Abstract
This study aimed to evaluate the diagnostic reliability of sentinel lymph node biopsy in patients with squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx by reviewing the published literature. A systematic literature review was performed using MEDLINE from 1970 to 2011. With Boolean search strings, search terms included sentinel node, supraglottic, supraglottis, tongue, head and neck, oral, pharynx, laryngeal, and larynx. Additional studies were identified through article references. Duplicate data and articles were excluded based on treating institution and study inclusion time period. Additional studies were excluded if the head and neck subsite or tumor stage was not specifically identified or if the sentinel lymph node biopsy occurred in previously treated necks. All patients had sentinel lymph node biopsy performed followed by a concurrent neck dissection. Twenty-six studies met our inclusion criteria (n = 766 patients). The pooled sensitivity and negative predictive value of SLNB for all head and neck tumors was 95 % (95 % CI 91–99 %) and 96 % (95 %CI 94–99 %), respectively. The overall sensitivity and negative predictive value of SLNB in the subset of oral cavity tumors (n = 631) was 94 % (95 % CI 89–98 %) and 96 % (95 % CI 93–99 %), respectively. One-hundred percent of oropharyngeal (n = 72), hypopharyngeal (n = 5), and laryngeal (n = 58) tumor sentinel lymph biopsy results correlated with subsequent neck dissections giving a negative predictive value of 100 %, showing that, sentinel lymph node biopsy is a valid diagnostic technique to correctly stage regional metastases in patients with head and neck squamous cell carcinoma.
Keywords: Sentinel lymph node, Head and neck, Oral, pharynx, Squamous cell carcinoma
Introduction
Head and neck squamous cell carcinoma (HNSCC) is predominantly a locoregional disease. Determining the presence of regional metastases (N+) is critical for staging, treatment, and prognosis. Metastasis to a single regional lymph node can transform a small stage I tumor to an advanced stage III or even stage IV head and neck cancer. The involvement of cervical lymph nodes can decrease disease specific survival by up to 50 % [1].
The treatment of patients without clinical or radio-graphic evidence of regional metastasis (N0) remains controversial. Elective neck dissections (END) are traditionally recommended when the tumor size and subsite confers at least a 15–20 % risk of lymphatic spread [2]. Performing ENDs for N0 patients can result in significant morbidity with questionable benefit for these patients confirmed by pathology [3, 4]. For instance, in HNSCC tumors that have a 20 % rate of nodal metastasis, the vast majority of these patients will undergo END with no evidence of lymph node metastasis.
A conservative trend in the treatment of HNSCC N0 patients has encouraged the application of sentinel lymph node biopsy (SLNB). Sentinel lymph node biopsy entails identifying and harvesting the initial node(s) to which the primary tumor drains, while limiting dissection and harm to vital structures. The advantages of implementing SLNB instead of END include decreased morbidity, operating room time, and length of postoperative stay [3, 4]. Prior HNSCC SLNB reviews and meta-analyses have supported the use of SLNB in oral and oropharyngeal squamous cell carcinoma (SCC) [5, 6]. In addition, the use of SLNB has since expanded to hypopharyngeal, and supraglottic tumors [7–11]. To further evaluate the diagnostic reliability of SLNB in squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx and supraglottis, a systematic review and meta-analysis of SLNB in N0 HNSCC patients was performed. To our knowledge, this is the largest meta-analysis of SLNB in patients with oral cavity and oropharyngeal SCC, and the first to include tumors of the hypopharynx and supraglottis.
Materials and methods
A systematic literature review was performed using MEDLINE from January 1, 1970 to December 31, 2011. Using Boolean search strings, search terms included the following key words: sentinel node, supraglottic, supraglottis, tongue, head and neck, oral, pharynx, laryngeal, and larynx. Additional studies were identified through article references. Duplicate data were identified based on the treating institution and study inclusion time period. Duplicate reports were subsequently excluded. Entire studies or individual patients were also excluded from our analysis, if the head and neck subsite or tumor stage could not be specifically identified. Studies investigating SLNB in previously treated necks were also excluded. All patients included in our meta-analysis had a concurrent END performed at the time of SLNB. Lymph nodes that demonstrated any evidence of carcinoma, including micrometastasis and tumor islet cells, were considered positive.
The pooled patient data were then categorized based on early tumor stage (T1–2) versus late tumor stage (T3–4), and by head and neck subsite including oral cavity, oropharynx, hypopharynx, and larynx. Categorized spreadsheets of specific subsites and tumor stage were then completed by including the following specific data points from each study: positive SLN, negative SLN, negative SLN with positive END, technique used for SLN localization [gamma probe with lymphoscintigraphy or single-photon emission computed tomography (SPECT)], method of pathological analysis (standard H&E, serial sectioning, immunohistochemistry), mean or median number of SLNs per patient, and cancer recurrence if reported.
Sensitivity and negative predictive values were calculated for each study. Sensitivity was the probability of a positive neck dissection given a positive SLNB, and the negative predictive value (NPV) was calculated as probability of a negative neck dissection after a negative SLNB. Meta- analyses were performed to calculate the combined sensitivities and negative predictive values using both a fixed-effects and a random-effects approach for all subsites combined and also for oral cavity tumors alone. The fixed-effects approach estimates the pooled test sensitivity and negative predicted values assuming the data came from a single study, that is, assuming no inter-study heterogeneity. The random-effects approach allows for inter-study heterogeneity. Heterogeneity among studies was evaluated by the Cochran Q statistic (considered significant for p values <0.10). Since three of our four meta-analysis calculations have a Q statistic p value <0.10, the random-effects method was chosen. Statistical analyses were performed using the meta package in R (version 2.14.2 www.r-project.org).
Results
A total of 26 studies met inclusion criteria [7–32]. The overall cohort totaled 766 patients (n = 766). Table 1 stratifies SLN status by tumor stage and subsite demonstrating the large majority of patients having early stage oral cavity tumors.
Table 1.
Cohort of all patients from the 26 studies included in this meta-analysis
| Tumor subsite and stage | SLN+a | SLN−/END–b | SLN−/END+c | Total patients |
|---|---|---|---|---|
| Oral cavity T1/T2 | 177 | 408 | 8 | 593 |
| Oral cavity T3/T4 | 7 | 27 | 4 | 38 |
| Oropharynx T1/T2 | 31 | 37 | 0 | 68 |
| Oropharynx T3/T4 | 2 | 2 | 0 | 4 |
| Hypopharynx T1/T2 | 0 | 0 | 0 | 0 |
| Hypopharynx T3/T4 | 3 | 2 | 0 | 5 |
| Larynx T1/T2 | 11 | 21 | 0 | 32 |
| Larynx T3/T4 | 5 | 21 | 0 | 26 |
Positive SLN
Negative SLN with negative END
Negative SLN with positive END
Sentinel lymph node localization and histopathology techniques utilized are summarized in Table 2. The most common method to preoperatively localize SLN included injecting a radioactive sentinel node tracer followed by lymphoscintigraphy, without the use of blue dye or lymphazurin. All studies utilized a gamma probe intraoperatively. In 17 of the 26 studies (65 %), the histopatho-logic examination consisted of serial sectioning with hematoxylin and eosin (H&E) staining, followed by immunohistochemistry staining for negative SLN.
Table 2.
Summary of the 26 studies' methodology for determining SLN
| Study characteristic | Number of studies |
|---|---|
| Sentinel node tracer | |
| Radionucleotide | 22 |
| Radionucleotide and blue dye | 4 |
| Sentinel node localization | |
| Gamma probe only | 1 |
| Gamma probe and lymphoscintigraphy | 21 |
| Gamma probe and SPECTa | 2 |
| Gamma probe and lymphoscintigraphy and SPECT | 2 |
| Pathology analysis | |
| Standard H&Eb | 7 |
| Standard H&E and IHCc | 2 |
| Standard H&E, IHC, and SSd | 17 |
Single-photon emission computed tomography
Hematoxylin and eosin
Immunohistochemistry
Serial sectioning
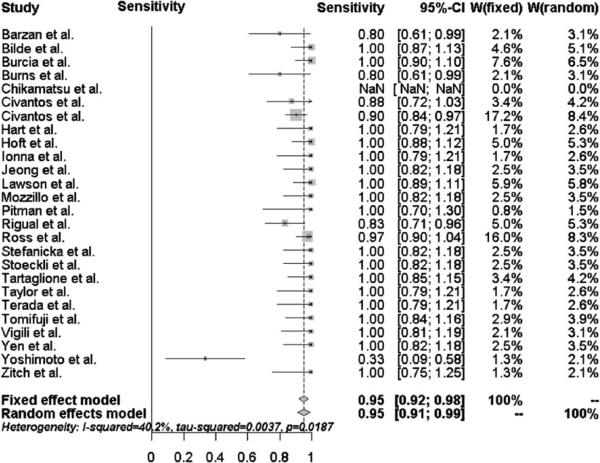
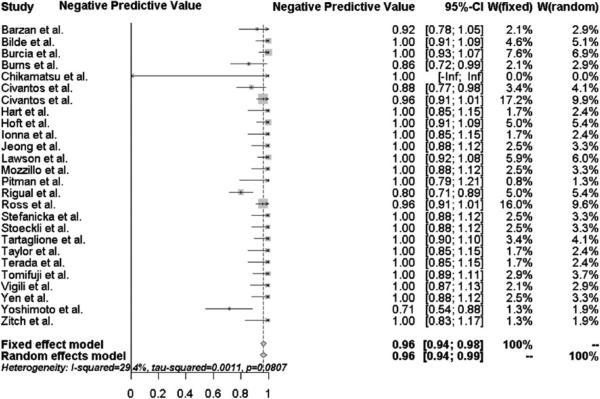
The pooled sensitivity and negative predictive value of SLNB for all head and neck subsites was 95 % [95 % confidence interval (95 % CI) 91–99 %] and 96 % (95 %CI 94–99 %), respectively, as shown in Figs. 1 and 2. Of the 766 patients, 236 (31 %) were upstaged by a positive sentinel lymph node biopsy. There were only 12 patients (<2 %) misclassified as N0 on SLNB that had a positive concurrent elective neck dissection. Eight of these 12 patients had early T1/T2 oral cavity tumors, and four patients had T3/T4 oral cavity tumors.
Fig. 1.
Forest plot of the sensitivity for all head and neck subsites
Fig. 2.
Forest plot of the negative predictive value for all head and neck subsites
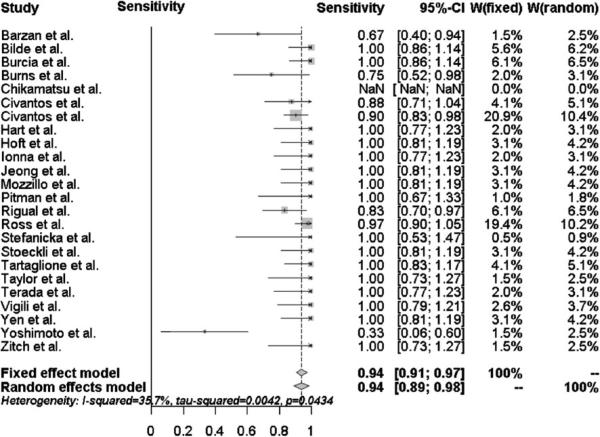
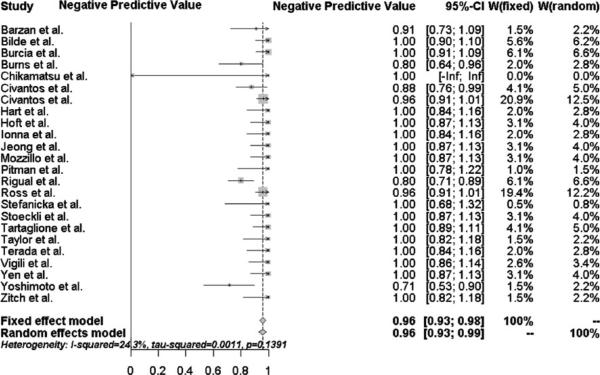
The overall sensitivity and negative predictive value of SLNB in the subset of oral cavity tumors (n = 631) were 94 % (95 % CI 89–98 %) and 96 % (95 % CI 93–99 %), respectively. as shown in Figs. 3 and 4. 184 of the 631 oral cavity tumors (29 %) were upstaged by positive SLNB. All 12 patients initially misclassified with a negative SLNB but positive END had oral cavity tumors. It is important to note that these patients represented only 2 % of the 631 patients with oral cavity tumors.
Fig. 3.
Forest plot of the sensitivity for oral cavity subsite
Fig. 4.
Forest plot of the negative predictive value for oral cavity subsite
100 % of oropharyngeal (n = 72), hypopharyngeal (n = 5), and laryngeal (n = 58) tumor SLNB results correlated with subsequent neck dissections giving a negative predictive value of 100 %. 33 patients with oropharyngeal SCC, 3 with hypopharyngeal SCC, and 16 with laryngeal SCC were accurately upstaged from N0 to N+ with SLNB.
Discussion
As Layland et al. [1] demonstrated in their review of over 3,000 HNSCC patients, disease specific survival decreases from 67.9 % for patients with N0 disease to 39.9 % with N+ disease. Clearly, regional lymphatic spread is a significant negative prognostic indicator in head and neck cancer [1]. Determining the presence of occult metastases in clinically and radiographically, N0 necks is critical not only for prognosis but for treatment [33]. As a major indicator of the need for adjuvant radiation therapy, the determination of positive lymph node status is of great import.
In our meta-analysis, 766 patients with HNSCC underwent SLNB followed by immediate END. A positive sentinel lymph node biopsy confirmed occult metastasis in 31 % of patients, which appropriately correlates with the 33 % occult metastatic rate reported by Shah et al. [34]. Overall, the sensitivity of SLNB in HNSCC was 95 %, with a NPV of 96 %.
Oral cavity tumors comprised the majority of the overall cohort (631/766, 82.4 %). The pooled sensitivity and NPV for oral cavity tumors alone were 94 and 96 %, respectively. Our data demonstrates quite clearly that oral cavity HNSCC patients with negative SLNBs can be assured to a very high degree of certainty that subsequent ENDs will also be negative. Remarkably, every SLNB from oropharyngeal (n = 72), hypopharyngeal (n = 5), and laryngeal (n = 58) tumors correlated with subsequent neck dissections, conferring a preliminary 100 % NPV for each sub-site. Although promising, the significance of the 100 % NPV of SLNB outside the oral cavity is limited, and further study is needed. Factors accounting for the 100 % NPV are that the number of reported cases from the hypopharynx and larynx are much smaller compared to the oral cavity, and the few surgeons reporting this preliminary data have extensive experience with SLNB.
Although often considered routine, ENDs are not without risks and morbidity [3, 4]. As Schiefke et al. [3] demonstrated, patients with oral cavity and oropharyngeal SCC who undergo SLNB have fewer swallowing problems, decreased impairment from cervical scars, decreased sensory dysfunction, and improved shoulder function than patients who undergo END. Murer et al. [4] have also shown that SLNB is associated with less shoulder dys-function, postoperative lymphedema, and significantly decreased injury to the lingual nerve or marginal mandibular branch of the facial nerve than END.
With the increased implementation of SLNB, the possible avoidance of adjuvant or elective chemoradiation should also not be underestimated. Radiation and chemo-therapy are generally advocated for patients as primary organ-sparing treatments or as adjuvant treatment for patients with neck disease [35]. Adjuvant radiation therapy to the neck has also been recommended for N0 patients who do not undergo END [36]. However, radiation therapy, with or without chemotherapy, is associated with significant morbidities [37–41]. 80 % of HNSCC patients receiving chemoradiation develop acute mucositis, which leads to substantial oral pain, dysphagia, increased hospitalizations, and feeding tube placement during treatments [37]. Long-term morbidities include progressive dysphagia and xerostomia [38–41]. Therefore, patients with negative SLNB can avoid adjuvant therapy, which would lessen patient morbidity. Radiation would also be reserved for a later time for treatment of possible second primaries or tumor recurrences.
In our literature review, five studies examined regional recurrence in oral cavity and oropharyngeal SCC patients who did not receive END following negative SLNB (Table 3) [23, 42–45]. The follow-up periods between the studies varied, but there were 11 documented regional recurrences from 200 total patients (5.5 %) with a mean of 6.7 % recurrence rate. This recurrence rate compares favorably to a recent prospective randomized study by Yuen et al. [46] that showed a 6 % cervical node recurrence rate in patients with T1/T2 N0 oral tongue SCC who underwent upfront END. Yuen et al. [46] compared disease recurrence and survival between patients with early tumor stage tongue cancer and clinically negative necks undergoing ENDs versus watchful waiting. They found a significant improvement with END in regional control, decreasing regional recurrence from 31 to 6 %. The 5-year disease specific survival was not significant between the two groups. Although a more formal randomized controlled study is needed to statistically compare disease recurrence and survival between patients receiving prophylactic END at diagnosis and those undergoing SLNB, Yuen's initial results support our findings that withholding an END from patients with a negative SLNB does not compromise disease recurrence.
Table 3.
Summary of the reported regional recurrences of patients with oral cavity squamous cell carcinoma who had a negative SLNB and no concurrent END
To date, the predominant clinical experience with SLNB has been with oral cavity tumors. There is still some debate in the literature regarding the accuracy of SLNB for floor of mouth tumors compared to other oral locations [11, 20, 21, 39]. The argument by those that report a lower sensitivity and negative predictive value for floor of mouth tumors compared to other locations is that tumors in the floor of mouth lie in very close proximity to level I nodes leading to difficulty in identifying and harvesting SLNs [21]. In our study, the specific location of oral cavity tumors was frequently not specified, which is one limitation of our analysis. A second limitation is that the quality of histopathological analysis of neck dissection specimens varies across institutions, and each individual lymph node likely does not undergo the scrutiny of serial sectioning and immunohistochemistry that as is common in the analysis of SLNs. Thus, tumor islet cells and micrometastases may not be identified, and consequently, our NPV for oral cavity tumors is likely overestimated. Another limitation revealed by this meta-analysis is the lack of multiple large clinical trials for SLNB in tumors of the oropharynx, hypopharynx, and larynx. Further study by experienced surgeons is needed and will likely clarify these clinical uncertainties.
Conclusion
Our meta-analysis reveals that SLNB is a valid diagnostic technique to correctly stage regional metastases in patients with HNSCC. Early oral cavity tumors have been the most widely studied to date, and implementation of SLNB in these patients can spare many of them the morbidity of END and primary chemoradiation therapy. Herein, we show for the first time that SLNB is also highly predictive in patients with oropharyngeal, hypopharyngeal, and laryngeal tumors. With time, additional cases of late tumor stage oral cavity tumors and cases from other head and neck subsites will be reported which can be included in future studies. Long-term follow-up will also be critical to better assess disease free and disease specific survival of SLNB patients.
Acknowledgments
The corresponding author, CFT, had full access to all the data in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Statistical analyses were supported by the UCLA Clinical and Translational Science Institute (grant UL1TR000124 and UL1RR033176).
Footnotes
Oral Presentation at the American Head and Neck Society 8th International Conference on Head and Neck Cancer, July 21–25, 2012; Toronto, Ontario, Canada.
Conflict of interest None.
Contributor Information
C. F. Thompson, David Geffen School of Medicine, UCLA Medical Center, 10833 Le Conte Ave, 62-132 CHS, Los Angeles, CA 90095-1624, USA
M. A. St. John, David Geffen School of Medicine, UCLA Medical Center, 10833 Le Conte Ave, 62-132 CHS, Los Angeles, CA 90095-1624, USA
G. Lawson, Université Catholique de Louvain Centre Hospitalier Universitaire de Mont-Godinne, Yvoir, Belgium
T. Grogan, David Geffen School of Medicine, UCLA Medical Center, 10833 Le Conte Ave, 62-132 CHS, Los Angeles, CA 90095-1624, USA
D. Elashoff, David Geffen School of Medicine, UCLA Medical Center, 10833 Le Conte Ave, 62-132 CHS, Los Angeles, CA 90095-1624, USA
A. H. Mendelsohn, David Geffen School of Medicine, UCLA Medical Center, 10833 Le Conte Ave, 62-132 CHS, Los Angeles, CA 90095-1624, USA
References
- 1.Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+. Laryngoscope. 2005;115(4):629–639. doi: 10.1097/01.mlg.0000161338.54515.b1. [DOI] [PubMed] [Google Scholar]
- 2.Pillsbury HC, Clark MA. Rationale for therapy of the N0 neck. Laryngoscope. 1997;107:1294–1315. doi: 10.1097/00005537-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Schiefke F, Akdemir M, Weber A, et al. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck. 2009;31(4):503–512. doi: 10.1002/hed.21001. [DOI] [PubMed] [Google Scholar]
- 4.Murer K, Huber GF, Haile SR, et al. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinoma. Head Neck. 2011;33(9):1260–1264. doi: 10.1002/hed.21622. [DOI] [PubMed] [Google Scholar]
- 5.Alvarez Amezaga J, Barbier Herrero L, Pajoan del Barrio JI, et al. Diagnostic efficacy of sentinel node biopsy in oral squamous cell carcinoma. Cohort study and meta-analysis. Med Oral Patol Oral Cir Bucal. 2007;12(3):E235–E243. [PubMed] [Google Scholar]
- 6.Paleri V, Rees G, Arullendran P, et al. Sentinel node biopsy in squamous cell cancer of the oral cavity and oral pharynx: a diagnostic meta-analysis. Head Neck. 2005;27(9):739–747. doi: 10.1002/hed.20228. [DOI] [PubMed] [Google Scholar]
- 7.Barzan L, Sulfaro S, Alberti F, et al. Gamma probe accuracy in detecting the sentinel lymph node in clinically N0 squamous cell carcinoma of the head and neck. Ann Otol Rhinol Laryngol. 2002;111(9):794–798. doi: 10.1177/000348940211100906. [DOI] [PubMed] [Google Scholar]
- 8.Höft S, Maune S, Muhle C, et al. Sentinel lymph-node biopsy in head and neck cancer. Br J Cancer. 2004;91(1):124–128. doi: 10.1038/sj.bjc.6601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson G, Matar N, Nollevaux MC, et al. Reliability of sentinel node technique in the treatment of N0 supraglottic laryngeal cancer. Laryngoscope. 2010;12(11):2213–2217. doi: 10.1002/lary.21131. [DOI] [PubMed] [Google Scholar]
- 10.Pitman KT, Johnson JT, Brown ML, et al. Sentinel lymph node biopsy in head and neck squamous cell carcinoma. Laryngoscope. 2002;112(12):2101–2113. doi: 10.1097/00005537-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Tomifuji M, Shiotani A, Fujii H, et al. Sentinel node concept in clinically n0 laryngeal and hypopharyngeal cancer. Ann Surg Oncol. 2008;15(9):2568–2575. doi: 10.1245/s10434-008-0008-x. [DOI] [PubMed] [Google Scholar]
- 12.Bilde A, von Buchwald C, Therkildsen MH, et al. Need for intensive histopathologic analysis to determine lymph node metastases when using sentinel node biopsy in oral cancer. Laryngoscope. 2008;118(3):408–414. doi: 10.1097/MLG.0b013e31815d8e15. [DOI] [PubMed] [Google Scholar]
- 13.Burcia V, Costes V, Faillie JL, et al. Neck restaging with sentinel node biopsy in T1-T2N0 oral and oropharyngeal cancer: Why and how? Otolaryngol Head Neck Surg. 2010;142(4):592.e1–597.e1. doi: 10.1016/j.otohns.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 14.Burns P, Foster A, Walshe P, O'Dwyer T. Sentinel lymph node biopsy in node-negative squamous cell carcinoma of the oral cavity and oropharynx. J Laryngol Otol. 2009;123(4):439–443. doi: 10.1017/S0022215108003514. [DOI] [PubMed] [Google Scholar]
- 15.Chikamatsu K, Kamada H, Ninomiya H, et al. A preliminary study on sentinel lymph node biopsy: feasibility and predictive ability in oral cavity cancer. Ann Nucl Med. 2004;18(3):257–262. doi: 10.1007/BF02985008. [DOI] [PubMed] [Google Scholar]
- 16.Civantos FJ, Gomez C, Duque C, et al. Sentinel node biopsy in oral cavity cancer: correlation with PET scan and immunohistochemistry. Head Neck. 2003;25(1):1–9. doi: 10.1002/hed.10213. [DOI] [PubMed] [Google Scholar]
- 17.Civantos FJ, Zitsch RP, Schuller DE, et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1–T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J Clin Oncol. 2010;28(8):1395–1400. doi: 10.1200/JCO.2008.20.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart RD, Nasser JG, Trites JR, et al. Sentinel lymph node biopsy in N0 squamous cell carcinoma of the oral cavity and oropharynx. Arch Otolaryngol Head Neck Surg. 2005;131(1):34–38. doi: 10.1001/archotol.131.1.34. [DOI] [PubMed] [Google Scholar]
- 19.Ionna F, Chiesa F, Longo F, et al. Follow-up after intra-operative sentinel node biopsy of N0 neck oral cancer patients. Eur Arch Otorhinolaryngol. 2011;268(3):429–435. doi: 10.1007/s00405-010-1364-2. [DOI] [PubMed] [Google Scholar]
- 20.Jeong HS, Baek CH, Son YI, et al. Sentinel lymph node radiolocalization with 99mTc filtered tin colloid in clinically node-negative squamous cell carcinomas of the oral cavity. J Korean Med Sci. 2006;21(5):865–870. doi: 10.3346/jkms.2006.21.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mozzillo N, Chiesa F, Caracò C, et al. Therapeutic implications of sentinel lymph node biopsy in the staging of oral cancer. Ann Surg Oncol. 2004;11(3 Suppl):263S–266S. doi: 10.1007/BF02523642. [DOI] [PubMed] [Google Scholar]
- 22.Rigual N, Douglas W, Lamonica D, et al. Sentinel lymph node biopsy: a rational approach for staging T2N0 oral cancer. Laryngoscope. 2005;115(12):2217–2220. doi: 10.1097/01.mlg.0000187870.82699.ed. [DOI] [PubMed] [Google Scholar]
- 23.Ross GL, Soutar DS, Gordon MacDonald D, et al. Sentinel node biopsy in head and neck cancer: preliminary results of a multicenter trial. Ann Surg Oncol. 2004;11(7):690–696. doi: 10.1245/ASO.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Stefanicka P, Profant M, Duchaj B, et al. Sentinel lymph node radiolocalization and biopsy in oral cavity and oropharynx mucosal squamous cell carcinoma. Bratisl Lek Listy. 2010;111(11):590–594. [PubMed] [Google Scholar]
- 25.Stoeckli SJ. Sentinel node biopsy for oral and oropharyngeal squamous cell carcinoma of the head and neck. Laryngoscope. 2007;117(9):1539–1551. doi: 10.1097/MLG.0b013e318093ee67. [DOI] [PubMed] [Google Scholar]
- 26.Taylor RJ, Wahl RL, Sharma PK, et al. Sentinel node localization in oral cavity and oropharynx squamous cell cancer. Arch Otolaryngol Head Neck Surg. 2001;127(8):970–974. doi: 10.1001/archotol.127.8.970. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglione G, Vigili MG, Rahimi S, et al. The impact of superficial injections of radiocolloids and dynamic lymphoscintigraphy on sentinel node identification in oral cavity cancer: a same-day protocol. Nucl Med Commun. 2008;29(4):318–322. doi: 10.1097/MNM.0b013e3282f4d399. [DOI] [PubMed] [Google Scholar]
- 28.Terada A, Hasegawa Y, Goto M, et al. Sentinel lymph node radiolocalization in clinically negative neck oral cancer. Head Neck. 2005;28(2):114–120. doi: 10.1002/hed.20305. [DOI] [PubMed] [Google Scholar]
- 29.Vigili MG, Tartaglione G, Rahimi S, et al. Lymphoscintigraphy and radioguided sentinel node biopsy in oral cavity squamous cell carcinoma: same day protocol. Eur Arch Otorhinolaryngol. 2007;264(2):163–167. doi: 10.1007/s00405-006-0150-7. [DOI] [PubMed] [Google Scholar]
- 30.Yen CY, Lee SY, Hsieh JF, et al. Radiolocalized sentinel lymph node biopsy in squamous cell carcinoma of the oral cavity and analysis of various parameters. Ann Surg Oncol. 2006;13(8):1130–1135. doi: 10.1245/ASO.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimoto S, Hasegawa Y, Matsuzuka T, et al. Sentinel node biopsy for oral and laryngopharyngeal squamous cell carcinoma: a retrospective study of 177 patients in Japan. Auris Nasus Larynx. 2012;39(1):65–70. doi: 10.1016/j.anl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Zitsch RP, 3rd, Todd DW, Renner GJ, et al. Intraoperative radiolymphoscintigraphy for detection of occult nodal metastasis in patients with head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. 2000;122(5):662–666. doi: 10.1016/S0194-5998(00)70192-6. [DOI] [PubMed] [Google Scholar]
- 33.Coughlin A, Resto VA. Oral cavity squamous cell carcinoma and the clinically N0 neck: the past, present, and future of sentinel lymph node biopsy. Curr Oncol Rep. 2010;12(2):129–135. doi: 10.1007/s11912-010-0090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah JP. Patterns of cervical lymph node metastasis from squamous cell carcinoma of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 35.Spriano G, Pellini R, Manciocco V, et al. Treatment of advanced neck metastases. Acta Otorhinolaryngol Ital. 2006;26(6):360–369. [PMC free article] [PubMed] [Google Scholar]
- 36.Shasha D, Harrison LB. Elective irradiation of the N0 neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Clin North Am. 1998;31(5):803–813. doi: 10.1016/s0030-6665(05)70088-8. [DOI] [PubMed] [Google Scholar]
- 37.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 1997;66(3):253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- 38.Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24(17):2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 39.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30:148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langendijk JA, Doornaert P, Verdonck-de Leeuw IM, Leemans CR, Aaronson NK, Slotman BJ. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26(22):3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 41.Pauloski BR, Rademaker AW, Logemann JA, et al. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head Neck. 2006;28(12):1069–1076. doi: 10.1002/hed.20459. [DOI] [PubMed] [Google Scholar]
- 42.Broglie MA, Haerle SK, Huber GF, Haile SR, Stoeckli SJ. Occult metastases detected by sentinel node biopsy in patients with early oral and oropharyngeal squamous cell carcinomas: impact on survival. Head Neck. 2012 doi: 10.1002/hed.23017. doi. doi:10.1002/hed.23017. [DOI] [PubMed] [Google Scholar]
- 43.Frerich B, Förster M, Schiefke F, et al. Sentinel lymph node biopsy in squamous cell carcinomas of the lips and the oral cavity—a single center experience. J Surg Oncol. 2007;95(2):97–105. doi: 10.1002/jso.20664. [DOI] [PubMed] [Google Scholar]
- 44.Terada A, Hasegawa Y, Yatabe Y, et al. Follow-up after intraoperative sentinel node biopsy of N0 neck oral cancer patients. Eur Arch Otorhinolaryngol. 2011;268(3):429–435. doi: 10.1007/s00405-010-1364-2. [DOI] [PubMed] [Google Scholar]
- 45.Yamauchi K, Fujioka Y, Kohno N. Sentinel node navigation surgery versus observation as a management strategy for early tongue carcinoma. Head Neck. 2012;34(4):568–572. doi: 10.1002/hed.21776. [DOI] [PubMed] [Google Scholar]
- 46.Yuen AP, Ho CM, Chow TL, et al. Prospective randomized study of selective neck dissection versus observation for N0 Neck of early tongue carcinoma. Head Neck. 2009;31(6):765–772. doi: 10.1002/hed.21033. [DOI] [PubMed] [Google Scholar]