cell Biology. 2005;17(5):459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 63.Wang MF, Kuo SH, Huang CH, et al. Exposure to environmental tobacco smoke, human E-cadherin C-160A polymorphism, and childhood asthma. Annals of Allergy, Asthma & Immunology. 2013;111(4):262–267. doi: 10.1016/j.anai.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Tan M, Xia S, Zhang Q, Zhu J, Bao E. The -160C>a polymorphism in e-cadherin is associated with the risk of nephrolithiasis. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0073109.e73109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villani AC, Lemire M, Thabane M, et al. Genetic risk factors for post-infectious irritable bowel syndrome following a waterborne outbreak of gastroenteritis. Gastroenterology. 2010;138(4):1502–1513. doi: 10.1053/j.gastro.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 66.Shin Y, Kim IJ, Kang HC. The E-cadherin −347G->GA promoter polymorphism and its effect on transcriptional regulation. Carcinogenesis. 2004;25(6):895–899. doi: 10.1093/carcin/bgh073. [DOI] [PubMed] [Google Scholar]
- 67.Zou XP, Dai WJ, Cao J. CDH1 promoter polymorphism (−347G→GA) is a possible prognostic factor in sporadic colorectal cancer. World Journal of Gastroenterology. 2009;15(42):5340–5345. doi: 10.3748/wjg.15.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B, Pan K, Liu Z, et al. Genetic polymorphisms of the E-cadherin promoter and risk of sporadic gastric carcinoma in Chinese populations. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(9):2402–2408. doi: 10.1158/1055-9965.EPI-08-0315. [DOI] [PubMed] [Google Scholar]
- 69.Zhan Z, Wu J, Zhang JF, et al. CDH1 gene polymorphisms, plasma CDH1 levels and risk of gastric cancer in a Chinese population. Molecular Biology Reports. 2012;39(8):8107–8113. doi: 10.1007/s11033-012-1658-0. [DOI] [PubMed] [Google Scholar]
- 70.Wu MS, Huang SP, Chang YT, et al. Association of the -160 C –> a promoter polymorphism of E-cadherin gene with gastric carcinoma risk. Cancer. 2002;94(5):1443–1448. doi: 10.1002/cncr.10371. [DOI] [PubMed] [Google Scholar]
- 71.Jenab M, McKay JD, Ferrari P, et al. CDH1 gene polymorphisms, smoking, Helicobacter pylori infection and the risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) European Journal of Cancer. 2008;44(6):774–780. doi: 10.1016/j.ejca.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 72.Medina-Franco H, Ramos-De la Medina A, Medina G, Medina-Franco JL. Single nucleotide polymorphisms in the promoter region of the E-cadherin gene in gastric cancer: case-control study in a young Mexican population. Annals of Surgical Oncology. 2007;14(8):2246–2249. doi: 10.1245/s10434-006-9054-4. [DOI] [PubMed] [Google Scholar]
- 73.Yamada H, Shinmura K, Ikeda S, et al. Association between CDH1 haplotypes and gastric cancer risk in a Japanese population. Scandinavian Journal of Gastroenterology. 2007;42(12):1479–1485. doi: 10.1080/00365520701478436. [DOI] [PubMed] [Google Scholar]
- 74.Humar B, Graziano F, Cascinu S, et al. Association of CDH1 haplotypes with susceptibility to sporadic diffuse gastric cancer. Oncogene. 2002;21(53):8192–8195. doi: 10.1038/sj.onc.1205921. [DOI] [PubMed] [Google Scholar]
- 75.Al-Moundhri MS, Al-Khanbashi M, Al-Kindi M, et al. Association of E-cadherin (CDH1) gene polymorphisms and gastric cancer risk. World Journal of Gastroenterology. 2010;16(27):3432–3436. doi: 10.3748/wjg.v16.i27.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corso G, Berardi A, Marrelli D, et al. CDH1 C-160A promoter polymorphism and gastric cancer risk. European Journal of Cancer Prevention. 2009;18(1):46–49. doi: 10.1097/CEJ.0b013e32830c8d70. [DOI] [PubMed] [Google Scholar]
- 77.Lu Y, Xu YC, Shen J, et al. E-cadherin gene C-160A promoter polymorphism and risk of non-cardia gastric cancer in a Chinese population. World Journal of Gastroenterology. 2005;11(1):56–60. doi: 10.3748/wjg.v11.i1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park WS, Cho YG, Park JY, et al. A single nucleotide polymorphism in the E-cadherin gene promoter-160 is not associated with risk of Korean gastric cancer. Journal of Korean Medical Science. 2003;18(4):501–504. doi: 10.3346/jkms.2003.18.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pharoah PD, Oliveira C, Machado JC, et al. CDH1 c-160a promotor polymorphism is not associated with risk of stomach cancer. International Journal of Cancer. 2002;101(2):196–197. doi: 10.1002/ijc.10590. [DOI] [PubMed] [Google Scholar]
- 80.Jonsson BA, Adami HO, Hagglund M, et al. -160C/A polymorphism in the E-cadherin gene promoter and risk of hereditary, familial and sporadic prostate cancer. International Journal of Cancer. 2004;109(3):348–352. doi: 10.1002/ijc.11629. [DOI] [PubMed] [Google Scholar]
- 81.Hajdinjak T, Toplak N. E-cadherin polymorphism—160 C/A and prostate cancer. International Journal of Cancer. 2004;109(3):480–481. doi: 10.1002/ijc.11705. [DOI] [PubMed] [Google Scholar]
- 82.Goto T, Nakano M, Ito S, Ehara H, Yamamoto N, Deguchi T. Significance of an E-cadherin gene promoter polymorphism for risk and disease severity of prostate cancer in a Japanese population. Urology. 2007;70(1):127–130. doi: 10.1016/j.urology.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 83.Kamoto T, Isogawa Y, Shimizu Y. Association of a genetic polymorphism of the E-cadherin gene with prostate cancer in a Japanese population. Japanese Journal of Clinical Oncology. 2005;35(3):158–161. doi: 10.1093/jjco/hyi040. [DOI] [PubMed] [Google Scholar]
- 84.Lindstrom S, Wiklund F, Jonsson BA, et al. Comprehensive genetic evaluation of common E-cadherin sequence variants and prostate cancer risk: strong confirmation of functional promoter SNP. Human Genetics. 2005;118(3-4):339–347. doi: 10.1007/s00439-005-0060-6. [DOI] [PubMed] [Google Scholar]
- 85.Bonilla C, Mason T, Long L, et al. E-cadherin polymorphisms and haplotypes influence risk for prostate cancer. Prostate. 2006;66(5):546–556. doi: 10.1002/pros.20374. [DOI] [PubMed] [Google Scholar]
- 86.Li HC, Albert JM, Shinohara ET, et al. E-cadherin promoter polymorphisms are not associated with the aggressiveness of prostate cancer in Caucasian patients. Urologic Oncology. 2006;24(6):496–502. doi: 10.1016/j.urolonc.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 87.Grunhage F, Jungck M, Lamberti C, et al. Association of familial colorectal cancer with variants in the E-cadherin (CDH1) and cyclin D1 (CCND1) genes. International Journal of Colorectal Disease. 2008;23(2):147–154. doi: 10.1007/s00384-007-0388-6. [DOI] [PubMed] [Google Scholar]
- 88.Pittman AM, Twiss P, Broderick P, et al. The CDH1-160C>A polymorphism is a risk factor for colorectal cancer. International Journal of Cancer. 2009;125(7):1622–1625. doi: 10.1002/ijc.24542. [DOI] [PubMed] [Google Scholar]
- 89.Porter TR, Richards FM, Houlston RS, et al Contribution of cyclin d1 (CCND1) and E-cadherin (CDH1) polymorphisms to familial and sporadic colorectal cancer. Oncogene. 2002;21(12):1928–1933. doi: 10.1038/sj.onc.1205245. [DOI] [PubMed] [Google Scholar]
- 90.Fei Y, Hu J, Liu S, Liu X, Wang F, Gong J. E-cadherin-160 C/A promoter polymorphism and risk of pancreatic carcinoma in Chinese population. Cancer Genetics and Cytogenetics. 2010;197(1):25–31. doi: 10.1016/j.cancergencyto.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Liang J, Kang S, et al. E-cadherin gene polymorphisms and haplotype associated with the occurrence of epithelial ovarian cancer in Chinese. Gynecologic Oncology. 2008;108(2):409–414. doi: 10.1016/j.ygyno.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 92.Ricketts C, Zeegers MP, Lubinski J, Maher ER. Analysis of germline variants in CDH1, IGFBP3, MMP1, MMP3, STK15 and VEGF in familial and sporadic renal cell carcinoma. PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0006037.e6037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang G, Hu X, Lu C, Su C, Luo S, Luo ZW. Promoter-hypermethylation associated defective expression of E-cadherin in primary non-small cell lung cancer. Lung Cancer. 2008;62(2):162–172. doi: 10.1016/j.lungcan.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 94.Chien MH, Yeh KT, Li YC, et al. Effects of E-cadherin (CDH1) gene promoter polymorphisms on the risk and clinicopathological development of hepatocellular carcinoma. Journal of Surgical Oncology. 2011;104(3):299–304. doi: 10.1002/jso.21929. [DOI] [PubMed] [Google Scholar]
- 95.Li XD, Wu LM, Xie HY, et al. No association exists between E-cadherin gene polymorphism and tumor recurrence in patients with hepatocellular carcinoma after transplantation. Hepatobiliary and Pancreatic Diseases International. 2007;6(3):254–258. [PubMed] [Google Scholar]
- 96.Shan K, Xiao-Wei M, Na W. Association of three single nucleotide polymorphisms of the E-cadherin gene with endometriosis in a Chinese population. Reproduction. 2007;134(2):373–378. doi: 10.1530/REP-07-0104. [DOI] [PubMed] [Google Scholar]
- 97.Song Y, Zhang S. Association of CDH1 promoter polymorphism and the risk of non-syndromic orofacial clefts in a Chinese Han population. Archives of Oral Biology. 2011;56(1):68–72. doi: 10.1016/j.archoralbio.2010.08.019. [DOI] [PubMed] [Google Scholar]


