Abstract
Background
Immunotherapeutic cancer protocols often rely on the ability to promote proliferative expansion of tumor-specific T-cell, but the influence of cancer on in vivo T-cell expansion remains largely undefined.
Methods
The ability of control and B16F10 melanoma-bearing C57BL/6 mice to expand lymphocytic choriomeningitis virus antigen-specific T-cell populations in response to acute viral infection was compared by using flow cytometric assays of splenocytes.
Results
The ability to expand virus-specific CD8+ and CD4+ T-cells was globally and markedly suppressed in tumor-bearing mice. Expanded cytotoxic T lymphocytes (CTLs) retained in vivo and in vitro functionality, suggesting that melanoma growth did not induce T-cell anergy. The magnitude of suppressed proliferative expansion was proportional to the extent of tumor burden. Melanoma-induced suppression of CTL expansion was correlated with upregulated apoptotic activity and hampered the induction of memory precursor effector cells. Adoptive transfer of resting LCMV antigen-specific T-cells before or after tumor establishment demonstrated that a critical period of in vivo exposure of resting T-cells to growing melanoma was responsible for the induction of suppressed expansion. This suppression was durable; surgical resection of melanoma after in vivo exposure to resting T-cells but before antigenic stimulation did not restore full expansion.
Conclusions
These data suggest that growing melanoma tumors exert a global, antigen-independent influence on resting T-cells that fundamentally reprograms their ability to undergo proliferative expansion in response to subsequent antigenic stimulation. This finding may have direct implications for T-cell-based immunotherapeutic strategies.
Efforts to mobilize the immune system against cancer have focused on CD8+ cytotoxic T lymphocytes (CTLs) for their ability to mediate antigen-specific tumor killing.1 Immunotherapeutic strategies to infuse and expand tumor-specific CTLs in vivo have shown great promise, but their overall clinical efficacy has been limited.2,3 Investigative attempts to explain and overcome these shortcomings have focused on the undesirable influences of cancer-induced immune suppression,4–9 but the specific influence of cancer on proliferative CTL expansion has not been fully explored.
In this report, we describe a novel quantitative animal model with which we can confirm and measure the suppressive influence of melanoma on proliferative T-cell expansion in vivo. By measuring the ability of immunocompetent C57BL/6 mice to expand antigen-specific T-cell populations in response to lymphocytic choriomeningitis virus (LCMV) infection, we demonstrate that melanoma growth nonspecifically suppresses antigen-driven T-cell expansion. The magnitude of this T-cell suppression is proportional to the extent of tumor burden. By modulating the duration of exposure of resting T-cells to growing tumor, we demonstrate that this suppression is exerted not at the moment of T-cell activation by antigen, but before antigen encounter; exposure of resting T-cells to melanoma weakens their ability to undergo proliferative expansion in response to future antigenic stimulation.
MATERIALS AND METHODS
Mice
Seven- to 8-week-old female C57BL/6/Ly5.2 mice (Taconic, Hudson, NY) and C57BL/6/Ly5.1 background P14 TCR transgenic mice with TCR specificity for the Db-restricted LCMV antigen peptide GP33 10 (obtained from M. Suresh) were maintained in pathogen-free conditions and handled in strict accordance with the guidelines of the University of Wisconsin and William S. Middleton Memorial VA Hospital Animal Care and Use Committees.
Tumor Cell Lines and Virus
B16F10, a poorly immunogenic melanoma cell line derived from C57BL/6 mice, was maintained in RPMI-1640 medium (Mediatech, Herndon, VA) supplemented with 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM of L-glutamine (Life Technologies, Inc., Grand Island, NY). Single inocula of 106 B16F10 cells suspended in serum-free RPMI1640 media were injected subcutaneously into mice. Mice were infected with 2×105 PFU of Armstrong strain LCMV by intraperitoneal injection. Levels of LCMV in explanted tissues were quantified using Vero cell-based plaque assays.11
Adoptive Transfer
Splenocytes were harvested from P14 mice and 103 to 104 CD8+ GP33-specific T-cells (Ly5.1+) were adoptively transferred into C57BL/6/Ly5.2 mice via tail vein injection.
Flow Cytometry
MHC class I tetramers loaded with various LCMV antigen peptides were prepared as previously described.12 Single cell splenocyte suspensions were stained with APC-labeled MHC class I (Db) tetramers loaded with class I-restricted LCMV epitope peptides, PE-labeled anti-CD8, and FITC-labeled anti-CD44 antibodies. Anti-CD62L, anti-CD 127, anti-Ly5.1, and anti-granzyme B antibodies also were used. Freshly harvested splenocytes (106 cells/well) were stimulated with or without LCMV epitope peptides (0.1 μg/mL) in the presence of brefeldin A and human recombinant IL-2 (10 U/well) at 37°C for 5 h in flat-bottomed 96-well plates. Cells were stained with anti-CD8 and anti-CD62L antibodies, and then permeabilized and stained for intracellular cytokines using anti-IFN-γ, anti-TNF-α, and anti-IL-2 antibodies using the Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA). Stained cells were acquired on a FACSCalibur flow cytometer (BD Biosciences) and resulting data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR). All reagents and antibodies were purchased from BD Biosciences with the exception of anti-CD127 (eBioscience, Inc., San Diego, CA) and anti-granzyme B antibodies (Invitrogen, Inc., Carlsbad, CA).
Statistical Analysis
Data were analyzed by using the SPSS 16.0 statistical analysis package (SPSS, Inc., Chicago, IL). Groups were compared by using the two-tailed Student’s t test, and significance was defined as p < 0.05. Comparisons between tumor mass and T-cell expansion were performed by using Pearson correlation coefficient.
RESULTS
Antigen-driven T-cell Expansion is Suppressed in the Presence of Melanoma
C57BL/6 mice were inoculated with media or 106 B16F10 cells on day 0 and were infected with LCMV on day 10. Splenocytes were harvested on day 18 (postinfection day 8, the point of maximal LCMV-specific CTL expansion).12 Tumor-bearing mice exhibited localized subcutaneous tumors without metastases or evidence of systemic illness, alterations in behavior, or weight loss on day 18 (data not shown). Tumor growth was comparable between uninfected and infected mice, and no inhibition of tumor growth was seen after LCMV infection (data not shown).
Acute LCMV infection induces marked lymphocyte proliferation manifested by splenic hyperplasia. Spleen cellularity on day 18 was diminished in tumor-bearing mice (Fig. 1a). Numbers of LCMV epitope-specific CD8+ T-cells as measured by MHC tetramer (Fig. 1b) or intracellular cytokine staining (Fig. 1c) were significantly lower among tumor-bearing mice. Melanoma-induced suppression affected expansion of T-cells specific to all immunodominant and subdominant LCMV peptide antigens tested (Fig. 1d, e). Expansion of CD4+ T-cells specific for the MHC class II I-Ab-restricted peptide GP61 also was suppressed in tumor-bearing mice (Fig. 1f, g). Mean fluorescence intensity of IFN-γ expression after in vitro stimulation with LCMV epitope peptides was modestly decreased among LCMV-specific CD8+ T-cells from tumor-bearing mice (Fig. 1h); however, no differences in granzyme B expression were noted between activated LCMV-specific CD8+ T-cells harvested directly ex vivo from control and tumor-bearing mice (Fig. 1i). Thus, proliferative expansion (but not individual function) of CD8+ and CD4+ T-cells in response to antigenic stimulation is suppressed by melanoma.
FIG. 1.
Expansion of LCMV-specific CD8+ T-cells is suppressed in tumor-bearing mice, a Numbers of splenocytes harvested on day 18 were significantly smaller in tumor-bearing mice (“tumor”) than in non-tumor-bearing mice (“control”) (n = 4–6 mice per group). b Splenocytes were harvested on day 18 and stained with anti-CD8 antibodies and Db MHC class I tetramers loaded with LCMV epitope peptides, and then analyzed by flow cytometry. These representative plots are gated on total viable splenocytes. c Splenocytes harvested on day 18 also were stimulated in vitro with Db MHC class I-restricted LCMV epitope peptides (or left unstimulated (“US”) as a negative control) for 5 h, and then stained with antibodies against CD8 and intracellular IFN-γ. These representative plots are gated on total viable splenocytes after stimulation with the LCMV epitope peptide NP396. d Significantly smaller absolute numbers of CD8+ CTLs (percent of tetramer-positive cells multiplied by total numbers of splenocytes) specific for NP396, GP33, and GP276 were observed in tumor-bearing mice (n = 4–6 mice per group; *indicates p < 0.05). e Significantly smaller absolute numbers of CD8+ CTLs (percent of IFN-γ-positive cells multiplied by total numbers of splenocytes) specific for the NP396, GP33, GP34, GP276, GP118, NP205 were observed in tumor-bearing mice (n = 4–6 mice per group; *indicates p < 0.05). f Splenocytes harvested on day 18 were stimulated in vitro with the MHC class II I-Ab-restricted LCMV epitope peptide GP61 (or left unstimulated) for 5 h, and then stained with antibodies against CD4 and intracellular IFN-γ. These representative plots are gated on total viable splenocytes. g Significantly smaller absolute numbers of CD4+ T-cells specific for GP61 were observed in tumor-bearing mice (n = 4 mice per group; *indicates p < 0.05). h Splenocytes harvested on day 18 were stimulated with NP396 peptide in vitro for 5 h; mean fluorescence intensity (MFI) of IFN-γ expression among activated NP396-specific T-cells was slightly lower in tumor-bearing mice (n = 4 mice per group; *indicates p < 0.05). i Levels of granzyme B expression among NP396-specific T-cells (identified by tetramer staining) harvested on day 18 were similar between control and tumor-bearing mice. (Light color represents isotype control; intermediate color represents control mouse; dark color represents tumor-bearing mouse.) All experiments were performed three to ten times with similar results
Magnitude of Suppression of T-cell Expansion is Proportional to Extent of Melanoma Burden
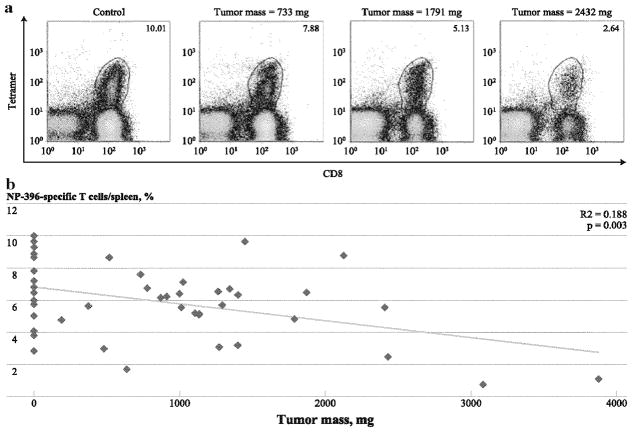
Despite the injection of fixed numbers of B16F10 tumor cells, variability in individual tumor weights was observed on day 18. When analyzed individually, an inverse correlation was observed between LCMV-specific T-cell population size and tumor weight (Fig. 2a, b). This relationship also was observed when tumor burden was compared using tumor dimensions, unifocal versus multifocal tumors, and nonexponential versus exponential tumor growth (data not shown). Thus, suppression of antigen-driven T-cell expansion appears to be proportional to tumor burden.
FIG. 2.
The magnitude of tumor-induced suppression of T-cell expansion is proportional to the extent of tumor burden. Splenocytes were harvested on day 18 and were analyzed by flow cytometry. a These representative plots are gated on total viable splenocytes and the numbers correspond to percentages of NP396-specific CD8+ T-cells among total viable splenocytes in control and tumor-bearing mice with various degrees of tumor burden, b Comparison of individual percentages of NP396-specific CTLs per total viable splenocytes and individual tumor weights identified a statistically significant correlation with an R2 value of 0.1535. Data points with tumor masses of 0 correspond to non-tumor-bearing control mice. These data were pooled from five experiments (n = 4–5 tumor-bearing mice per experiment)
Melanoma Does Not Alter the Timing of T-cell Expansion
The kinetics of T-cell activation involve an expansion phase of rapid antigen-driven proliferation, which ultimately results in antigen clearance followed by a contraction phase in which CTL populations involute through apoptotic cell death. A subset of memory precursor effector T-cells (MPEC), identifiable by high expression of IL-7R-α (CD127) and low expression of the senescence marker KLRG-1, survive and differentiate into mature memory T-cells capable of responding rapidly to antigen challenge. Eventual memory T-cell population size is directly proportional to clonal burst size at the time of maximal proliferative expansion.12,13 After LCMV infection, peak CTL expansion and viral clearance occur on postinfection day 8.12 To determine whether suppression of T-cell expansion by melanoma was accompanied by alterations in the timing of expansion and contraction, control, and tumor-bearing mice were sacrificed on days 16, 18, and 20 (postinfection days 6, 8, and 10). Timing of proliferative expansion and apoptotic contraction was similar between control and tumor-bearing mice, but fewer LCMV-specific T-cells were present in tumor-bearing mice on days 18 and 20 (Supplementary Fig. 1a, b). Proliferative activity among LCMV-specific T-cell populations (as measured by expression of the proliferation marker Ki-67) was similar (Supplementary Fig. 1c, d), but significantly more apoptotic activity (as measured by Annexin Vhigh expression) was seen in tumor-bearing mice on day 18 (Supplementary Fig. 1e, f). Concordant with the lower peak of CTL expansion in tumor-bearing mice, we identified smaller CD127high/KLRGlow MPEC populations among LCMV-specific T-cells in tumor-bearing mice on day 20 (Supplementary Fig. 1g). Thus, tumor-induced suppression of antigen-driven T-cell expansion is associated with augmented apoptotic activity, and may ultimately result in attenuated immunological memory.
Susceptibility of Naive Resting T-Cells to Melanoma-Induced Suppression of Antigen-Driven Expansion is Dependent on Timing of Adoptive Transfer
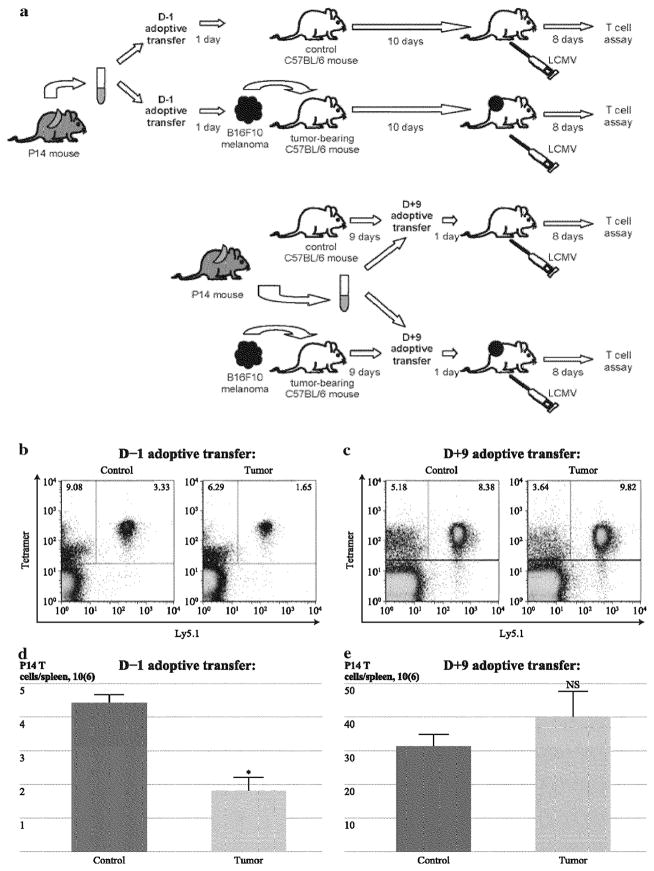
To follow the proliferative expansion of a clonal population of antigen-specific T-cells, adoptive transfer experiments were performed using naïve T-cells from uninfected P14 TCR transgenic mice (with TCR specificity for the Db restricted LCMV antigen peptide GP33). Differential expression of Ly5.1 and Ly5.2 permits discrimination between T-cells derived from P14 mice (Ly5.1+) and endogenous T-cells in wild-type C57BL/6 transfer recipients (Ly5.2+). 104 P14 T-cells were adoptively transferred one day before tumor inoculation (day −1); alternatively, 103 P14 T-cells were adoptively transferred on day 9, well after establishment of tumors (Fig. 3a). In this way, P14 T-cells transferred on day −1 were exposed to the growing tumor for the same duration as endogenous cells, whereas P14 T-cells transferred on day 9 were only minimally exposed to tumor before activation by GP33. A larger inoculum of cells was transferred on day −1 to compensate for attrition of in vivo cell viability anticipated during the 10 days between adoptive transfer and LCMV infection. Expansion of donor P14 T-cells transferred on day −1 in response to LCMV infection was suppressed in tumor-bearing mice (Fig. 3b, d), recapitulating the suppressed expansion of endogenous polyclonal T-cells (Fig. 1b–e). In contrast, P14 T-cells transferred on day 9 underwent similar expansion in control and tumor-bearing mice (Fig. 3c, e). Endogenous non-P14 Ly5.2+ CTL expansion was suppressed in tumor-bearing mice undergoing adoptive transfer at both time points (data not shown). Thus, the 9-day period of in vivo exposure of resting T-cells to tumor was necessary for the induction of melanoma-induced suppression of antigen-driven T-cell expansion.
FIG. 3.
Adoptively transferred resting T-cells not exposed to tumor undergo normal expansion in response to LCMV infection, a Spleno-cytes from Ly5.1 + P14 TCR transgenic mice (derived from a C57BL/6 background with TCR specificity for the LCMV epitope peptide GP33) were adoptively transferred as inocula of 104 or 103 GP33-specific CD8+ T-cells on day −1 or day 9, respectively, into wild-type Ly5.2 C57BL/6 mice. Recipient mice were inoculated with media or B16F10 on day 0 and infected with LCMV on day 10, and splenocytes were harvested on day 18 for comparative analysis of LCMV-specific T-cell expansion. Larger inocula of adoptively transferred cells were administered on day −1 because of the anticipated attrition of cell viability expected during the period of ten days before LCMV infection, b These representative plots are gated on CD8+ T-cells among total viable splenocytes in control and tumor-bearing mice that underwent adoptive transfer on day −1 (before tumor inoculation), c These representative plots are gated on CD8+ T-cells among total viable splenocytes in control and tumor-bearing mice that underwent adoptive transfer on day 9 (after tumor establishment and immediately before LCMV infection). d Comparison of absolute numbers of Ly5.1+ CD8+ T-cells after day −1 adoptive transfer identified significantly smaller populations of P14 CTLs in tumor-bearing mice (“tumor”) compared with non-tumor-bearing mice (“control”) (n = 4–5 mice per group), e Comparison of absolute numbers of Ly5.1 + CD8+ T-cells after day 9 adoptive transfer identified no significant differences in populations of P14 CTLs among tumor-bearing mice and non-tumor-bearing control mice (n = 4 mice per group. Each of these experiments was performed three times with similar results. (*Indicates/> < 0.05; NS indicates no significance and p>0.1)
Exposure of Resting T-Cells to Melanoma is Sufficient for Induction of Suppressed Antigen-Driven Expansion
To further test the possibility that melanoma-induced suppression of expansion may be mediated during exposure of resting T-cells to tumor, adoptive transfer experiments were repeated by transferring CD8+ T-cells from control and tumor-bearing P14 mice into control and tumor-bearing wild-type C57BL/6 mice on day 9 (Fig. 4a). In this way, GP33-specific T-cells harvested from tumor-bearing P14 mice were exposed to tumor in vivo for the same duration as endogenous T-cells or P14 T-cells transferred into tumor-bearing mice on day −1. Again, P14 T-cells harvested from control P14 mice did not undergo suppressed expansion when transferred into tumor-bearing recipients on day 9, but expansion of T-cells from tumor-bearing P14 mice was suppressed (Fig. 4b). Expansion of resting T-cells from tumor-bearing P14 mice was suppressed even when transferred into non-tumor-bearing recipients. Suppression was strongest when T-cells from tumor-bearing P14 mice were transferred into tumor-bearing recipients. There were no differences in numbers of P14 T-cells present among control and tumor-bearing P14 mice at the time of splenocyte harvest, suggesting that the influence of tumor on resting T-cells was not mediated by loss of CTL precursor frequency (data not shown). Thus, a critical period of in vivo exposure of resting T-cells to melanoma may fundamentally reprogram their ability to undergo full proliferative expansion in response to antigenic stimulation.
FIG. 4.
Exposure of resting T-cells to tumor before adoptive transfer restores suppressed expansion in response to LCMV infection. a Ly5.1+ P14 TCR transgenic mice and wild-type Ly5.2+ C57BL/6 mice were inoculated with media or B16F10 melanoma cells on day 0. Splenocytes from control and tumor-bearing Ly5.1+ P14 mice were adoptive transferred as inocula of 103 GP33-specific CD8+ T-cells on day 9 into control and tumor-free Ly5.2+ C57BL/6 mice. LCMV infection was performed on day 10, and splenocytes were harvested on day 18 for comparative analysis of LCMV-specific T-cell expansion. b Comparison of absolute numbers of Ly5.1+ CD8+ T-cells derived from tumor-free P14 donor mice identified similar populations of P14 CTLs in non-tumor–bearing recipient mice (“control/control”) and tumor-bearing recipient mice (“control/tumor”). Expansion of Ly5.1+ CD8+ T-cells derived from tumor-bearing P14 donor mice was suppressed in tumor-bearing recipient mice (“tumor/tumor”) compared with non-tumor–bearing recipient mice (“tumor/control”). Expansion of Ly5.1+ CD8+ T-cells derived from tumor-bearing P14 donor mice transferred into both groups (“tumor/control” and “tumor/tumor”) was suppressed compared with those derived from tumor-free P14 donor mice (“control/control” and “control/tumor”) (n = 4–5 mice per group). This experiment was performed two times with similar results. (*Indicates p < 0.05; NS indicates no significance and p > 0.1)
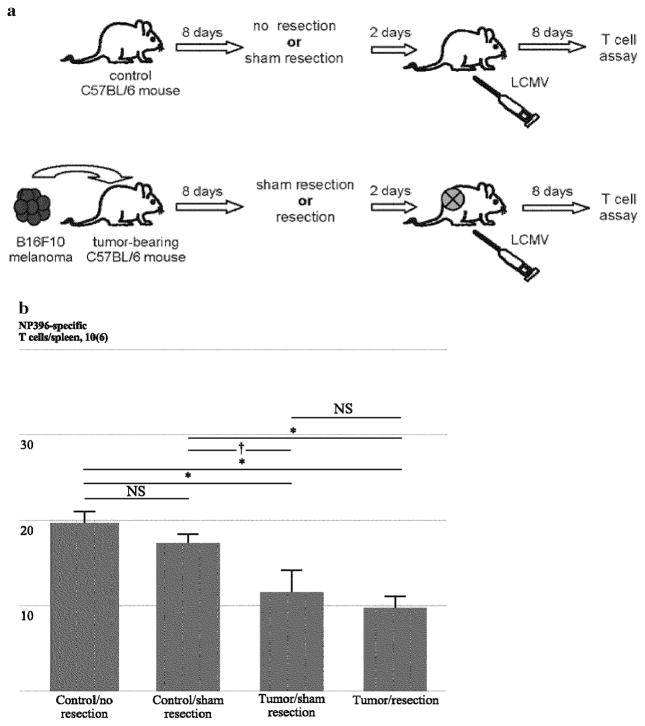
Surgical Resection of Established Melanoma does not Completely Reverse Suppression Antigen-Driven T-cell Expansion
To further test the possibility that in vivo exposure of resting T-cells was at least partially responsible for suppressed expansion, control and tumor-bearing mice underwent sham or actual surgical tumor resections on day 8 (Fig. 5a). Thus, established tumors were excised after in vivo exposure to resting T-cells but before activation of resting T-cells by LCMV. Sham resections performed in control mice did not alter T-cell expansion, and melanoma resection on day 8 did not reverse suppression of T-cell expansion in response to LCMV infection, suggesting that a period of in vivo exposure of resting T-cells to melanoma was sufficient to induce suppressed expansion (Fig. 5b). Thus, exposure of resting T-cells to melanoma may durably suppress their ability to undergo antigen-driven expansion even after melanoma removal has taken place.
FIG. 5.
Suppressed expansion of resting T-cells exposed to tumor is not prevented by tumor resection. a C57BL/6 mice were inoculated with media or B16F10 melanoma cells on day 0. Control mice underwent no resection or sham resection and tumor-bearing mice underwent no resection or tumor resection on day 8. LCMV infection was performed on day 10, and splenocytes were harvested on day 18 for comparative analysis of LCMV-specific T-cell expansion. Completeness of resection was confirmed at the resection sites at euthanasia. b Absolute numbers of NP396-specific CD8+ T-cells were compared in tumor-free mice undergoing no resection (“control/no resection”) and sham resection (“control/sham resection”) as well as tumor-bearing mice undergoing sham resection (“tumor/sham resection”) and resection (“tumor/resection”). Shem resection alone did not appear to influence CTL expansion (“control/no resection” vs. “control/sham resection”), and tumor-induced suppression of CTL expansion was only partially ameliorated by tumor resection (“tumor/sham resection” vs. “tumor/resection”) (n = 3–4 mice per group). This experiment was performed two times with similar results. (*Indicates p < 0.05; †indicates 0.05 <p< 0.1; NS indicates no significance and p > 0.1)
DISCUSSION
Immunotherapeutic strategies to augment antitumor immune responses have employed tumor antigen-specific CTLs for their ability to mediate immunological tumor rejection.1 Experimental animal models and clinical trials of adoptive T-cell immunotherapy in which tumor-specific CTLs are introduced into subjects with cancer, then expanded in vivo via antigenic stimulation, suggest that adjunctive immunomodulatory strategies, such as host lymphodepletion or cytokine-mediated immune stimulation, may be needed for adoptively transferred CTLs to achieve their potential efficacy.2,3 However, persistent shortcomings of T-cell–based cancer immunotherapies continue to motivate efforts to fully understand the influence of cancer on T-cell physiology.
In this study, we utilized a quantitative readout of T-cell expansion (proliferation of clonal LCMV epitope-specific CTL populations) to demonstrate that activation-induced T-cell expansion is weakened in the presence of melanoma. The absence of antigenic overlap between B16F10 and LCMV suggests that this melanoma-induced suppression of T-cell expansion is not antigen-specific. Indeed, a similar degree of suppression was seen for all clonal populations tested, regardless of the antigenicity of their target LCMV epitopes. This is compatible with recent observations of weakened CTL expansion in response to gp96-Ig-expressing vaccines in the presence of tumor,14,15 and suggests that cancers exert a globally suppressive influence on T-cell proliferation that is not restricted to tumor-antigen specific T-cells alone. The linear relationship between tumor burden and extent of suppression is in agreement with heightened deficits in cellular immunity observed among mice bearing bulky tumors and inverse relationships observed between tumor burden and responsiveness to T-cell vaccination strategies.15,16 These findings underscore the possibility that the optimal window of opportunity for effective clinical strategies in cancer immunotherapy may be in the setting of minimal disease.17
The kinetics and magnitude of activation-induced T-cell expansion and subsequent contraction are a function of antigenic stimulation and the resulting balance between cycle progression and apoptosis. The timing of LCMV-driven expansion and contraction did not differ between control and tumor-bearing mice. Analysis of explanted liver and lung tissue confirmed that both groups were capable of viremic clearance 8 days after LCMV infection (data not shown); thus, differences in T-cell expansion were not a result of differences in viral exposure. Rather, analysis of proliferative and apoptotic activity at various time points after LCMV infection suggest that the suppression of T-cell expansion in the presence of melanoma may be causally related to a pro-apoptotic milieu. Similar phenomena have been described among tumor-infiltrating lymphocytes, whose propensity toward apoptotic cell death in vitro is associated with downregulation of the antiapoptotic proteins Bcl-xL and Bcl-2.18 An eventual consequence of acute T-cell expansion in response to antigen is the induction of long-lived memory T-cells arising from MPEC populations.19 Because of the finite life expectancy of tumor-bearing mice, we were unable to measure mature memory T-cells in tumor-bearing mice at appropriate time points (>50 days) after LCMV clearance. However, the presence of fewer MPECs on postinfection day 10 in tumor-bearing mice suggests that melanoma-induced suppression of acute T-cell expansion may have durable implications throughout the longitudinal spectrum of activated T-cell homeostasis.
By transferring splenocytes from LCMV-naïve P14 TCR transgenic mice into control or tumor-bearing mice before or after tumor inoculation, we tested whether suppressed expansion in tumor-bearing mice was mediated before or at the time of antigenic stimulation. The observation of normal LCMV-driven expansion among P14 T-cells adoptively transferred after tumor establishment but immediately before viral exposure suggests that the 9 days of in vivo exposure of T-cells to growing melanoma in our model was necessary for the induction of suppressed expansion. Expansion of endogenous non-P14 polyclonal T-cells was suppressed in tumor-bearing animals regardless of the timing of adoptive transfer; therefore, it is unlikely that cotransferred populations of cells (e.g., mature dendritic cells) were responsible for allowing P14 T-cells transferred into tumor-bearing mice on day 9 to have expanded normally. Recapitulating tumor exposure by harvesting donor cells from tumor-bearing P14 mice 9 days after tumor inoculation restored the suppressed phenotype. Interestingly, this relatively brief exposure was sufficient to suppress expansion, as P14 T-cells transferred from tumor-bearing donors into tumor-free recipients immediately before LCMV infection remained suppressed (although not to the extent seen when transferred into tumor-bearing recipients). Resection of established tumors 8 days after melanoma inoculation (2 days before LCMV infection) was incapable of fully restoring normal T-cell expansion, and suppression among mice bearing resected tumors was less than that seen in melanoma-bearing mice that did not undergo resection. These parallel observations suggest that melanoma-induced inhibition of antigen-driven T-cell expansion is at least partly mediated by intrinsic suppression of resting T-cells occurring before antigenic stimulation.
Our observations suggest a causal relationship between melanoma growth and suppressed T-cell expansion. The mechanisms by which melanoma growth alters the ability of resting T-cells to undergo activation-induced expansion remain to be determined. The presence of similar numbers of resting P14 TCR transgenic T-cells in control versus tumor-bearing mice and the fact that tumors did not fundamentally alter baseline CD4+ and CD8+ T-cell populations in uninfected mice (data not shown) suggest that this suppression is not mediated by a reduction in CTL precursor frequency. Downregulated expression of the TCR signaling protein CD3ζ, which could impair TCR-mediated proliferation, has been observed among tumor-infiltrating lymphocytes.6,7 We were unable to identify evidence that expanded activated CTLs were functionally anergic in tumor-bearing mice, as evidenced by their ability to elaborate inflammatory cytokines in response to in vitro antigenic stimulation. Others also have observed restoration of functional activity of CTLs harvested from tumor-bearing using in vitro stimulation assays.15,20 However, the presence of similar levels of granzyme B expression among activated T-cells harvested directly ex vivo from control and tumor-bearing mice suggests that CTLs remained functionally competent in melanoma-bearing mice. Thus, the dominant consequence of melanoma on T-cells in our model is suppression of numbers, not functional activity.
These data offer evidence that growing melanoma tumors exert a globally suppressive influence on resting T-cells that intrinsically weakens their ability to undergo maximal proliferative expansion in response to antigenic stimulation. This phenomenon may have clinical implications for T-cell–based adoptive immunotherapy strategies designed to induce in vivo expansion of tumor antigen-specific CTL populations. Ongoing work in our laboratory seeks to elucidate the mechanisms underlying this suppression and identify strategies by which this clinically undesirable phenomenon may be overcome.
Supplementary Material
Acknowledgments
This work was supported by grant support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Science Research and Development Service, Career Development Award (CDA-2), American College of Surgeons Faculty Research Fellowship, and Central Surgical Association Foundation Grant to CSC.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1245/sl0434-011-1667-6) contains supplementary material, which is available to authorized users.
CONFLICTS OF INTEREST There are no potential conflicts of interest; the contents of this work do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marincola F, Wang E, Herlyn M, et al. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–12. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The three E’s of cancer im-munoediting. Ann Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 6.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockhart DC, Chan AK, Mak S, et al. Loss of T-cell receptor-CD3ζ and T-cell function in tumor-infiltrating lymphocytes but not in tumor-associated lymphocytes in ovarian carcinoma. Surgery. 2001;129:749–56. doi: 10.1067/msy.2001.114554. [DOI] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Ann Rev Immunol. 2007;25:267–95. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lizee G, Cantu MA, Hwu P. Less yin, more yang: confronting the barriers to cancer immunotherapy. Clin Cancer Res. 2007;13:5250–5. doi: 10.1158/1078-0432.CCR-07-1722. [DOI] [PubMed] [Google Scholar]
- 10.Grayson JM, Harrington LE, Lanier JG, et al. Differential sensitivity of naïve and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–70. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed R, Salmi A, Butler LD, et al. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice: role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–40. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altaian JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Hou S, Hyland L, Ryan KW, et al. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature. 1994;369:652–4. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 14.Oizumi S, Deyev V, Yamazaki K, et al. Surmounting tumor-induced immune suppression by frequent vaccination or immunization in the absence of B cells. J Immunother. 2008;31:394–401. doi: 10.1097/CJI.0b013e31816bc74d. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber TH, Deyev VV, Rosenblatt JD, et al. Tumor-induced suppression of CTL expansion and subjugation by gp96-Ig vaccination. Cancer Res. 2009;69:2026–33. doi: 10.1158/0008-5472.CAN-08-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danna EA, Sinha P, Gilbert M, et al. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205–11. doi: 10.1158/0008-5472.can-03-2646. [DOI] [PubMed] [Google Scholar]
- 17.Caldwell SA, Ryan MH, McDuffie E, et al. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402–12. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 18.Agrawal S, Marquet J, Delfau-Larue MH, et al. CD3 hyporesponsiveness and in vitro apoptosis are features of T cells from both malignant and nonmalignant secondary lymphoid organs. J Clin Invest. 1998;102:1715–23. doi: 10.1172/JCI3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaech SM, Tan JT, Wherry EJ, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 20.den Boer AT, van Mierlo GJD, Fransen MF, et al. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J Immunol. 2004;172:6074–9. doi: 10.4049/jimmunol.172.10.6074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.