Abstract
A noninvasive test was developed in rabbits based on fast adaptation measures for 2f1-f2 distortion-product otoacoustic emissions (DPOAEs). The goal was to evaluate the effective reflex activation, i.e., “functional strength,” of both the descending medial olivocochlear efferent reflex (MOC-R) and the middle-ear muscle reflex (MEM-R) through sound activation. Classically, it is assumed that both reflexes contribute toward protecting the inner ear from cochlear damage caused by noise exposure. The DP-gram method described here evaluated the MOC-R effect on DPOAE levels over a two-octave (oct) frequency range. To estimate the related activation of the middle-ear muscles (MEMs), the MEM-R was measured by monitoring the level of the f1-primary tone throughout its duration. Following baseline measures, rabbits were subjected to noise over-exposure. A main finding was that the measured adaptive activity was highly variable between rabbits but less so between the ears of the same animal. Also, together, the MOC-R and MEM-R tests showed that, on average, DPOAE adaptation consisted of a combined contribution from both systems. Despite this shared involvement, the amount of DPOAE adaptation measured for a particular animal's ear predicted that ear's subsequent susceptibility to the noise over-exposure for alert but not for deeply anesthetized rabbits.
I. INTRODUCTION
A major breakthrough in our understanding of the function of the olivocochlear-efferent system occurred when Liberman et al. (1996) developed a test in anesthetized cats based on distortion product otoacoustic emissions (DPOAEs). Using DPOAEs as a measure of outer hair cell activity, these investigators assessed the activation of the contralateral, as well as the ipsilateral olivocochlear descending neural projections on, what they termed, the “fast adaptive cochlear response.” Specifically, for a single f2 primary tone at 2 kHz, Liberman et al. (1996) reported that as a result of an acoustically induced activation of the olivocochlear pathway with long-duration f1 and f2 primary tones, DPOAEs in the ipsilateral test ear were maximally reduced at about 0.5 to 1 s after the onset of the primary tones. Moreover, this adaptive decrease in DPOAE levels was greater at about 10 dB in response to binaural than at 6 dB to monaural presentations of the primary tones. To ensure that the fast adaptive response they observed was specifically related to olivocochlear efferent-system activity, Liberman et al. (1996) also showed that the fast adaptive DPOAE effect was eliminated when the entire efferent nerve-fiber supply to the test ear was severed surgically. Maison and Liberman (2000) later extended these earlier findings in sedated cats to alert guinea pigs, and they further showed that this measure, as an index of olivocochlear-reflex strength, could be used to predict the ear's vulnerability to the adverse effects of a subsequent acoustic over-exposure.
The primary aim of the present study was to examine the combined effects of MOC-R and MEM-R activation, across a broad frequency range, to gain a more complete understanding of the effects that these two acoustic-activated reflex loops have on DPOAE adaptation in the alert compared to the anesthetized rabbit. An additional goal was to determine if the activity of the combined reflexes predicted the extent of subsequent cochlear dysfunction due to noise overstimulation. To accomplish these goals, a multifrequency test was employed spanning frequencies from 3 to 13 kHz, and an average DPOAE-adaptation index was calculated from these frequency-specific effects. In addition, to avoid a potential confounding influence on the DPOAE-adaptation index, contributions from the MEMs were measured in a similar manner. In fact, these combined measures of the DPOAE-adaptation index in the awake rabbit predicted the vulnerability of the cochlea to sound-induced dysfunction. In contrast, when the DPOAE-adaptation index was similarly determined in the same rabbits while deeply anesthetized, the measure failed to predict the cochlea's vulnerability to acoustic overstimulation.
II. METHODS
A. Animal subjects
Young (n = 10, 2–3 months old, 2–3 kg) New Zealand pigmented rabbits (Oryctolagus cuniculus) obtained from a local commercial supplier were used as experimental subjects. All rabbits initially underwent aseptic surgery under general anesthesia (40 mg/kg ketamine hydrochloride, 10 mg/kg xylazine, IM) to permanently affix to the dorsal surface of the skull a head-brace device consisting of an inverted stainless-steel screw. This brace supported the animal's head and also provided the stability necessary for obtaining repeated DPOAE measures, because it permitted the accurate replication of head position during different test periods. Rabbits were allowed to recover from the head-post surgery for 3 weeks before experimentation was initiated.
During the DPOAE-adaptation testing sessions, rabbits were examined while both awake and anesthetized, with the latter condition achieved using the anesthetic regimen noted above. Average DPOAE adaptation and MEM-R indices were determined in six alert rabbits. For these measures, five animals were assessed both monaurally and binaurally, and one only monaurally, to realize n = 11 test ears in all. In addition, in a set of preliminary experiments, the test/retest reliability of the DPOAE-adaptation index was determined in four other rabbits (n = 8 ears). In determining test/retest reliability, adaptation indices that were obtained initially and then 2 days later were compared statistically using correlational coefficients obtained from simple linear regression functions.
B. Measures of DPOAEs and DP-Grams
DPOAEs at the 2f1-f2 frequency were recorded conventionally using commercially available components. These elements included separate f1 and f2 ear-speakers (ER-2, Etymotic Research, Elk Grove Village, IL) and an acoustic probe/microphone assembly (ER-10A, Etymotic Research). As detailed in Martin et al. (1998), stimulus generation and response acquisition were controlled by a personal microcomputer (Apple, Macintosh Quadra 700, Cupertino, CA) through an on-board digital-signal processor (Audiomedia, Digidesign Inc, Palo Alto, CA) operated by customized software. In combination, this system was capable of obtaining DPOAEs in the form of routine DP-grams, i.e., DPOAE magnitudes as a function of the f2-test frequency at several constant primary-tone levels. That is, standard DP-grams were obtained initially between f2 frequencies of 1.6–18 kHz, in 0.1-octave steps (i.e., at n = 31 test frequencies), for equal-level (L1 = L2) primary tones ranging from 45–75 dB sound pressure level (SPL), in 10-dB steps, with the f2/f1 ratio set at 1.25. This protocol yielded four DP-grams per ear, which were used to confirm that experimental subjects exhibited emissions that were within the normal range based upon the laboratory's extensive database for rabbit DPOAEs. Also, the DP-grams at L1 = L2 = 55, 65, and 75 dB SPL served as pre-exposure measures used to determine post-exposure effects with respect to DPOAE levels at 3 weeks following the noise-exposure episode (see below).
C. Determination of the average DPOAE-adaptation index
The single-frequency fast adaptive measure developed by Liberman et al. (1996) was modified to include two distinct experimental stimulus-presentation protocols that were presented at three different levels of the primary tones, i.e., for L1 = L2 = 55, 65, and 75 dB SPL. As illustrated in Fig. 1(A), the first paradigm consisted of a monaural baseline DP-gram condition involving the simultaneous presentation of the 46-ms f1 and f2 tonebursts to the test ear, with a 2.5-s interstimulus interval (ISI). Measurements (n = 8 synchronously averaged samples) between the selected frequency range of about 3–13 kHz (i.e., f2 = 3.162–12.647 kHz, n = 11 f2 frequencies) were initiated at a time interval that was about 5.7 ms after the onset of the ramped primary tones. This frequency range was chosen, because it spanned over 2 oct and encompassed the frequency region oct above the subsequent octaveband-noise (OBN) exposure centered at 2 kHz, as this region would exhibit the greatest noise damage (Lonsbury-Martin and Meikle, 1978; Engdahl and Kemp, 1996; Harding and Bohne, 2004).
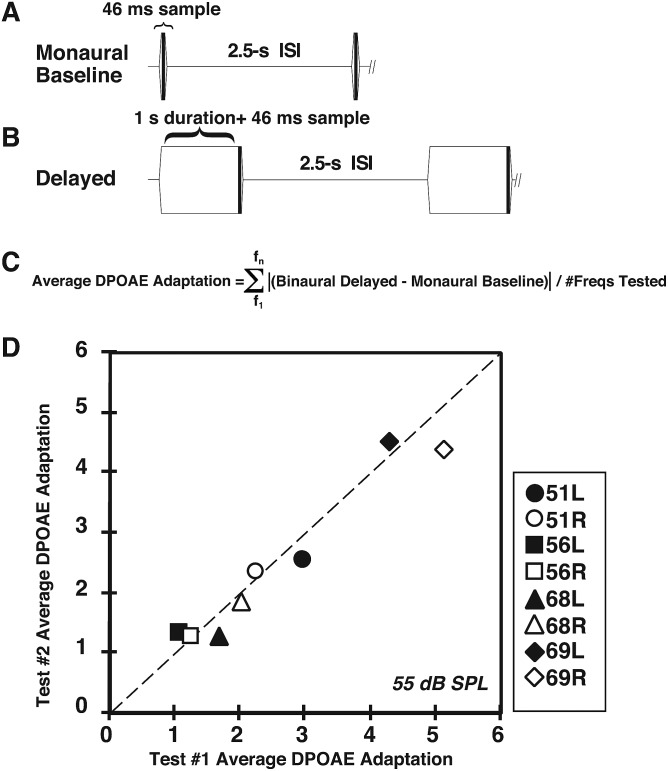
FIG. 1.
Determination of the average DPOAE adaptation index. (A) Schematic representation of the monaural DPOAE-adaptation measurement protocol. Essentially, two condensed DP-grams (f2 = 3–12.7 kHz) were obtained consisting of the monaural baseline measure and either the delayed-monaural or binaural measure. In the baseline condition, DPOAEs were routinely elicited monaurally from the test ear and their magnitudes measured at 46 ms after the ramped onset of the f1 and f2 primary tones. (B) Schematic showing the delayed DPOAE-adaptation condition in which DPOAEs were measured either monaurally or binaurally at about 1046 ms (1 s + 46 ms) after the presentation of the continuous f1 and f2 primary tones. For both the baseline and delayed-monaural or binaural conditions, a 2.5-ISI was interspersed between each pair of primary-tone presentations. (C) Equation showing the computation of the average DPOAE-adaptation index. That is, the absolute value of the difference between the monaural-baseline DPOAE level and the DPOAE level for either the delayed-monaural or binaural conditions was determined for each f2 frequency tested. This difference was then summed across the test frequencies and divided by the number of frequencies tested (n = 11) over a selected f2 range to yield the average DPOAE-adaptation index. (D) Scatterplot illustrating repeatability of determining the average DPOAE adaptation Index within each rabbit and the correspondence between right and left ears of an individual rabbit. The dotted diagonal line at 45° represents the condition in which the amount of the average DPOAE change from f2 = 3.1–12.3 kHz was identical between the right and left ears of the same rabbit.
The second stimulus-presentation or delayed paradigm illustrated in Fig. 1(B) consisted of an adaptive measure in which the f1 and f2 primaries were presented simultaneously, either monaurally to the test ear, or binaurally for 1046 ms, resulting in a repetition rate of about once every 3.5 s, with the DPOAE being measured (n = 8 synchronously averaged samples) from the test ear only during the final 46 ms of this lengthy stimulation period. For both the monaural baseline and what is termed here as either the delayed monaural or binaural adaptive protocols, each stimulus presentation was separated by a 2.5-s ISI. In this manner, sufficient time was permitted for the adaptive-related efferent-induced effects to dissipate before the next set of primary tones was presented. It was expected that the second protocol based on either the delayed monaural or binaural presentation of the long duration f1 and f2 tone bursts would maximize the DPOAE adaptive effect.
As indicated by the equation displayed in Fig. 1(C), an average DPOAE-adaptation index was calculated by initially computing the values representing the differences between DPOAE levels obtained during the monaural baseline and either the longer-lasting delayed monaural or binaural condition for each of the 11 selected DP-gram frequencies. These differences as absolute values were then summed and divided by the number of test frequencies (n = 11) in each 2-oct DP-gram to obtain a measure referred to as the average DPOAE-adaptation index. Absolute values were used to compute differences between baseline and delayed DPOAE measures, because underlying nonlinear sources at times affected the primary tones uniquely, thus, resulting in complex effects in which DPOAE levels were enhanced rather than suppressed (Meinke et al., 2005). Regardless, the DPOAE-adaptation metric reflected the average amount of activated olivocochlear-efferent system feedback to the test ear in terms of its functional ability to modify DPOAE levels.
D. Determination of the average MEM reflex index
The potential contribution that the primary tones made by an acoustic activation of the MEMs was estimated similarly to the mean DPOAE-adaptation index. That is, the amount of change to the middle-ear conduction apparatus introduced by the acoustic stimulus was estimated as an alteration in middle-ear immittance, which was indexed by monitoring changes in the level of the f1-primary tone in the outer ear canal of the test ear. Thus, the differences between the levels of the f1 tones measured during the monaural baseline stimulation period compared to f1-primary tone levels during either the subsequent delayed monaural or delayed binaural stimulation interval were computed for each of the 11 frequencies that comprised the condensed 2-oct DP-gram function. These difference values were then summed and averaged by dividing the sum by the number (n = 11) of test frequencies, thus, yielding the average MEM-R index.
E. Noise exposure paradigm
One day after control measures of the delayed (adapted) monaural and binaural DPOAEs were determined, rabbits were placed inside a sound-reverberant, noise-exposure chamber, where they were allowed free access to food and water. The sound exposure consisted of a 105-dB SPL OBN centered at 2 kHz that was presented for 6 h/d over a 2 consecutive-day period (Martin et al., 1998). Following a 3-week recovery period, post-exposure DPOAEs were measured as expanded full-frequency standard DP-grams (i.e., n = 31 test frequencies), so that pre-vs post-exposure DPOAE levels could be compared. In this manner, the amount of permanent noise-induced change in DPOAE levels was determined.
F. Determination of the average DPOAE loss and DPOAE “threshold”-shift indices
To quantify the loss in DPOAE levels due to the noise over-exposure, an additional measure termed the average DPOAE-loss index was devised. This measure represented the mean difference between the pre-vs post-DP-grams elicited by 65-dB SPL primaries at the 21 test frequencies making up a selected-frequency DP-gram for f2's ranging from 3.162 to 12.647 kHz that was extracted from the 31-frequency standard DP-gram. These difference values were then summed and divided by 21 to calculate the average DPOAE-loss index. In addition, changes in iso-response DPOAE detection “thresholds” were determined using a 10-dB SPL level criterion. Toward this end, DPOAE input/output (I/O) or response-growth functions were obtained at these 21 test frequencies by decreasing primary-tone levels in 5-dB steps from 75 to 45 dB SPL. Mean DPOAE “threshold” shifts were then computed by comparing the stimulus levels necessary to elicit post-vs pre-DPOAE 10-dB SPL detection “thresholds” over the shortened DP-gram test range. Again, the average DPOAE threshold-shift index was computed by summing these difference values and dividing this sum by 21, i.e., the number of test frequencies within the selected (3.162–12.647 kHz) frequency range of the 31-frequency DP-gram.
G. Form of data and statistical analyses
The DPOAE responses illustrated below are typically displayed in the form of DP-grams that describe the absolute levels of the individual DPOAE magnitudes as a function of the f2 test frequency. However, when comparing MOC-R and MEM-R activity within the same animal, difference plots were used (see Fig. 3). Difference plots simply describe the amount of change in dB that occurred in response to the monaural baseline vs delayed monaural or binaural stimulation condition. Thus, if the delayed presentations of the longer lasting primary-tone bursts did not affect either the emission level or the level of the f1-primary tone as measured in the ear canal, the function representing each response type would lie parallel to the x axis at “0” dB on the ordinate, thus, representing a “no change” outcome. It should be noted that the plots illustrating the major results of this study consisting of DP-grams or “difference”-grams are shown below only for the delayed binaural stimulation condition. However, the trends for the delayed monaural data were essentially the same as those for the binaural results as can be appreciated from comparing the monaural vs binaural delayed adaptive measures listed in Table I.
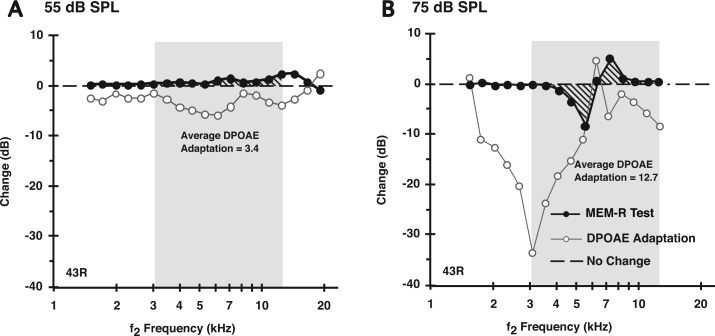
FIG. 3.
DP-gram difference plots showing that the average DPOAE adaptation in the alert rabbit elicited by either 55 -(A) or 75-dB SPL (B) primaries was likely composed of both a presumed olivocochlear efferent reflex (MOC-R, open circles) plus a middle-ear muscle reflex (MEM-R, filled circles) component. The presumed “MEM-R” contribution to DPOAE adaptation measured with an f1 primary-tone constancy test (see text) increased with the higher 75-dB SPL sound pressure levels, yet was still detectable at 55 dB SPL, which is below the reported acoustic threshold for the MEM response in alert rabbits (see text). The bold dashed line at “0” on the ordinate represents a “no change” outcome in which the level of f1 stayed at a constant level between the monaural baseline and, in this case, the delayed-binaural condition.
TABLE I.
Average DPOAE adaptation/MEM-R values measured in alert rabbits predicted susceptibility to OBN-induced decrements in DPOAE levels in both the delayed-monaurally and binaurally tested conditions. Right most column displays average DPOAE loss determined over the same frequency region that DPOAE adaptation/MEM-R was calculated (see text). Last three rows show significance values when DPOAE adaptation/MEM-R values are compared with DPOAE losses, for each animal (right and left ears averaged). Note in deeply anesthetized animals, DPOAE adaptation/MEM-R values were not predictive of the OBN-induced reductions in DPOAE levels.
| Rabbit Ear/Statistics for avg. DPOAE adaptation index/MEM-R (dB) vs DPOAE Loss @ 65 dB SPL | Monaural Awake | Binaural Awake | Monaural Anesthetized | Binaural Anesthetized | Avg. OBN- Induced DPOAE Loss in dB for | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary Level (dB SPL) | 55 | 65 | 75 | 55 | 65 | 75 | 55 | 65 | 75 | 55 | 65 | 75 | 65-dB SPL primaries |
| 42L | 2.0/0.2 | 2.4/0.5 | 3.5/1.4 | 3.0/0.4 | 4.4/1.9 | 12.8/3.4 | 3.9/1.2 | 2.8/1.2 | 3.6/1.1 | 7.7/2.3 | 6.3/2.4 | 7.5/2.3 | 23.3 |
| 42R | 1.9/1.9 | 2.3/2.1 | 3.5/3.0 | 3.2/3.2 | 5.6/4.6 | 11.6/5.9 | 0.5/0.1 | 0.7/0.1 | 1.0/0.1 | 0.6/0.1 | 1.0/0.1 | 1.1/0.1 | 19.7 |
| 43L | 1.0/0.6 | 1.4/0.5 | 1.7/0.7 | 1.2/1.1 | 3.1/1.4 | 12.4/2.8 | 0.2/0.2 | 0.2/0.2 | 0.2/0.2 | 0.8/0.6 | 1.5/0.6 | 3.1/0.8 | 18.3 |
| 43R | 1.3/0.7 | 5.6/2.1 | 8.0/2.8 | 6.8/3.0 | 15.7/3.8 | 14.5/4.0 | 0.8/0.1 | 1.7/0.1 | 1.4/0.3 | 1.4/0.4 | 4.2/1.2 | 8.8/2.2 | 16.0 |
| 44L | 1.2/0.2 | 1.9/0.2 | 1.3/0.2 | 1.4/0.4 | 3.2/0.4 | 5.1/0.7 | 3.9/0.7 | 2.7/0.8 | 3.3/0.8 | 6.9/1.2 | 5.1/1.2 | 6.4/1.3 | 22.9 |
| 44R | 1.3/0.5 | 2.0/0.4 | 3.5/1.1 | 2.1/1.4 | 3.9/2.0 | 11.1/3.8 | 1.2/0.1 | 2.1/0.1 | 1.4/0.1 | 10.9/4.7 | 9.0/4.8 | 5.4/5.1 | 23.7 |
| 45L | 1.3/0.2 | 1.8/0.6 | 3.9/1.2 | 3.4/0.9 | 5.4/1.3 | 12.7/2.5 | 0.7/0.1 | 0.8/0.1 | 1.1/0.0 | 1.1/0.1 | 1.1/0.1 | 1.3/0.3 | 10.1 |
| 45R | 0.7/0.8 | 1.5/0.7 | 0.9/0.7 | 1.5/1.7 | 2.8/1.6 | 10.1/2.4 | 0.5/0.4 | 0.5/0.4 | 0.8/0.4 | 0.8/0.3 | 1.0/0.3 | 2.4/0.5 | 14.8 |
| 65L | 1.0/0.9 | 4.9/2.4 | 9.8/3.5 | 8.6/4.2 | 18.4/5.5 | 15.1/6.2 | 1.0/0.5 | 2.6/0.6 | 1.8/0.6 | 1.6/0.7 | 6.4/1.7 | 5.6/2.6 | 24.5 |
| 65R | 1.1/0.3 | 1.5/0.3 | 1.8/0.3 | 1.3/0.6 | 2.0/0.6 | 5.9/0.8 | 0.9/0.6 | 1.1/0.6 | 1.5/0.6 | 1.3/1.0 | 1.5/1.0 | 1.9/1.0 | 23.7 |
| 66R | 1.0/0.3 | 0.8/0.2 | 1.5/0.2 | 1.2/0.3 | 2.8/0.7 | 10.0/1.3 | 1.8/0.3 | 1.5/0.3 | 2.0/0.3 | 2.6/0.5 | 2.6/0.5 | 3.3/0.5 | 24.6 |
| R2 (n=6 animals) | 0.10/0.55 | 0.82/0.95 | 0.89/0.98 | 0.92/0.92 | 0.84/0.97 | 0.58/0.81 | 0.20/0.17 | 0.03/0.13 | 0.06/0.07 | 0.07/0.036 | 0.04/0.003 | 0.37/0.068 | |
| p value | 0.54/0.09 | 0.02/<0.001 | 0.01/<0.001 | 0.003/0.002 | 0.01/0.001 | 0.07/0.02 | 0.38/0.42 | 0.73/0.48 | 0.66/0.65 | 0.59/0.72 | 0.69/0.92 | 0.47/0.62 | |
| Significance | -/- | */* | */* | */* | */* | -/* | -/- | -/- | -/- | -/- | -/- | -/- | |
The relationships between the four indices representing average DPOAE-adaptation, MEM-R activity, DPOAE loss, and DPOAE “threshold” shifts for each animal were analyzed using commercial software (StatView v.4.5, Abacus Concepts, Piscataway, NJ) to determine various linear-regression coefficients and their associated p-values. As noted above, for the test/retest preliminary study, correlation coefficients were obtained from simple linear-regression calculations. Comparisons across subjects (see Fig. 5) were made based on the mean value of each rabbit's right and left ears to avoid having highly dependent measures from related ears influencing statistical correlations. A probability value of <0.05 represented the adopted level of statistical significance.
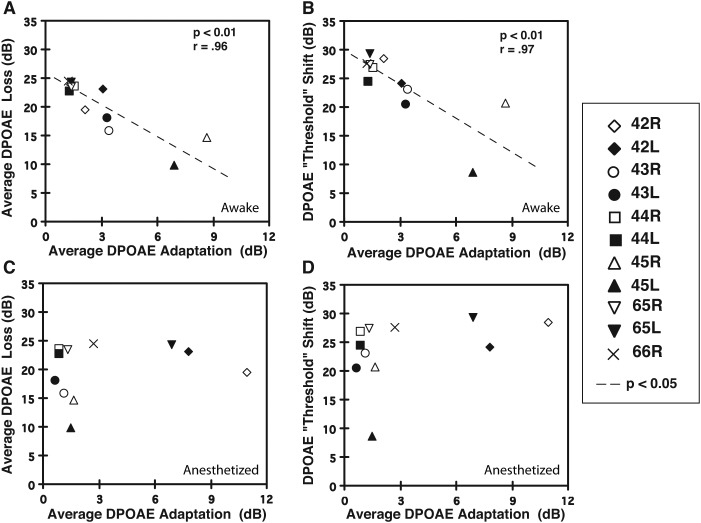
FIG. 5.
Relationship of the average DPOAE-adaptation index to the average DPOAE-loss value that estimated the amount of adverse effects on emissions produced by the OBN exposure. (A) For all rabbits, the average DPOAE-adaptation index computed while awake predicted the amount of noise-induced decrement in DPOAE levels in that there was an inverse correlation between the adaptive-index value and the value representing the average DPOAE loss. (B) A similar outcome occurred when measuring the OBN-induced DPOAE “threshold” shift. That is, the average DPOAE-adaptation index measured for the awake rabbit was inversely related to the amount of “threshold” shift, with larger indices (e.g., rabbit 45 L = filled triangle) associated with small threshold shifts, and smaller indices (e.g., rabbit 44 R = open square) associated with large threshold shifts. (C) In anesthetized rabbits, the average DPOAE-adaptation index did not predict the amount of noise-induced loss in DPOAE levels. (D) Similarly, DPOAE-adaptation indices determined while the animal was anesthetized, did not predict the amount of DPOAE threshold shift. Note that statistical correlations were based on the mean value for the left and right ears of individual rabbits. Symbols missing in these plots represent instances in which the overlay of data points prevented data for individual ears to be distinguished.
The University of Miami's Miller School of Medicine's Institutional Animal Care and Use Committee approved all procedures performed on the rabbit subjects.
III. RESULTS
In the present study, the fast adaptive method developed by Liberman et al. (1996) to test olivocochlear efferent system function at a specific frequency of 2 kHz in the anesthetized cat was modified for application to the alert rabbit over a greater frequency range. The scatterplot of Fig. 1(D) illustrates, for the representative f1 = f2 = 55-dB SPL primary-tone condition, the excellent stability of the average DPOAE-adaptation index for four rabbits [n = 8 ears represented by the unique symbols noted in the boxed legend at right (filled symbols = L ear, open symbols = R ear)]. Here, values obtained during two unique test sessions separated by 2 d, i.e., during session #1 (abscissa) vs session #2 (ordinate), are compared. Note the respectable correlation (r = 0.98) between the two sets of data. In addition, the general similarity of the DPOAE-adaptive index between the ears of the same animal, indicated by the identical filled and open symbol types, is also apparent. For example, note the low DPOAE-adaptive index values at around 1.5 for the left (filled square) and right (open square) ears of rabbit #56, i.e., 56 L and 56 R, respectively, compared to the higher values at >4 for the left (filled diamond) and right (open diamond) ears of rabbit #69, i.e., 69 L and 69 R, respectively.
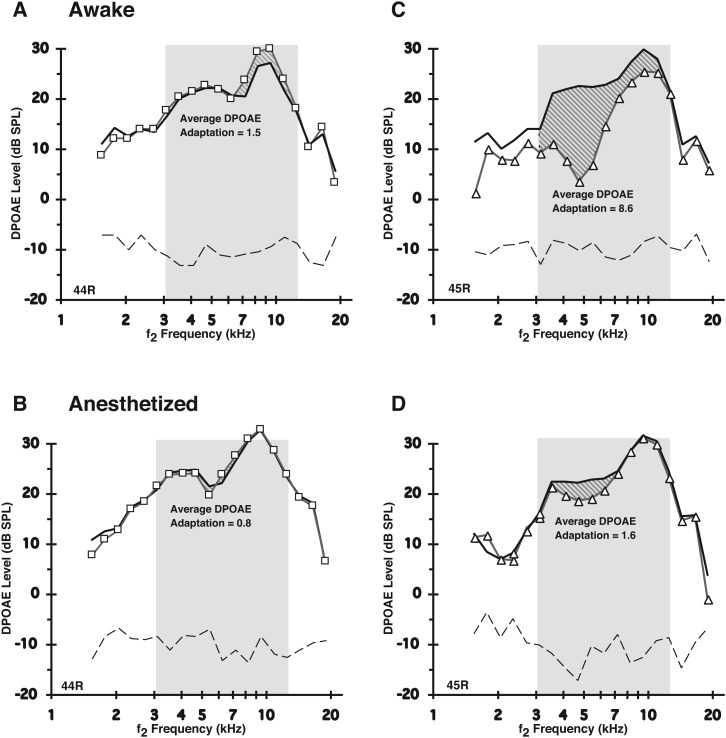
The identical average DPOAE-adaptation indices were measured for other rabbits that were anesthetized. As illustrated for the right ears of rabbits #44 (44R) and #45 (45R), Fig. 2 compares DPOAE-adaptation measures for both the awake [Figs. 2(A) and 2(C)] vs anesthetized [Figs. 2(B) and 2(D)] states. Note the reduction in the observed amount of average DPOAE adaptation for the anesthetized compared to the same alert animal, and that diminution was most prominent for rabbit #45 [Figs. 2(C) and 2(D)]. To summarize, in the alert condition [Fig. 2(A)], rabbit 44R [Fig. 2(A)] demonstrated a weak adaptive index of 1.5, while rabbit 45 R [Fig. 2(C)] displayed a much stronger adaptive index of 8.6. Clearly, the corresponding data in the plots of Figs. 2(B) and 2(D) representing the anesthetized condition show that anesthesia reduced the DPOAE-adaptive effect to 0.8 for rabbit 44R and to 1.6 for rabbit 45R. Thus, the diminished effect was more severe for rabbit 45R displaying the greater amount of olivocochlear-efferent system related activity in the awake state. That is, whereas the strong average DPOAE-adaptation index was reduced by anesthesia by about 81% for rabbit 45R, it was reduced by about only 47% for rabbit 44R, which showed a less robust average DPOAE-adaptation index in the awake state. In addition to the appreciable reduction in the amount of DPOAE adaptation under anesthesia for most rabbits, there was also a noted decrease in the amount of variability of the adaptive index between rabbits. In other words, anesthesia appeared to make different rabbits more alike with respect to their corresponding average DPOAE-adaptive indices than they were in the awake state, affecting rabbits with lower DPOAE adaptation indexes less so than rabbits with higher DPOAE adaptations, presumably because there was less MOC-Rs and MEM-Rs to be suppressed under anesthesia.
FIG. 2.
Anesthesia suppressed the magnitude of the average DPOAE-adaptation index measured in alert rabbits. Shaded regions show, for two ears from different awake rabbits (A = right ear of rabbit 44 R; C = right ear of rabbit 45 R), the absolute DPOAE levels elicited by 55-dB SPL primaries for the baseline monaural (thick linewithout symbols) condition vs the delayed binaural condition (line with symbols), for f2 frequencies tested over the selected 2-oct interval designated by the slanted-line region in each plot. (B and D) These show counterpart responses for the same rabbits illustrated in (A) and (C), respectively, while under deep anesthesia. Note only the slight variability in baseline DPOAE levels between the two states of consciousness in contrast to the decreased adaptation index, i.e., for A (8.6) vs B (1.6) for rabbit 45 R, and for C (1.5) vs D (0.8) for rabbit 44 R. Also, note that anesthesia tended to make the average DPOAE-adaptation indices more similar between different rabbit subjects (see also numeric data in Table I).
For each rabbit and ear, these data are presented quantitatively in Table I, which compares the average DPOAE-adaptation indices determined for both the delayed-monaural and binaural conditions, at all three primary-tone levels, under the awake and anesthetized states. Several trends are apparent for both the awake and anesthetized conditions including the observation that the adaptation index usually became larger as the primary-tone levels increased from 55 to 75 dB SPL. Moreover, indices based on the delayed-binaural presentation of the eliciting stimuli tended to be larger than their corresponding values determined using delayed monaural stimulation. Finally, in comparing the numerical details for each rabbit, it is clear that anesthesia tended to reduce the average DPOAE-adaptation indices from the values measured in their counterpart awake condition.
It is certain that in the alert rabbit at least two processes can potentially contribute to feedback mechanisms that affect the level of the measured DPOAE response. These processes include the activation of the MOC-R as well as the MEM-R pathways. Thus, to estimate the involvement of the MEM-R during the short-duration monaural and long-duration delayed monaural-and binaural-adaptive protocols, the levels of the f1-primary tones in the ear canal of the test ear were also monitored. This control procedure was based on the expectation that if an MEM-R was triggered by the eliciting f1 and f2 stimuli, a modification in ear-canal immittance would reflect such activation, since there would be a related change in the level of the primary tones.
In general, this measure in the form of the average MEM-R index showed that at sound pressure levels, which were even below the reported threshold for MEM activation in the alert rabbit, i.e., at around 55 dB SPL (Whitehead et al., 1992), a change in the levels of the monitoring f1-primary tone was detected between the short-lasting monaural and the longer-lasting delayed-monaural or binaural protocols. For example, the DP-gram difference plots of Fig. 3 for rabbit #43 R compare the amount of DPOAE adaptation (open circles) and related MEM-R activity (solid circles) as a function of the f2 frequency in response to two levels of stimulation consisting of L1 = L2 = 55 [Fig. 3(A)] and 75 dB SPL [Fig. 3(B)].
It is clear from these plots that the MEM-R was activated under both stimulus conditions as signified by the stippled regions around the horizontal dashed “no change” line indicating alterations to DPOAE levels between the routine monaural vs delayed-binaural paradigms. In this case, in response to the 55-dB SPL primary tones shown in Fig. 3(A), MEM-R activity increased the levels of the f1 primary, especially, for frequencies greater than about 5 kHz. In contrast, the more intense 75-dB SPL primaries [Fig. 3(B)] produced a more complex effect. That is, for frequencies from ∼4–6 kHz, the MEM-R related activity reduced the level of the f1 primary tone by up to 10 dB. In comparison, from about 6–8 kHz, the 75-dB SPL primaries caused the opposite effect in that an MEM-R mediated an increase of ∼5 dB for the level of the f1-primary-tone. Interestingly, in both cases, an appreciable amount of reduction in the corresponding DPOAE levels occurred due to the olivocochlear-efferent system's adaptive effects. Clearly, then, in the rabbit, the average DPOAE-adaptation index reflected a mixture of both MOC-R and MEM-R mediated activities.
Following determination of the adaptive measures, to establish the utility of the average DPOAE-adaptation index for an individual ear with respect to predicting its susceptibility to acoustic over-exposure, alert rabbits were exposed to a 105-dB SPL, 2-kHz OBN for 6 h/d, on 2 consecutive days. The animals were then allowed to recover from the acoustic overstimulation for 3 weeks after which pre-vs post-exposure DPOAE levels were compared.
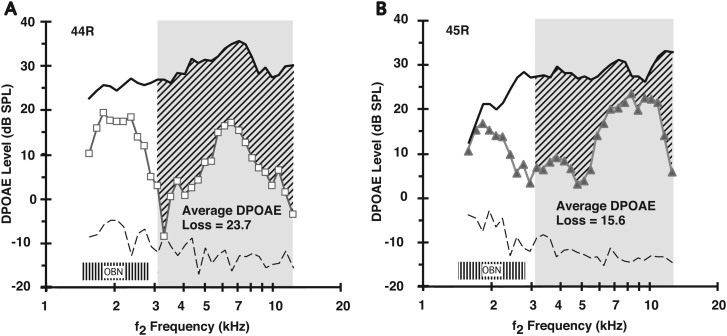
Figures 4(A) and 4(B) illustrate for the right ears of two rabbits, i.e., 44 R and 45 R, respectively, the variability in the effects of the overstimulation episode on the DP-gram, here elicited by 65-dB SPL primary tones. These particular effects translated into corresponding mean DPOAE-loss indices of 23.7 dB for 44 R [Fig. 4(A)] and 15.6 dB for 45 R [Fig. 4(B)]. Note that rabbit 45 R exhibiting less of a noise-induced reduction in DPOAE levels [Fig. 4(B)], demonstrated the more robust mean DPOAE adaptation index, i.e., 8.6 in Fig. 2(C). Conversely, rabbit 44 R, which was more affected by the noise exposure as indicated by the low DPOAE levels of the post-noise DP-gram (open squares) of Fig. 4(A), displayed a weak mean DPOAE-adaptation index during the pre-exposure period, i.e., 1.5 in Fig. 2(A). Such observations of the relationship of the average DPOAE-loss indices to the average DPOAE-adaptation indices occurred across rabbit subjects, as indicated by comparing the DPOAE-loss values in the right-hand column of Table I to the DPOAE-adaptation indices noted in the remaining columns to the left. This association was apparent even though, as shown in Fig. 4 for 65-dB SPL primaries, animals exhibited similar levels of their pre-exposure DPOAEs (solid black curve). That is, for this level of acoustic stimulation, control levels of DPOAEs in different rabbits and in different ears tended to be about 25–30 dB SPL for f2 frequencies ranging from around 3–13 kHz.
FIG. 4.
Average amount of noise-induced loss in DPOAE levels was variable in different rabbits. The average DPOAE loss was plotted as a function of the f2 frequency to compute a DPOAE-loss index for each rabbit, here shown for both 44 R (A: open squares) and 45 R (B: closed triangles). Rabbit 44 R, which exhibited an average DPOAE-adaptation index of 1.5 [see Fig. 2(A)], showed an appreciable OBN-induced DPOAE loss at 23.7 dB. In contrast, rabbit 45 R, which displayed a more hardy average DPOAE-adaptation index of 8.6 [Fig. 2(C)], showed a lesser noise-induced average loss of 15.6 dB. The shaded “OBN” box around 2 kHz represents the 2-kHz OBN exposure.
Figure 5(A) showing data points representing the mean value for each subject's right and left ears, illustrates that across the six animals the average DPOAE-adaptation and loss indices in the awake condition were inversely correlated. That is, large mean average DPOAE-adaptation indices were correlated with small mean DPOAE-loss values (r = 0.96, p < 0.01, n = 6 animals). Further, as shown in Fig. 5(B), the adaptation and threshold-shift indices were also inversely correlated, (r = 0.97, p < 0.01, n = 6 animals). Specifically, the larger the average DPOAE-adaptation index, the smaller the DPOAE-threshold shift value and vice versa.
As would be expected, based on the poor correspondence for mean DPOAE-adaptation indices determined under awake vs anesthetized conditions, there was no correlation between the adaptation index measured in the anesthetized rabbit and either the DPOAE-loss [Fig. 5(C)] or DPOAE threshold-shift [Fig. 5(D)] indices. Also, as shown in Table I, note this same trend under the anesthetized condition when the individual data for each rabbit (average values for both ears) and each ear are examined. Table I also summarizes the statistical outcomes of comparing, for each primary-tone level, the association of the average amount of DPOAE loss (measured in response to 65-dB SPL primary tones) to the corresponding average DPOAE-adaptation or MEM-R index in response to either delayed-monaural or binaural stimulation, under either awake or anesthetized conditions. It is clear from the statistical significances indicated in the bottom row of Table I that in response to most primary-tone levels, both the average DPOAE-adaptation and MEM-R indices predicted the amount of OBN-induced loss in DPOAE levels. In contrast, as can be appreciated from listing of the comparable data obtained under the anesthetized state, there was no significant correlation between either the average DPOAE-adaptation or MEM-R indices, and the subsequent mean DPOAE-loss values under the influences of anesthesia.
IV. DISCUSSION
The present results demonstrate that a broad DPOAE-frequency range in alert rabbits can be assessed for the capacity of ear-related feedback systems to influence cochlear activity as measured by emitted responses. In addition, the observed findings indicate that the fast adaptive-efferent effect in awake rabbits, measured at multiple frequencies, was similar to that described earlier for the anesthetized cat by Liberman et al. (1996) at a single frequency. In the prior study, Liberman et al. (1996) showed that the fast adaptive effect on DPOAEs was predominantly due to the efferent-feedback system as the olivocochlear-efferent system-induced reductions were mostly eliminated when the efferent-nerve fibers supplying the cochlea were surgically severed. Interestingly, following the elimination of the olivocochlear bundle, a much smaller, slower adaptation remained in the cat that could not be attributed to efferent-related activity. Clearly, some intrinsic-cochlear effect must have accounted for the small adaptation observed by these investigators following abolition of the efferent system.
There was no deliberate attempt in the present study to eliminate the efferent-fiber system. However, experiments in deeply anesthetized rabbits were performed using xylazine, a known pharmacological depressant of brainstem neurons that include a region encompassing the cell bodies of the descending olivocochlear-efferent system (Smith and Mills, 1989; Astl et al., 1996; Harel et al., 1997). The present experiments in acutely anesthetized rabbits resulted in less DPOAE adaptation than in the awake state as shown both in Figs. 2(B) and 2(D), and by comparing the numeric counterparts of these data in Table I. Thus, even though it is uncertain whether intrinsic cochlear-adaptation processes were affected in the sedated rabbit, it is clear that both the magnitude of the MEM-R and the MOC-R were greatly reduced. And, given that there was no relationship between the amount of DPOAE adaptation and the consequences of acoustic overstimulation under anesthetic conditions, it would seem that adaptation from both reflexes is much greater than adaptation due to intrinsic cochlear effects, at least, in the rabbit.
It would be straightforward to surgically eliminate the MEMs in rabbits to eliminate the influence of the MEM-R system on the DPOAE-adaptive index in the awake preparation. Unfortunately, prior studies in our laboratory showed that severing the MEMs in rabbits causes the DPOAEs to be immeasurable by 2 days postsurgery (personal observations). This inability to measure DPOAEs after sectioning the MEMs is due presumably to irreversible changes in the reverse-conduction pathway for emissions caused by reduced tension (i.e., no MEMs) in the ossicular chain. Acute experiments in which either the MEMs or the olivocochlear bundle are severed to better isolate the untainted efferent-adapted effect require deep anesthetic levels. And, unfortunately, such anesthetic levels have been shown in the present study to suppress both the efferent- and MEM-related effects. Thus, working in acute preparations to measure olivocochlear-efferent system activity is not an option, in any event, in the rabbit.
It is well known that species differences in DPOAE-adaptation effects exist. For example, in the anesthetized rat, DPOAE adaptation, at least at 8 kHz, was composed mainly of the MEM-R response, with the MOC-R system contributing little to emission adaptation (Relkin et al., 2005). Further, in the anesthetized mouse, Sun and Kim (1999) measured small “fast” DPOAE-adaptation effects of 1–2 dB for single frequencies. However, these authors made no effort to determine what component of the DPOAE-adaptation response represented unique contributions from either the MOC-R or MEM-R systems, or other intrinsic cochlear processes. In addition, Maison and Liberman (2000) reported in the anesthetized mouse a similar low-level DPOAE adaptation effect in response to primary-tone levels below 70 dB SPL. Their result was not eliminated by surgically sectioning the efferent fibers, by eliminating the efferent system pharmacologically with strychnine, or by targeted gene deletion of the relevant α9/α10 cholinergic receptors (Maison et al., 2012). However, these investigators (Chambers et al., 2012) later compared the emission-reducing effects of contralateral acoustic stimulation (CAS) in anesthetized vs alert mice and demonstrated much larger MOC-R effects in alert mice. Specifically, while a <1-dB reduction in DPOAE level was observed on average in anesthetized mice, the single-frequency CAS-induced DPOAE suppression in awake, but restrained mice, was about 8 dB.
Moreover, each species investigated, to date, with respect to efferent-related decrements in DPOAE levels has exhibited a unique frequency range over which the efferent-induced effects were greatest. This frequency was found to be 2 kHz in the anesthetized cat (Liberman et al., 1996), 10 kHz in the alert guinea pig (Maison and Liberman, 2000), between about 3–13 kHz for both the sedated guinea pig and alert rabbit (Luebke et al., 2002), and between 1–2 kHz in humans (Meinke et al., 2005). Maison and Liberman (2000) using a monaural single-frequency, DPOAE-based measure of efferent strength in alert guinea pigs discovered that the varied values they observed predicted susceptibility to a subsequent acoustic over-exposure. Thus, awake guinea pigs with larger efferent effects showed less noise-induced decrements in DPOAE levels than did animals with smaller amounts of efferent-related activity. In their experiment, however, the change in ear-canal sound pressure that likely reflects MEM-R related activity was not deliberately monitored. Thus, it was not clear, in the Maison and Liberman (2000) study what proportion of the protection from noise exposure was due to a MEM-R or MOC-R activity. However, it is important to note that these authors used monaural stimulation to measure efferent activity, and it is well established that the threshold for activation of the monaural MEM-R is higher than for binaural activation. This evidence, along with the results of other experiments reported by Maison and Liberman (2000) in which the MEMs were deliberately sectioned, suggest that the MEM-R likely does not contribute significantly to the protective effects observed in prior studies in the alert guinea pig.
Other DPOAE-adaptation experiments in our laboratory on lightly sedated guinea pigs used a combined ketamine/acepromazine anesthetic regimen to demonstrate that when MEM-Rs were assayed with the MEM-R test, they were undetectable (Luebke and Foster, 2002). This latter work also demonstrated that the greater the efferent strength, as measured with the multifrequency averaged adapted-DPOAE measure detailed here, the less susceptible the sedated guinea pig was to acoustic overexposure (Luebke and Foster, 2002). In an earlier study, Patuzzi and Thompson (1991) noted that when anesthetized guinea pigs were exposed to damaging noises, the variability in noise susceptibility was reduced thus inferring that perhaps either MOC-R or MEM-R related activity, which would be expected to be less active in anesthetized animals, contributed less to any innate susceptibilities. Also, Zheng et al. (1997) showed that, when the olivocochlear bundle was severed in chinchillas, the de-efferented ears were more susceptible to noise damage, which supports the significance of the olivocochlear-efferent system as a protector against the adverse effects of excessive sound in alert preparations.
While in the awake rabbit, DPOAE adaptation measurements are somewhat contaminated by the MEM-R and, thus, are not pure measures of the strength of the MOC-R, they are still predictive of susceptibility to noise-induced cochlear dysfunction. However, due to the lower-level stimuli needed to elicit them, stimulus-frequency OAEs (SFOAEs) are not contaminated by MEM activation (Guinan et al., 2003; Backus and Guinan, 2007). Despite this dual contribution of the DPOAE-adaptation measurements, the combined feedback systems, i.e., the MEM-R and MOC-R, in anesthetized rabbits, do not predict the adverse effects of noise exposure, presumably because both systems are suppressed by anesthesia. Moreover, the multi-frequency DPOAE-adaptation method used here is feasible in human testing (Meinke et al., 2005), although the findings of Kim et al. (2001) suggest the possibility of intrinsic-adaptation effects that are greater in humans than in animal models. Nevertheless, applying this technique to predict susceptibility to NIHL caused by regular exposure to extreme sounds in the workplace may be a promising approach toward early detection of occupational-related hearing loss.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Public Health Service (DC003086, DC003114, DC000613).
This study was conducted while the authors were at the University of Miami Ear Institute at the Miller School of Medicine.
REFERENCES
- 1.Astl, J., Popelar, J., Kvasnak, E., and Syka, J. (1996). “Comparison of response properties of neurons in the inferior colliculus of guinea pigs under different anesthetics,” Audiology 35, 335–345 10.3109/00206099609071954 [DOI] [PubMed] [Google Scholar]
- 2.Backus, B. C. , and Guinan, J. J. , Jr. (2007). “Measurement of the distribution of medial olivocochlear acoustic reflex strengths across normal-hearing individuals via otoacoustic emissions,” J. Assoc. Res. Otolaryngol. 8, 484–496 10.1007/s10162-007-0100-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers, A. R. , Hancock, K. E. , Maison, S. F. , Liberman, M. C. , and Polley, D. B. (2012). “Sound-evoked olivocochlear activation in unanesthetized mice,” J. Assoc. Res. Otolaryngol. 13, 209–217 10.1007/s10162-011-0306-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engdahl, B., and Kemp, D. T. (1996). “The effect of noise exposure on the details of distortion product otoacoustic emissions in humans,” J. Acoust. Soc. Am. 99, 1573–1587 10.1121/1.414733 [DOI] [PubMed] [Google Scholar]
- 5.Guinan, J. J. , Jr., Backus, B. C. , Lilaonitkul, W., and Aharonson, V. (2003). “Medial olivocochlear efferent reflex in humans: Otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs,” J. Assoc. Res. Otolaryngol. 4, 521–540 10.1007/s10162-002-3037-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding, G. W. , and Bohne, B. A. (2004). “Temporary DPOAE level shifts, ABR threshold shifts and histopathological damage following below-critical-level noise exposures,” Hear. Res. 196, 94–108 10.1016/j.heares.2004.03.011 [DOI] [PubMed] [Google Scholar]
- 7.Harel, N., Kakigi, A., Hirakawa, H., Mount, R. J. , and Harrison, R. V. (1997). “The effects of anesthesia on otoacoustic emissions,” Hear. Res. 110, 25–33 10.1016/S0378-5955(97)00061-0 [DOI] [PubMed] [Google Scholar]
- 8.Kim, D. O. , Dorn, P. A. , Neely, S. T. , and Gorga, M. P. (2001). “Adaptation of distortion product emission in humans,” J. Assoc. Res. Otolaryngol. 2, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberman, M. C. , Puria, S., and Guinan, J. J. , Jr. (1996). “The ipsilaterally evoked olivocochlear reflex causes rapid adaptation of the 2f1-f2 distortion product otacoustic emission,” J. Acoust. Soc. Am. 99, 3572–3584 10.1121/1.414956 [DOI] [PubMed] [Google Scholar]
- 11.Lonsbury-Martin, B. L. , and Meikle, M. B. (1978). “Neural correlates of auditory fatigue: Frequency-dependent changes in the activity of single cochlear nerve fibers,” J. Neurophysiol. 41, 987–1006 [DOI] [PubMed] [Google Scholar]
- 12.Luebke, A. E. , and Foster, P. K. (2002). “Variation in inter-animal susceptibility to noise damage is associated with alpha 9 acetylcholine receptor subunit expression level,” J. Neurosci. 22, 4241–4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luebke, A. E. , Foster, P. K. , and Stagner, B. B. (2002). “A multifrequency method for determining cochlear efferent activity,” J. Assoc. Res. Otolaryngol. 3, 16–25 10.1007/s101620010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maison, S. F. , and Liberman, M. C. (2000). “Predicting vulnerability to acoustic injury with a noninvasive assay of olivocochlear reflex strength,” J. Neurosci. 20, 4701–4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maison, S. F. , Usubuchi, H., Vetter, D. E. , Elgoyhen, A. B. , Thomas, S. A. , and Liberman, M. C. (2012). “Contralateral-noise effects on cochlear responses in anesthetized mice are dominated by feedback from an unknown pathway,” J. Neurophysiol. 108, 491–500 10.1152/jn.01050.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin, G. K. , Jassir, D., Stagner, B. B. , Whitehead, M. L. , and Lonsbury-Martin, B. L. (1998). “Locus of generation for the 2f1-f2 vs 2f2-f1 distortion-product otoacoustic emissions in normal-hearing humans revealed by suppression tuning, onset latencies, and amplitude correlations,” J. Acoust. Soc. Am. 103, 1957–1971 10.1121/1.421347 [DOI] [PubMed] [Google Scholar]
- 17.Meinke, D. K. , Stagner, B. B. , Martin, G. K. , and Lonsbury-Martin, B. L. (2005). “Human efferent adaptation of DPOAEs in the L1, L2 space,” Hear. Res. 208, 89–100 10.1016/j.heares.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 18.Patuzzi, R. B. , and Thompson, M. L. (1991), “Cochlear efferent neurones and protection against acoustic trauma: Protection of outer hair cell receptor current and interanimal variability,” Hear. Res. 54, 45–58 10.1016/0378-5955(91)90135-V [DOI] [PubMed] [Google Scholar]
- 19.Relkin, E. M. , Sterns, A., Azeredo, W., Prieve, B. A. , and Woods, C. I. (2005). “Physiological mechanisms of onset adaptation and contralateral suppression of DPOAEs in the rat,” J. Assoc. Res. Otolaryngol. 6, 119–135 10.1007/s10162-004-5047-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith, D. I. , and Mills, J. H. (1989). “Anesthesia effects: Auditory brain-stem response,” Electro-encephalogr. Clin. Neurophysiol. 72, 422–428 10.1016/0013-4694(89)90047-3 [DOI] [PubMed] [Google Scholar]
- 21.Sun, X. M. , and Kim, D. O. (1999). “Adaptation of 2f1-f2 distortion product otoacoustic emission in young-adult and old CBA and C57 mice,” J. Acoust. Soc. Am. 105, 3399–3409 10.1121/1.424668 [DOI] [PubMed] [Google Scholar]
- 22.Whitehead, M. L. , Lonsbury-Martin, B. L. , and Martin, G. K. (1992). “Evidence for two discrete sources of 2f1-f2 distortion-product otoacoustic emission in rabbit: I. Differential dependence on stimulus parameters,” J. Acoust. Soc. Am. 91, 1587–1607 10.1121/1.402440 [DOI] [PubMed] [Google Scholar]
- 23.Zheng, X. Y. , Henderson, D., Hu, B. H. , Ding, D. L. , and McFadden, S. L. (1997). “The influence of the cochlear efferent system on chronic acoustic trauma,” Hear. Res. 107, 147–159 10.1016/S0378-5955(97)00031-2 [DOI] [PubMed] [Google Scholar]