Abstract
Extracorporeal Photochemotherapy (ECP) is a widely used therapy for cutaneous T cell lymphoma (CTCL). Although the mechanism of clinical action of ECP is not precisely established, previous studies have shown evidence of induction of dendritic cells (DC). Here we show that, under flow conditions similar to those in post-capillary venues, ECP promotes platelet immobilization and activation, initiating stepwise receptor-ligand interactions with monocytes, which then differentiate into DC. These findings clarify how ECP directly stimulates DC maturation; suggest a new clinically applicable approach to the obtainment of DC; and identify a novel mechanism that may reflect physiological induction of DC.
Introduction
The potential for cellular immunity to control or therapeutically eliminate cancer has long aroused considerable interest [1]. For example, an important immunoprotective role for antitumor T cells in colorectal, melanoma and prostate cancers is supported by recent evidence [2-4]. However, once a cancer is clinically progressive, it has already overcome immunologic defenses. Therefore, the substantial challenge to the cancer immunotherapist is that a functional tolerance to the malignant cells must be broken.
Extracorporeal Photochemotherapy (ECP), a therapy with expanding use in more than 500 centers in Europe and America, induces clinical responses in the majority of immunocompetent cutaneous T cell lymphoma (CTCL) patients, including some complete and long term remissions[2-4]. Although its mechanism of action is complex and not clearly elucidated, its clinical efficacy and advantageous safety profile have attracted much investigative interest[5-11]. In ECP therapy, the patient's blood is extracorporeally routed, as a thin film, between parallel transparent plates, permitting site-focused targeting of passaged leukocytes by UVA irradiation, followed by intravenous return of the treated blood. While the initial rationale was that rapidly-dividing lymphoma cells were killed by UV-induced DNA damage, we recently reported that ECP induces extracorporeally passaged monocytes to enter the DC differentiation pathway within a single day of treatment[12].
ECP causes both clinically potent anti-CTCL T cell responses and functional DCs suggesting a linkage between the two phenomena. Since ECP's induction of monocyte-to-DC maturation does not require addition of cytokine growth factors, but merely monocyte passage through the ECP flow chamber, we reasoned physiologic signaling is likely responsible. We further reasoned that, if the basis for that phenomenon could be deciphered and then harnessed, it might then become possible to not only enhance ECP's clinical potency for CTCL, but also to apply the underlying therapeutic principle to treatment of a broader range of cancers.
To interrogate this possibility, we hypothesized that ECP directly promotes DC differentiation. Based initially on dynamic imaging of leukocytes passing through an experimental photopheresis chamber, we have identified and report a process by which platelets adhere to the device chamber wall, become activated, engaging monocytes in a P-selectin-dependent interaction that promotes DC differentiation in a manner dependent on platelet density and shear stress. These findings support the hypothesis that ECP is a potent initiator of immune activation; they suggest a new clinically applicable approach to the obtainment of DC that does not depend on supraphysiologic concentrations of cytokines; and they identify a functional, shear-stress-dependent platelet monocyte interaction that may reflect a physiologic method of induction of DC.
Materials and Methods
Procurement of leukocytes and platelets
All samples were acquired from young, healthy subjects not taking medications known to influence platelet function. Samples were obtained under the guidelines of the Yale Human Investigational Review Board, and informed consent was provided according to the Declaration of Helsinki. Peripheral blood specimens were collected into syringes containing heparin, then separated using Ficoll-Hypaque (Gallard-Schlessinger). The mononuclear leukocyte fraction was collected and washed then resuspended in RPMI-1640 medium (GIBCO) to a final concentration of 5 × 106 mononuclear cells/ml.
Preparation of Platelet-rich-Plasma
Whole blood was centrifuged at 150 g for 15 min at room temperature. The platelet-rich-plasma (PRP) layer was collected and centrifuged at 900 g for 5 min, and the platelet pellet resuspended in RPMI 1640 to the desired concentration.
Preparation of Parallel-Plates
Two types of parallel-plate flow chambers were used to model the flow dynamics of ECP. Experiments involving the assessment of cell phenotype post-flow were conducted using the larger Glycotech system (Glycotech). This system consisted of a volumetric flow path measuring 20000 × 10000 × 254 microns (length × width × height). The bottom plate in this system was composed of a 15mm petri dish (BD Biosciences) separated by a gasket and vacuum-connected to an acrylic flow deck, which formed the upper plate. For experiments requiring the plates to be pre-coated with platelets, prior to assembling the flow chamber, 20 drops of the desired concentration of PRP was placed in the center of the petri dish and platelets allowed to settle for 20 minutes at room temperature. The petri dish was washed twice with 2ml of RPMI, and the flow chamber then assembled.
Experiments not requiring the collection and phenotyping of cells post-flow were carried out using Vena8 biochips (Cellix Ltd), a parallel-plate system made of acrylic chips with channels measuring 20000 × 400 × 100 microns (length × width × height). Protein coating of these chips is described in the appropriate section below.
Experiments using Parallel-Plates
The parallel-plate flow chamber was mounted on the stage of a phase contrast optical microscope (CK40) with a 10× objective. All runs were performed at room temperature. A uniform laminar flow field was simulated by use of a syringe pump (KD Scientific) capable of generating near-constant volumetric flow rates.
All experiments were viewed in real time, recorded at 15.2 frames per second using a DP 200 digital camera and software (DeltaPix).
Overnight culture
Cells were cultured at a concentration of 5 × 106 cells/ml in RPMI-1640 medium (GIBCO) supplemented with 15% AB serum (Gemini Bio-Products) for 18 hours at 37° C in 5% CO2.
Immunophenotyping
Monoclonal antibodies for immunophenotyping were obtained from Beckman Coulter or Sigma and used at their pre-determined optimal dilutions. Immunofluorescence was analyzed using a FC500 flow cytometer (Beckman Coulter). Two-color membrane staining was performed using standard protocol.
Quantitative real-time PCR
Gene expression was compared between cells exposed during flow through the parallel plates to low (10 ± 5/low power field [lpf]) versus high (102 ± 32/lpf) levels of platelets, followed by overnight culture. Cell RNA was isolated using RNeasy Mini Kit columns with on-column DNase I treatment (QIAGEN). RNA yield and purity were measured using a NanoDrop ND-1000 Spectrophotometer and an Agilent 2100 Bioanalyzer. RNA was reverse transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). TaqMan real-time PCR was used to detect transcripts of DC-LAMP, CD40, ADAM Decysin, Lox1, CCR7, CD80, CD83, CD86, FPRL2, and GPNMB. Primers and probes for each sequence were obtained as inventoried Taqman Gene Expression Assays (Applied Biosystems). HPRT1 was used as a reference gene.
Co-cultures of Platelets with Monocytes
Following Ficoll-Hypaque separation, mononuclear cells were resuspended in 30% AB serum/RMPI to a final concentration of 10 × 106 cells/ml. An additional 500 μl of platelets (suspended in RPMI, at 2× the desired final concentration) or RPMI without platelets was then added to each well. To activate platelets, 500 μl containing 2 units of thrombin was added to half the wells, and 500 μl of RPMI was added to the others to balance the volume.
Platelet Adhesion Studies
Platelet adhesion experiments were performed using the Vena8 flow chamber. Fibrinogen and fibronectin (Sigma) were dissolved in PBS to a final concentration of 200mcg/ml. Channels of the Vena8 chips were incubated at room temperature in a humidified chamber for 2 hours with the protein solution, autologous plasma, or PBS alone. The channels were washed with 5× the volume RPMI. Platelet-rich-plasma was then perfused through the protein-coated channel at the indicated shear-stress, held constant. For each channel, still images were acquired exactly 90 seconds into the experiment at fields centered at 2500, 7500, 12500, and 17500 microns from the start point of infusion.
Some experiments involved pre-treating platelet-rich-plasma with protein fragments prior to infusion through the channels. Small RGD peptides, containing the amino-acid sequence Arg-Gly-Asp-Ser; DRG peptides, contain the amino-acid sequence Ser-Asp-Gly-Arg; or fragment 400-411 of fibrinogen, containing the amino-acid sequence His-His-Leu-Gly-Gly-Ala-Lys-Gln-Ala-Gly-Asp-Val, were incubated at a concentration of 2mM with PRP for 20 minutes at room temperature. The PRP was then perfused through the channels as previously described.
Receptor-Ligand Studies
Platelet-coated Vena8 channels were pre-treated with either 40 μg/ml anti-p-selectin (R&D Systems) or 40 μg/ml of an isotype control for 30 minutes at room temperature, then washed with 5× the volume RPMI. Mononuclear cell suspensions were pre-treated with either RGD or DGR peptides at a concentration of 2.5 mM. Video samples lasting 400 frames (26.3 seconds) were recorded 60 seconds after commencement of flow using a lower power field of view spanning 400 microns and centered at 7500 microns from the flow start point.
β-1 integrin conformation was assessed using the Glycotech flow chamber. 15mm platelet-coated petri dishes (described above) were pre-treated with 40 μg/ml anti-P-selectin or an isotype control for 20 minutes at room temperature, then washed with 5× the volume RPMI. Immediately following perfusion through the platelets, cells were immunophenotyped with anti-CD29 HUTS-21 (BD Biosciences), an antibody that specifically binds to the active (open) conformation of β1 integrins.
Results
Monocytes in flow transiently interact with immobilized platelets
ECP involves the flow of leukapheresed blood between large transparent plastic parallel-plates separated by 1 mm. To permit detailed analysis of the flow dynamics involved during ECP, independent of UVA/8-MOP exposure, we reproduced the flow conditions of ECP using miniature parallel plates with surface area of only 0.8 mm2, separated by 100 microns. This model permitted visualization using digital microscopy. Studies using the model revealed the following sequence occurring in ECP (see Supplement 1 for video): initial adherence of platelets from the flow stream to the plastic plate, followed by transient binding of passaged monocytes to the immobilized platelets.
DC induction correlates with the number of monocyte-platelet interactions
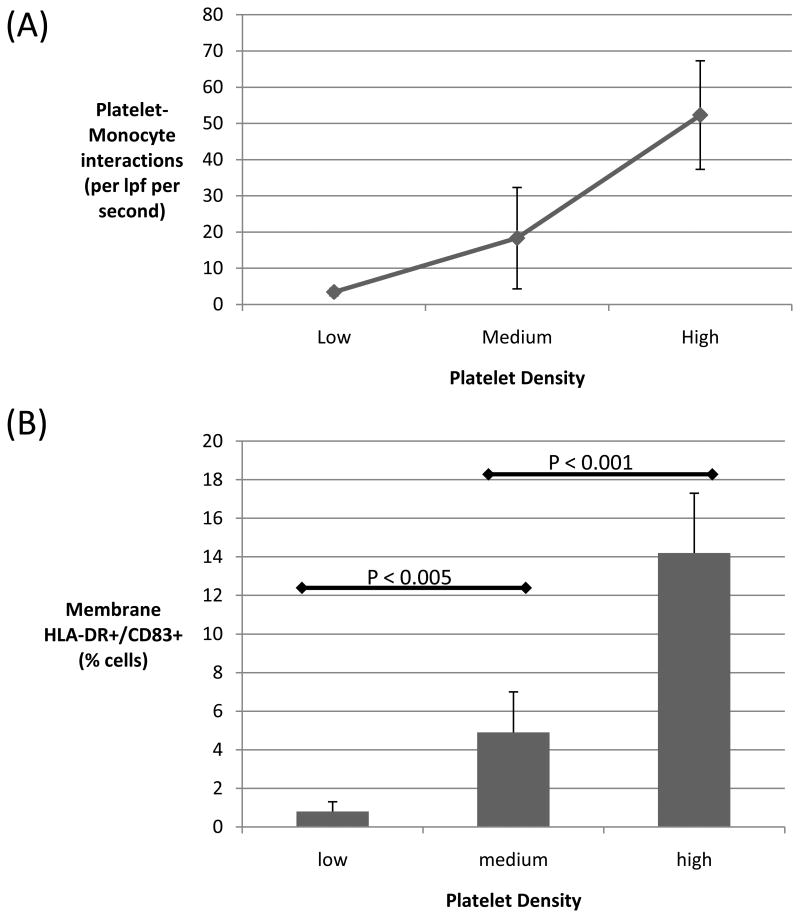
Platelets were hypothesized to induce monocyte-to-DC differentiation under conditions of flow. To test the influence of platelets on monocyte-to-DC differentiation, monocytes were passed between parallel plates pre-coated with autologous platelets at low (10±5/low power field [lpf]), medium (44±20/lpf), and high (102±32/lpf) densities. Cells were passed through the plates at a flow rate producing a wall shear stress of 0.5 dyne/cm2, analogous to the wall shear stress in post-capillary venules. The number of monocyte-platelet interactions per unit time increased in proportion to augmented density of platelets (see Supplement 2 for videos). An average of 52.3 ⩲ 15 monocyte-platelet interactions per lpf per second were observed with the high-density plate, dropping to 18.3 ⩲ 14 and 3.4 ⩲1 interactions per second with the medium and low-density plates, respectively (Figure 1A).
Figure 1. Effect of platelet density on number of monocyte-platelet interactions and subsequent monocyte phenotype.
Monocytes were passed through parallel plates coated with platelets at low, medium, or high density. (A) The number of monocyte-platelet interactions increased substantially for plates coated with higher densities of platelets. (B) After overnight incubation, monocytes which were exposed to high levels of platelets were significantly more likely to develop a phenotype consistent with DC differentiation, as assessed by expression of membrane CD83 and HLA-DR (high versus medium or low density: p <0.0001; medium versus low density: p < 0.005). Data shown are the means (± SD) of at least 6 independent experiments. lpf, low power field.
Percentage of monocytes developing a DC phenotype correlated with frequency of observed platelet interactions (Figure 1B). An increasing number of monocyte-platelet interactions correlated with increasing proportion of cells expressing markers consistent with DC differentiation, membrane HLA-DR and CD83. In 6 independent experiments, an average of 14.2% of monocytes exposed to the high-density platelet-coated plate were simultaneously HLA-DR+ and CD83+ after overnight incubation, compared to 4.9% and 0.8% of monocytes exposed to plates coated with medium and low levels of platelets, respectively.
Monocyte exposure to platelets results in changes in gene expression
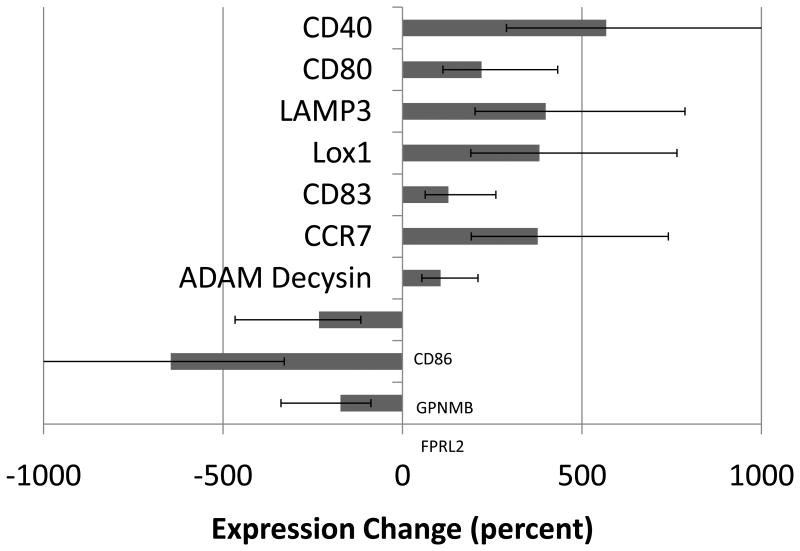
RT-PCR was performed to assess for changes in gene expression. Monocytes were passed through parallel plates coated with high or low densities of platelets as described in the previous section. Following overnight incubation, RNA was extracted and RT-PCR performed to determine level of expression for 10 genes associated with DC (Figure 2). CD40, a costimulatory molecule with known expression on mature DCs [13], was found to be upregulated by more than five-fold (565%) in monocytes exposed to high densities of platelets relative to monocytes exposed to low levels. LAMP3, a marker specific to DC differentiation [14], was upregulated four-fold (398%). CD80, a costimulatory molecule known to be upregulated upon APC activation [15], was found upregulated two-fold (220%) in monocytes exposed to high levels of platelets. CCR7, a chemokine receptor known to play a role in DC migration to lymphoid organs, was upregulated nearly four-fold (376%). LOX1, CD83, CCR7, and ADAM Decysin, all genes associated with DC [16], were also upregulated in the monocytes exposed to high levels of platelets. FPRL2, GPNMB, and CD86 were all downregulated in monocytes exposed to high levels of platelets. FPRL2 is a receptor that when activated is known to inhibit DC maturation [17]; GPNMB is a protein involved in decreasing cytokine production [18]; CD86 is a costimulatory molecule expressed by APCs.
Figure 2. Gene expression following exposure to platelets.
Monocytes were exposed to high or low levels of platelets in flow. Following overnight incubation, cells were assessed for differences in gene expression using RT-PCR. Figure shows gene expression changes in monocytes exposed to high levels of platelets relative to those exposed to low levels. Seven genes associated with DC-differentiation and/or function were found to be upregulated, while three were downregulated. Of the genes downregulated, GPNMB and FPRL2 have known functions in decreasing cytokine production and inhibiting DC maturation, respectively. Of the genes upregulated, all have either pro-immune functions or miscellaneous roles in DC biology. See text for specific description of genes. Data shown are the means (± SE) of 2 independent experiments.
DC induction in the presence of platelets does not occur under static conditions
Platelets could potentially influence monocytes through direct receptor-ligand interaction, or via cytokines and other secreted mediators. Furthermore, it is recognized that formation of receptor-ligand bonds in flowing systems is influenced by shear forces [19, 20]. To determine whether the platelet induction of monocyte-to-DC differentiation requires flow dynamics, we tested the role of platelets under static conditions in three independent experiments. Monocytes were co-cultured with low (<50,000/mm3), medium (100-200,000/mm3) and high (>400,000/mm3) concentrations of platelets, with platelets in either an inactive or active state (induced by the addition of thrombin). After overnight incubation in static conditions (shear stress = 0), we found that neither activated nor non-activated platelets were capable of inducting DC differentiaton of monocytes in the absence of flow (Supplemental Figure 1).
Platelets suspended in flow bind to serum proteins adsorbed onto the plate
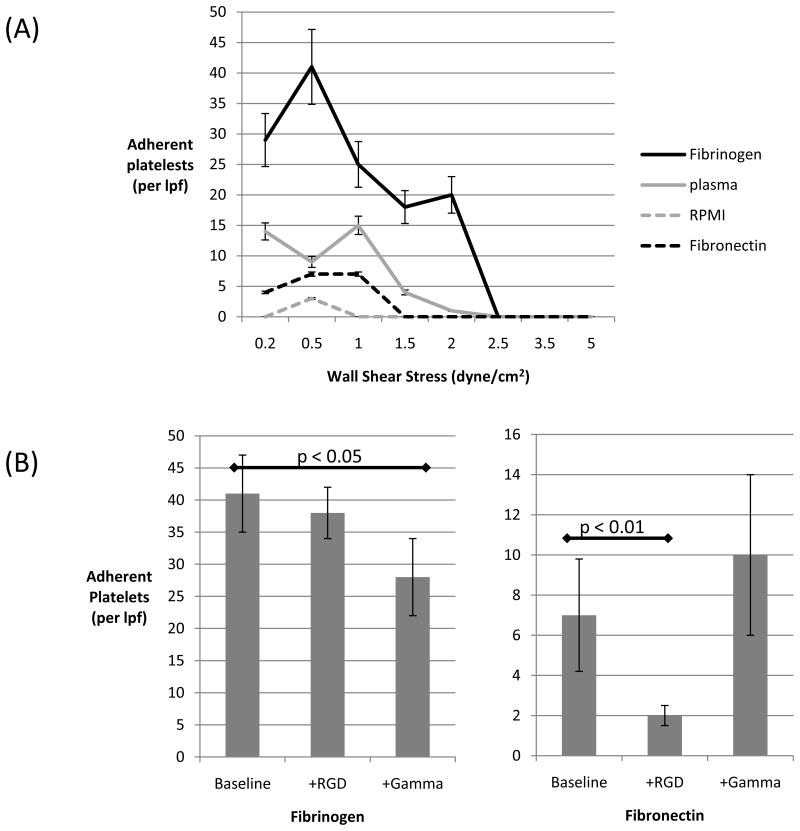
Since fibronectin and fibrinogen adsorb onto glass and plastic surfaces, we assessed the contribution of plastic-adherent plasma proteins to platelet adhesion and activation. Plastic parallel plates were pre-coated with fibrinogen, fibronectin, plasma, or saline. Unactivated platelets were passed through at shear rates producing wall shear stresses ranging from 0.2 to 6.0 dyne/cm2 encompassing the relevant ECP force ranges. The highest concentrations of platelets adhered to plates coated with fibrinogen (Figure 3a). Adhesion to fibronectin-coated was observed as well, but to a significantly lower extent (p < 0.05). In the absence of flow, platelet adherence was equivalent on all protein substrates (Supplemental Figure 2).
Figure 3. Plasma protein influence on platelet adhesion to plastic plates.
(A) Platelets were passed through plates coated with fibrinogen (blue), plasma (red), fibronectin (purple), or RMPI (green) at the shear stress level indicated by the x-axis. Platelets in flow adhered optimally to fibronectin. For all proteins, platelet adhesion occurred maximally between 0.5 and 1.0 dyne/cm2. (B) Platelets were either untreated (baseline), or pretreated with either RGD fragments (+RGD) or (gamma fragments (+Gamma) and their subsequent adhesion to fibrinogen (left panel) and fibronectin (right panel) was assessed. Platelet binding to fibrinogen was decreased by gamma fragments (p < 0.05), while binding to fibronectin was decreased by RGD peptides (p < 0.001). lpf, low power field. Data shown are the means (± SD) of at least 2 independent experiments.
Both fibrinogen and fibronectin contain segments with the amino acid sequence arginine(R)-glycine(G)-aspartate(D), RGD. RGD segments interact with many integrin receptors, particularly the I/A domain of beta subunits, which are exposed when the integrins are in the active conformation [21]. In experiments using fibrinogen-coated plates, platelet adhesion was not significantly altered by pre-incubation of platelets with RGD peptides; however, adhesion was significantly decreased (p < 0.05) by pre-incubation of platelets with peptide fragments corresponding to amino acids 400-411 of fibrinogen, the gamma component of the protein (Figure 3b). In experiments using fibronectin-coated plates, pre-incubating platelets with RGD peptides decreased adhesion significantly (Figure 3c). Unlike the I/A domain of integrins, which is known to interact with RGD domains of proteins, the region of the integrin found to interact with the gamma component of fibrinogen is exposed in the integrin's inactive state [22]. Therefore, this data suggests that unactivated platelets in flow bind to the γ-chain of fibrinogen adherent to coated plates.
Platelets are activated by adhesion to the plate
Platelets physiologically circulate in an inactive state, with an array of proteins stored in intracellular granules. Upon encountering stimuli such as damaged endothelium or thrombin, platelets become activated and almost instantaneously translocate these intracellular proteins to the plasma membrane [23]. We postulated that platelet adhesion to the plastic plate/absorbed proteins caused platelet activation similar to that caused by well-known stimuli.
To test this hypothesis, we assessed surface expression of P-selectin, a well-known marker of platelet activation, before and after adhesion in three independent experiments. Prior to adhesion, 6 ⩲3% of platelets were found to express P-selectin, with a mean fluorescence intensity (MFI) of 12.4 ⩲ 6.9; following adhesion, P-selectin positivity increased to 64 ⩲ 13% (MFI 98.2 ⩲ 14). The positive control, platelets activated with thrombin, was 71 ⩲ 18 % P-selectin positive (MFI 108.3 ⩲ 23). Since the clinical ECP procedure can often last longer than 60 minutes, expression of P-selectin was further assessed at 30, 60, and 90 minutes following platelet adhesion; P-selectin expression remained stable at all time points, with 72 ⩲ 11% of platelets P-selectin positive 90 minutes after adhesion, indicating that platelets remain in an active state for the duration of the clinical procedure. Similar trends were found in assessment of αIIb-β3, a fibrinogen-binding integrin, with surface expression of this protein increasing from 4 ⩲ 3% prior to adhesion, to 49 ± 18% post-adhesion.
Monocytes interact with P-selectin and RGD-containing ligands expressed on activated platelets
The monocyte-platelet interactions observed on video were divided into two categories: (1) short-acting, arbitrarily defined as contact occurring for less than 3 seconds (46 frames), and (2) long-acting, defined as contact longer than 3 seconds, including stable binding. Since activated platelets express an array of proteins including P-selectin and RGD containing proteins (e.g. fibronectin, fibrinogen, and vitronectin), we sought to determine the role of these proteins in short and long-duration monocyte interactions. Plastic plates were pre-coated with platelets, and four conditions tested: (1) platelets pre-treated with an irrelevant isotype control; (2) platelets pre-treated with an irrelevant isotype control, and monocytes pre-incubated with RGD peptides; (3) platelets pre-treated with anti-P-selectin, and monocytes untreated; (4) platelets pre-treated with anti-P-selectin, and monocytes pre-treated with RGD peptides. Thus, the four conditions tested represent each permutation with the two platelet ligands, P-selectin and RGD-containing-proteins. The videos reveal that blocking with anti-P-selectin alone abolished both short and long monocyte-platelet interactions almost completely (Supplement 3 for video). Superimposing blockage of RGD simultaneously was not additive, indicating a dominant role for P-selectin in these interactions. .
Based on the pattern of interactions observed in each of the four conditions, these data suggest: (1) P-selectin is predominantly responsible for the short-duration interactions; (2) RGD-containing proteins expressed by the platelet are involved in long-duration interactions, but not short-duration interactions; (3) monocyte interaction with P-selectin must occur upstream of monocyte interaction with RGD-containing proteins expressed by platelets. This last conclusion is based on the observation that conditions of P-RGD+ decreased both short and long duration interactions to near zero, while P+RGD- conditions only decreased long-duration interactions. If the interactions were not sequential, conditions of P-RGD+ would likely have produced similar results to P+RGD+ in terms of long-duration interactions. Furthermore, the ordering of the interactions, i.e. that P-selectin acts upstream of RGD-interactions, is indicated by the finding that conditions of P+RGD- only influenced long duration interactions, while conditions of P-RGD+ produced similar results to those of P-RGD-.
Monocyte exposure to P-selectin results in downstream monocyte integrin-activation
Integrin receptors, in their open conformation, are known to interact with RGD-containing ligands [24]. Using an antibody that recognizes an epitope exposed only when the β1 integrin is in its open conformation, we assessed monocyte integrins before and after flow through the ECP model in three independent experiments. As the number of short-acting monocyte-platelet interactions increased, there was corresponding increase in the percentage of monocytes expressing integrins in their open conformation immediately post-flow (Figure 4). An average of 71% of monocytes which had received a high number of platelet-interactions (> 61⩲19/lpf × sec) expressed β1 in the active form (black line), statistically greater (P < 0.05) compared to 9% of monocytes which had received a low number of platelet interactions (< 5.1 ⩲ 2 /lpf × sec). These results were not significantly affected by pre-treating the adherent platelets with an irrelevant isotype control (gray line). In contrast, pre-treating platelets with anti-P-selectin reduced the monocyte-platelet interactions to near zero, and monocytes emerging from flow in these conditions (dashed line) displayed low levels of active β1 integrins, irrespective of the density of platelets to which they were exposed. It is noteworthy that all cell populations prior to passage through the plates demonstrated similar low levels of baseline integrin activation (<10%); therefore, differences seen in short-duration monocyte-platelet interactions were not the result of differences in integrin conformation pre-flow.
Figure 4. Effect of p-selectin exposure on monocyte integrins.
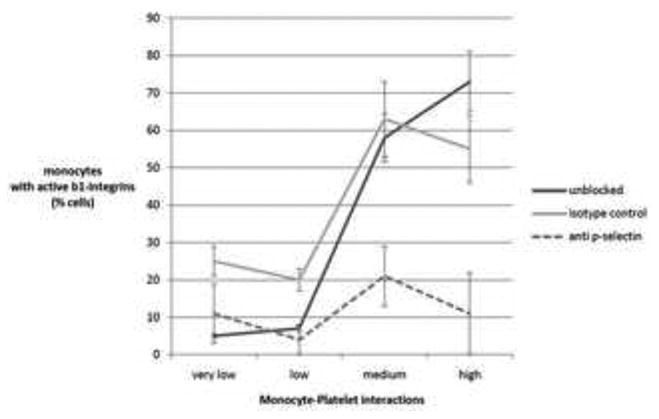
Plastic plates were coated with platelets at the relative density indicated by the x-axis. Platelets were then pretreated with anti p-selectin (dashed line) or an isotype control (gray line), or received no pretretment (black line). Monocytes were passed through the plates at 0.5 dyne/cm2 and then immediately assessed by flowcytometry for expression of active β1 integrins. The y-axis indicates the percent of monocyte which bound an antibody directed at an epitope only exposed when the integrin is in the open confirmation. Data shown are the means (+/- SD) of 3 independent experiments.
Monocyte exposure to P-selectin is required for DC differentiation
Given the dependence of monocyte-platelet interactions on platelet P-selectin, we set out to determine if there was a relationship between monocyte exposure to P-selectin at time 0, and the phenotype later developed by the monocyte after overnight incubation, time 18-hours (Figure 5). Monocytes were passed through parallel plates coated with high densities (108⩲36/lpf) of platelets that were either untreated (unblocked), or pretreated with either anti-P-selectin or an isotype control. 15.5 ⩲ 4 % of monocytes exposed to unblocked platelets became co-expressers of membrane HLA-DR and CD83 (markers of maturing DC) after overnight incubation, and 13 ⩲ 4 % of the those exposed to platelets blocked with the irrelevant isotype control. In contrast, only 3 ⩲ 2% of the monocytes exposed to platelets blocked with anti-P-selectin became HLA-DR+/CD83+ after overnight incubation.
Figure 5. Effect of P-selectin exposure on monocyte phenotype after overnight incubation.
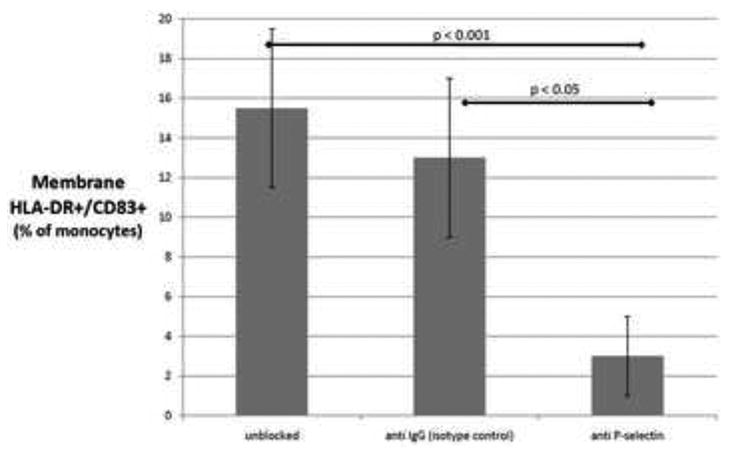
Platelet-coated plates were either untreated (first column), or pretreated with an isoptype control (second column) or anti-P-selectin (third column). Monocytes were passed through the plates at 0.5 dyne/cm2 then incubated overnight. The y-axis indicates the percent of monocytes which developed a phenotype consistent with DC differentiation, i.e., membrane HLA-DR+/CD83+. Data shown are the means (+/- SD) of 3 independent experiments.
Discussion
The present studies reveal that ECP promotes DC differentiation through direct engagement of monocytes by ligands at the surface of activated platelets. These findings suggest a new clinically applicable approach to the procurement of autologous DC that does not depend on supraphysiologic concentrations of cytokines. ECP has been widely used for three decades in the treatment of patients with leukemic cutaneous T-cell lymphoma (CTCL), graft versus host disease, and organ transplant rejection[2, 5, 25]. While previous studies on ECP had revealed that the procedure causes monocyte-to-DC differentiation[12], a lack of mechanistic knowledge precluded introduction of scientifically based modifications that could potentially expand its therapeutic scope to encompass a much broader range of cancers beyond CTCL.
To our knowledge, we have presented the first evidence suggesting that platelets are capable of driving DC differentiation. Our results are consistent with the following mechanistic sequence in platelet-driven induction of monocyte-to-dendritic cell differentiation (Figure 6): (1) plasma fibrinogen first coats the plastic surface of the flow chamber; (2) through its αIIbβ3 receptor, unactivated platelets bind and adhere to the plastic-immobilized fibrinogen; (3) platelets become activated and instantaneously express preformed P-selectin; (4) passaged monocytes transiently bind P-selectin via PSGL-1, causing partial monocyte activation and integrin receptor conformational changes; (5) partially-activated monocytes, now capable of further interactions, bind additional platelet-expressed ligands, including those containing RGD domains; (6) finally, so influenced, monocytes efficiently enter the DC maturational pathway. In-vivo, step (1) above could simply be replaced by the pro-platelet adhesion state of inflamed endothelium.
Figure 6. Proposed mechanism for induction of monocyte-to-DC differentiation.
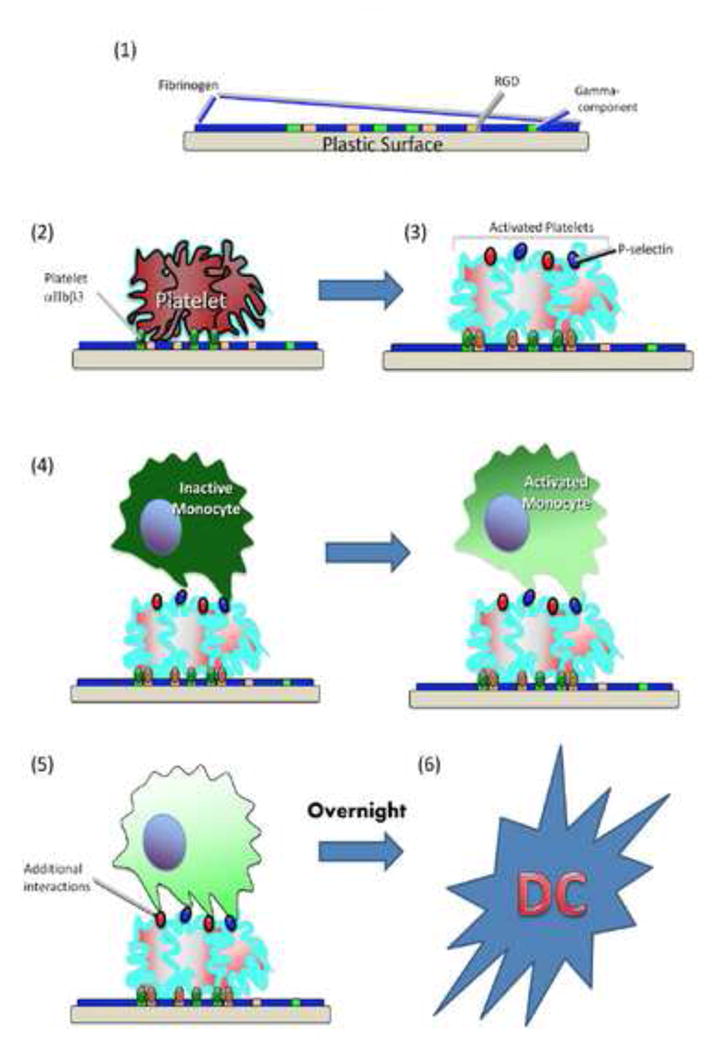
Based on data presented in this manuscript, the following sequence of events is postulated: (1) plasma fibrinogen coats the plastic surface of the flow chamber; (2) through their αIIbβ3 receptor, unactivated platelets bind to the gamma-component of immobilized fibrinogen; (3) platelets become activated and instantaneously express preformed P-selectin and other surface proteins; (4) passaged monocytes transiently bind P-selectin via PSGL-1, causing partial monocyte activation and integrin receptor conformational changes; (5) partially-activated monocytes, now capable of further interactions, bind additional platelet-expressed ligands, including those containing RGD domains; (6) finally, so influenced, monocytes efficiently enter the DC maturational pathway within 18 hours. Note that, in-vivo, step (1) above may be replaced physiologically by inflammatory signals from tissue acting on local endothelium, causing it to recruit and activate platelets in a similar manner (see text).
The contribution of platelets to DC maturation may have multiple practical, as well as scientific, implications. For example, it may be possible with future advancements to generate DC for treatment of other hematologic malignancies beyond CTCL, as well as for solid tumors.
The central role of DCs in the immune system has placed them at the forefront of cancer immunotherapy. DCs have the specialized capacity to internalize, process, and cross-present cancer-specific antigens to CD8+ T-cells. Recent years have seen an increase in attempts at generating DC for the purpose of inducing clinical immunity against various cancers [26-28]. The vast majority of protocols are based on generating DC from monocytes by administering suprapharmacologic doses of cytokines and growth factors (e.g. GCM-CSF and IL-4, followed by TNF-alpha for variable periods) [29]. The ex vivo differentiation, and therefore selection, of DC under conditions not normally reproducible in vivo may handicap the function and even survival of the induced DC after reintroduction of the derived cells for therapeutic purposed.
The findings reported in this paper identify several special features of ECP that likely contribute to its immunotherapeutic efficacy. First, since the triggering of monocyte-to-DC maturation occurs during a one-hour ex vivo plate passage phase of the treatment procedure, maturation of the induced DCs is synchronized, thereby producing a more homogeneous population than that yielded by cytokine maturation. Second, since ECP induces DCs via physiologic platelet signaling, without addition of the supra-physiologic cytokine concentrations, the reinfused developing DCs are not dependent on conditions that cannot be reproduced in vivo and are therefore better prepared to survive and function in treated patients. Third, ECP produces both a long-lived population of immunizing mature DCs and a short-lived population of tolerizing DCs, providing a potential explanation of how the treatment immunizes against tumor antigens in CTCL, while tolerizing in the transplant setting. Maturation of the fraction of ECP-induced DCs that is minimally exposed to photoactivated 8-methoxypsoralen (8-MOP) while flowing through the ECP exposure plate uniformly proceeds uninhibited and yields immunizing DCs (33, 34). In contrast, the fraction of ECP-induced DCs that receives maximal exposure to activated 8-MOP yields maturationally truncated tolerizing DCs.
ECP's induction of DCs through physiologic platelet signaling of monocyte precursors, along with its capacity to skew the resulting DCs toward immunizing or tolerizing function, suggests intriguing therapeutic possibilities. It will now be important to determine whether these underlying principles can be applied to the management of a broader range of immunogenic cancers and whether a modified “tunable” device can be tailored to either immunization or tolerance induction, as clinically indicated.
Supplementary Material
Acknowledgments
This work was supported by grants from: NIH-NCI Spore grant 1 P50 CA121974; NIH Cancer Center Support Grant 3 P30 CA 16359-28S1; and the NY Cardiac Foundation. We thank Eve Robinson, Renata Filler and Julia Lewis for their expert technical assistance.
Footnotes
Conflict of Interest Statement: Yale University owns patents deriving from the dendritic cell research of RT and RE. Although no products have been derived from these laboratory studies, it is possible that, along with their parent institution, these two authors could personally benefit from commercialization of these discoveries in the future.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 2.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New England Journal of Medicine. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 3.Lipson EJ, Drake CG. Ipilimumab: An Anti-CTLA-4 Antibody for Metastatic Melanoma. Clinical Cancer Research. 2011;17:6958–62. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New England Journal of Medicine. 363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy-Preliminary-results. New England Journal of Medicine. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 6.Knobler R, Duvic M, Querfeld C, Straus D, Horwitz S, Zain J, et al. Long-term follow-up and survival of cutaneous T-cell lymphoma patients treated with extracorporeal photopheresis. Photodermatol Photoimmunol Photomed. 2012;28:250–7. doi: 10.1111/j.1600-0781.2012.00689.x. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb SL, Wolfe JT, Fox FE, DeNardo BJ, Macey WH, Bromley PG, et al. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: A 10-year experience at a single institution. Journal of the American Academy of Dermatology. 1996;35:946–57. doi: 10.1016/s0190-9622(96)90119-x. [DOI] [PubMed] [Google Scholar]
- 8.Couriel DR, Hosing C, Saliba R, Shpall EJ, Anderlini P, Rhodes B, et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood. 2006;107:3074–80. doi: 10.1182/blood-2005-09-3907. [DOI] [PubMed] [Google Scholar]
- 9.Greinix HT, Volc-Platzer B, Rabitsch W, Gmeinhart B, Guevara-Pineda C, Kalhs P, et al. Successful use of extracorporeal photochemotherapy in the treatment of severe acute and chronic graft-versus-host disease. Blood. 1998;92:3098–104. [PubMed] [Google Scholar]
- 10.Malawista SE, Trock DH, Edelson RL. Treatment of rheumatoid arthritis by extracorporeal photochemotherapy - A pilot-study. Arthritis and Rheumatism. 1991;34:646–54. doi: 10.1002/art.1780340604. [DOI] [PubMed] [Google Scholar]
- 11.Vowels BR, Cassin M, Boufal MH, Walsh LJ, Rook AH. Extracorporeal photochemotherapy induces the production of tumor-necrosis-factor-alpha by monocytes - implications for the treatment of cutaneous T-cell lymphoma and systemic-sclerosis. Journal of Investigative Dermatology. 1992;98:686–92. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- 12.Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4+CD25+GITR+Foxp3+CD62L+functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–9. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 13.DiRenzo M, Rubegni P, DeAloe G, Paulesu L, Pasqui AL, Andreassi L, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology. 1997;92:99–103. doi: 10.1046/j.1365-2567.1997.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–7. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 15.Berger C, Hoffmann K, Vasquez JG, Mane S, Lewis J, Filler R, et al. Rapid generation of maturationally synchronized human dendritic cells: contribution to the clinical efficacy of extracorporeal photochemotherapy. Blood. 2010;116:4838–47. doi: 10.1182/blood-2009-11-256040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cella M, Scheidegger D, PalmerLehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. Journal of Experimental Medicine. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Saint-Vis B, Vincent J, Vandenabeele S, Vanbervliet B, Pin JJ, Ait-Yahia S, et al. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–36. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 18.Slavik JM, Hutchcroft JE, Bierer BE. CD80 and CD86 are not equivalent in their ability to induce the tyrosine phosphorylation of CD28. Journal of Biological Chemistry. 1999;274:3116–24. doi: 10.1074/jbc.274.5.3116. [DOI] [PubMed] [Google Scholar]
- 19.Berger C, Hoffmann K, Vasquez JG, Mane S, Lewis J, Filler R, et al. Rapid generation of maturationally synchronized human dendritic cells: contribution to the clinical efficacy of extracorporeal photochemotherapy. Blood. 2010;116:4838–47. doi: 10.1182/blood-2009-11-256040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang HK, Lee HY, Kim MK, Park KS, Park YM, Kwak JY, et al. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met inhibits human monocyte-derived dendritic cell maturation via formyl peptide receptor and formyl peptide receptor-like 2. Journal of Immunology. 2005;175:685–92. doi: 10.4049/jimmunol.175.2.685. [DOI] [PubMed] [Google Scholar]
- 21.Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. Gpnmb is induced in macrophages by IFN-gamma and lipopolysaccharide and acts as a feedback regulator of proinflammatory responses. Journal of Immunology. 2007;178:6557–66. doi: 10.4049/jimmunol.178.10.6557. [DOI] [PubMed] [Google Scholar]
- 22.Chen SQ, Springer TA. Selectin receptor-ligand bonds: Formation limited by shear rate and dissociation governed by the Bell model. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:950–5. doi: 10.1073/pnas.98.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas WE. Understanding the counterintuitive phenomenon of catch bonds. Current Nanoscience. 2007;3:63–83. [Google Scholar]
- 24.Xiong JP, Stehle T, Zhang RG, Joachimiak A, Frech M, Goodman SL, et al. Crystal structure of the extracellular segment of integrin alpha V beta 3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296:151–5. doi: 10.1126/science.1069040. [DOI] [PubMed] [Google Scholar]
- 25.Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the Platelet Membrane Glycoprotein-IIB-IIIA Complex and its Interaction with Fibrinogen and Other Ligands by Electron-Microscopy. Journal of Biological Chemistry. 1992;267:16637–43. [PubMed] [Google Scholar]
- 26.Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins - studies on release and subcellular-localization. Blood. 1979;53:604–18. [PubMed] [Google Scholar]
- 27.Ruoslahti E. RGD and other recognition sequences for integrins. Annual Review of Cell and Developmental Biology. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 28.Hivelin M, Siemionow M, Grimbert P, Lantieri L. Extracorporeal photopheresis: From solid organs to face transplantation. Transplant Immunology. 2009;21:117–28. doi: 10.1016/j.trim.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 29.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annual Review of Immunology. 2000;18:245–73. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 30.Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Investigation. 2003;21:873–86. doi: 10.1081/cnv-120025091. [DOI] [PubMed] [Google Scholar]
- 31.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Current Opinion in Immunology. 2003;15:138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 32.Ovali E, Dikmen T, Sonmez M, Yilmaz M, Unal A, Dalbasti T, et al. Active immunotherapy for cancer patients using tumor lysate pulsed dendritic cell vaccine: a safety study. Journal of Experimental & Clinical Cancer Research. 2007;26:209–14. [PubMed] [Google Scholar]
- 33.Girardi M, Berger CL, Wilson LD, Christensen IR, Thompson KR, Glusac EJ, et al. Transimmunization for cutaneous T cell lymphoma: A phase I study. Leukemia & Lymphoma. 2006;47:1495–503. doi: 10.1080/10428190600581419. [DOI] [PubMed] [Google Scholar]
- 34.Futterleib J, Feng H, Tigelaar R, Choi J, Edelson R. Activation of GILZ Gene by Photoactivated 8-Methoxypsoralen: Potential Role of Immunoregulatory Dendritic Cells in Extracorporeal Photochemotherapy. Transfusion and Apheresis Sci. 2014 doi: 10.1016/j.transci.2013.10.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.