Abstract
Objective
To evaluate the therapeutic efficacy of a novel modular polymer platform in the treatment of HNSCC.
Study Design
in vivo study.
Setting
Academic research laboratory.
Subjects and Methods
C3H/HeJ mice and SCID Beige mice were randomized to receive implantation of (1) no polymer; (2) plain polymer; (3) plain polymer with local cisplatin injection; (4) cisplatin polymer. The two groups of mice implanted with cisplatin polymer or no polymer were further randomized to receive (1) 4 Grays external beam radiation for 4 days; (2) no radiation. Tumor size was measured until the mice were euthanized. At necropsy, the tumors were excised and weighed.
Results
Our results using this novel polymer platform demonstrate a significant reduction in tumor growth. The cisplatin secreting polymer effectively reduced human head and neck tumor growth in SCID mice by 17 fold (P < 0.01); and SCCVII/SF tumors in the C3H/HeJ mice by over 16-fold (P < 0.01) as compared to control, plain polymer, and plain polymer + intratumoral cisplatin injection groups. We also observed a statistically significant lower tumor weight among mice treated with cisplatin polymer and concomitant radiation compared to the radiation alone group and the control group.
Conclusion
Herein we demonstrate the efficacy of a novel polymer platform in delivering cisplatin to a partially resected SCC in a murine model. Our results indicate that this polymer may represent a new therapeutic modality for patients with HNSCC. Once this polymer platform is optimized we will plan for validation in the context of a prospective trial in patients with unresectable advanced or recurrent HNSCC.
INTRODUCTION
Head & Neck Squamous Cell carcinoma (HNSCC) is the sixth most common cancer in the world. Patients with HNSCC are at considerable risk of mortality, with more than 300,000 deaths attributable to the disease annually.1 Aggressive surgical resection, with or without adjuvant chemoradiation (CRT) is the cornerstone of treatment for early disease. In many patients, the necessary surgery can be disfiguring and may also affect everyday functioning, with a profound impact on quality of life.2 During the past 30 years, the 3-to 5-year survival rate of patients with advanced T3 and T4 HNSCC has remained poor (20–30%) despite considerable advances in surgical techniques and irradiation delivery and improvement in chemotherapeutic strategies. Because 50% of the patients with advanced and unresectable disease fail primary management, salvage these patients is of paramount importance.3 Many of these patients receive radiation (RT) as definitive or as adjuvant therapy, which makes retreatment a challenge. Currently, the standard of care for recurrent disease is surgical salvage. Unfortunately, many advanced head and neck cancers are unresectable due to their proximity to vital structures such as the carotid artery or the skull base. Although palliation by chemotherapy is often attempted, systemic toxicity and its impact on the quality of life of patients prevents its wider clinical application.4 Given these dismal figures, new advances are needed in the effective treatment of HNSCC.
The science of polymer technology for drug delivery has evolved considerably since 1990.5 Polymers have been developed to deliver different types of drugs, including anticancer agents and antibiotics.6,7,8 Because most head and neck cancers and their cervical metastatic nodes are clinically accessible, local treatment with a polymer matrix may have significant clinical applications.
We have developed a novel modular drug delivery device that reproducibly reduces tumor growth in vivo. In this study, we have used a partial tumor resection model in the mouse, replicating the difficult situation we see in our patients in which the entire tumor is not resectable. The polymer platform we have developed is a flexible sheet that is designed to be applied intraoperatively to the surgical bed after removing or debulking the tumor, and is engineered to adapt and adhere to the surgical resected tissue contours. Cisplatin has been widely used in combination with radiation as a radiosensitizer in preclinical and clinical studies.7 We hypothesized that the local delivery of cisplatin is expected to maximize therapeutic index, minimize systemic side effects, and enhance post-operative radiation treatment.
METHODS
Polymer Fabrication
The cisplatin-releasing polymer was designed to be adequately flexible to adapt to irregular tissue contours without tearing. To meet this requirement, a wide range of mixing ratios involving two polymers: poly-ε-caprolactone (PCL) and a co-polymeric blend of poly (DL-lactide-co-ε-caprolactone) (PLCL), were evaluated in a pilot study. A 70:30 ratio of PLCL:PCL was found to offer the optimal flexibility and malleability of the PCL sheet, allowing for more facile handling by surgeons during implantation in vivo. Both PCL and PLCL were obtained from Boehringer Ingelheim and are manufactured under GMP and ISO-certified facilities, qualifying these materials’ availability for future clinical testing. Polymers were dissolved in chloroform in 70:30 ratio of PLCL:PCL for 24 hours until gentle mixing9. Fresh cisplatin (4mg/kg) was added to the polymer solution prior to spreading on a glass or polyethylene surface to form the thin sheets. After spreading, the sheets were dried overnight vacuum packed, and protected from ambient light. This polymer device is designed to degrade gradually overtime to prevent extrusion and long-term giant cell foreign body response. Furthermore, material selection was limited to those that are used in FDA-approved devices. Therefore, both the intact polymer as well as its degradation products is known to be well tolerated in humans.
Mouse Model
The mice used in this study were 6-week-old C3H/HeJ mice (Jackson labs, Bar Harbor, Maine), or 6-week-old SCID Beige mice (Jackson labs, Bar Harbor, Maine). The care and use of the animals is in accordance with the guidelines of the Animal Research Committee of the University of California at Los Angeles. The mice were maintained under specific pathogen-free conditions, and sterilized food and water is available ad libitum. Animals were maintained in the University of California, Los Angeles vivarium according to NIH guidelines, and all procedures were conducted under institutionally approved animal protocols.
Animal Model Surgical Procedure
For testing the polymer platform, two animal models were used: (1) 5 × 106 cells from the well established human OSCC line TU68610 were injected into 6-week-old SCID/Beige mice; and (2) 4 × 105 cells from the well established C3H/HeJ mouse SCCA cell line SCCVII/SF were injected into 6-week-old C3H/HeJ mice. The SCC VII/SF cell line is a spontaneously arising squamous cell carcinoma syngeneic to C3H/HeJ mice11. The TU686/SCID model allowed us to test the efficacy of the polymer against a human OSCC cell line. Eight mice were injected in each group unless otherwise specified. All mice were injected subcutaneously over the right flank. Tumor growth was assessed with calipers three times/week following polymer implantation for 18–31 days to evaluate the antitumor efficacy of the different treatments. (Tumor growth was assessed for 18–31 days as this was the time point at which control animals required euthanization as determined by the Animal Research Committee of the University of California at Los Angeles due to tumor burden.) The length, width, and height (in mm) of the tumors were measured and tumor volume (mm3) was calculated according to the formula:
When tumors reached an average size of 0.5–1 cm3, all animals underwent surgery to debulk their tumors by 50% to approximate the surgical situation when a patient’s tumor is unresectable, and some tumor is left behind prior to polymer therapy. Animals were then randomly assigned to the different treatment groups. The treatment groups included: (1) no polymer; (2) plain polymer; (3) plain polymer with local cisplatin injection; (4) cisplatin polymer. No systemic cisplatin was given. Each tumor bed was completely covered with the polymer according to the treatment group. The polymer was placed over each tumor and draped over the tumor edges.
Radiation therapy
After tumor debulking (described above) animals were assigned to various treatment groups. For the RT experiments, the treatment groups were: (1) No treatment (no polymer addition, surgical debulking only); (2) No treatment (no polymer addition, surgical debulking only) + RT; (3) cisplatin polymer alone; and (4) cisplatin polymer + RT. On postsurgical day number three, the mice were anaesthetized and positioned in a Lucite jig with lead shielding the body, except for the tumor site, which was irradiated using a Gamma cell 40 irradiator (Cs-137 source; Atomic Energy of Canada Ltd., Ottawa, Canada) at a dose rate of approximately 0.6Gy/min. Tumors were irradiated with a total dose of 16Gy given in 4Gy fractions on 4 consecutive days. Dosimetry was performed using Harshaw TLD-100H (LiF:Mg, Cu, P) and film (GAFCHROMIC EBT2, International Specialty Products, Wayne, NJ) and calibrated against a clinical cobalt-60 irradiator (Theratron-1000, MDS Nordion, Ontario) indicating that leakage and scatter of gamma rays to the shielded areas reached about 10.9–14.1% of the total dose. Dose fractionation was chosen to allow for repair, which is required for radiosensitization by cisplatin. The size of dose was based primarily on the fact that these tumors grow considerably more rapidly than in the clinic and standard 2 Gy doses are inadequate to compensate for rapid cell division. In addition, doses of >2 Gy per fraction are becoming increasing popular clinically either as planned homogeneous or inhomogeneous dose distributions, so the use of 4 Gy fraction sizes is not clinically irrelevant12. Cisplatin is a known radiation sensitizer and the polymer platform releasing 4mg/kg cisplatin within the radiation field will allow us to amplify radiation dose in a localized manner13, 14. We were studying the benefits of cisplatin as a radiation sensitizer and therefore were not administering the drug systemically. The length, width and height (mm) of each tumor were measured with calipers three times a week. After the animals were sacrificed, a gross necropsy and histopathological examination of the tissues surrounding the implant site was conducted. The inflammatory response to the implanted polymer was determined based on the average number of cell types present in the surrounding tissue. Tissue responses were found to be minimal (data not shown). The histopathological examination of all tissues and tumors was performed with the assistance of the pathologists at the University of California, Los Angeles.
Statistics
Tumor growth curves were compared between treatment arms using repeated measures analysis of variance (ANOVA) models. These models contained terms for time, treatment effects and the interaction between time and treatment terms. For tumor growth experiments in which there was a significant treatment by time interaction effects (demonstrating differences in tumor growth rates between groups), we compared the mean tumor sizes between treatment arms of interest at specific time points using Bonferroni corrected two-sample t-tests. Tumor weight was compared between groups with a two-way ANOVA model containing terms for cisplatin polymer and radiation. In both types of tumor models the log transformation was used to reduce the influence of outliers. P < 0.05 was considered significant.
RESULTS
The Modular Polymer Device is Safe and Biocompatible in the Animal Model and is Facile for Surgical Use
Although the tissue biocompatibility of the backbone of our polymers is well documented in the literature15, we initially tested the safety and biocompatibility of the Chemotherapeutic Layer of our device in our mouse model. In order to accelerate clinical translation, we selected materials that are currently used in medical devices with known characteristics in terms of toxicity, hypersensitivity, mutagenesis, and inflammatory responses. We initially tested the safety and biocompatibility of the Chemotherapeutic Layer of our device in our mouse model.
We used the partial tumor resection model in the mouse. After implantation, the mice were observed daily for overt toxicity. The animals tolerated the implant well and were not observed to pick or scratch at the polymer. No signs of bleeding, swelling, or infection were noted at the incision site. When the animals were sacrificed, a histopathological examination of the tissues surrounding the implant site was conducted. The inflammatory response to the implanted polymer was found to be minimal (data not shown).
Cisplatin Secreting Polymer Reduces Tumor Burden in Head and Neck Cancer
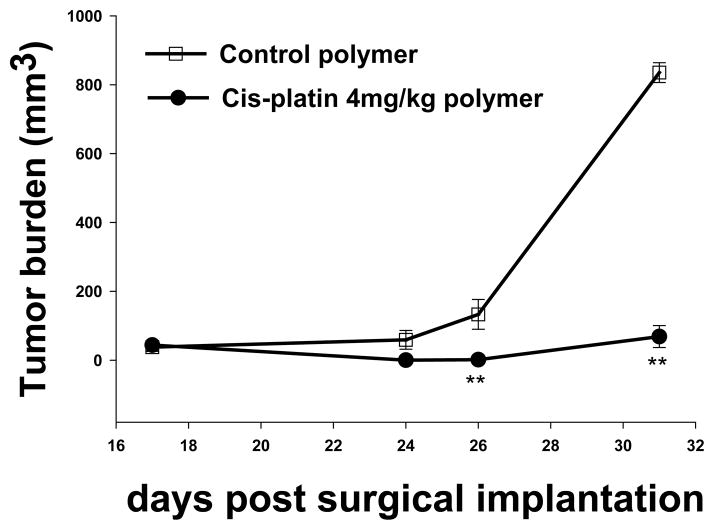
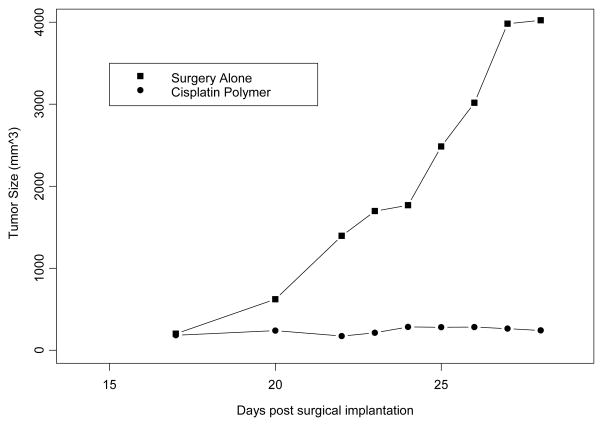
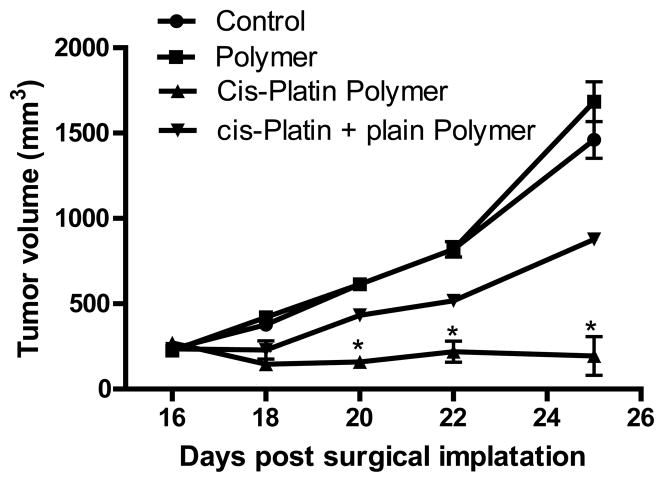
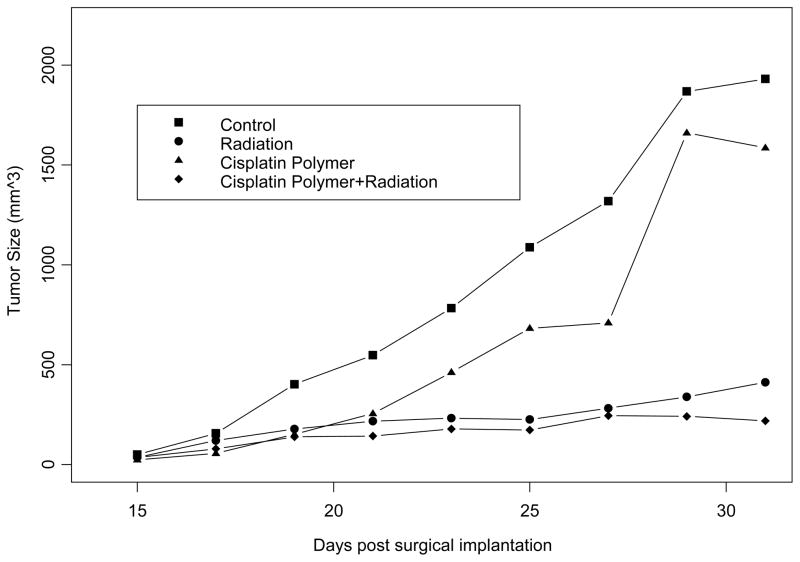
We evaluated the antitumor efficacy of the chemotherapeutic layer of the polymer platform in murine models of head and neck cancer. Our results using this novel polymer platform demonstrate a significant reduction in tumor growth. The cisplatin secreting polymer effectively reduced TU686 human head and neck tumor growth in SCID mice by 17 fold on day 31 (P < 0.01) (Figure 2); and SCCVII/SF tumors in the C3H/HeJ mice by over 10-fold on day 25 (P < 0.01) (Figure 3) as compared to control (surgical debulking only), plain polymer, and plain polymer + intratumoral cisplatin injection groups (P < 0.01). We were able to confirm this decrease in tumor burden by excising the tumors that had been treated with the cisplatin polymer (Figure 4). Our data shows that our cisplatin-secreting polymer is more effective against head and neck cancer than the plain polymer plus cisplatin given as an intratumoral bolus injection.
Figure 2. Cisplatin Release Polymer.
Cisplatin release is sustained for 6 weeks by the drug delivery platform, which consists of a blend of pure poly(ε-caprolactone) and a copolymeric blend of poly(ε-caprolactone) and poly(lactic acid).
Figure 3. Cisplatin Polymer Effectively Reduces the Growth of Human HNSCC.
5×106 TU686 HNSCC cells were inoculated in SCID beige mice. The implanted cisplatin secreting polymer significantly reduced tumor growth kinetics compared to control polymer. n=8 mice/group,** p<0.01 compared to control polymer.
Figure 4. Cisplatin Polymer Effectively Reduces the Growth of Mouse SCCVII/SF.
4×105 SCVII/SF tumor cells were inoculated in C3H mice. The implanted cisplatin polymer significantly reduced tumor growth by 16 fold compared to controls. n=8 mice/group,** p<0.01 compared to surgery only.
Cisplatin Secreting Polymer Enhances the Efficacy of Radiation Therapy
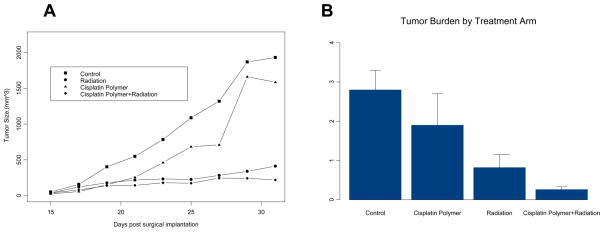
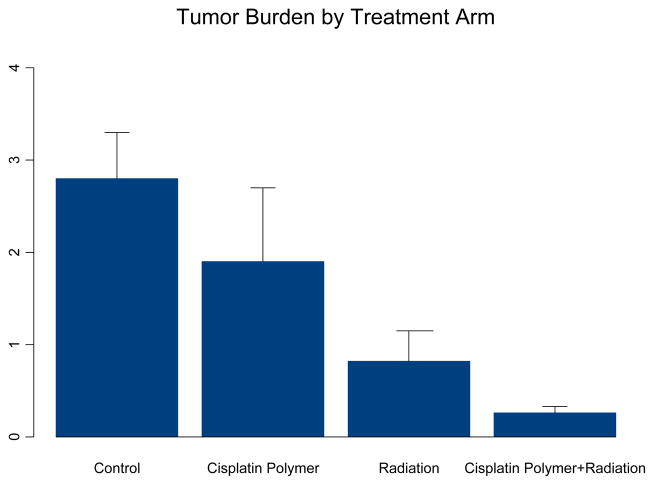
Mice treated with radiation, with or without cisplatin polymer implantation, demonstrated significant reduction in tumor regrowth compared to the control group (radiation alone, 22% the size of control, P= 0.005; radiation with cisplatin polymer 12% the size of control, P= 0.003). A closer comparison between the two different radiation treatment groups revealed lower tumor burden in the group also implanted with cisplatin polymer (Table 1, Figure 5) (53% the size of the radiation alone group). An overall analysis of the four treatment arms found that both cisplatin polymer and radiation resulted in significantly reduced tumor size over time (p=0.03 and 0.001, respectively). This observation was corroborated by the statistically significant lower tumor weight noted among mice treated with cisplatin polymer and concomitant radiation compared to the radiation alone group and the control group (Figure 6) ( 32% the weight of the radiation alone group, P=0.05; 9% the weight of the control group treated with debulking alone, P=0.0002). These results are quite promising that the polymer platform can direct the highest dose of radiation therapy to the tumor and spare surrounding normal tissues.
Table 1.
Final Tumor Weight by treatment group
| Mean Tumor Weight (SD) | |
|---|---|
| Control | 2.79 (1.19) |
| Cisplatin Polymer | 1.90 (1.91) |
| XRT | 0.82 (0.73) |
| Cisplatin Polymer + XRT | 0.26 (0.17) |
Figure 5. Cisplatin Polymer is More Effective at Reducing Tumor Growth Than Cisplatin Injection.
The implanted cisplatin polymer significantly reduced tumor growth kinetics compared to plain polymer and cisplatin injection plus plain polymer. n=8 mice/group,** p<0.01 compared to control polymer.
FIGURE 6. Time-series Plot of Tumor Size in the Four Treatment Arms.
The effects of treatments were assessed with a repeated measures ANOVA model. Treatment with cisplatin polymer and radiation resulted in significantly reduced tumor size over time (p=0.03 and 0.001, respectively).
DISCUSSION
We have developed a novel modular drug delivery device that reproducibly reduces tumor growth and enhances the efficacy of RT in vivo after partial tumor resection. We have used this partial tumor resection model in the mouse, replicating the difficult situation we see in our patients in which the entire tumor is not resectable. The device we have developed is a flexible sheet that is designed to be applied intraoperatively to the surgical bed after removing or debulking the tumor, and is engineered to adapt and adhere to the surgical resected tissue contours.
The combined use of radiotherapy and chemotherapy has been effective in improving the therapeutic index of radiation therapy for a variety of human cancers14. Cisplatin is a highly effective anticancer agent and has been widely used in combination with radiation as a radiosensitizer in preclinical and clinical studies14–16. The successful use of chemotherapeutic agents as radiosensitizers is dependent on enhanced tumor cell killing without increased normal tissue toxicity. The local delivery of synergistic agents is expected to maximize therapeutic index, minimize systemic side effects, and enhance post-operative radiation treatment. Our data above shows that our cisplatin-secreting polymer is more effective against head and neck cancer than the plain polymer plus cisplatin given as an intratumoral bolus injection. This enhanced antitumor activity is likely due to a more durable sustained release of cisplatin from the polymer platform increasing the interaction time with the tumor cells.
Some advantages of this polymer system over conventional brachytherapy are: better control of dose distribution, elimination of radioprotection and safety issues for the patient and the patient’s family, and treating personnel. An important psychological factor is that the patient’s daily activities are not restricted during the entire treatment time. An additional benefit of this polymer system includes prophylaxis against tumor recurrence following resection. Viable squamous cell carcinoma (SCC) cells have been recovered from the surgical wound following neck dissection and were shown to be capable of growing as colonies in vitro; theoretically, these may implant and cause cancer recurrence17. Therefore, exposing such cells to this polymer system in combination with EBRT, before they implant (while they are isolated and fragile), may decrease the chance of implantation.
Prior studies in oral and head and neck cancer have looked at combining cisplatin with collagen as a protein carrier. Kanekal et al18 noted that this combination allowed for higher intratumor drug levels for up to 48 h. Additionally, Yapp et al19 evaluated the use of biodegradable polymer implants to deliver cisplatin as compared to delivery by systemic injection and by osmotic pump. In contrast to these latter two systems, cisplatin in the tumor were found to be higher than those in the blood and kidney when the drug was delivered using the polymer implant. Herein, we have introduced the advantages of the polymer platform in directing the highest dose of radiation therapy to the tumor and sparing surrounding normal tissues. Another attractive addition to our modular platform is the concept of polymer delivery of immunomodulators, which will increase the efficiency of tumor cell killing by the host’s immune system. Patients with HNSCC have been well documented to exhibit local immunosuppression with depressed T-cell-mediated responses as well as depressed NK cell and antibody-dependent cell cytotoxic effects20. Gene therapy approaches include replacement gene therapy, suicide gene therapy, and immunotherapy. Despite these many approaches, gene therapy still has been limited significantly4. The role of cytokines in tumor regression is now well established20. The major limitation for the clinical use of cytokines is the lack of a simple and effective protocol for the local and sustained delivery of cytokines to the tumor milieu.
Although the polymer used herein is currently used in FDA-approved devices, and both the intact polymer as well as its degradation products are known to be well tolerated in humans, the exact polymer-tissue interactions in the vicinity of a dynamic tumor environment is unknown. In this current work, we did not explicitly evaluate biocompatibility in such an environment, and instead focused on demonstrating the overall utility of this modular approach. Now that feasibility has been demonstrated, the next step of our research program is to optimize the polymer platform to improve handling, cell delivery, and drug delivery.
We propose to develop the optimal combination of the bilayers to improve the outcome for patients with advanced or recurrent HNSCC. The modular nature of this polymer platform provides an elegant approach to future dosing modifications and device improvements, allowing us to incorporate changes into one layer without altering the chemical-physical properties of the other layer. Our C3H/HeJ mouse model has an intact functional immune system permitting further optimization of the polymer platform with potentially synergistic immunomodulators, unlike previous xenograft models in immunodeficient mice used for other polymers16. The robustness of this design will also enable us to dissect underlying mechanisms of immune activation and expansion, which will in turn help us design additional strategies to block the inactivation and death of the cytotoxic effectors in patients with aggressive HNSCC. We have obtained promising results from our pilot in vivo studies using a single layer polymer releasing cisplatin only. We are in the process of developing the optimal combination of the bilayers to improve the outcome for patients with advanced or recurrent HNSCC. Once this polymer platform is optimized in an in vivo model, we will plan for the ultimate validation in the context of a prospective trial in patients with unresectable advanced or recurrent HNSCC.
Figure 1. Treatment Arms.
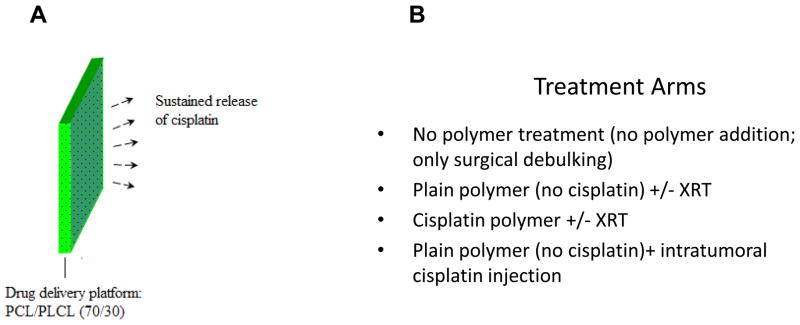
When tumors reached an average size of 0.5–1 cm3, all animals underwent surgery to debulk their tumors by 50%. Animals were then randomly assigned to the different treatment groups.
FIGURE 7. Tumor Burden at Sacrifice in the Four Treatment arms.
Analysis of variance models determined that both the cisplatin polymer and radiation significantly reduced tumor weight (p=0.04 and 0.0001 respectively).
Acknowledgments
This study was supported by the American Academy of Otolaryngology-American Head & Neck Society Surgeon Scientist Career Development Award (MSJ), the Tobacco-Related Disease Research Program of the University of California (MSJ), the STOP Cancer Foundation (MSJ), The Jonsson Cancer Center, and NIDCR K23 (MSJ).
Footnotes
This data was presented at the 2011 AHNS 2010 Research Workshop on Biology, Prevention & Treatment of Head & Neck Cancer, October 29, 2010, Arlington, VA.
References
- 1.Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2000: Cancer incidence, mortality and prevalence worldwide, version 1.0. IARC Cancer Base No 5 [Google Scholar]
- 2.Shikani AH, Domb AJ. Polymer chemotherapy for head and neck cancer. Laryngoscope. 2000;110:907–17. doi: 10.1097/00005537-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ross DA, Hundal JS, Son YH, et al. Microsurgical free flap reconstruction outcomes in head and neck cancer patients after surgical extirpation and intraoperative brachytherapy. Laryngoscope. 2004;114:1170–6. doi: 10.1097/00005537-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Xian J, Yang H, Lin Y, et al. Combination nonviral murine interleukin 2 and interleukin 12 gene therapy and radiotherapy for head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2005;131:1079–85. doi: 10.1001/archotol.131.12.1079. [DOI] [PubMed] [Google Scholar]
- 5.Domb AJ, editor. Polymeric site-specific pharmacotherapy. New York: John Wiley; 1994. pp. 1–464. [Google Scholar]
- 6.Domb AJ, Kost J, Wiseman D, editors. Handbook for biodegradable polymers. Amsterdam: Harwood Academic Publishers; 1997. pp. 1–513. [Google Scholar]
- 7.Cohen EM, Ding H, Kessinger CW, Khemtong C, Gao J, Sumer BD. Polymeric micelle nanoparticles for photodynamic treatment of head and neck cancer cells. Otolaryngol Head Neck Surg. 2010;143:109–15. doi: 10.1016/j.otohns.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Colella G, Cannavale R, Vicidomini A, Rinaldi G, Compilato D, Campisi G. Efficacy of a spray compound containing a pool of collagen precursor synthetic aminoacids (l-proline, l-leucine, l-lysine and glycine) combined with sodium hyaluronate to manage chemo/radiotherapy-induced oral mucositis: preliminary data of an open trial. Int J Immunopathol Pharmacol. 2010;23:143–51. doi: 10.1177/039463201002300113. [DOI] [PubMed] [Google Scholar]
- 9.Wang L. MS thesis. UCLA Biomedical Engineering; Sep, 2010. Sequential Layer Polymer Deposition. [Google Scholar]
- 10.Zhang X, Chen ZG, Choe MS, et al. Tumor growth inhibition by simultaneously blocking epidermal growth factor receptor and cyclooxygenase-2 in a xenograft model. Clin Can Res. 2005;11:6261–9. doi: 10.1158/1078-0432.CCR-04-2102. [DOI] [PubMed] [Google Scholar]
- 11.O’Malley BW, Jr, Cope KA, Johnson CS, et al. A new immunocompetent murine model for oral cancer. Arch Otol. 1997;123:20–4. doi: 10.1001/archotol.1997.01900010022003. [DOI] [PubMed] [Google Scholar]
- 12.Stuschke M, Pottgen C. Altered fractionation schemes in radiotherapy. Frontiers of radiation therapy and oncology. 2010;42:150–156. doi: 10.1159/000262470. [DOI] [PubMed] [Google Scholar]
- 13.Deurloo MJ, Kop W, van Tellingen O, Bartelink H, Begg AC. Intratumoural administration of cisplatin in slow-release devices: II. Pharmacokinetics and intratumoural distribution. Cancer Chemother Pharmacol. 1991;27:347–53. doi: 10.1007/BF00688856. [DOI] [PubMed] [Google Scholar]
- 14.Ning S, Yu N, Brown DM, Kanekal S, Knox SJ. Radiosensitization by intratumoral administration of cisplatin in a sustained-release drug delivery system. Radiother Oncol. 1999;2:215–23. doi: 10.1016/s0167-8140(98)00134-0. [DOI] [PubMed] [Google Scholar]
- 15.Gőpferich A. Mechanisms of Polymer Degradation and Erosion. Biomaterials. 1996;17:103. doi: 10.1016/0142-9612(96)85755-3. [DOI] [PubMed] [Google Scholar]
- 16.Yu NY, Conley FK, Luck EE, et al. Response of murine tumors to matrix-associated cisplatin intratumoral implants. NCI Monogr. 1988;6:137–40. [PubMed] [Google Scholar]
- 17.Vikram B, Mishra S. Permanent iodine-125 implants in postoperative radiotherapy for head and neck cancer with positive surgical margins. Head Neck. 1994;16:155–7. doi: 10.1002/hed.2880160209. [DOI] [PubMed] [Google Scholar]
- 18.Kanekal S, Joo J, Bublik M, et al. Retention of intratumor injections of cisplatinum in murine tumors and the impact on laser thermal therapy for cancer treatment. Eur Arch Otorhinolaryngol. 2009 Feb;266(2):279–84. doi: 10.1007/s00405-008-0736-3. [DOI] [PubMed] [Google Scholar]
- 19.Yapp DT, Lloyd DK, Zhu J, Lehnert SM. Cisplatin delivery by biodegradable polymer implant is superior to systemic delivery by osmotic pump or i.p. injection in tumor-bearing mice. Anticancer Drugs. 1998 Oct;9(9):791–6. doi: 10.1097/00001813-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Jewett A, Cacalano NA, Teruel A, et al. Inhibition of nuclear factor kappa B (NFkappaB) activity in oral tumor cells prevents depletion of NK cells and Increases their functional activation. Cancer Immunol Immunother. 2006;55:1052–63. doi: 10.1007/s00262-005-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]