Abstract
Background
Patients with Major Depressive Disorder (MDD) often experience unexpected relapses, despite achieving remission. This study examines the utility of a single multidimensional measure that captures variance in patient-reported Depressive Symptom Severity, Functioning, and Quality of Life (QOL), in predicting MDD relapse.
Methods
Complete data from remitted patients at the completion of 12 weeks of citalopram in the STAR*D study were used to calculate the Individual Burden of Illness index for Depression (IBI-D), and predict subsequent relapse at six (n = 956), nine (n = 778), and twelve months (n = 479) using generalized linear models.
Results
Depressive Symptom Severity, Functioning, and QOL were all predictors of subsequent relapse. Using Akaike information criteria (AIC), the IBI-D provided a good model for relapse even when Depressive Symptom Severity, Functioning, and QOL were combined in a single model. Specifically, an increase of one in the IBI-D increased the odds ratio of relapse by 2.5 at 6 months (β = 0.921 ± 0.194, z = 4.76, p < 2 × 10−6), by 2.84 at 9 months (β = 1.045 ± 0.22, z = 4.74, p < 2.2 × 10−6), and by 4.1 at 12 months (β = 1.41 ± 0.29, z = 4.79, p < 1.7 × 10−6).
Limitations
Self-report poses a risk to measurement precision. Using highly valid and reliable measures could mitigate this risk. The IBI-D requires time and effort for filling out the scales and index calculation. Technological solutions could help ease these burdens. The sample suffered from attrition. Separate analysis of dropouts would be helpful.
Conclusions
Incorporating patient-reported outcomes of Functioning and QOL in addition to Depressive Symptom Severity in the IBI-D is useful in assessing the full burden of illness and in adequately predicting relapse, in MDD.
Keywords: Major depressive disorder, Burden of illness, Functioning, Quality of life, STAR*D, Mood disorders
1. Introduction
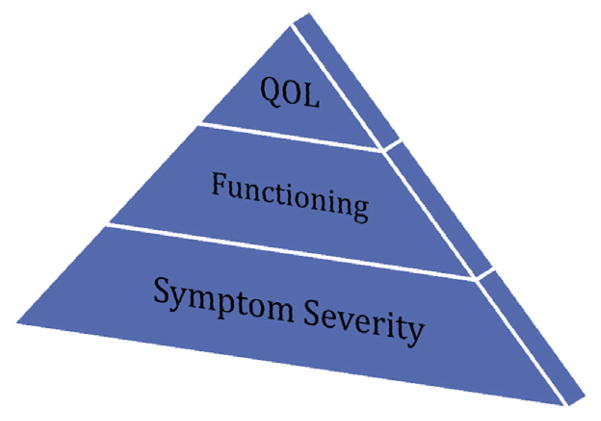
Depression affects 350 million people worldwide according to the latest statistics from the World Health Organization (WHO, 2012). Patients with Major depressive disorder (MDD) not only suffer from symptoms of depression, but also from impairments in quality of life (QOL) and function that lead to increased suffering with negative consequences for families as well as for society at large (Mathers and Loncar, 2006; IsHak et al., 2011; Rupp et al., 1997). In prior work, the concept of an MDD patient’s individual burden of illness was introduced to accurately capture this suffering by incorporating symptom severity (intensity, frequency, duration), impairment in functioning (occupational, social, and leisure activities), and reduction in quality of life (QOL) (satisfaction with health, occupational, social, and leisure activities), as depicted in Fig. 1.
Fig. 1.
The burden of illness components.
The Individual Burden of Illness Index for Depression (IBI-D) was developed and validated as a means of providing a single measure that would accurately reflect the degree to which an individual patient is suffering from depression (IsHak et al., 2013). Following on the above conceptualization, the IBI-D was the name given to the first and only statistically significant principal component obtained from a principal component analysis (PCA) of well-validated patient-reported outcomes of depressive symptom severity, functioning, and QOL: the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) (Rush et al., 2003), the Work and Social Adjustment Scale (WSAS) (Mundt et al., 2002), and the Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q) (Endicott et al., 1993), respectively. The initial exploratory PCA was based on the patient-reported measures from MDD patients in the Cedars-Sinai Psychiatric Treatment Outcome Registry (IsHak et al., 2013) and the confirmatory analysis was based on the patient-reported measures of patients enrolled in Level 1 of the NIMH Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial study at their time of entry (Rush et al., 2004; Fava et al., 2003). More recently the single IBI-D number was shown to provide an accurate accounting of the multidimensional impact of antidepressant treatment (Cohen et al., 2013).
In the present study we examined whether this multidimensional measure of depression offers advantages over the more traditional one-dimensional measures that emphasize symptom severity. The evaluation rests on the examination of the relapse rates of STAR*D Level 1 citalopram treated remitted patients (QIDS-SR score of 5 or less) after 6, 9, and 12 months. We hypothesized that remitted patients with higher IBI-D scores, i.e., higher burden of illness, would be more likely to relapse over the ensuing 12 months. As all remitted patients have relatively low and similar symptom severity ratings, higher IBI-D values primarily reflect residual impairments in QOL and/or functioning. Finding such a relationship would provide strong support for (1) The need to focus on improvements in QOL and function in addition to reductions in symptom severity if we are to provide adequate treatment to MDD patients and, (2) The usefulness of the IBI-D as a clinical indicator in the assessment of antidepressant treatments and relapse potential.
2. Methods
2.1. Population
The patient sample for this study was derived from the STAR*D trial. STAR*D is an NIMH-funded study, conducted at 18 primary care settings and 23 psychiatric care settings in the United States, from 2001 to 2007, that enrolled 4041 treatment-seeking out-patients from 18 to 75 years old who had a primary diagnosis of MDD, for the purpose of evaluating response and remission using a sequential approach of medication regimens: Level 1 through 4. The full details of the study are described elsewhere (Fava et al., 2003; Rush et al., 2004). The authors obtained NIMH Data Use Certificate to use the STAR*D dataset (STAR*D Pub Ver3). This analysis focused on patients with complete severity, functioning, and QOL data, collected by the Interactive Voice Response system, at six (n = 956), nine (n = 778), and twelve (n = 479) months of the follow-up phase of the study who met criteria for remission at exit after completing Level 1 treatment as defined by QIDS-SR of 5 or less. Follow-up patients were instructed to stay on the same medication at the same dose of the acute treatment and have follow-up visits every two months.
2.2. Outcome measures
The measures used to calculate the IBI-D consisted of the following three instruments: (a) Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR) (Rush et al., 2003) for depressive symptom severity, (b) Work and Social Adjustment Scale (WSAS) (Mundt et al., 2002) for functioning, and (c) Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (Q-LES-Q) (Endicott et al., 1993) for QOL.
The QIDS-SR measures depressive symptom severity with scores ranging from 0 (not depressed) to 27 (most depressed). Remission is defined as a score of 5 or less, which is equivalent to a score of 7 or less on the Hamilton Rating Scale for Depression. Scores of 6–10 are used for mild, 11–15 for moderate, 16–20 for severe, and > 20 for very severe depression (Rush et al., 2003).
The WSAS was used to measure functioning, with scores ranging from 0 (no impairment) to 40 (severe impairment). Scores above 20 indicate moderate to severe impairment, 10–20 for significant impairment, and < 10 for subclinical impairment. The WSAS has fairly strong psychometric properties, with a Cronbach’s alpha ranging from 0.70 to 0.94, and a test–retest reliability of r = 0.73 (Mundt et al., 2002).
QOL was assessed using the Q-LES-Q; with a score range of 0–100 where 0 is the lowest QOL score and 100 is the highest. Community norm samples have an average Q-LES-Q score of 78.3 (SD = 11.3) and scores within 10% of this value are considered within-normal (Q-LES-Q> = 70.47), whereas Q-LES-Q scores greater than 2 SD below the community norm indicate severe impairment, i.e., Q-LES-Q scores less than or equal to 55.7 are considered severely impaired. The Q-LES-Q also enjoys strong psychometric properties, with a Cronbach’s alpha of 0.90 and a test–retest reliability of 0.74 (Endicott et al., 1993).
2.3. Calculation of IBI-D index
Each scale is converted to a z-score according to the development and validation study (5). For the QIDS-SR: zQIDS-SR = (QIDS-SR-15.6)/5.1, for the WSAS: zWSAS = (WSAS-23.9)/9.3, and for the Q-LES-Q: zInvQ-LES-Q = (41.4-Q-LES-Q)/15.3. The IBI-D index is calculated using the following formula:
2.4. Defining remission and relapse
Remission was defined as QIDS-SR = < 5 at exit after completing Level 1 treatment. Relapse was defined as QIDS-SR equal or more than three standard deviations above the mean for remitters, i.e., QIDS-SR > 7 at each follow-up point of 6, 9, and 12 months. Although QIDS-SR > = 11 was used in examination of relapse in some STAR*D analyses, 11 seems to be a relatively high score (equivalent to moderate depression) that would encompass a variety of patients who are well into full blown depressive episodes, so we opted to a definition that include patients experiencing even mild depressive symptoms.
2.5. Statistical analyses
Summary values are expressed as means (SD) for continuous variables, and frequencies (%) for categorical variables. χ2 Analysis was used to compare the percentage of patients who relapsed to those who maintained remission at 6, 9, and 12 months. P values of less than 0.05 were considered statistically significant. Analyses were performed using generalized linear models in the open source R programming language version # 2.10.1 (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
The demographic characteristics of the STAR*D sample were previously reported (Rush et al., 2004; Fava et al., 2003). Briefly, the sample majority is Caucasian (> 80%), with two-thirds women, one-third graduated from college and a little more than one-half employed. The relapse rates of MDD patients in the follow-up phase were 23.7%, 28.2%, and 24.7% at 6, 9, and 12 months respectively (Table 1).
Table 1.
Remitted patients with follow-up at 6, 9 and 12 months.
| Level 1 patients
| |||||
|---|---|---|---|---|---|
| Months | Total in F/U | Initially remitted | Maintained | Relapsed | % Relapsed |
| 6 | 956 | 379 | 289 | 90 | 23.7 |
| 9 | 778 | 301 | 216 | 85 | 28.2 |
| 12 | 479 | 178 | 134 | 44 | 24.7 |
Remission is defined as a score of 5 or less on the QIDS-SR.
Relapse is defined as a score more than 7 on the QIDS-SR.
Subjects who relapsed at 6, 9, and 12 months rated themselves as significantly worse on patient-reported outcomes of depressive symptom severity functioning, and quality of life (higher QIDS-SR and WSAS, and lower Q-LES-Q scores) compared to patients who maintained remission following exit from Level 1.
3.1. Predicting relapse at follow-up using the IBI-D
With the exception of the WSAS at 6 months, the Level 1 exit QIDS-SR, Q-LES-Q, and WSAS ratings of remitted patients were predictive of their QIDS-SR scores at the 6, 9, and the 12-month follow-up visits when assessed with a Gaussian linear model. Nevertheless, using the IBI-D alone proved to be as good a model for predicting follow-up symptom severity, e.g., the goodness of fit Akaike information criteria (AIC) measure with IBI-D alone was 2168 at 6 months, 1746 at 9 months and 1028 at 12 months whereas the AIC for the QIDS-SR+WSAS+Q-LES-Q model was not significantly better at 2167 at 6 months, 1748 at 9 months and 1029 at 12 months (Table 2).
Table 2.
Using the IBI-D alone compared to the combination of the rating scales reflecting symptom severity, quality of life and function.
| IBI-D alone
|
AIC | Adj R2 | QIDS-SR
|
Q-LES-Q
|
WSAS
|
AIC | Adj R2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coeff | t | p | Coeff | t | p | Coeff | t | p | Coeff | t | p | |||||
| 6 mo. | 2.28 | 6.611 | < 2 × 10−10 | 2168 | 0.102 | 0.49 | 3.26 | < 0.002 | −0.044 | 2.1 | < 0.05 | 0.077 | 1.77 | < 0.08 | 2167 | 0.108 |
| 9 mo. | 2.81 | 6.8 | < 6 × 10−11 | 1747 | 0.131 | 0.27 | 1.48 | NS | −0.035 | −1.35 | NS | 0.19 | 3.67 | < 0003 | 1748 | 0.133 |
| 12 mo. | 3.14 | 6.43 | < 1.2 × 10−9 | 1028 | 0.186 | 0.63 | 2.78 | < 0.006 | −0.039 | −1.23 | NS | 0.16 | 2.58 | 0.01 | 1029 | 0.194 |
AIC = Akaike information criteria; IBI-D = individual burden of illness for depression; QIDS-SR = Quick Inventory of Depressive Symptomatology-Self Report; Q-LES-Q = Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form; WSAS = Work and Social Adjustment Scale.
Although it was somewhat surprising that the QIDS-SR at exit would prove to be predictive of subsequent symptom severity given the definition of remission as a QIDS-SR of less than or equal to 5, given the finding that it was, we were led to test whether the IBI-D would significantly contribute to a Gaussian linear model for follow-up symptom severity if the exit symptom severity was entered first into the same model. We found that it did. For example, at 6 months in the combined model of exit QIDS-SR and exit IBI-D, the QIDS-SR had a t value of 2.17, p = 0.03 and the IBI-D a t of 4.27, p < 2.5 × 10−5, at 9 months, the t-value of the QIDS-SR was insignificant while the IBI-D had a t-value of 5.45, p < 1.1 × 10−7, and at 12 months, the QIDS-SR was also not significant with a t-value of 1.71, p < 0.09 while the IBI-D had a t-value of 4.38, p = 2 × 10−5.
Next, we evaluated whether higher values of IBI-D were predictive of relapse as defined by a QIDS-SR score of 3 standard deviations higher than the mean value of remitted subjects, i.e., QIDS-SR > 7 as the cutoff for relapse. Using a binomial model, with relapse as the dependent variable and the IBI-D as the independent variable, the coefficient for IBI-D was 0.921 ± 0.194 (z = 4.76, odds ratio [OR] = 2.51, p < 2 × 10−6) for relapse at 6 months, 1.045 ± 0.22 (z = 4.74, OR = 2.84, p < 2.2 × 10−6) for relapse at 9 months and 1.41 ± 0.29 at 12 months (z = 4.79, OR = 4.1, p < 1.7 × 10−6). To further demonstrate that the IBI-D adds valuable information to our ability to predict relapse at 6, 9, and 12 months as compared to depression severity at-exit, we entered the exit QIDS-SR first in a logistic model followed by the exit IBI-D. Entering the QIDS-SR at-exit first yields a non-significant coefficient of 0.13 (z = 1.35, p = 0.18), but a significant IBI-D coefficient of 0.77 (z = 3.36, p < 0.0008) at 6 months, a non-significant QIDS-SR coefficient of −0.025 (z = 0.11, p = 0.8) and a significant IBI-D coefficient of 1.08 (z = 4.06, p < 5 × 10−5) at 9 months and a significant coefficient of 0.32 (z = 2.04, p < 0.05) for the QIDS-SR and a 1.07 (z = 3.41, p = 0.001) for the IBI-D at 12 months.
Findings supporting the importance of the IBI-D at the time of a patient’s exit from Level 1 of the STAR*D trial in predicting a patient’s severity of depressive symptoms at follow-up are illustrated in Tables 3 and 4. The statistically significant independent Student t-test differences between the way remitted patients destined to maintain remission rate themselves with respect to depressive symptom severity (QIDS-SR), functioning (WSAS) and quality of life (Q-LES-Q) compared to those destined to relapse are depicted in Table 3.
Table 3.
Comparison of rating scales at level 1 exit in subjects who maintained remission to those who relapsed (relapse was defined as QIDS-SR > 7).
| Months |
Maintained Mean (SD) |
Relapsed Mean (SD) |
t-Value | p Value |
|---|---|---|---|---|
| QIDS-SR | ||||
| 6 mo. | 2.76 (1.56) | 3.48 (1.55) | 3.76 | < 0.0003 |
| 9 mo. | 2.76 (1.57) | 3.28 (1.52) | 2.64 | < 0.01 |
| 12 mo. | 2.68 (1.56) | 3.84 (1.23) | 5.05 | < 2.3 × 10−6 |
| QLESQ | ||||
| 6 mo. | 79.30 (12.33) | 71.19 (15.50) | 4.15 | < 6.2 × 10−5 |
| 9 mo. | 79.34 (12.61) | 71.82 (14.15) | 4.28 | < 3.5 × 10−5 |
| 12 mo. | 79.33 (12.50) | 68.75 (16.24) | −3.95 | 0.0002 |
| WSAS | ||||
| 6 mo. | 4.25 (5.95) | 7.02 (7.64) | 3.16 | 0.002 |
| 9 mo. | 3.55 (5.08) | 7.4 (8.25) | 4.02 | 0.0001 |
| 12 mo. | 3.80 (5.43) | 9.80 (9.52) | 3.97 | 0.0002 |
| IBI-D | ||||
| 6 mo. | −2.73 (0.56) | −2.35 (0.74) | 4.4 | < 2.4 × 10−5 |
| 9 mo. | −2.76 (0.54) | −2.37 (0.69) | 4.68 | < 8 × 10−6 |
| 12 mo. | −2.76 (0.54) | −2.15 (0.79) | 4.73 | < 2 × 10−5 |
Table 4.
χ2 analysis comparing the percentage of remitted patients who relapsed compared to remitted patients who maintained their remission at the 6, 9, and 12 months, based on abnormal versus normal functioning and QOL.
| n | Relapsed | Maintenance | % Relapsed | χ2 | Odds ratio | p-Value | |
|---|---|---|---|---|---|---|---|
| 6 Months | |||||||
| Total | 379 | 90 | 289 | 23.75 | |||
| Normal WSAS | 306 | 61 | 245 | 19.93 | 11.68 | 2.65 | 0.0006 |
| Abnormal WSAS | 73 | 29 | 44 | 39.73 | |||
| Normal QLESQ | 281 | 51 | 230 | 18.15 | 17.63 | 2.98 | < 3 × 10−5 |
| Abnormal QLESQ | 98 | 39 | 59 | 39.80 | |||
| 9 Months | |||||||
| Total | 301 | 85 | 216 | 28.24 | |||
| Normal WSAS | 246 | 55 | 191 | 22.36 | 21.42 | 4.16 | 3.7 × 10−6 |
| Abnormal WSAS | 55 | 30 | 25 | 54.55 | |||
| Normal QLESQ | 220 | 49 | 171 | 22.27 | 13.28 | 2.79 | 2.7 × 10−4 |
| Abnormal QLESQ | 81 | 36 | 45 | 44.44 | |||
| 12 Months | |||||||
| Total | 178 | 44 | 134 | 24.72 | |||
| Normal WSAS | 137 | 25 | 112 | 18.25 | 11.92 | 3.86 | 0.00056 |
| Abnormal WSAS | 41 | 19 | 22 | 46.34 | |||
| Normal QLESQ | 125 | 20 | 105 | 16.00 | 15.61 | 4.34 | 7.8 × 10−5 |
| Abnormal QLESQ | 53 | 24 | 29 | 45.28 | |||
IBI-D = individual burden of illness for depression; QIDS-SR = Quick Inventory of Depressive Symptomatology-Self Report; Q-LES-Q = Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form; WSAS = Work and Social Adjustment Scale.
Maintenance is defined as QIDS-SR score = < 5 (Rush et al., 2003). Relapse is defined as QIDS-SR score > 7. Normal functioning is defined as WSAS < 10 (Mundt et al., 2002).
Normal quality of life is defined as Q-LES-Q > = 70.47 (within 10% of community norms, Endicott et al., 1993).
The odds ratio for the likelihood of relapse at 6, 9 and 12 months for remitted patients that either rate themselves as having abnormal quality of life or functioning are displayed in Table 4.
4. Discussion
The main findings in this study are: (1) nearly one in four remitted patients relapsed between 6 months and 1 year after achieving remission on SSRI treatment, (2) greater burden of illness as measured by the IBI-D significantly contributes to the likelihood of relapse at 6, 9, and 12 months from the completion of acute treatment, (3) the IBI-D is of greater value in predicting relapse than depressive symptom severity at-exit as measured by the exit QIDS-SR. More specifically, remitted patients that rate themselves as having abnormal function and/or quality of life are at 2.5–4.1 times greater risk of relapse.
This analysis confirms that a sizeable proportion of patients who achieve remission are not immune from relapse of symptoms relatively shortly (within one year) following acute treatment completion despite being instructed to continue on the same medications/doses as the acute treatment, and follow-up at least on bimonthly basis. Our findings are compatible with long-term studies of relapse after remission onset (O’Leary et al., 2000). The above data strongly support the importance of the IBI-D at the time of a patient’s exit from Level 1 of the STAR*D trial in predicting a patient’s severity of depressive symptoms at follow-up as well as a patient’s likelihood to relapse. The latter is of particular importance given that relapse continues to be a significant challenge to clinicians (Keller et al., 1982; Dobson and Ottenbreit, 2004). A number of biopsychosocial factors have been implicated in increasing relapse risk including genetic loading with mood disorders, co-morbid medical and psychiatric conditions and substance use, psychosocial stressors, and personality coping styles (Klein et al., 2004; Milne et al., 2009; Zisook et al., 2004; Riise and Lund, 2001; Kivelä et al., 2000). Furthermore, baseline symptom severity, duration of illness, number of depressive episodes, duration of depressive episode, and number of medication trials until remission play an important role as well (Pintor et al., 2004; Mulder et al., 2006; Holma et al., 2008; Greer et al., 2010; Rush et al., 2012). However, low functioning level and quality of life impairments have been significantly under-studied despite emerging evidence of their ability to increase the risk of relapse in remitted patients (IsHak et al., 2011; Holma et al., 2008). Further, it may well be that baseline symptom severity, duration of illness, number of depressive episodes, duration of depressive episode, and number of medication trials until remission influence relapse risk primarily through their effects on the patient’s QOL and level of functioning, even after symptom reduction. Although it is seemingly intuitive that QOL and level of functioning would contribute to relapse risk, in contrast to symptom severity there have been few studies examining this relationship (Greer et al., 2010). We chose to examine this relationship using the IBI-D as it reduces the three dimensions of symptom severity, quality of life and level of function into a single dimension that we have labeled as burden of illness. Using a binomial model, this analysis showed that the IBI-D, was able to predict relapse. With each one-point increase on the IBI-D, patients were about two and half to four times more likely to relapse. Furthermore, when the QIDS-SR at exit was entered first in the model, non-significant coefficients for symptom severity at 6 and 9 months were obtained, whereas the IBI-D coefficient was significant at all three intervals, confirming that the IBI-D was the better predictor of relapse. Only the 6-month data appears to provide any support that knowledge of symptom severity may improve prediction of relapse at follow up over and above what can be achieved with the IBI-D itself.
The need for adequate measurement of long-term recovery in psychiatric disorders cannot be overemphasized, especially when patients, their families, advocacy groups, regulators, and the public, are all expecting more than just symptom remission (Insel and Scolnick, 2006). Studies examining which outcomes MDD patients considered important, identified the following three aspects: experiencing positive mental health (such as optimism and self-confidence), returning to one’s usual/normal self, and return to normal levels of functioning (at home, work, or school), as more important than absence of depressive symptoms (Zimmerman et al., 2006). Recent studies of the global burden of disease consistently confirm the elevated prevalence of high role impairment in psychiatric disorders, especially in depression (Kessler et al., 2009). Efforts such as the European Pact for Mental Health and Well-Being and the EU-Compass for Action on Mental Health and Well-Being, have not only emphasized treatment of depression and prevention of suicide, but have also identified the critical need for innovation in both how we measure and intervene to improve level of functioning and quality of life (European Commission, 2010).
Assessing the burden of illness, not only in populations at large but also in individuals, offers a promising approach by going beyond symptom improvement and incorporating functioning and QOL to achieve restoration of health (WHO, 1948), as the primary goal of healthcare interventions.
5. Clinical application
How should these findings inform clinical practice? Clinicians treating depressed patients must assess not only depressive symptoms, but also level of functioning and QOL even in the context of a successful reduction in depressive symptoms. In instances in which levels of functioning and/or QOL are still poor even after depressive symptom reduction, additional interventions should be considered. For example, psychotherapy (individual, group, and family), social work and case management interventions (to improve financial, residential, and health care access problems), vocational rehabilitation programs, occupational therapy services, and recreational therapy opportunities may improve level of functioning (Siskind et al., 2012). Nutrition and nutritional supplements, exercise, massage, meditation, yoga, humor, music, dopaminergic agents, and future-directed therapy (Vilhauer et al., 2013) may improve quality of life (IsHak et al., 2011). The above and other forms of enrichment may be considered important elements of long-term recovery.
Clinicians could use the IBI-D for a more accurate assessment of the overall impact of depression, treatment efficacy, and risk of relapse in their depressed patients. We suggest the following as one possible approach based on our experience in evaluating, treating, and tracking the outcome of outpatients in the Cedars-Sinai Psychiatric Treatment Outcome Registry (IsHak et al., 2012), as detailed in AHRQ Registries User’s Guide (AHRQ, 2013). Patients complete the QIDS-SR, WSAS and Q-LES-Q prior to their clinician interviews either in the waiting room (with staff assistance if necessary), or in the future using secure online access. The measures are scored manually or electronically, and the IBI-D is calculated in less than one minute using a calculator as outlined under Calculation of IBI-D Index above. In the near future, clinicians should be able to simply input the raw scores into a website or mobile application which will calculate IBI-D instantaneously. The individual measures, and the IBI-D scores are then entered in the medical record and interpreted. This process is repeated in subsequent visits, monthly, quarterly, or guided by the clinical presentation. As explained in previously published work, an IBI-D index of 0 indicates an average burden of illness for an individual seeking treatment for depression, an IBI-D index of −2 indicates a remarkably low burden of illness that is less than 98% of depressed patients seeking treatment, and an index of 2 indicates a remarkably high burden of illness that is exceeded by only 2% of depressed patients (IsHak et al., 2013). We know that patients with high burden of illness have a lower probability of response to standard SSRI antidepressant treatment. Therefore these patients might benefit from higher levels of care (e.g., inpatient admission partial hospitalization, or intensive outpatient programs), or more complex interdisciplinary treatment interventions as detailed above. Successful treatment should lead to a trending down of the IBI-D. Because a one-point increase in IBI-D is associated with 2.5-fold increase in odds ratio of relapse at 6 months and even higher odds at 9 and 12 months, patient’s with low depressive symptoms (QIDS-SR = < 5) who continue to have high IBI-D indices should alert the clinician to the need for more frequent visits to identify the earliest signs of relapse and additional interventions to reduce the likelihood of relapse.
6. Limitations
The limitations of this study include measurement constraints using the IBI-D, the challenges of patient-reported outcomes (PRO) in terms of precision, validity and reliability, and the nature of the follow-up STAR*D sample. The limitations of the IBI-D were highlighted before (IsHak et al., 2013; Rush et al., 2004). Briefly, the IBI-D requires time and effort for a patient to fill out the underlying scales (20–30 min), in addition to the measures and index calculation (5–10 min of clinician time). The authors are turning to technological solutions such as tablet/smart phone applications to ease this burden. Validation in different languages and cultures is also needed. Self-report challenges include bias, negative influence of MDD on rating other aspects of patient’s life, and attribution errors (Rush et al., 2004). However, PROs are gaining momentum worldwide as a crucial source of clinical information influencing medical care with significant efforts underway in the United Kingdom (UK NHS Patient-Reported Outcome Measures—PROMs), in the United States (NIH Patient-Reported Outcomes Measurement Information System—PROMIS), and globally by the WHO (International Classification of Functioning, Disability and Health—ICF). Moreover, the measures contained in the IBI-D have a well-established validity and reliability (Rush et al., 2003; Mundt et al., 2002; Endicott et al., 1993). Like many other large studies, the follow-up sample suffered from attrition. For the purpose of studying relapse, information about dropouts is important to examine. Separate analysis of dropouts using the last observation carried forward, would be helpful in this regard.
7. Strengths
Strengths of the present study include the STAR*D’s adequate sample size in the follow-up phase despite significant attrition. Moreover, because the STAR*D study recruited patients from primary care and psychiatric clinics it is likely that findings from the present study are applicable to everyday clinical practice.
8. Future directions
Although the approach highlighted in this study has the potential to be clinically useful in assessing long-term recovery in MDD, psychiatric measures generally are still suffering from issues of precision, difficulties in and resistance to implementation, and lack of effective training (IsHak et al., 2002). Clinicians and medical educators will need to embrace technology-facilitated measurement-based care as implemented in other medical specialties and in psychiatric research. Alternatively, psychiatric measurement teams (analogous to phlebotomy and pathology services) may be utilized in larger systems of care. Measuring the burden of illness in individuals will also need to be linked to specific interventions to improve functioning and ameliorate quality of life. Finally, we need a better understanding of the underlying factors that are responsible for the continued poor levels of functioning and quality of life that persist in some patients despite significant depressive symptom reduction and how these factors influence relapse.
9. Conclusions
This study shows that relapse in remitted patients with MDD during follow-up is more likely in patients experiencing higher burden of illness as evidenced by IBI-D values. These findings suggest that symptom reduction in MDD without substantial improvements in QOL and functioning is insufficient to secure continued remission and that poor QOL and functioning impairment themselves may have causative roles in depression. Therefore, the IBI-D may prove to be a useful measure that clinicians can utilize in the determination of and the justification for the application of additional therapeutic interventions to restore functioning and improve QOL, even in patients who are remitted by the usual depressive symptom based standards.
Acknowledgments
Role of funding source
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial was funded by the United States National Institute of Mental Health (NIMH) # NCT00021528. The current analysis in this manuscript is not funded.
Data used in the preparation of this article were obtained from the limited access datasets distributed from the NIH-supported “Sequenced Treatment Alternatives to Relieve Depression” (STAR*D). STAR*D focused on non-psychotic major depressive disorder in adults seen in outpatient settings. The primary purpose of this research study was to determine which treatments worked best if the first treatment with medication does not produce an acceptable response. The study was supported by NIMH Contract # N01MH90003 to the University of Texas Southwestern Medical Center. The ClinicalTrials.gov identifier is NCT00021528. This manuscript reflects the views of the authors and may not reflect the opinions or views of the STAR*D Study Investigators or the NIH.
Footnotes
Conflict of interest
Dr. IsHak received research grant support from NARSAD: Quality of Life in Major Depressive Disorder, 2005–2007; Pfizer: Ziprasidone monotherapy for Major Depressive Disorder, 2007–2011. Drs. Cohen and Greenberg do not have any conflicts of interest.
Contributor Information
Waguih William IsHak, Email: Waguih.IsHak@cshs.org.
Jared M. Greenberg, Email: JMGreenberg@mednet.ucla.edu.
Robert M. Cohen, Email: rmcohe2@emory.edu.
References
- Agency for Healthcare Research and Quality’s (AHRQ) Registries for Evaluating Patient Outcomes: A User’s Guide. (3) 2013 < http://effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=531#toc>. [PubMed]
- Cohen RM, Greenberg JM, IsHak WW. Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the individual burden of illness index for depression (IBI-D) to measure treatment impact and recovery in major depressive disorder. JAMA Psychiatry (formerly Archives of General Psychiatry) 2013;70:343–350. doi: 10.1001/jamapsychiatry.2013.286. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Ottenbreit ND. Tertiary intervention for depression and prevention of relapse. In: Dozois DJ, Dobson KS, editors. The prevention of anxiety and depression: theory, research and practice. American Psychological Association Press; Washington DC: 2004. pp. 233–260. [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumental R. Quality of life enjoyment and satisfaction questionnaire: A new measure. Psychopharmacology Bulletin. 1993;29:321–326. [PubMed] [Google Scholar]
- European Commission. [accessed 15.01.13];EU-Compass for Action on Mental Health and Well being. 2010 Available from < http://ec.europa.eu/health/mental_health/eu_compass/index_en.htm>.
- Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, Quitkin FM, Wisniewski S, Lavori PW, Rosenbaum JF, Kupfer DJ. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatric Clinics of North America. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- Greer TL, Kurian BT, Trivedi MH. Defining and measuring functional recovery from depression. CNS Drugs. 2010;24:267–284. doi: 10.2165/11530230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Holma KM, Holma LA, Melartin TK, Rytsälä HJ, Isometsä ET. Long-term outcome of major depressive disorder in psychiatric patients is variable. Journal of Clinical Psychiatry. 2008;69:196–205. doi: 10.4088/jcp.v69n0205. [DOI] [PubMed] [Google Scholar]
- Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Molecular Psychiatry. 2006;11:11–17. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IsHak WW, Burt T, Sederer LI. Outcome Measurement in Psychiatry: A Critical Review. American Psychiatric Publishing Inc; Washington, DC: 2002. [Google Scholar]
- IsHak WW, Greenberg JM, Balayan K, Kapitanski N, Jeffrey J, Fathy H. Quality of life: The ultimate outcome measure of interventions in major depressive disorder. Harvard Review of Psychiatry. 2011;19:229–239. doi: 10.3109/10673229.2011.614099. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Balayan K, Bresee C, Greenberg JM, Fakhry H, Christensen S, Rapaport MH. A descriptive analysis of quality of life using patient-reported measures in major depressive disorder in a naturalistic outpatient setting. Quality of Life Research. 2013;22:585–596. doi: 10.1007/s11136-012-0187-6. [DOI] [PubMed] [Google Scholar]
- IsHak WW, Greenberg JM, Saah T, Mobaraki S, Fakhry H, Wu QV, Ngor E, Yu F, Cohen RM. Development and validation of the individual burden of illness index for major depressive disorder (IBI-D) Administration and Policy in Mental Health and Mental Health. 2013;40:76–86. doi: 10.1007/s10488-011-0376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MB, Shapiro RW, Lavori PW, Wolfe N. Relapse in major depressive disorder: analysis with the life table. Archives of General Psychiatry. 1982;39:911–915. doi: 10.1001/archpsyc.1982.04290080031005. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustün TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiologia e Psichiatria Sociale. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivelä SL, Viramo P, Pahkala K. Factors predicting the relapse of depression in old age. International Journal of Geriatric Psychiatry. 2000;15:112–119. doi: 10.1002/(sici)1099-1166(200002)15:2<112::aid-gps83>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Klein DN, Shankman AS, Lewinsohn PM, Rohde P, Seeley JR. Family study of chronic depression in a community sample of young adults. American Journal of Psychiatry. 2004;161:646–653. doi: 10.1176/appi.ajp.161.4.646. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne BJ, Caspi A, Harrington H, Poulton R, Rutter M, Moffitt TE. Predictive value of family history on severity of illness: the case for depression, anxiety, alcohol dependence, and drug dependence. Archives of General Psychiatry. 2009;66:738–747. doi: 10.1001/archgenpsychiatry.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder RT, Joyce PR, Frampton CM, Luty SE, Sullivan PF. Six months of treatment for depression: outcome and predictors of the course of illness. American Journal of Psychiatry. 2006;163:95–100. doi: 10.1176/appi.ajp.163.1.95. [DOI] [PubMed] [Google Scholar]
- Mundt JC, Marks IM, Shear MK, Greist JH. The work and social adjustment scale: a simple measure of impairment in functioning. British Journal of Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- O’Leary D, Costello F, Gormley N, Webb M. Remission onset and relapse in depression. An 18-month prospective study of course for 100 first admission patients. Journal of Affective Disorders. 2000;57:159–171. doi: 10.1016/s0165-0327(99)00086-5. [DOI] [PubMed] [Google Scholar]
- Pintor L, Torres X, Navarro V, Matrai S, Gastó C. Is the type of remission after a major depressive episode an important risk factor to relapses in a 4-year follow up? Journal of Affective Disorders. 2004;82:291–296. doi: 10.1016/j.jad.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Riise T, Lund A. Prognostic factors in major depression: a long-term follow-up study of 323 patients. Journal of Affective Disorders. 2001;65:297–306. doi: 10.1016/s0165-0327(00)00260-3. [DOI] [PubMed] [Google Scholar]
- Rupp A, Narrow W, Regier D, Sirovatka P. Epidemiology and costs of depression: a hidden burden. Disease Management and Health Outcomes. 1997;2:134–140. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB. The 16-item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, Thase ME, Nierenberg AA, Quitkin FM, Kashner TM, Kupfer DJ, Rosenbaum JF, Alpert J, Stewart JW, Mcgrath PJ, Biggs MM, Shores-Wilson K, Lebowitz BD, Ritz L, Niederehe G. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Controlled Clinical Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Wisniewski SR, Zisook S, Fava M, Sung SC, Haley CL, Chan HN, Gilmer WS, Warden D, Nierenberg AA, Balasubramani GK, Gaynes BN, Trivedi MH, Hollon SD. Is prior course of illness relevant to acute or longer-term outcomes in depressed out-patients? A STAR*D report. Psychological Medicine. 2012;42:1131–1149. doi: 10.1017/S0033291711002170. [DOI] [PubMed] [Google Scholar]
- Siskind D, Harris M, Pirkis J, Whiteford H. Personalised support delivered by support workers for people with severe and persistent mental illness: a systematic review of patient outcomes. Epidemiology and Psychiatric Sciences. 2012;21:97–110. doi: 10.1017/s2045796011000734. [DOI] [PubMed] [Google Scholar]
- Vilhauer JS, Cortes J, Moali N, Chung S, Mirocha J, IsHak WW. Improving Quality of Life for Patients with Major Depressive Disorder by Increasing Hope and Positive Expectations with Future Directed Therapy (FDT) Innovations in Clinical Neuroscience. 2013;10:12–22. [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) [accessed 15.01.13];Constitution of the World Health Organization. 1948 Available from < http://www.who.int/governance/eb/who_constitution_en.pdf>.
- World Health Organization (WHO) [accessed 15.01.13];Depression, fact sheet no 369. 2012 Available from < http://www.who.int/mediacentre/factsheets/fs369/en/>.
- Zimmerman M, Mcglinchey JB, Posternak MA, Friedman M, Attiullah N, Boerescu D. How should remission from depression be defined? American Journal of Psychiatry. 2006;163:148–150. doi: 10.1176/appi.ajp.163.1.148. [DOI] [PubMed] [Google Scholar]
- Zisook S, Rush AJ, Albala A, Alpert J, Balasubramani GK, Fava M, Husain M, Sackeim H, Trivedi MH, Wisniewski S. Factors that differentiate early vs. later onset of major depression disorder. Psychiatry Research. 2004;129:127–140. doi: 10.1016/j.psychres.2004.07.004. [DOI] [PubMed] [Google Scholar]