1. Introduction
Involuntary contractions are common in muscles paralyzed by spinal cord injury (SCI) and represent one component of spasticity (Hiersemenzel et al., 2000). While some individuals find these spasms useful, others indicate they disrupt sleep (Biering-Sorensen et al., 2009), care, mobility, and rehabilitation (Little et al., 1989; Sköld et al., 1999; Adams and Hicks, 2005). Clinical assessments require that people with SCI count their spasms. Spasms are rated as severe when more than 10 occur per hour (Penn et al., 1989) or per day (Snow et al., 1990). To our knowledge, there are no official guidelines for counting muscle spasms. Thus, the nature of the contractions, their duration, intensity, source, or the context in which they occur may influence whether someone includes a contraction in their spasm count. Contractions may not be perceived if the injury results in sensory deficits, which may lead to underestimates in the count. Spasms cannot be counted during sleep. Thus, an objective record of muscle activity is needed to reveal the typical occurrence of involuntary contractions after SCI.
Our long-term objective is to use 24-hour electromyographic (EMG) recordings from multiple muscles to characterize involuntary muscle contractions after SCI. Quantification of spasm count and duration based on EMG would provide objective measurements for comparison to subject counts, evaluation of clinical standards of care, and assessment of intervention effectiveness. To interpret analog EMG recordings as spasm events requires an operational definition for identifying an individual spasm, as well as a measure for quantifying its duration and intensity. Criteria are also needed to classify different types of involuntary contractions that commonly occur in paralyzed muscles (e.g. clonus, motor unit potentials, tonic EMG, myoclonus; Little et al., 1989; Thomas and Ross, 1997; De Mello et al., 2002; Gorassini et al., 2004; Benz et al., 2005; Wallace et al., 2012; Zijdewind and Thomas, 2012). Continuous recordings of EMG during voluntary contractions have been digitized, partitioned into events and rest intervals, and the length of each contraction and rest measured (Kern et al., 2001; Mork and Westgaard, 2005). In practice, however, any given algorithm would have to be developed to capture the suite of interpretable features in the EMG signal that human observers would agree are important for spasm characterization. Consequently, our aim was to develop a spasm identification and classification approach for EMG. To evaluate the approach, we compared how well two knowledgeable observers independently identified and classified spasm events in the same dataset using the same set of analysis criteria. Comparisons of rater agreements and discrepancies provide invaluable information to guide development of software to automate future analyses. The amount of agreement between raters also sets a standard that an automated algorithm may be expected to attain. Furthermore, development of a standard for quantifying spasm measurements from EMG gives a baseline for comparison to subjective counts of spasms.
2. Methods
2.1. Subject information
The subjects (one woman, 3 men; 27-52 years) had a chronic (4-33 years) cervical SCI at C4 (n= 1) or C6 (n=3) due to a diving (n=3) or a sports accident. All procedures were approved by the Investigational Review Board at the University of Miami. Subjects gave informed written consent before participation.
2.2. Experimental setup
2.2.1. Muscles and electrode placements
Bipolar surface EMG was recorded from 8 muscles (right and left medial gastrocnemius, MG; tibialis anterior, TA; biceps femoris, BF; vastus lateralis, VL) of each subject for 24 hours (Klein et al., 2010; Mummidisetty et al., 2012). To record EMG, three electrodes (2.5×1 cm) were taped to each muscle, 4 cm apart (Superior Silver Electrodes, Uni-patch, MN). The distal electrode for TA and MG was just proximal to the respective tendon-muscle interface; 12 cm proximal to the patella for VL and BF, the latter posterior on the leg. The two distal electrodes were active. The proximal electrode was ground (Mummidisetty et al., 2012).
2.2.2. Experimental protocol
Before and after the 24-hour EMG recording, maximal muscle compound action potentials (M-waves) were recorded from each MG, TA and VL muscle in the laboratory in response to supramaximal stimulation of the respective tibial, common peroneal and femoral nerves. The sciatic nerve is too deep to reliably excite BF using surface stimulation. Single pulses (50 μs duration) of increasing intensity (10 mA steps) were delivered to each nerve until no further EMG increment occurred. EMG were sampled online (3,200 Hz) using a SC/Zoom system (Umeå University, Sweden). For each muscle, the M-wave area in 10 ms was used to normalize the 24-hour EMG.
For the 24-hour EMG recording, the electrodes from each muscle were connected to a battery-powered, microcontroller-based, digital data-logger that amplified (Model Z03, Motion Labs Systems, Baton Rouge, LA), filtered (10-500 Hz) and sampled the data continuously at 1000 Hz for 24 hours (Mummidisetty et al., 2012). The system was carried in a hip pack throughout the recording. Offline, spasms were analyzed using custom routines written in MATLAB and DADiSP (Klein et al., 2010).
2.2.3. EMG processing
All EMG were involuntary because signals were recorded from muscles paralyzed by SCI (under no voluntary control). The records were rectified, and integrated every 10 ms, which corresponds to the average duration of motor unit potentials, the smallest signal we expect to record (Häger-Ross et al., 2006; Thomas et al., 2006). EMG signals were distinguished from baseline noise by calculating a threshold. Five 30 s periods of quiet time (no EMG) in different hours of each 24-hour recording were rectified, integrated every 10 ms, and the mean and standard deviation (SD) of the highest 10% of baseline integrals calculated. The threshold for spasm detection was defined as the mean+3SD of this baseline integral (Klein et al., 2010).
2.3. Protocol for spasm characterization
Two raters (authors) with similar experience in EMG recognition independently identified and classified all the spasms in 48 hours of data, and marked the spasm starts and ends. Three hours of data from the right MG, TA, VL, and BF muscles (12 different hours, spread across the 24-hour recording) were analyzed per subject (n=4), which involved analysis of ~1000 total spasms.
2.3.1. Identification and count
Raters identified spasms by the presence of EMG (defined as above-threshold integrals), its timing, and pattern. To include EMG in the spasm count, there had to be ≥50 ms of above-threshold integrals in ≥ 100 ms, and this EMG had to be preceded and followed by at least 1 s of rest (below-threshold integrals; Fig. 1A). The only exception was myoclonus (see, 2.3.3. Classification). The four motor unit potentials in Fig. 1B also constitute a spasm because each potential generated two above-threshold integrals (80 ms of EMG, total) over >100 ms and were preceded and followed by 1 s of below-threshold integrals (not shown).
Fig. 1.
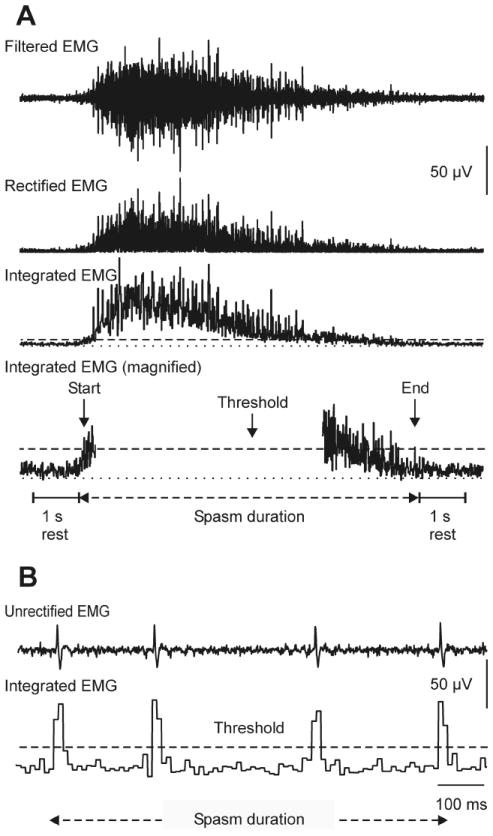
(A) EMG data were software-filtered (cascaded 30 Hz high-pass; 60 Hz FIR filters; basic windowed linear-phase Finite Impulse Response digital filters of order 472; Mummidisetty et al., 2012), rectified, and integrated every 10 ms. Integrals above-threshold (dashed line) distinguished EMG from baseline. There was one second of rest between spasms. Spasm duration was determined by threshold crossings. EMG formed an interference pattern, indicating a tonic spasm. (B) Distinct motor unit potentials signified a unit spasm.
2.3.2. Duration and intensity
Spasm start (time the first integral crossed threshold) and end (time the last integral went below threshold) were marked by each rater using DADiSP cursors. The difference between these times represented spasm duration (e.g. 7.25 s and 875 ms, Fig. 1A and B, respectively). Below-threshold integrals were always included in spasm duration. Only above-threshold integrals, from spasm start to end, were averaged offline to provide mean spasm intensity. To compare results across spasms, muscles and experiments, data were normalized to the respective maximal M-wave.
2.3.3. Classification
Each spasm was classified as one of five types based on the duration, timing, and pattern of EMG. These types were termed: 1) Unit spasm, provided the EMG included distinct motor unit potentials (Fig. 1B, Fig. 2A,B); 2) Tonic spasm, if the motor unit potentials overlapped to form an interference pattern (Fig. 1A, 2C,D); 3) Clonus, provided there were ≥4 bursts of EMG, each typically lasting 40-80 ms and occurring ~80-250 ms apart (Fig. 2E,F; Mummidisetty et al., 2012); 4) Myoclonus, if the spasm involved ≥4 contractions that repeated at a rate <4 Hz. As this criterion introduced an exception to the 1 s rest rule used to define a spasm, classification of myoclonus depended on recognition of the relatively rhythmic nature of the bursts of EMG (Fig. 2G,H). Myoclonus ended when no contraction occurred in twice the time between the initial 3 contractions (up to 65 s); 5) a Mixed spasm, if EMG included at least three spasm types. For example, spasms often began with motor unit potentials, that then formed an interference pattern so became tonic, transitioned into clonus, followed by motor unit potentials (Fig. 2I,J). While spasms involving tonic EMG, clonus or myoclonus often began, included, and/or ended with discrete motor unit potentials (Fig. 2C,F,G), they retained their original classification unless a third contraction type emerged.
Fig. 2.
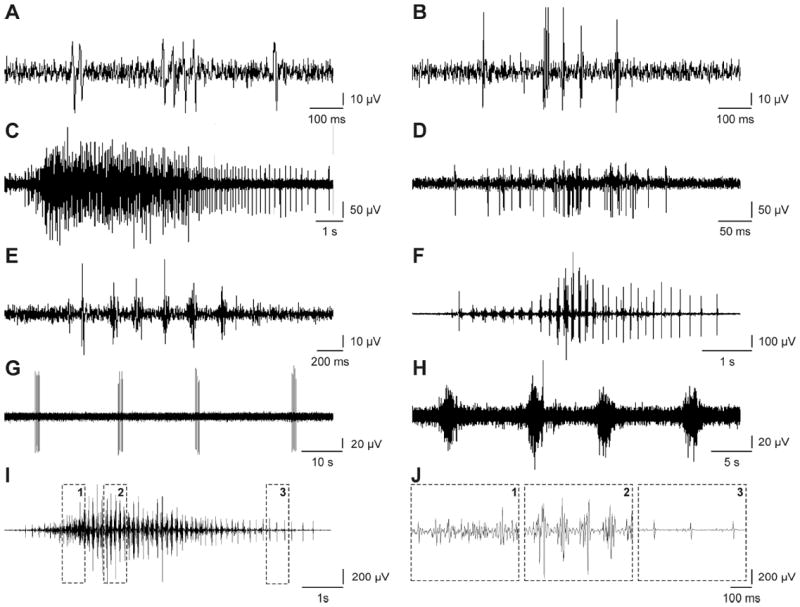
Examples of unit (A: BF data, 4-5pm; B: MG, 8-9am), tonic (C: BF, 2-3am; D: VL, 4-5am), clonus (E: MG, 6-7am; F: MG, 3-4pm), myoclonus (G: MG, 4-5am; H: MG, 7-8pm) and a mixed spasm (I: MG, 9-10am). (J) Regions within the mixed spasm are expanded to show tonic EMG (box 1), clonus (box 2) and unit potentials (box 3).
2.3.4. Evaluation of rater analysis
Agreement on spasm count occurred when two raters marked the same spasm. Agreement on spasm duration was the time overlap in these same spasms. Agreement on spasm classification occurred when both raters gave the same label to a given spasm or the same data (one rater labeled EMG as one spasm but the other rater split these same data into multiple spasms of the same type).
Reliability between raters for spasm count and duration was expressed by the intraclass correlation coefficient (ICC). A linear model with random effects for subjects, muscles within subjects, and raters was fit since inference beyond the current two raters is of interest. An ICC for absolute reliability (ICC(A,1), McGraw and Wong, 1996), which includes variation from all sources in the model, was estimated using mean squares (Weir, 2005). An F test was used to assess systematic effects between raters. muscles within subjects, and subjects.
The Bowker (1948) test for symmetry was used to test for agreement between raters on spasm classification, with spasms classified as tonic, unit or other.
3. Results
3.1. Total spasm counts
In 48 hours of data, Rater 1 and 2 marked a total of 935 and 1049 spasms, respectively. When total counts were compared for each muscle (n=4) and subject (n=4), most data lay near the unity line, indicating that the total count for a given muscle and subject was similar for each rater (Fig. 3A). An estimated ICC of 0.902 suggests high rater reliability for spasm count.
Fig. 3.
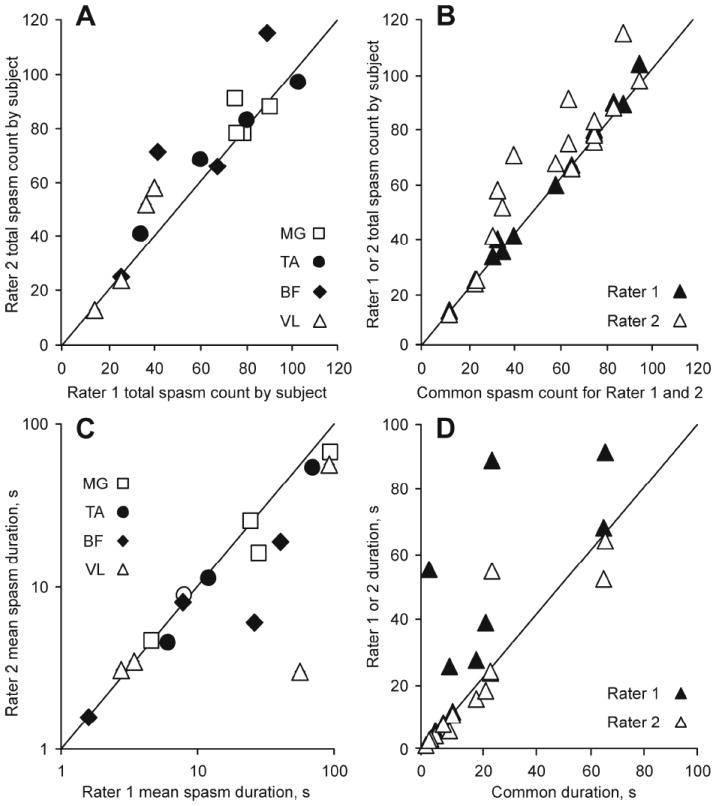
(A) Total spasm count and (C) mean spasm duration (logarithmic axes) for Rater 1 versus Rater 2 by muscle. (B) Common spasm count versus Rater 1 and 2 total count. (D) Mean spasm duration common to both raters compared to durations for Rater 1 and 2. Total spasm counts and mean spasm durations were the same when symbols lay on the unity line.
Most variability in the total spasm count was due to differences among muscles within subjects (84%) and among subjects (6.5%). For example, the total count for MG ranged from 75 to 90 across subjects for Rater 1 and from 78 to 91 for Rater 2 (open squares, one/subject), whereas the corresponding data for VL was 14 to 40 and 13 to 58 spasms (open triangles, one/subject), respectively (Fig. 3A). In contrast, variation in the total spasm count due to differences between raters was 2.6%. Thus, among several sources of variation that contribute to the total spasm count, variation between raters was by far the smallest.
3.2. Two independent raters marked the same spasms most of the time
Agreement on marking spasms was high across raters because the data showing the total count per muscle (n=4) and subject (n=4) for each rater (n=16 symbols) versus the common count (where both raters marked the same spasm), lie near the unity line (Fig. 3B). Both raters marked the same spasms in 896 cases. Discrepancies arose because one of the two raters split a spasm into two or more events (n=31), different spasms were combined as one event (n=15), spasms were missed (n=18), or marked events did not meet spasm criteria (n=19).
3.3. Spasm duration and intensity
The mean duration of spasms marked by Rater 1 were positively related to the mean duration of spasms identified by Rater 2 (Fig. 3C). An ICC of 0.764 is consistent with good rater reliability for spasm duration measurements. Like spasm counts, variability in spasm duration was largely due to muscle within subjects (58.4%) and subject (18.0%) rather than measurement differences between raters (7.4%). For example, mean spasm duration for MG muscles varied from 5 s to 94 s across subjects (open squares).
Most of the spasm duration measured by each rater overlapped (common duration, indicating similar spasm start and end times) since data for the individual versus common measurements of each rater lie on or close to the unity line (Fig. 3D). Since each rater largely marked the same spasms, with similar starts and ends, mean spasm intensity was also comparable across raters (intensity range across muscles: 0.5-6.1% and 0.5-5.5% maximal M-wave for Rater 1 and 2, respectively; R2=0.99).
3.4 Differences in spasm count and duration
There were systematic differences among raters for spasm count (F1,15=6.88, p=0.019) and duration (F1,15=8.34, p=0.011) relative to residual error in ANOVA. The absolute differences in spasm duration increased when one rater marked EMG as a single spasm while the other rater split the same data into multiple spasms. For example, in a myoclonus spasm, all of the rest times between the contractions contributed to spasm duration for Rater 1 (600 s, Fig. 4A). Rater 2 separated these data into two spasms (durations: 332 s and 92 s), thereby excluding the rest times between the events. Rater 2 marked multiple spasms more often (count ratio <1) than Rater 1 (ratio >1; Fig. 4B).
Fig. 4.
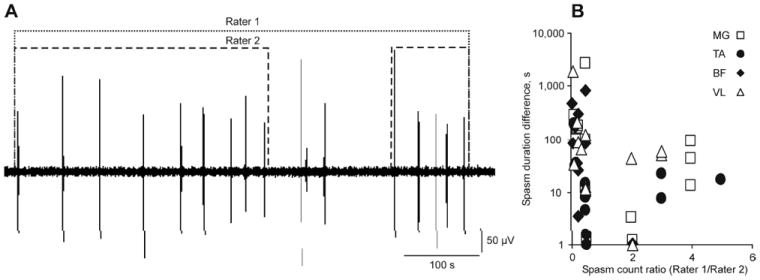
(A) EMG marked as myoclonus by Rater 1 was separated into 2 myoclonus spasms by Rater 2 resulting in different spasm counts and durations (MG data, 4-5am). (B) Differences in spasm count, expressed as the ratio between the spasm counts for Rater 1 versus Rater 2, versus differences in spasm durations. A count ratio >1 or <1 meant Rater 1 or 2 split EMG into multiple events, respectively.
3.5. Spasm classification
Figure 5 compares spasm classifications of Rater 1 and 2 with their classification agreement for four muscles. Agreement was higher when the symbol for each rater was close to the corresponding bar. Both raters classified most spasms as trains of motor unit potentials, then tonic spasms, irrespective of muscle. Clonus, myoclonus and mixed spasms were less frequent.
Fig. 5.
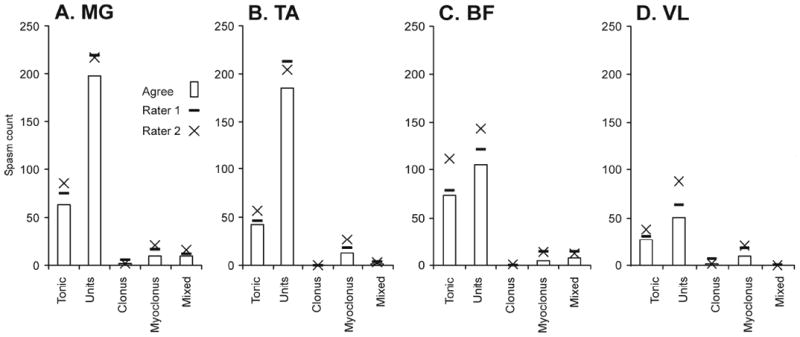
Number of spasms classified as unit, tonic, clonus, myoclonus or mixed by rater and muscle (A-D), compared to classification agreement between raters (bars).
3.6. Two raters classified most spasms the same way
Agreement on spasm classification was high. For example, Rater 1 and 2 counted 214 and 215 unit spasms in MG, respectively, but agreed on the classification for 195 spasms (Fig. 5A). For all of MG, two raters agreed on the classification of 278 spasms. Of the 24 disagreements, 10 (42%) arose from one rater marking EMG as one event while the other rater broke the same data into multiple spasms. Different classifications most often involved tonic versus mixed (n=8), unit (n=6), or myoclonus spasms (n=3).
Most spasms (89%) were classified into the same category by Rater 1 and 2 (x2= 31.57, 3df, p<0.001; Table 1). Overall, Rater 1 identified more spasms as tonic and fewer spasms as units or other than Rater 2.
Table 1.
Frequency of spasm classification
| Tonic Rater 2 n=220 | Unit Rater 2 n=574 | Other Rater 2 n=102 | |
|---|---|---|---|
| Tonic Rater 1 n=261 | 205 | 35 | 21 |
| Unit Rater 1 n=558 | 6 | 532 | 20 |
| Other Rater 1 n=77 | 9 | 7 | 61 |
4. Discussion
Our results show that two raters performed similarly when identifying muscle spasms, marking the beginning and end of contractions, and classifying different types of spasms. Agreement was high for spasms recorded from different muscles and subjects. Discrepancies in the spasm count, duration and type largely arose because the raters marked a series of contractions as either one or several events. These results provide the basis from which to design an algorithm to automate muscle spasm analysis. Automated analysis would eliminate observer variation in interpretation of spasm signatures and reduce the time needed to manually analyze large datasets generated with long-term EMG recordings.
4.1. Different raters count spasms similarly
The rules used here to identify muscle spasms were implemented reliably because only 2.6% of the variance in spasm count was attributable to raters. Far more variation in spasm count came from analysis of data from different muscles within a subject (84%) and from different subjects (6.5%; Fig. 3). Most of the designated spasm duration was also common to both raters, so the proportion of total variance due to raters was low (7.6%; Fig. 3). High inter-rater reliability and agreement on spasm count and duration was attributable to recognition of EMG and careful examination of the time over which signals exceeded a predetermined threshold for EMG. While raters missed some small motor unit potentials, or marked a few events that did not meet spasm criteria, most discrepancies in the count arose from one rater marking EMG as one event while the other rater split the same data into multiple events (Fig. 4). Any rests contributing to the duration of a single event were excluded from multiple events, resulting in smaller spasm durations. These inter-rater discrepancies can drive design of software that automates this analysis. An algorithm that applied the 1 s rule universally may improve accuracy because it would eliminate the spasm count and duration differences that arose from splitting contractions into multiple spasms. Automated analysis would also catch all small motor unit potentials that were occasionally missed by each rater and relieve the tedious process of manual evaluation. The rater would then only have to choose whether or not to combine contractions belonging to myoclonus or remove noise that sometimes contaminates long-term EMG recordings.
Rater differences in spasm counts were small across subjects (Fig. 3). How these results would compare to the spasm counting approach used by individuals with SCI is unclear. We counted spasms by muscle contractions that were 1 s apart and EMG that spanned at least 100 ms. Examination of EMG from different muscles shows complex patterns of activation. Simultaneous activation of multiple muscles was common but different muscles did not necessarily begin to contract together or fall silent at the same time. Subjective counts of spasms may take a more global view, particularly if contractions of certain muscle groups disrupt tasks (Little et al., 1989). Subjects may count contractions in muscle groups versus individual muscles, or only problematic contractions rather than all spasms as defined here. In this case, subjective counts would underestimate the number of contractions and overall muscle activity. This may explain why more than 10 spasms per hour are rated as severe in clinical studies (Penn et al., 1989), whereas our spasm counts per muscle were often more than double this. Future studies need to compare spasms identified from EMG to the involuntary muscle activity counted by individuals with SCI. Documenting which muscle contractions are counted, and why, would facilitate identification of those spasms that need clinical management.
4.2. Raters reliably classify different spasm types
Rater agreement on spasm classification was high (89%, Table 1). Across muscles, each rater classified most of the spasms as motor unit potentials, followed by spasms involving tonic EMG. The experience each rater had in EMG recognition enabled them to distinguish between EMG that included distinct motor unit potentials (unit spasm) versus an interference pattern (tonic spasm) most of the time (Fig. 5). It would be more difficult to program an algorithm to interpret these signal features. One intermediate solution tested here, after these analyses, was to define an EMG interference pattern by the number of consecutive above-threshold integrals. Close examination of spasms that both raters classified as tonic spasms showed that they typically included 5 or more consecutive integrals, whereas unit spasms had less. One EMG potential usually generated one or two above-threshold integrals, requiring 3 to 10 closely-spaced potentials for signal interference. These potentials are also likely to belong to multiple motor units since firing rates reached 34 Hz during tonic spasms (Thomas and Ross, 1997). Adding these criteria resolved the inter-rater differences in classification of tonic and unit spasms, and is a strategy that would be easy to implement with an algorithm.
Agreement was reduced on less frequent spasm types (clonus, myoclonus, mixed), all of which involve rhythmic contractions. Bursts of EMG occur at 4 to 12 Hz during clonus, largely dependent on the reflex arc length (Iansek, 1984; Wallace et al., 2012). Contractions repeat at lower frequencies during myoclonus (De Mello et al., 2002; Zucconi et al., 2006) and classification, count and duration discrepancies arose largely from raters grouping versus separating contractions. The EMG in all of these spasm types also share common features beyond the presence of motor unit potentials, another factor that contributes to rater discrepancies. For example, most of the repeated bursts of EMG during myoclonus in themselves meet the criteria for classification as a unit or tonic spasm (Fig. 2G,H).
Both the agreement and discrepancies seen here can guide design of an algorithm to automate spasm classification. To eliminate the different grouping (or separation) of contractions, all of the contractions could be marked individually using time and integral threshold crossing criteria, including the repetitive contractions of myoclonus. An expert would then only have to identify when spasms involving repetitive contractions start and end, and combine them. Furthermore, 93% of the spasms agreed on by both raters were classified as unit or tonic (Fig. 5). Thus, an automated, dual-choice classification approach (unit or tonic) would type most contractions appropriately. Other features seen by a rater would be more difficult to automate. For example, motor unit potentials often occur between bursts of EMG in clonus (Mummidisetty et al., 2012) and myoclonus. Similarly, only a rater would identify above-threshold EMG versus noise. Thus, the long-term goal of automated analysis would be to mimic what a person sees and recognizes in the EMG itself. More complex approaches such as machine learning may be valuable. Nevertheless, intermediate strategies that use time and threshold-based automation would ensure consistent analysis of muscle spasms and dramatically reduce analysis time from days to hours.
4.3. Clinical implications
Long-term (24-hour) EMG recordings can provide objective and quantifiable measures of activity in paralyzed muscles. This includes when different types of spasms occur, how often, the contraction duration, intensity, and patterns of muscle activation. These data have no clinical filter, however. A key issue is to identify the contraction types and features that are important to a person with SCI. They may define spasms as those that either disrupt or facilitate completion of a task. Thus, future studies need to link spasms identified from EMG records to subject interpretation of the same spasms. While a person can count spasms during wake time, other contraction features, spasm types or the context in which the contractions occur (e.g. daytime versus sleep) may provide more informative identifiers of spasm importance and improve our understanding of involuntary muscle activity that has functional consequences. This new information may assist in clinical spasm evaluation, show which spasm types need treatment, and how they respond to intervention.
Acknowledgments
This work was supported by the Craig Nielsen Foundation (#83848); National Institutes of Health (NS-30226; R24 A#1F51C43 under contract to the Rehabilitation Institute of Chicago); and The Miami Project to Cure Paralysis.
Biographies
Christine K. Thomas received her PhD degree from the University of Alberta, Canada, in 1986. She is a Professor at The Miami Project to Cure Paralysis, at the University of Miami. Her research examines the consequences of spinal cord injury on skeletal muscle. including muscle atrophy, weakness, denervation, fatigue and spasticity.

Marine Dididze received her MD degree from Tbilisi State Medical University in 1990 and a PhD in 2001. Currently she is an Associate Scientist at The Miami Project to Cure Paralysis, at the University of Miami Miller School of Medicine. Her research interests focus on neurotrauma and interventions for the acutely damaged human spinal cord, including hypothermia, and Schwann cell transplantation.

Adriana Martinez received a B.Sc. in Mathematics from Havana University, Havana, Cuba. She is currently a graduate student in Statistics at George Mason University, Fairfax, VA and a Research Support Specialist at The Miami Project to Cure Paralysis, Miami, FL.

Richard W. Morris received a Masters in Statistics and a Ph.D. in Biomathematics from the Department of Statistics at NC State University. He is a research statistician at Social & Scientific Systems, Inc., Durham NC. His interests include toxicology, genomics and clinical studies.

Footnotes
Conflicts of interest
None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MM, Hicks AL. Spasticity after spinal cord injury. Spinal Cord. 2005;43(10):577–86. doi: 10.1038/sj.sc.3101757. [DOI] [PubMed] [Google Scholar]
- Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86(1):52–9. doi: 10.1016/j.apmr.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Jennum P, Laub M. Sleep disordered breathing following spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):165–70. doi: 10.1016/j.resp.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Bowker AH. A test for symmetry in contingency tables. J Am Stat Assoc. 1948;43(244):572–4. doi: 10.1080/01621459.1948.10483284. [DOI] [PubMed] [Google Scholar]
- De Mello MT, Silva AC, Esteves AM, Tufik S. Reduction of periodic leg movement in individuals with paraplegia following aerobic physical exercise. Spinal Cord. 2002;40(12):646–9. doi: 10.1038/sj.sc.3101381. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127(10):2247–58. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Hager-Ross CK, Klein CS, Thomas CK. Twitch and tetanic properties of human thenar motor units paralyzed by chronic spinal cord injury. J Neurophysiol. 2006;96(1):165–74. doi: 10.1152/jn.01339.2005. [DOI] [PubMed] [Google Scholar]
- Hiersemenzel LP, Curt A, Dietz V. From spinal shock to spasticity: neuronal adaptations to a spinal cord injury. Neurology. 2000;54(8):1574–82. doi: 10.1212/wnl.54.8.1574. [DOI] [PubMed] [Google Scholar]
- Iansek R. The effects of reflex path length on clonus frequency in spastic muscles. J Neurol Neurosurg Psychiatry. 1984;47(10):1122–4. doi: 10.1136/jnnp.47.10.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol. 2001;91(5):2224–32. doi: 10.1152/jappl.2001.91.5.2224. [DOI] [PubMed] [Google Scholar]
- Klein CS, Peterson LB, Ferrell S, Thomas CK. Sensitivity of 24-h EMG duration and intensity in the human vastus lateralis muscle to threshold changes. J Appl Physiol. 2010;108(3):655–61. doi: 10.1152/japplphysiol.00757.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JW, Micklesen P, Umlauf R, Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil. 1989;68(1):32–6. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Mork PJ, Westgaard RH. Long-term electromyographic activity in upper trapezius and low back muscles of women with moderate physical activity. J Appl Physiol. 2005;99:570–8. doi: 10.1152/japplphysiol.00198.2005. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychol Meth. 1996;1:30–46. [Google Scholar]
- Mummidisetty CK, Bohorquez J, Thomas CK. Automatic analysis of EMG during clonus. J Neurosci Methods. 2012;204(1):35–43. doi: 10.1016/j.jneumeth.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RD, Savoy SM, Corcos D, Latash M, Gottlieb G, Parke B, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–21. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- Sköld C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80(12):1548–57. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Tsui JK, Bhatt MH, Varelas M, Hashimoto SA, Calne DB. Treatment of spasticity with botulinum toxin: a double-blind study. Ann Neurol. 1990;28(4):512–5. doi: 10.1002/ana.410280407. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Ross BH. Distinct patterns of motor unit behavior during muscle spasms in spinal cord injured subjects. J Neurophysiol. 1997;77(5):2847–50. doi: 10.1152/jn.1997.77.5.2847. [DOI] [PubMed] [Google Scholar]
- Thomas CK, Johansson RS, Bigland-Ritchie B. EMG changes in human thenar motor units with force potentiation and fatigue. J Neurophysiol. 2006;95(3):1518–26. doi: 10.1152/jn.00924.2005. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Ross BH, Thomas CK. Motor unit behavior during clonus. J Appl Physiol. 2005;99(6):2166–72. doi: 10.1152/japplphysiol.00649.2005. [DOI] [PubMed] [Google Scholar]
- Wallace DM, Ross BH, Thomas CK. Characteristics of lower extremity clonus after human cervical spinal cord injury. J Neurotrauma. 2012;29(5):915–24. doi: 10.1089/neu.2010.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–40. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Thomas CK. Firing patterns of spontaneously active motor units in spinal cord-injured subjects. J Physiol. 2012;590(Pt 7):1683–97. doi: 10.1113/jphysiol.2011.220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucconi M, Ferri R, Allen R, Baier PC, Bruni O, Chokroverty S, et al. The official World Association of Sleep Medicine (WASM) standards for recording and scoring periodic leg movements in sleep (PLMS) and wakefulness (PLMW) developed in collaboration with a task force from the International Restless Legs Syndrome Study Group (IRLSSG) Sleep Med. 2006;7(2):175–83. doi: 10.1016/j.sleep.2006.01.001. [DOI] [PubMed] [Google Scholar]


