Abstract
Background
Abnormal levels of CD8+ T-cell activation persist in HIV-1-infected patients on suppressive antiretroviral therapy (ART) and may be deleterious.
Methods
CD8+ T-cell activation (%co-expressing CD38/HLA-DR) was analyzed on blood specimens from 833 HIV-1-infected patients on ART for ≥96 weeks with concurrent plasma HIV RNA (vRNA) ≤200 copies/mL. Factors associated with CD8+ T-cell activation were assessed using generalized estimating equations to incorporate longitudinal measurements (median 4/participant).
Results
Participants were 84% male, 47% white, 28% black, 22% Hispanic; with median pre-ART age 38 years and median ART exposure 144 weeks. CD8+ T-cell activation was higher at timepoints when vRNA was 51-200 copies/mL versus ≤50 copies/mL (mean CD8+ T-cell activation 23.4% vs. 19.7%; adjusted difference: 1.7% [95%CI 0.1%–3.4%], p=0.042). Restricting to vRNA≤50 copies/mL, multivariable models showed the following factors associated with higher CD8+ T-cell activation: older age (≥45 vs. ≤30 years: 3.6% [1.4%–5.7%], p=0.004), HCV antibody positivity (3.6% [0.9%–6.2%], p=0.032), Hispanic vs. White (7.2% [5.3%–9.0%], p<0.001), lower concurrent CD4 count (≤200 vs. >500 cells/mm3: 2.2% [0.7%–3.7%], p<0.001), lower concurrent CD4/CD8 ratio (−2.6% [−3.7%– −1.5%] per 0.5 unit increase, p<0.001), and higher pre-ART CD8+ T-cell activation (2.0% [1.6%–2.5%] per 10% higher, p<0.001).
Conclusions
In participants included in our analysis, residual low-level viremia between 51-200 copies/ml during ART was shown to be associated with greater CD8+ T cell activation than full suppression to <50 copies/ml. Older age, HCV positivity, race/ethnicity, higher pre-ART CD8+ T-cell activation, and lower concurrent CD4/CD8 ratio and CD4 cell count also contribute to greater CD8+ T-cell activation during suppressive ART.
Keywords: CD8+ T-cell activation, viral suppression, age, HCV, CD4+ cell count
Introduction
Antiretroviral treatment (ART) leads to virologic suppression, increases in circulating CD4+ T-cells, and a decline in T-cell activation in most HIV-infected patients, but levels of activation do not normalize to the levels of HIV-uninfected individuals1–3. Persistent CD8+ T-cell activation also appears to predict poor CD4+ T-cell recovery during ART4 and may play a role in the development of non-AIDS and AIDS-related complications in patients on ART5–8, albeit the risk of non-AIDS events has been associated primarily with other markers of immune activation (e.g., IL-6)7. In addition, persistent T-cell activation may facilitate replenishment of the viral reservoir and impede decay of proviral DNA9, and it has been associated with increased risk of viral blips10. For these reasons, defining risk factors for persistent CD8+ T-cell activation may help identify both novel targets for interventions and populations at highest risk.
Previous studies have identified potential risk factors for persistent CD8+ T-cell activation, but our knowledge of the determinants of persistent activation is incomplete. Factors associated with the level of CD8+ T-cell activation include pre-treatment nadir CD4+ T-cell count11; duration of ART-mediated viral suppression2,11, HCV co-infection11–14, CMV and/or other herpesvirus co-infection15, CD4/CD8 ratio16–17, and possibly statin use18. However these findings were often based on populations including viremic subjects off or on ART, had not fully examined multiple co-factors in adjusted models, or had been relatively underpowered to assess some risk factors. In addition, the influence of the degree of virologic suppression on CD8+ T-cell activation has not been fully addressed; viral blips below 400 copies/mL have been reported not to change CD8+ T-cell activation levels significantly10, but the impact of persistent viremia of 50–200 copies/mL has yet to be elucidated. It is critical to fill this knowledge gap partly because current United States Department of Health and Human Services (DHHS) guidelines suggest that the threshold for virologic failure in clinical practice should be HIV-1 RNA > 200 copies/mL19. Finally, the relationship between T-cell activation and the magnitude of CD4+ T-cell (CD4) restoration in patients experiencing prolonged viral suppression is not certain. Although this issue has been investigated by several groups, conflicting results have been published. A cross-sectional study suggested that CD8+ T-cell activation influences the extent of gains in CD4 cell counts from 3 months onwards in virally suppressed patients while on ART11. However, Benito et al. found no association between the extent of decline in CD8+ T-cell activation and the extent of CD4 cell recovery in a longitudinal study20. Both studies had small sample sizes (99 and 31, respectively) and follow-up ranged from 27 – 42 months after initiating ART. Two AIDS Clinical Trials Group [ACTG] investigations with larger samples reported that higher CD8+ T-cell activation after starting ART was associated with lower CD4 cell count increases2,4.
Using CD8+ T-cell activation data from several large ACTG studies, we examined whether degree of virologic suppression influences CD8+ T-cell activation by comparing the levels of CD8+ T-cell activation between timepoints with HIV-1 RNA ≤ 50 copies/mL to those with low-level viremia (51-200 copies/mL). Furthermore we evaluated several factors as potential correlates of CD8+ T-cell activation after long-term viral suppression. We believe this is the largest such analysis ever undertaken.
Methods
Study population and CD8+ T-cell activation
The study population was drawn from four ACTG treatment studies of initially ART-naive HIV-infected individuals (A384, A388, A5014 and A5095)21–24, and the analysis used additional data and follow-up from the ACTG Longitudinal Linked Randomized Trials (ALLRT) observational cohort25 where CD8+ T-cell activation measurements were obtained longitudinally. To be included in the analyses, participants had to have at least one CD8+ T-cell activation (% CD8+ T-cells co-expressing CD38/HLA-DR) measurement at a timepoint when HIV-1 RNA ≤ 200 copies/mL after ≥ 96 weeks of ART (regimen changes permitted).
The CD8+ T-cell activation was measured longitudinally at 16–48 week intervals on freshly obtained blood specimens (collected in 2000–2003) at different ACTG laboratories using a standardized ACTG protocol2. HIV-1 RNA was measured using Roche Ultrasensitive assay (lower detection limit of 50 copies/mL) run centrally in the Johns Hopkins University laboratory.
Statistical methods
The objective of this analysis was to identify important factors associated with CD8+ T-cell activation in virally suppressed subjects who have been on long-term ART. There were two aims. The first aim was to assess whether the degree of virologic suppression influences CD8+ T-cell activation. Levels of CD8+ T-cell activation between timepoints with HIV-1 RNA ≤ 50 copies/mL and 51-200 copies/mL were compared using the generalized estimating equation (GEE) method and an exchangeable correlation structure to take into account repeated measures and within-subject correlations26. Analysis was restricted to timepoints after 96 weeks of ART with concurrent and previous HIV-1 RNA ≤ 200 copies/mL. Univariate and multivariable GEE models were used to adjust for demographics (age, sex, race/ethnicity), other pre-treatment factors (CD4 cell count, HIV-1 RNA level, injection drug use) and on-treatment factors (concurrent CD4 cell count, ART class, HCV antibody status, statin use, body mass index (BMI) category, duration of prior viral suppression).
As a second aim, we assessed factors associated with CD8+ T-cell activation when HIV-1 RNA was ≤ 50 copies/mL, using univariate and multivariable GEE models and correlations. For this aim, we restricted the analyses to timepoints at or after 96 weeks of ART when both the concurrent and previous HIV-1 RNA were ≤ 50 copies/mL. Due to a large number of assay laboratories contributing data, all models were adjusted for assay laboratory by including a categorical variable with each level representing a laboratory (measurements without laboratory information were assigned to a level with missing laboratory). Highly correlated factors were evaluated separately in multivariable models. Sensitivity analyses were carried out to assess the robustness of our results.
Results
A total of 833 participants were included in the analyses. The majority of participants were male (84%); 47% were white non-Hispanic, 28% were black non-Hispanic, 22% were Hispanic and 2% were other race/ethnicity (Asian/American Indian/Alaskan Native). The median (Q1, Q3) pre-ART age was 38 (32, 44) years. Median (Q1, Q3) pre-treatment HIV-1 RNA was 5.2 (4.5, 5.6) log10 copies/mL and CD4 cell count was 222 (51, 390) cells/mm3. Thirty-six percent of participants initiated a protease inhibitor (PI) + nucleoside reverse transcriptase inhibitors (NRTIs) regimen, 31% started a PI+ non-nucleoside reverse transcriptase inhibitor (NNRTI) regimen, 28% initiated a NNRTI+NRTIs regimen, and 5% started a NRTI-only regimen (Table 1). Pre-ART characteristics were generally similar among the parent studies with the exception that ACTG 388 participants had more advanced HIV disease (this study only enrolled those with CD4 count ≤ 200 cells/mm3 or HIV-1 RNA ≥ 80,000 copies/mL).
Table 1.
Participant characteristics at parent study entry.
| Characteristic | Parent Study | |||||
|---|---|---|---|---|---|---|
| Total (N=833) | 384 (N=553) | 388 (N=178) | A5014 (N=27) | A5095 (N=75) | ||
| Age at parent study entry (years) | Mean (s.d.) | 38.2 (9.3) | 37.6 (9.0) | 39.5 (9.8) | 39.0 (10.9) | 39.3 (8.7) |
| Median (Q1, Q3) | 37 (32, 44) | 36 (31, 43) | 38 (33, 46) | 36 (30, 45) | 38 (34, 45) | |
| <= 30 | 164 (20%) | 118 (21%) | 27 (15%) | 7 (26%) | 12 (16%) | |
| 31–44 | 475 (57%) | 321 (58%) | 101 (57%) | 12 (44%) | 41 (55%) | |
| 45+ | 194 (23%) | 114 (21%) | 50 (28%) | 8 (30%) | 22 (29%) | |
| Sex | M | 701 (84%) | 463 (84%) | 155 (87%) | 23 (85%) | 60 (80%) |
| Race/Ethnicity | White | 395 (47%) | 258 (47%) | 93 (52%) | 6 (22%) | 38 (51%) |
| Black | 232 (28%) | 171 (31%) | 33 (19%) | 7 (26%) | 21 (28%) | |
| Hispanic | 184 (22%) | 109 (20%) | 48 (27%) | 13 (48%) | 14 (19%) | |
| Other | 22 (3%) | 15 (3%) | 4 (2%) | 1 (4%) | 2 (3%) | |
| IV drug history | Never | 770 (92%) | 512 (93%) | 162 (91%) | 26 (96%) | 70 (93%) |
| Ever | 63 (8%) | 41 (7%) | 16 (9%) | 1 (4%) | 5 (7%) | |
| Pre-ART HIV-1 RNA (log10 copies/mL) | Mean (s.d.) | 5.0 (0.8) | 4.9 (0.9) | 5.5 (0.6) | 4.3 (0.8) | 5.0 (0.7) |
| Median (Q1, Q3) | 5.2 (4.5, 5.6) | 5.0 (4.3, 5.5) | 5.5 (5.2, 5.9) | 4.3 (4.1, 4.8) | 4.8 (4.5, 5.5) | |
| Pre-ART CD4 (cells/mm3) | Mean (s.d.) | 249 (213) | 278 (219) | 139 (153) | 318 (162) | 273 (214) |
| Median (Q1, Q3) | 222 (51, 390) | 266 (77, 422) | 79 (27, 189) | 344 (172, 416) | 252 (87, 388) | |
| 0 – 50 | 208 (25%) | 116 (21%) | 78 (44%) | 2 (7%) | 12 (16%) | |
| 51 – 200 | 185 (22%) | 104 (19%) | 57 (32%) | 5 (19%) | 19 (25%) | |
| 201 – 350 | 187 (22%) | 135 (24%) | 23 (13%) | 7 (26%) | 22 (29%) | |
| 351 – 500 | 144 (17%) | 111 (20%) | 13 (7%) | 9 (33%) | 11 (15%) | |
| >500 | 109 (13%) | 87 (16%) | 7 (4%) | 4 (15%) | 11 (15%) | |
| ARV drug class | PI | 301 (36%) | 177 (32%) | 112 (63%) | 12 (44%) | 0 (0%) |
| NNRTI | 235 (28%) | 179 (32%) | 0 (0%) | 3 (11%) | 53 (71%) | |
| PI/NNRTI | 258 (31%) | 192 (35%) | 64 (36%) | 2 (7%) | 0 (0%) | |
| NRTI | 39 (5%) | 5 (1%) | 2 (1%) | 10 (37%) | 22 (29%) | |
CD8+ T-cell activation was analyzed after 96 weeks of ART (median 144 weeks, ranging from 96 to 256 weeks), with a median (Q1, Q3) of 4 (2, 6) measurements per participants. The median (Q1, Q3) duration of virologic suppression (≤200 copies/mL) prior to CD8+ T-cell activation measurements was 112 (84, 146) weeks. Among the 3382 CD8+ T-cell activation measurements, 3071 (91%) were at timepoints with both current and previous plasma HIV-1 RNA level ≤ 50 copies/mL, 136 (4%) at timepoints with current HIV-1 RNA ≤ 50 copies/mL and previous RNA between 51-200 copies/mL and 175 (5%) at timepoints with current HIV-1 RNA between 51-200 copies/mL and previous HIV-1 RNA ≤ 200 copies/mL. The estimated correlation between CD8+ T-cell activation measurements within the same participant was 0.66 (based on the null GEE model).
Among the 3382 CD8+ T-cell activation measurements included in the analyses, 78% were assayed in 29 different laboratories (22% did not have testing laboratory information). The number of CD8+ T-cell activation measurements contributed by each laboratory ranged from 3 to 289 with the number of participants ranging from 1 to 82. Seven laboratories contributed 41% of the measurements with similar contributions between the two large parent studies. The median CD8+ T-cell activation reported by the 7 largest laboratories ranged from 10% to 25% with overlapping interquartile ranges. All testing laboratories followed the same standardized operating procedure when testing CD8+ T-cell activation.
Virologic suppression and CD8+ T-cell activation
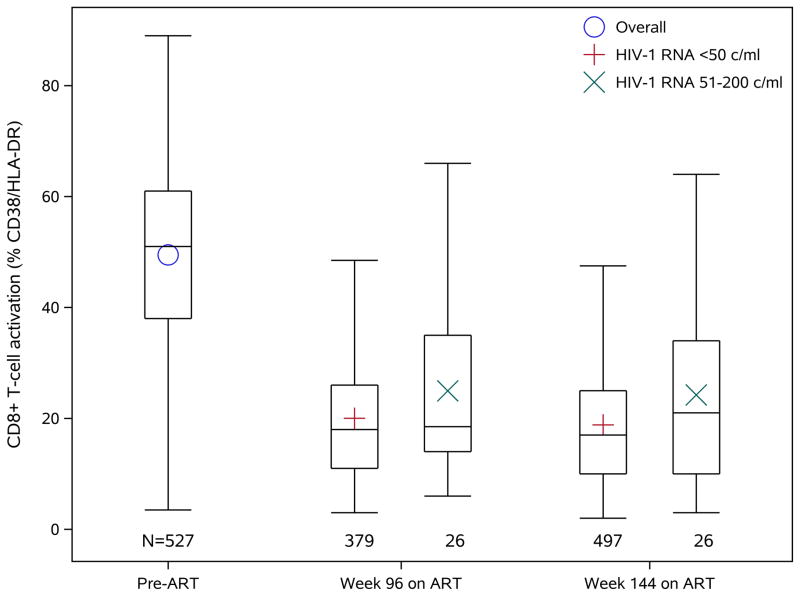
As expected, CD8+ T-cell activation markedly declined after initiation of ART (Figure 1). Levels of CD8+ T-cell activation were compared between timepoints with both current and previous HIV-1 RNA ≤ 50 copies/mL versus concurrent HIV-1 RNA between 51-200 copies/mL and previous HIV-1 RNA ≤ 200 copies/mL. CD8+ T-cell activation was higher at timepoints when concurrent HIV-1 RNA was 51-200 copies/mL than at timepoints when HIV-1 RNA was ≤50 copies/mL (mean CD8+ T-cell activation 23.4% vs. 19.7%, model-estimated difference 1.8%, 95% confidence interval [0.2%, 3.3%], p=0.026). After adjusting for demographics (age, sex, race/ethnicity), other pre-treatment factors (Hepatitis C virus antibody status, statin use, injection drug use, CD4 cell count, HIV-1 RNA, BMI category) and on-treatment factors (concurrent CD4 cell count, ARV class, duration of viral suppression and assay laboratory), CD8+ T-cell activation remained higher for measurements with concurrent HIV-1 RNA between 51-200 copies/mL (adjusted estimated difference 1.7% [0.1%, 3.4%], p=0.042). We further evaluated the association in the smaller dataset restricted to participants who had pre-ART CD8+ T-cell activation results available (513 participants with 1916 CD8+ T-cell activation measures) using the same multivariable model as above but with additional adjustment for pre-ART CD8+ T-cell activation. Higher CD8+ T-cell activation was still associated, albeit marginally, with HIV-1 RNA between 51-200 copies/mL (2.0% [−0.1%, 4.1%, p=0.067).
Figure 1.
CD8+ T-cell activation (% co-expressing CD38/HLA-DR) levels pre-ART and at 96 and 144 weeks on ART. Top and bottom of the box represent the lower and upper quartiles. The bar and the symbol inside the box represent the median and the mean, respectively. The whiskers extend to the furthest measurement within 1.5 times the interquartile range from the upper or lower quartile.
Factors associated with CD8+ T-cell activation when HIV-1 RNA is ≤ 50 copies/mL
To assess pre-treatment and treatment related factors associated with higher CD8+ T-cell activation in participants with HIV-1 RNA ≤ 50 copies/mL, we restricted analyses to CD8+ T-cell activations at timepoints with both current and previous HIV-1 RNA below this threshold (3071 measurements from 797 participants). Pre-ART characteristics of the participants were similar to those for the overall analysis population.
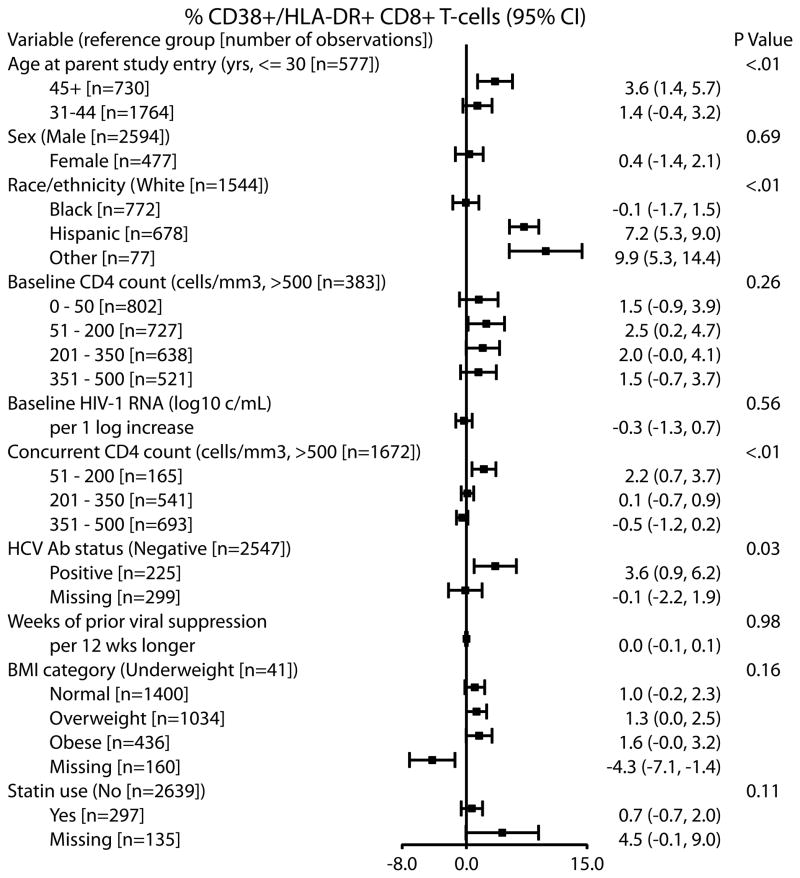
In the multivariable model adjusting for concurrent ARV class and assay laboratory, the following factors were independently associated with higher CD8+ T-cell activation (Figure 2): older age (difference in CD8+ T-cell activation between those ≥45 vs. ≤30 years: 3.6% [1.4%, 5.7%], p=0.004), Hispanic or other race/ethnicity (Hispanic vs. white: 7.2% [5.3%, 9.0%]; other vs. white: 9.9% [5.3%, 14.4%], p<0.001), HCV antibody status (positive vs. negative, 3.6% [0.9%, 6.2%], p=0.032) and lower concurrent CD4 cell count (51-200 vs. > 500 cell/mm3: 2.2% [0.7%, 3.7%], p<0.001). Sex, pre-ART viral load, statin use, BMI and duration of viral suppression were not associated with CD8+ T-cell activation (all p ≥ 0.11). Lower pre-ART CD4 cell count was significantly associated with higher CD8+ T-cell activation in the univariable model (p=0.006), but the association did not persist when concurrent CD4 cell count was included in the multivariable model (p=0.26). The effects of other factors were similar in univariable models compared to the multivariable model. Due to high correlation between injection drug use (IDU) and HCV antibody status, IDU was evaluated in a separate multivariable model which did not include HCV antibody status and was not found to be significantly associated with higher CD8+ T-cell activation (p=0.22). IDU was also not significantly associated with CD8+ T-cell activation in the univariable model (p=0.34). We also examined the association between age and CD8+ T-cell activation treating age as a continuous variable in a comparable multivariable model and found a significant association (per 10 years older: 1.4% [0.7%, 2.2%], p<0.001).
Figure 2.
Associations of demographics and clinical factors with CD8+ T-cell activation in subjects on ART ≥ 96 weeks with concurrent and previous HIV-1 RNA ≤ 50 copies/mL. Results are from a multivariable repeated measure model; the effects of each factor are adjusted for all the other listed factors, as well as concurrent ART class and assay laboratory.
Due to the nature of our analysis population and pooling of data from multiple testing laboratories, two sets of sensitivity analyses were carried out. The first set attended to potential differences among subpopulations from the 4 parent studies. We first adjusted for parent study in the multivariable model and then evaluated the associations separately in the two large parent studies (the number of participants from the other two parent studies was not large enough to allow us to carry out the multivariable analysis). The above identified associations remained significant in both sensitivity analyses. The second set of sensitivity analyses attempted to address the issues related to pooling data from various testing laboratories. We first carried out the same multivariable model without adjustment for testing laboratories, then in a subset restricted to testing laboratories with high within-person correlations (r≥ 0.45), and in a subset restricted to CD8+ T-cell activation measures with available testing laboratory information, and finally in a subset restrict to the 7 largest contributing laboratories. Similar results were obtained in all the sensitivity analyses, i.e., similar statistical significance and magnitude of the effects.
In a subgroup analysis of 491 participants with pre-ART CD8+ T-cell activation data, there was also a significant association between pre-treatment CD8+ T-cell activation and CD8+ T-cell activation on long-term ART (2.0% [1.6%, 2.5%] per 10% higher pre-ART CD8+ T-cell activation, p<0.001), when added to the multivariable model of the factors above. In this model including pre-ART CD8+ T-cell activation, older age (≥45 vs. ≤30 years: 3.2% [0.8%, 5.5%], p=0.019), Hispanic or other race/ethnicity vs. white (Hispanic vs. white: 5.0% [2.7%, 7.3%]; other vs. white: 8.5% [2.2%, 14.8%], p<0.001), HCV antibody status (positive vs. negative: 5.0% [2.3%, 7.6%], p=0.003), and lower concurrent CD4 cell count (51-200 vs. > 500 cell/mm3: 2.7% [0.9%, 4.5%], p=0.001) remained significant.
In a separate subgroup analysis of 426 participants with concurrent CD4+ T-cell activation (% co-expressing CD38/HLA-DR), CD4+ activation was significantly associated with CD8+ T-cell activation (0.58% [0.42%, 0.73%] per 1% higher concurrent CD4+ T-cell activation, p<0.001) in the multivariable model of the same factors. Concurrent CD4 cell count was no longer associated with CD8 T-cell activation when CD4+ T-cell activation was added to the model.
To determine the association between CD4/CD8 ratio and CD8+ T-cell activation, pre-ART and concurrent CD4/CD8 ratio were evaluated in a multivariable model (adjusted for ARV regimen class and assay laboratory) with demographics (age, sex, race/ethnicity), pre-treatment HIV-1 RNA level and on-treatment factors (HCV antibody status, statin use, BMI category, duration of prior viral suppression). Concurrent CD4/CD8 ratio (over all observations median = 0.68; Q1-Q3: 0.45–1.01) was significantly associated with CD8+ T-cell activation (−2.6% [−3.7%, −1.5%] per 0.5 unit increase, p<0.001). Pre-ART CD4/CD8 ratio was significantly associated with on-treatment CD8+ T-cell activation in a univariable model, but was no longer significant in the multivariable model when concurrent CD4/CD8 ratio was present. When concurrent CD8+ T-cell count was evaluated in a separate multivariable model adjusting for pre-treatment and concurrent CD4 count as well as the same factors above, concurrent CD8+ T-cell count was significantly associated with CD8+ T-cell activation (0.3% [0.2%, 0.5%] per 100 cells increase, p<0.001).
To describe on a common scale above identified associations with CD8+ T-cell activation, a resampling method27 was used to estimate univariate associations in terms of Spearman correlations between CD8+ T-cell activation (after ≥96 weeks of ART with both current and previous HIV-1 RNA ≤50 copies/mL) and age, pre-ART CD4 cell count, concurrent CD4 cell count and concurrent CD4/CD8 ratio. The strongest correlation was observed between CD8+ T-cell activation and concurrent CD4/CD8 ratio (r= −0.20), followed by concurrent CD4 cell count (r= −0.18), age (r= 0.12) and pre-treatment CD4 cell count (r= −0.10).
Discussion
Using a large dataset of CD8+ T-cell activation measurements in HIV-infected participants on long-term suppressive ART, we evaluated factors associated with CD8+ T-cell activation. In our analyses, compared to timepoints with stable plasma HIV-1 RNA levels ≤ 50 copies/mL, HIV-1 RNA between 51-200 copies/mL during ART was associated with higher CD8+ T-cell activation, albeit of a small magnitude. This supports the possible immunologic consequences of less than complete virologic suppression28–29. When restricted to timepoints when HIV-1 RNA was ≤ 50 copies/mL, older age, HCV positivity, Hispanic race, lower concurrent CD4/CD8 ratio, and lower concurrent CD4 cell count were independently associated with higher CD8+ T-cell activation. While the association between viremia of 51-200 copies/mL and higher CD8+ T-cell activation identified in our study may suggest that activated T-cells are a source of low-level viremia during ART (to the extent that CD8+ T-cell activation reflects CD4+ T-cell activation), or that low-level viremia directly activates CD8+ T-cells, a causal relationship cannot be assumed. Our findings may also be consistent with alternative explanations. For example, it is possible that low-level viremia and CD8+ T-cell activation are caused or mediated by common factors (i.e., co-infections, vaccinations, etc.) or that low-level viremia is a consequence of impaired immunity rather than a cause. Regardless of its nature, the association urges evaluation of other potential effects of low-level viremia. A recently published cohort study showed that persistent low-level viremia of 51-200 copies/mL for 6 months was associated with an increased risk of virologic failure when compared to undetectable viral load for the same period30. Studies are needed to determine whether viremia of 51-200 copies/mL affects levels of soluble markers of immune activation and monocyte activation, which are more predictive of non-AIDS events during ART than CD4+ or CD8+ T-cell activation7, 31. A better understanding of the effect of low-level viremia on resistance evolution, virologic failure and clinical outcomes is needed as well. These data are necessary to determine whether viremia of 51-200 copies/mL should be tolerated in clinical practice, as suggested by the DHHS guidelines, or whether viral suppression below 50 copies/mL should be the treatment target in all patients.
Heightened immune activation may be one of the mechanisms for premature aging of HIV-infected patients despite ART. Indeed, age has been shown in HIV-uninfected individuals to be associated with increased CD38/HLA-DR expression on CD8 cells32. Our study is the first to clearly demonstrate that in HIV-infected patients with HIV-1 RNA ≤ 50 copies/mL on ART, CD8+ T-cell activation is higher in older subjects than in younger participants (per 10 years increase: 1.4% [0.7%, 2.2%], p<0.001). Among older (≥ 50 years old) individuals with HIV RNA ≤ 200 copies/mL, a recent study, with study population drawn from the same cohort of ours, reported a significant association between elevated on-ART CD8+ T-cell activation (%CD38+HLA-DR+) and incidence of an AIDS or non-AIDS event in years 2–5 on ART on suppressive ART, with an odds ratio (OR) of 1.42 per 10 percentage point higher CD8+ T-cell activation; a significant association was also seen among all subjects, with OR=1.225.
A recently published study has shown the association between lower CD4/CD8 ratio and higher CD8 T-cell activation in treated HIV-infected patients with undetectable viral load and high CD4+ T-cell count16–17. This association was replicated across different CD4 count levels in our study population, although the observed association seems to be weaker than was reported for CD4> 500 cells/mm3 population in the previous study (−0.20 vs. −0.51).
We also confirmed that HCV co-infection is associated with increased CD8+ T-cell activation, which was shown in several other studies11–14. However due to limited data, we could not further evaluate the association of HCV RNA level and CD8+ T-cell activation.
We did not find an association between pre-treatment HIV-1 RNA level and CD8+ T-cell activation during viral suppression. The association between lower pre-treatment CD4 cell count and increased CD8+ T-cell activation was significant in univariable analysis, but no longer significant in the multivariable analysis when concurrent CD4 cell count was present. This is consistent with previously reported associations between persistent CD8+ T cell activation and blunted ART-mediated CD4+ T cell recovery2, 4, 11.
The observed association of Hispanic or other race/ethnicity with increased CD8+ T-cell activation is surprising and puzzling. In sensitivity analyses, the association between Hispanic race and increased CD8+ T-cell activation remained significant (Hispanic vs. White: 7.1% [5.3%, 8.9%] in the parent study adjusted analysis; 6.1% [3.8%, 8.3%] in ACTG 384 subpopulation and 10.1% [6.8%, 13.4%] in ACTG 388 subpopulation). To our knowledge there is no known clinical correlation for this finding. Differences in clinical outcomes between Hispanics and others have not been studied extensively in large studies that have the ability to control for potential confounders33. Of the few studies that evaluated Hispanic ethnicity, almost all did not see a racial/ethnic difference in clinical progression of HIV33–36. One exception is a large study that reported greater mortality in Blacks and Hispanics, compared to Whites, in analyses that adjusted for age. The investigators’ finding may be due to confounding factors since Blacks and Hispanics in the study population had significantly more medical comorbidities37. If the racial/ethnic differences in CD8+ T-cell activation observed in our study are confirmed, one possible explanation is that they may be driven by some unmeasured differences between subjects or underlying genetic factors. Future studies to explore genetic determinants of CD8+ T-cell activation may be worth pursuing.
Our study has a number of strengths. First, we examined over 800 individuals and more than 3000 CD8+ T-cell activation measurements, giving us the power to detect important associations that may have been missed by smaller studies. In our second aim, we focused our analyses on participants with sustained viral suppression of HIV-1 RNA ≤ 50 copies/mL. This enabled us to evaluate the factors associated with CD8+ T-cell activation without the potential confounder of uncontrolled viremia.
There are several limitations for our study. First, some of the CD8+ T-cell activation measurements were taken when participants were on antiretroviral regimens no longer recommended due to suboptimal virologic efficacy (25% on unboosted PI regimen, 31% on unboosted PI+NNRTI regimen and 4% on NRTIs only regimen at week 96). Hence we did not formally evaluate the impact of specific ARVs on CD8+ T-cell activation; instead, we adjusted for ARV drug class in the multivariable model. Second, we had limited data on HCV RNA levels, and no information on cytomegalovirus and herpes simplex virus, which constrained our ability to fully assess the effects of these co-infections. Finally, the CD8 activation measures were contributed by a large number of ACTG assay laboratories. Although all laboratories used an ACTG standardized consensus protocol for the assay in terms of processing times, processing procedures, reagents, analysis, etc., only a small subgroup (4 laboratories) underwent an external quality assurance program. To interrogate the impact of pooling assay data generated from various laboratories on our findings, we adjusted for the testing laboratories in both the univariable and multivariable models. Furthermore, we carried out a set of sensitivity analyses to evaluate the robustness of our findings and obtained similar results. Nevertheless, we cannot definitively exclude the possibility that measurement bias contributed to our results, so it would be valuable to have our findings confirmed by a study using a central laboratory for CD8+ T-cell activation testing.
In conclusion, our results suggest that in participants included in our analysis, HIV-1 RNA between 51-200 copies/mL duing ART was associated with higher CD8+ T-cell activation compared with levels ≤ 50 copies/mL. Among persons with viral load ≤ 50 copies/mL, older age, HCV antibody positivity, Hispanic race, higher pre-ART CD8+ T-cell activation, lower concurrent CD4/CD8 ratio and lower concurrent CD4 cell count were independently associated with higher CD8+ T-cell activation. Although it is not clear that intervention to reduce CD8+ T-cell activation would have any clinical benefit, mechanisms for the observed associations should be studied and clinical correlates of these factors should be delineated.
Acknowledgments
We thank the chairs and teams of the contributing ACTG studies and the study staff and volunteers for their time and effort.
Source of Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [AI-68636, AI-68634, AI-38858, AI-38855, and AI-69434].
Footnotes
The results of this analysis were previously presented at the XIX International AIDS Conference (Washington D.C., USA) on July 22–27, 2012.
Potential Conflicts of Interest
B.T. has served as an advisor and/or received research support (to Northwestern University) from Janssen, Pfizer, GlaxoSmithKline and ViiV. R.T.G. has received institutional research grant support from Abbott, Viiv and Janssen. A.C.C. receives research support from Merck and past research support from Schering-Plough; previously owned stock in Abbott, Bristol-Myers-Squibb, Johnson and Johnson, and Pfizer; and was a DSMB member for a Merck-sponsored study. C.F. reports receiving research grant support from Boehringer-Ingelheim and GlaxoSmithKline for research unrelated to this study; and serving as a consultant to Bristol-Myers Squibb, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Roche, Schering-Plough, Tobira Therapeutics, Tibotec, Vertex, Virostatics, and ViiV Healthcare. All other authors report no potential conflict.
Contributor Information
Lu Zheng, Harvard School of Public Health, Boston, MA, USA.
Babafemi Taiwo, Division of Infection Diseases, Northwestern University, Chicago, IL, USA.
Rajesh T. Gandhi, Massachusetts General Hospital and Ragon Institute, Boston, MA, USA.
Peter W. Hunt, School of Medicine, University of California at San Francisco, San Francisco, CA, USA.
Ann C. Collier, Division of Infectious Diseases; Department of Medicine; University of Washington, Seattle, WA, USA.
Charles Flexner, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Ronald J. Bosch, Harvard School of Public Health, Boston, MA, USA.
References
- 1.Valdez H, Connick E, Smith KY, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 2.Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42(4):426–434. doi: 10.1097/01.qai.0000226789.51992.3f. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PW, Landay AL, Sinclair E, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One. 2011;6(1):e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang X, Hunt PW, Hammer SM, Cespedes MS, Patterson KB, Bosch RJ. Immune Activation While on Potent Antiretroviral Therapy Can Predict Subsequent CD4+ T-Cell Increases Through 15 Years of Treatment. HIV Clin Trials. 2013;14(2):61–67. doi: 10.1310/hct1402-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lok JJ, Hunt PW, Collier AC, et al. The impact of age on the prognostic capacity of CD8+ T-cell activation during suppressive antiretroviral therapy. AIDS. 2013;24;27(13):2101–10. doi: 10.1097/QAD.0b013e32836191b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T-cell activation on CD4+ T-cell recovery and mortality in HIV-infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123–2131. doi: 10.1097/QAD.0b013e32834c4ac1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt PW, Rodriguez B, Shive C, et al. Gut Epithelial Barrier Dysfunction, Inflammation, and Coagulation Predict Higher Mortality during Treated HIV/AIDS [abstract 278]. Presented at: The 19th Conference on Retroviruses and Opportunistic Infections; 2012; Seattle, WA, USA. [Google Scholar]
- 8.Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of Functional Impairment with Inflammation and Immune Activation in HIV Type 1-Infected Adults Receiving Effective Antiretroviral Therapy. J Infect Dis. 2013;208(2):249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013;254(1):326–342. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taiwo B, Hunt PW, Gandhi RT, et al. CD8+ T-cell activation in HIV-1-infected patients experiencing transient low-level viremia during antiretroviral therapy. J Acquir Immune Defic Syndr. 2013;63(1):101–104. doi: 10.1097/QAI.0b013e3182895af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4 T cell gains in human immunodeficiency virusinfected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 12.Valdez H, Anthony D, Farukhi F, et al. Immune responses to hepatitis C and non-hepatitis C antigens in hepatitis C virus infected and HIV-1 coinfected patients. AIDS. 2000;14(15):2239–2246. doi: 10.1097/00002030-200010200-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovacs A, Karim R, Mack WJ, et al. Activation of CD8 T cells predicts progression of HIV infection in women coinfected with hepatitis C virus. J Infect Dis. 2010;201(6):823–834. doi: 10.1086/650997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt PW, Martin JN, Sinclair E, et al. Valganciclovir Reduces T Cell Activation in HIV-infected Individuals With Incomplete CD4+ T Cell Recovery on Antiretroviral Therapy. J Infect Dis. 2011;203(10):1474–1483. doi: 10.1093/infdis/jir060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serrano-Villar S, Gutiérrez C, Vallejo A, et al. The CD4/CD8 ratio in HIV-infected subjects is independently associated with T-cell activation despite long-term viral suppression. J Infect. 2013 Jan;66(1):57–66. doi: 10.1016/j.jinf.2012.09.013. Epub 2012 Oct 6. [DOI] [PubMed] [Google Scholar]
- 17.Serrano-Villar S, Sainz T, Lee S, et al. A Low CD4/CD8 Ratio During Effective ART Predicts Immunosenescence and Morbidity/Mortality. Conference on Retroviruses and Opportunitistic Infections; March 3–6, 2014; Boston, Massachusetts, USA. 2014. Abstract #242. [Google Scholar]
- 18.Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–764. doi: 10.1093/infdis/jiq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services; [Assessed October 1, 2013]. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 20.Benito JM, Lopez M, Lozano S, et al. CD4+ T cell recovery beyond the first year of complete suppression of viral replication during highly active antiretroviral therapy is not influenced by CD8+ T cell activation. J Infect Dis. 2005;192(12):2142–2146. doi: 10.1086/498168. [DOI] [PubMed] [Google Scholar]
- 21.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischl MA, Ribaudo HJ, Collier AC, et al. A randomized trial of 2 different 4-drug antiretroviral regimens versus a 3-drug regimen, in advanced human immunodeficiency virus disease. J Infect Dis. 2003;188(5):625–634. doi: 10.1086/377311. Epub 2003 Aug 15. [DOI] [PubMed] [Google Scholar]
- 23.Landay AL, Spritzler J, Kessler H, et al. Immune reconstitution is comparable in antiretroviral-naive subjects after 1 year of successful therapy with a nucleoside reverse-transcriptase inhibitor- or protease inhibitor-containing antiretroviral regimen. J Infect Dis. 2003;188(10):1444–1454. doi: 10.1086/379041. Epub 2003 Nov 13. [DOI] [PubMed] [Google Scholar]
- 24.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350(18):1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 25.Smurzynski M, Collier AC, Koletar SL, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang KY, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 27.Hoffman EB, Sen PK, Weinberg CR. Within-cluster Resampling. Biometrika. 2001;88:1121–1134. [Google Scholar]
- 28.Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS. 2013;8(3):211–216. doi: 10.1097/COH.0b013e32835f9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buzón MJ, Massanella M, Llibre JM, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16(4):460–465. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 30.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: Results from 12 years of observation. Clin Infect Dis. 2013 doi: 10.1093/cid/cit529. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Tenorio A, Zheng Y, Bosch R, et al. Soluble Markers of Inflammation and Coagulation, but Not T Cell Activation, Predict Non--AIDS--defining Events during Suppressive ART [abstract 790]. Presented at: The 20th Conference on Retroviruses and Opportunistic Infections; 2013; Atlanta, GA, USA. [Google Scholar]
- 32.Kalayjian RC, Landay A, Pollard RB, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187(12):1924–1933. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med. 2009 Sep;24(9):1065–72. doi: 10.1007/s11606-009-1049-y. Epub 2009 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastos K, Schneider MF, Gange SJ, et al. The association of race, sociodemographic, and behavioral characteristics with response to highly active antiretroviral therapy in women. J Acquir Immune Defic Syndr. 2005;39:537–44. [PubMed] [Google Scholar]
- 35.Tedaldi EM, Absalon J, Thomas AJ, Shlay JC, van den Berg-Wolf M. Ethnicity, race, and gender. Differences in serious adverse events among participants in an antiretroviral initiation trial: results of CPCRA 058 (FIRST Study) J Acquir Immune Defic Syndr. 2008;47:441–8. doi: 10.1097/QAI.0b013e3181609da8. [DOI] [PubMed] [Google Scholar]
- 36.Chen NE, Gallant JE, Page KR. A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. J Immigr Minor Health. 2012 Feb;14(1):65–81. doi: 10.1007/s10903-011-9497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinnis KA, Fine MJ, Sharma RK, et al. Understanding racial disparities in HIV using data from the veterans aging cohort 3-site study and VA administrative data. Am J Public Health. 2003;93:1728–33. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]