Abstract
BACKGROUND
Whole blood donation in the United States is restricted in volume to 10.5 mL/kg or less in an effort to prevent hypovolemic reactions, but still may exceed more than 15% of a donor’s estimated blood volume (EBV). We analyzed the association of EBV with prefaint and systemic vasovagal reactions (SVRs) among whole blood donors and the potential impact of an EBV-based deferral policy.
STUDY DESIGN AND METHODS
Independent predictors for prefaint reactions and SVRs were assessed by multivariate logistic regression analysis on 591,177 unique donors participating in the Retrovirus Epidemiology Donor Study-II study.
RESULTS
Young age (16 years old odds ratio [OR], 3.70; 95% confidence interval [CI], 2.78-4.94), low EBV (<3.5 L OR, 3.30; 95% CI, 2.57-4.23), and first-time donation status (OR, 2.33; 95% CI, 2.03-2.67) were the strongest predictors for SVRs, with similar trends seen for prefaint reactions. Sex, height, race, blood center, and donation site were weakly associated predictors. A total of 5.6% of all donors had an EBV of less than 3.5 L and experienced 12.5% of all prefaint reactions and 14.5% of SVRs. The highest reaction rates were seen in donors less than 23 years old with an EBV of less than 3.5 L who comprised 2.7% of all donors, who were mostly female (99.9%), and who experienced 8.8% of prefaint reactions and 11.0% of SVRs.
CONCLUSION
Young age, low EBV, and first-time donation status are the major correlates of prefaint reactions and SVRs, suggesting that high school and college donors are at particular risk. Deferral of donors with low EBV who are less than 23 years old may offer a rational approach to protecting donors at greater risk of reactions without jeopardizing the adequacy of the blood supply.
To increase the number of blood components available for distribution, blood collection agencies have expanded the use of donor incentives to attract new donors, encouraged current or lapsed donors to give more frequently, increased the collection of multiple components by apheresis from individual donors, aggressively pursued donation by high school and college donors, and supported state legislation to decrease the minimum age for a blood donation to 16 years of age.1 Of concern, however, is whether any of these steps, particularly collecting from young donors, results in increased risk of adverse events for the individual donor. Blood collection facilities have the dual obligation to make the donation process as safe as possible for the donor and to provide an adequate blood supply to the community; consequently, they should design measures to minimize risk to donors as they strive to increase the number of blood components available for transfusion.2,3
Blood donation is generally well tolerated by most individuals; however, some people will experience adverse events after the collection of whole blood by manual methods. The most common complications are small hematomas and prefaint (presyncope) reactions, occurring in up to 16 and 5% of donors, respectively.4 Potentially more significant adverse events range from vasovagal syncope (loss of consciousness) with occasional injuries from falling or accidents, phlebotomy-related reactions such as hematomas, nerve irritation/injuries, arterial punctures, phlebitis, and thrombosis and skin infections to rare systemic reactions, allergy/anaphylaxis, and myocardial infarction. A voluntary blood donation has not been causally implicated in a donor’s death, based on reports to the US Food and Drug Administration.5
Prefaint reactions and loss of consciousness after donation are of particular concern, because they may lead to falls, injuries, and accidents and reduce the likelihood that a donor will return to donate again.1,6 Many studies have highlighted young age, first-time donation status, female sex, low weight, and Caucasian race as important risk factors for reactions, and high school students are at particular risk.1,3,7,8 Eder and colleagues1 reported that donors aged 16 and 17 years have higher rates of vasovagal reactions than older donors and that high school–age donors experiencing adverse reactions are less likely to donate blood in the future. Younger donors also had significantly higher rates of donor reactions needing additional medical care than adult donors (5.8 reactions/ 10,000 collections for donors age 16-17 years vs. 2.9 reactions/10,000 collections for donors aged ≥ 20 years).
Wiltbank and coworkers3 also reported the risk factors for vasovagal reactions in donors 17 years or older and showed that estimated blood volume (EBV) and body mass index are risk factors for mild (pallor, sweating, anxiety, or reactions lasting < 15 min), moderate (vomiting, loss of consciousness, hypotension, or reactions lasting 10-30 min), and severe (chest pain, incontinence, convulsions, delayed recovery, or reactions lasting > 30 min) donor reactions among whole blood donors. Donors with an EBV of less than 3.5 L had an odds ratio (OR) for prefaint and faint reactions of 2.88 (95% confidence interval [CI], 2.57-3.23).3 A low body mass index (<18.5) was also associated with a high risk for prefaint and faint reactions (OR, 2.51; 95% CI, 1.99-3.15). These authors noted that donors with an EBV of less than 3.5 L are at risk of the loss of more than 15% of their blood volume with the donation of a standard unit of blood under current AABB Standards that limit blood loss to 10.5 mL/kg (525 mL from a 50-kg [110-lb] donor). This study led the authors to implement additional donor eligibility criteria requiring that male and female donors ages 16 to 22 years have a total blood volume of 3.5 L or more.9
With this background, we conducted a study of the relationship of risk factors such as weight, height, and EBV, with prefaint and systemic vasovagal reactions (SVRs) among donors of allogeneic whole blood as young as 16 years old, in two American Red Cross (ARC) regions participating in the NIH-sponsored Retrovirus Epidemiology Donor Study-II (REDS-II). The study focused on the potential benefit and impact of implementing donor restrictions based on EBV.
MATERIALS AND METHODS
Study design, population, and sites
This was a cross-sectional study of prefaint and SVRs among 591,177 whole blood donors who made allogeneic whole blood donations during 2006 through 2007 at the Southern (Atlanta, GA; SARC) and New England (Dedham, MA; NEARC) Regions of ARC Blood Services. The two ARC centers are participating in the National Heart, Lung, and Blood Institute REDS-II, a multicenter study designed to research blood safety and availability issues in the United States. These blood centers were selected for the study among the six blood centers participating in the REDS-II because they are the only two REDS-II blood centers that share a uniform blood donation reaction classification scheme. A proportion of the data collected in 2006 has been included in prior reports.1,2 The study protocol was approved by the institutional review board at each participating blood center and the central coordinating center, Westat, Inc. (Rockville, MD).
Data collection
Information on demographic characteristics (age, sex and race/ethnicity, body weight and height, first-time or repeat donor status) and blood drive site has been collected by the REDS-II program continuously since January 2006. The height and weight are self-reported by the donors at the time of donation and are not measured by staff. The blood centers routinely send their donation data with encrypted donor identification to Westat, Inc., where they are compiled for analysis. Informed consent was obtained from all donors at the time of blood collection and parental permission for donation was obtained for all 16-year-old donors.
Manual blood collection
Using 500-mL blood collection bags (Fenwal, Inc., Lake Zurich, IL; Pall Corp., East Hills, NY), the acceptable volume of whole blood is 500 mL ± 10% (450-550 mL); however, collection scales are calibrated and set so that the calculated maximum volume of all blood collected from a donor, which includes the collection bag, sample tubes, and collection set tubing, does not exceed 525 mL. The minimum donor weight for whole blood donations is 110 lb (50 kg), so that the maximum calculated blood loss does not exceed 10.5 mL/kg, in keeping with AABB Standards.10 The removal of 525 mL of blood represents 15% of blood volume from a donor with an EBV of 3.5 L and 13% of a donor with an EBV of 4.0 L.
Donor reaction classification
All blood donor reactions were classified by the ARC blood center staff using standard protocols as previously described.2 The classification scheme for donation reactions did not change during the study period. This analysis focused on systemic (vasovagal-type) donor reactions and the selected categories were organized as prefaint and “systemic vasovagal reactions” (short loss of consciousness [lasting less than 1 min], long loss of consciousness [lasting 1 min or more], loss of consciousness or prefaint with injury, or prolonged recovery; see Table 1.) Because donors could only be coded for a single adverse reaction category, those that suffered other adverse reactions (e.g., phlebotomy-related reactions) were not at risk of being attributed a prefaint reaction or SVR and were excluded from further analysis.
TABLE 1.
Donor reaction classification and incidence in this study
| Complication | Number | Proportion of donors (%) |
|---|---|---|
| No reaction | 562,529 | 95.15 |
| Minor discomforts | ||
| Prefaint | 22,385 | 3.79 |
| Hematoma, small (≤2 × 2 in.) | 4,578 | 0.77 |
| Subtotal | 26,963 | 4.56 |
| Systemic vasovagal | ||
| Loss of consciousness, short (<1 min) | 856 | 0.14 |
| Loss of consciousness, long (≥1 min) | 136 | 0.02 |
| Prolonged recovery (≥30 min) | 232 | 0.04 |
| Prefaint/loss of consciousness with injury | 108 | 0.02 |
| Subtotal | 1,332 | 0.22 |
| Major phlebotomy-related | ||
| Nerve injury | 119 | 0.02 |
| Arterial puncture | 86 | 0.01 |
| Hematoma, large (>2 ×2 in.) | 62 | 0.01 |
| Subtotal | 267 | 0.04 |
| Other reactions | ||
| Other, minor reaction | 35 | 0.01 |
| Citrate, minor reaction | 20 | 0.00 |
| Allergic, minor reaction | 17 | 0.00 |
| Other, major reaction | 10 | 0.00 |
| Allergic, major reaction | 3 | 0.00 |
| Citrate, major reaction | 1 | 0.00 |
| Subtotal | 86 | 0.01 |
| Total | 591,177 | 100 |
Statistical analysis
In this study, each donor is represented only once in the analytical data set, even if they made multiple donations. In other words, only the first allogeneic whole blood donation of each donor during the 2-year period at the two ARC blood centers was included. First-time versus repeat donor status was ascertained using donor self-report, as well as retrospective examination of the blood center database for previous donations and/or infectious disease test results relating to the same person.
The following factors for predicting prefaint reactions and SVRs were analyzed: 1) sex (male and female); 2) age (16, 17, 18, 19-22, 23-69, and ≥70 years, where 16-18 years approximates the ages of high school students and 19-22 years reflects the ages of college students); 3) donor status (first-time and repeat); 4) race/ethnicity (Asian, black, Hispanic, white, and other); 5) region (NEARC and SARC); 6) blood drive sites (high school, college, and all others); 7) weight (110-119, 120-129, 130-139, 140-149, 150-174, 175-199, and ≥200 lb); 8) height (<60 inch, 60-61, 62-63, 64-65, and ≥66); and 9) EBV calculated using weight, height, and sex (<3.5, 3.5 to <4.0, 4.0 to <4.5, 4.5 to <5.0, and ≥5.0 L) according to the formula of Nadler and colleagues.11,12 Some of the 18-year-old donors could have been enrolled at a college at their time of their donation and some of the young donors could have donated blood at sites other than schools, colleges, or universities.
Overall donor reaction rates by reaction category were calculated. Stratified SVR and prefaint reaction rates by EBV, body weight, height, age, sex, race/ethnicity, first-time/repeat donor status, region, and blood drive type were computed. An m × n chi-square test was used to assess the overall difference of the frequency distribution of reactions among subgroups.
To measure the independent effect of each of these nine factors, adjusted ORs were derived from multivariate logistic regression (Figs. 1 and 2). The binary dependent variables were prefaint versus no reaction and SVR versus no reaction.
Fig. 1.
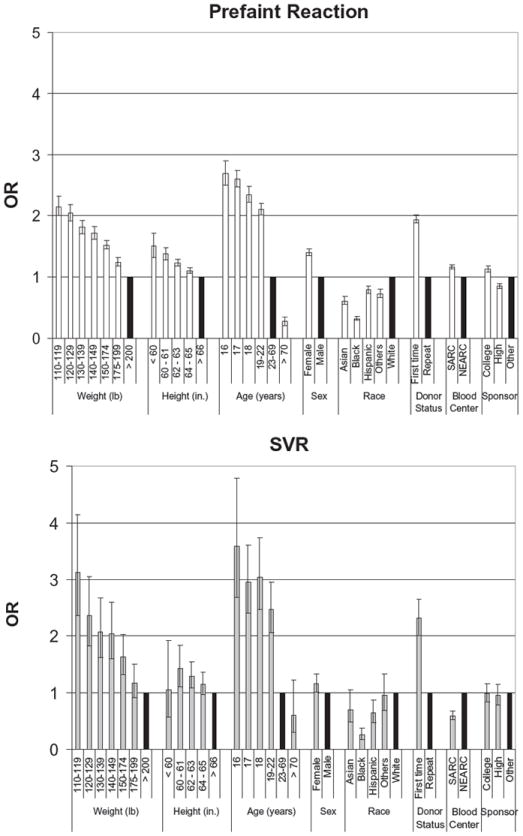
Multivariate logistic regression analysis of risk factors to predict prefaint reactions and SVRs incorporating weight and height. Adjusted OR and 95% CI, shown for each variable. (■) OR = 1 of control group for each variable.
Fig. 2.
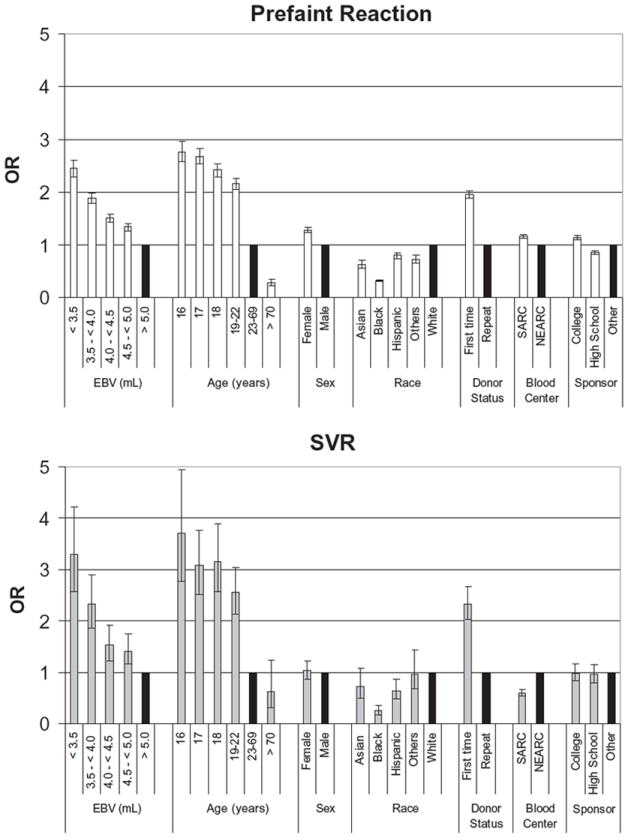
Multivariate logistic regression analysis of risk factors to predict prefaint and SVRs replacing weight and height with EBV. Adjusted OR and 95% CI, shown for each variable. (■) OR = 1 of control group for each variable.
Approximately 14.4% of the donors did not provide their body weight and/or height due to either their refusal to provide all the requested demographic information or their specific refusal to provide body weight and/or height. To avoid losing important information, these donors were retained in the multivariate logistic regression model by including them in a “missing” category for body weight and height as well as the derived variable, EBV (Table 2). Computer software (SAS/STAT, Version 9.1.2; SAS Institute, Inc., Cary, NC) was used for the data analysis in this study.
TABLE 2.
Donor demographic characteristics and the distribution of prefaint and major SVR events
| Characteristic | Total number of donors | Proportion of donors (%)* | Donors without any reaction | Prefaint events
|
SVRs
|
|||
|---|---|---|---|---|---|---|---|---|
| Number of prefaint events | Proportion of events (%)† | Number of SVRs | Proportion of events (%)† | |||||
| Total donors | 586,246 | 562,529 | 22,385 | 3.83 | 1,332 | 0.24 | ||
| Sex | ||||||||
| Male | 272,009 | 46.40 | 264,343 | 7,202 | 2.65 | 464 | 0.18 | |
| Female | 314,234 | 53.60 | 298,184 | 15,182 | 4.84 | 868 | 0.29 | |
| Missing | 3 | 0.00 | 2 | 1 | 0.5 | 0 | 0.00 | |
| Age (years) | ||||||||
| 16 | 15,647 | 2.67 | 14,142 | 1,420 | 9.12 | 85 | 0.60 | |
| 17 | 61,498 | 10.49 | 55,973 | 5,168 | 8.45 | 357 | 0.63 | |
| 18 | 35,519 | 6.06 | 32,770 | 2,559 | 7.24 | 190 | 0.58 | |
| 19-22 | 59,761 | 10.19 | 55,990 | 3,557 | 5.97 | 214 | 0.38 | |
| 23-69 | 402,793 | 68.71 | 392,703 | 9,612 | 2.39 | 478 | 0.12 | |
| ≥70 | 11,028 | 1.88 | 10,951 | 69 | 0.63 | 8 | 0.07 | |
| Race/ethnicity | ||||||||
| White | 483,498 | 82.47 | 463,291 | 19,071 | 3.95 | 1,136 | 0.24 | |
| Asian | 9,765 | 1.67 | 9,367 | 371 | 3.81 | 27 | 0.29 | |
| Black | 48,568 | 8.28 | 47,706 | 832 | 1.71 | 30 | 0.06 | |
| Hispanic | 19,805 | 3.38 | 18,805 | 953 | 4.82 | 47 | 0.25 | |
| Others | 11,000 | 1.88 | 10,516 | 448 | 4.09 | 36 | 0.34 | |
| Missing | 13,610 | 2.32 | 12,844 | 710 | 5.53 | 56 | 0.44 | |
| Donor status | ||||||||
| Repeat donors | 335,686 | 57.26 | 327,879 | 7,438 | 2.22 | 369 | 0.11 | |
| First-time donors | 245,076 | 41.80 | 229,328 | 14,790 | 6.06 | 958 | 0.42 | |
| Missing | 5,484 | 0.94 | 5,322 | 157 | 2.95 | 5 | 0.09 | |
| ARC center | ||||||||
| NEARC | 320,407 | 54.65 | 307,404 | 12,093 | 3.79 | 910 | 0.30 | |
| SARC | 265,839 | 45.35 | 255,125 | 10,292 | 3.88 | 422 | 0.17 | |
| Blood drive sites | ||||||||
| College | 73,708 | 12.57 | 68,452 | 4,930 | 6.72 | 326 | 0.47 | |
| High school | 83,745 | 14.28 | 77,820 | 5,562 | 6.67 | 363 | 0.46 | |
| Others | 428,793 | 73.14 | 416,257 | 11,893 | 2.78 | 643 | 0.15 | |
| Height (in.) | ||||||||
| <60 | 3,881 | 0.66 | 3,602 | 268 | 6.93 | 11 | 0.30 | |
| 60-61 | 21,314 | 3.64 | 19,884 | 1,347 | 6.34 | 83 | 0.42 | |
| 62-63 | 62,158 | 10.60 | 58,432 | 3,511 | 5.67 | 215 | 0.37 | |
| 64-65 | 85,460 | 14.58 | 81,166 | 4,054 | 4.76 | 240 | 0.29 | |
| ≥66 | 332,019 | 56.63 | 321,038 | 10,368 | 3.13 | 613 | 0.19 | |
| Missing | 81,414 | 13.89 | 78,407 | 2,837 | 3.62 | 170 | 0.22 | |
| Weight (lb) | ||||||||
| 110-119 | 17,846 | 3.04 | 16,070 | 1,651 | 9.32 | 125 | 0.77 | |
| 120-129 | 34,333 | 5.86 | 31,585 | 2,591 | 7.58 | 157 | 0.49 | |
| 130-139 | 45,485 | 7.76 | 42,521 | 2,795 | 6.17 | 169 | 0.40 | |
| 140-149 | 48,966 | 8.35 | 46,243 | 2,560 | 5.25 | 163 | 0.35 | |
| 150-174 | 123,520 | 21.07 | 118,434 | 4,805 | 3.9 | 281 | 0.24 | |
| 175-199 | 104,182 | 17.77 | 101,364 | 2,682 | 2.58 | 136 | 0.13 | |
| ≥200 | 127,795 | 21.80 | 125,277 | 2,393 | 1.87 | 125 | 0.10 | |
| Missing | 84,119 | 14.35 | 81,035 | 2,908 | 3.59 | 176 | 0.22 | |
| Blood volume (L) | ||||||||
| <3.5 | 28,173 | 4.81 | 25,573 | 2,432 | 8.68 | 168 | 0.65 | |
| 3.5 to <4.0 | 94,988 | 16.20 | 88,949 | 5,688 | 6.01 | 351 | 0.39 | |
| 4.0 to <4.5 | 90,788 | 15.49 | 86,693 | 3,897 | 4.3 | 198 | 0.23 | |
| 4.5 to <5.0 | 79,287 | 13.52 | 76,297 | 2,821 | 3.57 | 169 | 0.22 | |
| ≥5.0 | 208,438 | 35.55 | 203,552 | 4,617 | 2.22 | 269 | 0.13 | |
| Missing | 84,572 | 14.43 | 81,465 | 2,930 | 3.6 | 177 | 0.22 | |
Column percent.
Row percent.
RESULTS
Characteristics of all donors
During the period from January 2006 to December 2007, a total of 591,177 unique donors made whole blood donations to the SARC and NEARC. On the day of their first donation during this study period, the majority of donors (95.15%; Table 1) did not experience a reaction. The most commonly recorded adverse events were prefaint reactions (3.79% donors), small hematoma (0.77%), and loss of consciousness, less than 1 minute (0.14%). Overall, a SVR occurred in 0.22% of donors, while phlebotomy-related (0.04%) and other reactions (0.01%) were rare.
Characteristics of donors with prefaint reactions, SVRs, or no reactions
We focused our analysis on prefaint reactions and SVRs as the most frequent events that likely correlate with donor characteristics amenable to avoidance through EBV-related donor deferral policies, especially in high school (mostly 16-18 years old) or college-age (19-22 years old) donors. The focused analysis included 586,246 donors with no reactions, prefaint reactions, or SVRs and excluded donors who experienced other reactions; the result of the focused analysis indicates that 3.83% of the donors in the focused analysis had prefaint reactions and 0.24% of the donors in the same analysis experienced SVRs (Table 2). These donors were more likely to be female (53.6% vs. 46.4%), white (82.5%), and repeat donors (57.3%). A total of 112,664 (19.2%) donors were 16 to 18 years old, while 59,761 (10.2%) were 19 to 22 years old. A total of 28,173 (4.8%) donors had an EBV of less than 3.5 L. EBV could not be calculated for 84,572 (14.4%) donors due to missing height or weight data, such that donors with an EBV of less than 3.5 L comprised 5.6% of the evaluable donors for which EBV could be calculated.
Frequency of prefaint reactions and SVRs by donor and site characteristics
Analysis of the incidence of prefaint reactions and SVRs using the chi-square test revealed that prefaint reactions and SVRs were unevenly distributed among subgroups of sex, age, race/ethnicity, donor status, blood drive sites, height, weight, and EBV (p < 0.0001, data not shown). The NEARC blood services region had higher rate of SVRs (p < 0.0001), but not prefaint reactions (p = 0.07, data not shown) than the SARC.
For example, Table 2 shows that 9.32% of the donors weighing 110 to 119 lb had a prefaint reaction compared to 1.87% of the donors weighing 200 lb or more. Younger donors had higher rates of prefaint reactions than older donors (9.12% in donor age 16 years old vs. 2.39% in donors 23-69 years old). Male donors had lower prefaint rates than female donors (2.65% vs. 4.84%). Hispanic donors had the highest reaction rates (4.82%) compared to donors of any racial or ethnic group; nevertheless, information on the race / ethnicity was not available from 2.32% of donors, 5.53% of whom experienced prefaint reactions and 0.44% SVRs. It is unclear why the reaction rate should be high in this group. First-time donors had more prefaint reactions than repeat donors (6.06% vs. 2.22%). Donors giving at college drives (6.72%) and at high schools (6.67%) had more prefaint reactions than other collection sites (2.78%). Donors with low EBVs of less than 3.5 L had the highest prefaint reaction rates (8.68%) when compared to donors with EBVs of 5.0 L or more (2.22%). In general, the SVR rates mirrored the prefaint rates but occurred at approximately 10- to 20-fold lower rates.
Association between donor characteristics and prefaint reactions and SVRs
A multivariate logistic regression model was constructed using weight, height, age, sex, race, donor status, blood center, and blood drive sites as independent variables for prefaint reactions and SVRs. Table 2 indicates that prefaint reactions and SVRs are more common in female versus male donors, first-time versus repeat donors, Hispanic and white versus black donors, and donors who weigh close to the minimum acceptable donor weight in the United States (110 lb). There was a clear monotonic decrease in both prefaint reaction and SVR rates with increasing body weight and increasing age, and a significant difference was seen in both prefaint reaction and SVR rates reported in the New England and Southern regions. For the risk-adjusted analysis, reference groups were chosen based on unadjusted ORs as follows: donors age 23 to 69 years old, donors weighing 200 lb or more, male donors, repeat donors, donors with a height of 66 in. or more, white donors, NEARC donors, and donors donating at sites other than high schools or colleges (see Figs. 1 and 2).
After controlling for each factor associated with prefaint reactions, adjusted ORs show that ages below 23 years were the strongest risk factors (Fig. 1), with 16-year-olds having the greatest risk (OR, 2.69; 95% CI, 2.50- 2.90), weight below 130 lb (<120 lb OR, 2.15; 95% CI, 2.00-2.32; <130-120 lb OR, 2.05; 95% CI, 1.92-2.18), and first-time donation status (OR, 1.94; 95% CI, 1.88-2.01) as the next highest risk factors. Height (<60 in. OR, 1.51; 95% CI, 1.32-1.72) and female sex (OR, 1.40; 95% CI, 1.35-1.45) were both positively associated with prefaint reactions while black donors were least affected (OR, 0.32; 95% CI, 0.30-0.35). The NEARC was more likely to record a prefaint reaction (OR, 1.16; 95% CI, 1.13-1.20) than the SARC, and these reactions were more likely to be seen at college drives (OR, 1.13; 95% CI, 1.08-1.18) than high school drives (OR, 0.85; 95% CI, 0.81-0.89).
In this analysis, SVRs showed trends similar to those of prefaint reactions (Fig. 1), with the age of 16 years (OR, 3.58; 95% CI, 2.68-4.78); weight of 110 to 119 lb (OR, 3.13; 95% CI, 2.36-4.14); age of 17, 18, and 19 to 22 years (OR, 2.95-2.47); weight of 120 to 129 lb (OR, 2.36; 95% CI, 1.82-3.05); and first-time donors (OR, 2.31; 95% CI, 2.01-2.65) at highest risk. Black (OR, 0.26; 95% CI, 0.18-0.38) and Hispanic (OR, 0.64; 95% CI, 0.48-0.87) donors had the lowest risk in the race category while the drive site was not a significant risk factor. Height (60-61 in. OR, 1.43; 95% CI, 1.11-1.84) and female sex (OR, 1.16; 95% CI, 1.01-1.34) were significant but less important risk factors. Finally, the SARC recorded fewer SVRs than the NEARC (OR, 0.60; 95% CI, 0.52-0.68).
A separate multivariate logistic regression analysis was performed using the calculated EBV variable, with the exclusion of height and weight (Fig. 2), because EBV is dependent on these variables. The EBV reference group was an EBV of 5.0 L or more; the reference groups for the rest of the variables were the same with the first model. In this analysis, age 16 years, EBV of less than 3.5 L, and first-time donation status were the major risk factors for prefaint (16 years old OR, 2.76; 95% CI, 2.57-2.97; EBV < 3.5 L OR, 2.45; 95% CI, 2.29-2.61; first-time status OR, 1.95; 95% CI, 1.89-2.02) and SVR events (16 years old OR, 3.70; 95% CI, 2.78-4.94; EBV < 3.5 L OR, 3.30. 95% CI, 2.57-4.23; first-time donor OR, 2.33; 95% CI, 2.03-2.67). Black race was protective for prefaint reactions (OR, 0.32; 95% CI, 0.30-0.34) and SVRs (OR, 0.26; 95% CI, 0.18-0.37). Of interest is that prefaint appears to be at a linearly increasing risk as EBV decreases below 5 L (Fig. 2), while a dramatically increased risk for SVR is seen for donors below 4.0 L EBV (<3.5 L OR, 3.30; 95% CI, 2.57-4.23; 3.5 to <4.0 L OR, 2.32; 95% CI, 1.86-2.89). Female sex remains a minor risk factor for prefaint reactions (OR, 1.28; 95% CI, 1.23-1.33) but is not significant for SVR (OR, 1.03; 95% CI, 0.87-1.22).
Potential impact of donor deferrals based on EBV and age
Our analyses demonstrate that age, EBV, weight, first-time donation status, and race are the most important predictors of prefaint reactions and SVRs. These data support the strategy of developing deferral criteria that selectively reduce the incidence of reactions, especially in high school and college drives where young, first-time, and low-EBV donors are most likely to donate. Broad-based deferral criteria based on age, first-time donation status, and race are not practical because such measures might indiscriminately eliminate an unacceptable number of donors and impair the sustainability of the blood supply. We therefore explored the possibility of restrictions based on both EBV and age in a stratified analysis of reactions. Figure 3 shows the non–risk-adjusted rates of prefaint reactions and SVRs by EBV group and confirms that the highest risk of prefaint reactions is seen with 16- (13.7%), 17- (13.7%), and 18- (12.5%) year-old donors with an EBV of less than 3.5 L, while donors have the highest rates of SVRs in the 18- (1.24%) and 19- to 22- (0.99%) year-old age groups.
Fig. 3.
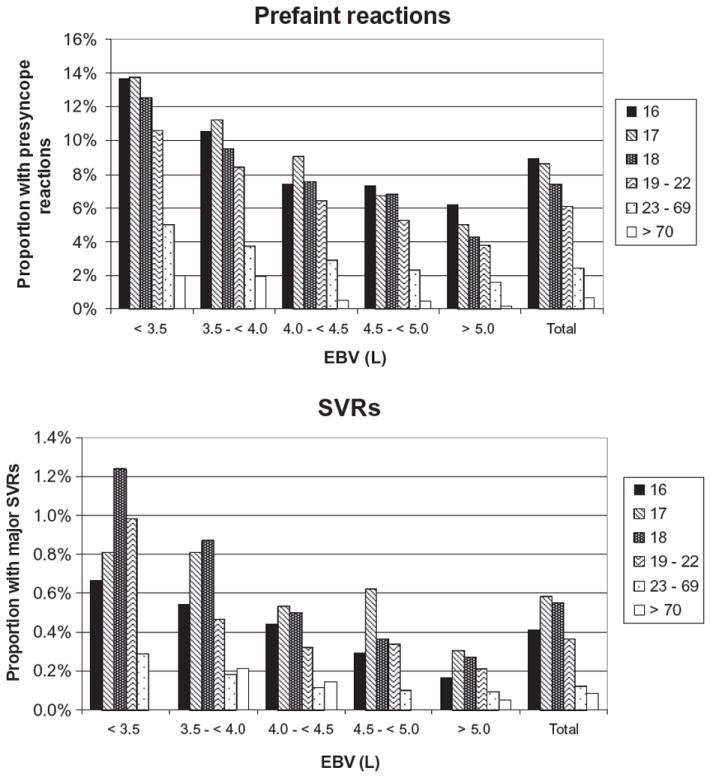
Non–risk-adjusted rates of prefaint reactions and SVRs broken down by age and EBV.
Of note is that donors with an EBV of 3.5 to less than 4.0 L also show substantial rates of prefaint reactions in the 16- to 18-year-old groups (9.5%-10.6%) and for SVRs in the 17- to 18-year-old groups (0.81%-0.87%). In contrast, donors 23 years and older had substantially lower rates of prefaint reactions and SVRs in all EBV groups, suggesting that restriction of an EBV-based donor deferral to donors less than 23 years old would prevent the reactions occurring at the highest rates while minimizing donor loss.
Donor deferral criteria based on EBV (<3.5 L) in all age groups would lead to deferral of 5.6% of currently presenting donors, with a potential avoidance of 14.5% of SVRs and 12.5% of all prefaint reactions (Table 3). In contrast, restriction of EBV-based deferral to donors less than 23 years of age and an EBV of less than 3.5 L would prevent donation by 2.7% of otherwise acceptable donors and avoid 8.8% of all prefaint reactions and 11.0% of all SVRs. A total of 99.9% (13,580/13,588) of the donors with an EBV of less than 3.5 L and an age of less than 23 years old prevented from donation would be female; these donors would represent 9.1% of all evaluable donors less than 23 years old and 16.5% of evaluable females less than 23 years old. The more effective strategy at reducing adverse events would be a restriction on all donors less than 23 years of age with an EBV of less than 4.0 L, which would avoid 31.9% of SVRs and 26.7% of prefaint reactions; however, 9.7% of presenting donors would become ineligible to donate (Table 3).
TABLE 3.
Estimated proportion of all donors and donors less than 23 years old, with an EBV of less than 3.5 or less than 4.0 L, and the proportion of prefaint and SVR events in each group*
| Criteria | EBV criteria (L) | Number of donors | Proportion of all donors† (%), n = 501,674 | Number of prefaint events | Proportion of prefaint events (%)‡, n = 19,455 | Number of SVR events | Proportion of SVR events (%)†, n = 1155 |
|---|---|---|---|---|---|---|---|
| All donors | <3.5 | 28,173 | 5.6 | 2,432 | 12.5 | 168 | 14.5 |
| <4.0 | 123,161 | 24.6 | 8,120 | 41.7 | 519 | 44.9 | |
| All female donors | <3.5 | 28,154 | 5.6 | 2,430 | 12.5 | 168 | 14.5 |
| <4.0 | 122,526 | 24.4 | 8,054 | 41.4 | 510 | 44.2 | |
| All male donors | <3.5 | 19 | 0.0 | 2 | 0.0 | 0 | 0.0 |
| <4.0 | 635 | 0.1 | 66 | 0.3 | 9 | 0.8 | |
| <23 years old | <3.5 | 13,588 | 2.7 | 1,715 | 8.8 | 127 | 11.0 |
| <4.0 | 48,777 | 9.7 | 5,197 | 26.7 | 368 | 31.9 | |
| <23 years old, female | <3.5 | 13,580 | 2.7 | 1,714 | 8.8 | 127 | 11.0 |
| <4.0 | 48,349 | 9.6 | 5,143 | 26.4 | 360 | 31.2 | |
| <23 years old, male | <3.5 | 8 | 0.0 | 1 | 0.0 | 0 | 0.0 |
| <4.0 | 428 | 0.1 | 54 | 0.3 | 8 | 0.7 |
Calculation of the proportions excludes missing data. EBV data were missing in 84,572 donors, leaving 501,674 donors who sustained 19,455 prefaint and 1155 SVR events evaluable in this analysis.
Column percent.
Row percent.
DISCUSSION
Young donors (16-22 years old) contribute an increasing proportion of the overall US blood supply as the post–World War II generation ages and are unable to donate, blood drives in industrial and manufacturing settings diminish, and an increasing number of states allow donation by both 16- and 17-year-old donors.1,13 Blood donation is promoted at high schools and colleges as an important vehicle to increase voluntary service and contribute to the community, with the rationale that students given multiple opportunities to donate at an early age may be more likely to mature into lifelong blood donors. The dilemma raised by recruiting high school students is that, while most donors in every age group have a satisfactory experience and feel good about donating, the youngest age groups are well documented to be the most likely to suffer adverse events related to donation that threaten their well-being and reduce their likelihood of returning. Eder and colleagues1 documented a higher rate of adverse events in 16- to 17-year-olds compared to donors 20 years and older, including SVRs and phlebotomy-related injuries such as hematomas, nerve irritation, and arterial punctures. Most concerning was the rare occurrence (5.9 per 10,000 donations), but 14.5-fold higher rate (OR, 14.5; 95% CI, 10.4-20.0) of injuries after falls or accidents related to prefaint or loss of consciousness reactions in 16- to 17-year-olds compared to adults. Donors who suffer either minor or major adverse events were significantly less likely to return to donate again.1,2 In this setting, substantial effort has been expended to devise programs to protect donors and reduce risks. The measures to improve safety for young donors were summarized by an ad hoc AABB work group14 and fall into distinct categories addressing education before and after donation and control of the drive environment and adequate staffing and specific interventions, such as modified selection criteria for whole blood donation.9,14-19
Herein we explore the possibility that risk may be moderated by judicious restrictions on donor eligibility based on predictors of reactions identified by multivariate logistic regression modeling. Analysis of 591,177 donations in two ARC blood centers participating in the REDS-II program that use the same standardized classification scheme for adverse reactions reveals young age, low weight, low EBV, first-time donation status, and Caucasian race as the strongest predictors of prefaint and SVRs. Sex, height, blood center, and site of donation were less important predictors. The combination of age and EBV (Fig. 3), in particular, provides a powerful means to identify those donors at highest risk. Donors 23 years and older had the lowest rates of prefaint reactions and SVRs, while younger donors with low EBV (<3.5 L) had the highest rates, with 13.7% of 16- and 17-year-olds experiencing prefaint reactions and 1.24% of 18-year-olds experiencing SVRs.
Our findings corroborate those recently reported by Wiltbank and coworkers3 using a different adverse reaction classification scheme in a separate blood donation program and extend the analysis to demonstrate the particular susceptibility of 16-year-old donors with low EBV to donation-related vasovagal-type reactions. We also examined only the first donation in our study period, to reduce the bias that repeat donation by donors who may be less likely to incur adverse reactions could introduce into the analysis. Nevertheless, together our data raise the question whether US donor qualifications sufficiently protect young donor with low blood volumes.
The EBV of an adult can be calculated with a simple body weight formula that assumes that an adult has approximately 70 mL/kg11 of blood or with a more accurate formula that accounts for the height, weight, and sex of the donors such as proposed by Nadler and colleagues.12 Since data on the weight and height of the study subjects were available to us, we used Nadler’s formula. Applying this formula to our donor population reveals that as many as 5.6% of donors, almost all of whom are female (>99.9%), have an EBV of less than 3.5 L.12 Indeed, a female donor weighing 110 lb and 4 ft 10 in. height has an EBV of 2980 mL and a 525-mL donation represents 17.6% of her EBV. Furthermore, more recent measurements that take into account the gradually increasing body mass index of the US population over time suggests that the Nadler formula may overestimate blood volume by as much as 12%.20 If so, then more than 5.6% of donors are at risk for donating more than 15% of their blood volume and the smallest donors may lose 20% of their EBV (525 mL from a donor with an EBV of 2625 mL).
The AABB Standards for Blood Banks and Transfusion Services limit the total amount of whole blood collected per donation to 10.5 mL/kg.10 Combining the minimum weight requirement of 110 lb (50 kg) for blood donations and blood volume requirements results in a maximum blood loss per donation of 525 mL for a 50-kg donor. The AABB Standards are based on the general assumption that an average adult in the United States has an EBV of 70 mL/kg,11 so that 10.5 mL/kg represents 15% or less of the EBV of the smallest eligible donor (525 mL from a donor with an EBV of 3500 mL). In the past decade, US blood collection agencies have increased the size of the bags used for blood collections (from 450- to 500-mL bags) with intent to increase the amount of plasma that can be harvested for further manufacturing purposes and to compensate for the loss of red blood cells retained by leukoreduction filters. However, collectors have to account for an additional 40- to 60-mL blood loss in samples taken for viral marker testing and in tubing attached to the bag. These constraints allow the maximum collection of approximately 465 to 485 mL of blood in the bag with in a total blood loss that is very close to the allowable blood loss of 525 mL per donation. It is notable that the European Commission21 requires member countries to enforce a minimum donor weight of 50 kg and a maximum volume of blood donation of 13% EBV, based on height, weight, and sex.22 In our analysis, a 13% blood loss restriction for a 525-mL donation roughly equates to a lower limit of EBV of 4.0 L (525 mL is 13.1% of an EBV of 4.0 L).
Possible interventions to prevent donors from donating an excessive proportion of their EBV include preventing whole blood donation by donors with an EBV below a predetermined limit for a given volume of collection (525 mL) or reducing the amount of whole blood collected in each donation.
We present the analysis of the impact of an EBV-based deferral and show that such limits are only necessary for young donors less than 23 years of age. In the two blood centers studied, a deferral policy based on age less than 23 years and EBV of less than 3.5 L identifies 2.7% of the presenting donors, of which 99.9% are female. These donors account for 8.8% of prefaint reactions and 11.0% of SVRs in the overall evaluable donor pool. The more stringent restriction based on deferral of donors less than 23 years old with an EBV of less than 4.0 L would defer 9.7% of current donors while preventing 26.7% of prefaint reactions and 31.9% of SVRs (Table 3). This can be compared to the potential impact of the European standard (<13% of the EBV of donors of all ages) which would require an EBV of more than 4.0 L and the deferral of 24.6% of current donors, potentially avoiding 41.7% of prefaint reactions and 44.9% of SVRs (Table 3). Again, our data suggest that there is little need for such a draconian restriction in donors more than 23 years old. One limitation of our proposed EBV-based donor deferral policy for both first-time and repeat donors is that it could result in the deferral of repeat donors with a low EBV who had never experienced a prefaint reaction or a SVR; however, our data suggest that these donors (repeat donors with an EBV of ≤3.5 L) are still at a higher risk of adverse events that repeat donors with an EBV of more than 3.5 L.
An alternative approach would be to reduce the volume of blood collected from each US blood donor of less than 23 years of age. Collecting no more than 450 mL from the smallest female donor would ensure that 15% of EBV is not exceeded, according to the formula of Nadler and coworkers12 (assuming a 110-lb female with a height of 4 ft 10 in. with an EBV of 2980 mL) and no EBV-based donor restriction would be necessary. Furthermore, every donor would lose approximately 14% less blood than is the current practice, which may reduce the overall risk of reactions, although our data cannot predict the magnitude of this effect. The introduction of a two-tiered blood volume collection system with the collection of lower blood volumes from high school and college-aged students is amenable to study and should be considered as an intervention to protect these most vulnerable donors.
Our study has a number of potential weaknesses. As in previous reports, we rely on self-reported donor weights and there may be pressure on young, first-time donors to exaggerate their weight to qualify for donation. A second issue is the presence of missing data. A total of 14.4% of donors in our study failed to provide sufficient height and/or weight data to allow calculation of an EBV and this was not evenly distributed: 33.1% (5173/15,647) of 16-year-olds and 10.9% to 11.9% of 17-, 18-, and 19- to 22-year-olds failed to provide complete data. Nevertheless, we do not believe that the results of the study are affected by selection bias since the donors provided their demographic information before the time when the majority of donation reactions take place—during phlebotomy. In our multivariate analyses we included a “missing” category to account for these donors. Nevertheless, the disproportionate number of 16-year-olds with missing data suggests that our analysis may misestimate the potential benefit of a deferral of donors with an EBV of less than 3.5 L and proportion of donors that would be deferred based on a 3.5-L EBV deferral criterion.
The actual volume of whole blood collected from each donor was not recorded, and a direct correlation between EBV and blood volume loss was not established. For donors with an EBV of less than 3.5 L, however, the standard blood draw is likely to exceed 15% of their total blood volume. Whether a smaller proportionate blood loss in a susceptible donor can account for most of the reactions remains undetermined.
EBV was calculated using a formula that accounts for the sex, height, and weight of the donors. Therefore, the effects of EBV on prefaint reactions and SVRs are somewhat dependent on the influence of sex on the calculation of EBV. Nevertheless, our multivariate analysis revealed that sex remains as an independent predictor of prefaint reactions, but not SVRs. The effect on sex on prefaint reactions (when adjusting for EBV, age, race, donor status, blood center, and sponsor) is small compared to the effect of an EBV less than 3.5 L (with donors with an EBV of ≥5.0 L as the reference group).
In this study of blood donation reactions, we only used the donor’s first donation to calculate the rates and risks for donation reactions. It is possible for the same donor to have experienced more than one reaction during the 2-year study period and that a subsequent reaction was not accounted for. Furthermore, the study was limited to allogeneic donations, and the results cannot be generalized to the autologous donations since these donations have different eligibility requirements. The study was conducted using data from two ARC blood centers located in the Northeast and Southeast regions of the United States and differences in reaction rates between the two centers were observed. For example, the OR for SVR among blood donors from the New England Region was higher than the OR for the same type of reaction from donors from the Southern Region. This difference in OR for SVR among these two blood centers could be partially explained by the inherent subjectivity in the recognition and reporting of blood donor reactions.2 It could be that a similar study of risk factors for prefaint reactions and SVRs at another blood collection agency will reveal different rates for the factors associated with donor reactions, although our overall conclusions are in line with those reported by others.3
Finally, this study corroborates the findings of Wiltbank and coworkers3 of a strong relationship between prefaint reactions and SVRs with low EBV and the risk of physiologic hypovolemic symptoms after donation. We found that prefaint reactions and SVRs are strongly associated with younger-age first-time donors with low EBV who tend to be Caucasian and female. No single value for age, weight, or EBV can be identified that would allow selective and definitive reduction in the risk through selective deferral of at-risk donors. Nevertheless, we propose that blood centers consider interventions to protect young donors (<23 years old) with low EBV (<3.5 L), the most vulnerable members of an important group of future blood donors.
Acknowledgments
The authors thank the staff at the New England and Southern Regions of the ARC. Without their help, this study would not have been possible.
This work was supported by NHLBI REDS-II Contracts N01-HB-47169, -47170, and -47175.
ABBREVIATIONS
- ARC
American Red Cross
- EBV
estimated blood volume
- NEARC
New England Region of the American Red Cross Blood Services
- REDS-II
Retrovirus Epidemiology Donor Study-II
- SARC
Southern Region of the American Red Cross Blood Services
- SVR(s)
systemic vasovagal reaction(s)
The Retrovirus Epidemiology Donor Study (REDS)-II is presently the responsibility of the following persons:
Blood Centers
American Red Cross Blood Services, New England Region: R. Cable, J.A. Rios, R.J. Benjamin
American Red Cross Blood Services, Southern Region/Emory University: C.D. Hillyer, K.L. Hillyer, J.D. Roback
BloodCenter ofWisconsin: J.L. Gottschall, A. E. Mast
Hoxworth Blood Center, University of Cincinnati Academic Health Center: R.A. Sacher, S.L. Wilkinson, P.M. Carey
Regents of the University of California/Blood Centers of the Pacific/BSRI: E.L. Murphy, M.P. Busch, B. Custer
The Institute for Transfusion Medicine (ITxM)/LifeSource Blood Services: D.J. Triulzi, R.M. Kakaiya, J. Kiss
Central Laboratory
Blood Systems Research Institute: M.P. Busch, P.J. Norris
Coordinating Center
Westat, Inc.: J. Schulman, M.R. King, D.J. Wright, T.L. Simon, S.H. Kleinman, P.M. Ness
National Heart, Lung, and Blood Institute, NIH
G.J. Nemo, S. Glynn, T.H. Mondoro
Steering Committee Chairman
R.Y. Dodd
Footnotes
CONFLICT OF INTEREST
None.
References
- 1.Eder AF, Hillyer CD, Dy BA, Notari EP, IV, Benjamin RJ. Adverse reactions to allogeneic whole blood donation by 16- and 17-year-olds. JAMA. 2008;299:2279–86. doi: 10.1001/jama.299.19.2279. [DOI] [PubMed] [Google Scholar]
- 2.Eder AF, Dy BA, Kennedy JM, Notari EP, Strupp A, Wissel ME, Reddy R, Gibble J, Haimowitz MD, Newman BH, Chambers LA, Hillyer CD, Benjamin RJ. The American Red Cross donor hemovigilance program: complications of blood donation reported in 2006. Transfusion. 2008;48:1809–19. doi: 10.1111/j.1537-2995.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiltbank TB, Giordano GF, Kamel H, Tomasulo P, Custer B. Faint and prefaint reactions in whole-blood donors: an analysis of predonation measurements and their predictive value. Transfusion. 2008;48:1799–808. doi: 10.1111/j.1537-2995.2008.01745.x. [DOI] [PubMed] [Google Scholar]
- 4.Newman BH. Donor reactions and injuries from whole blood donation. Transfus Med Rev. 1997;11:64–75. doi: 10.1016/s0887-7963(97)80011-9. [DOI] [PubMed] [Google Scholar]
- 5.Center for Biologics Evaluation and Research, Food and Drug Administration. Fatalities reported to FDA following blood collection and transfusion, Annual Summary for Fiscal Years 2005 and 2006. Rockville (MD): Food and Drug Administration; [2009 Jul 4]. Available from: URL: http://www.fda.gov. [Google Scholar]
- 6.Newman BH, Pichette S, Pichette D, Dzaka E. Adverse effects in blood donors after whole-blood donation: a study of 1000 blood donors interviewed 3 weeks after whole-blood donation. Transfusion. 2003;43:598–603. doi: 10.1046/j.1537-2995.2003.00368.x. [DOI] [PubMed] [Google Scholar]
- 7.Newman BH, Satz SL, Janowicz NM, Siegfried BA. Donor reactions in high-school donors: the effects of sex, weight, and collection volume. Transfusion. 2006;46:284–8. doi: 10.1111/j.1537-2995.2006.00713.x. [DOI] [PubMed] [Google Scholar]
- 8.Tomasulo PA, Anderson AJ, Paluso MB, Gutschenritter MA, Aster RH. A study of criteria for blood donor deferral. Transfusion. 1980;20:511–8. doi: 10.1046/j.1537-2995.1980.20581034503.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts P. AABB News. Bethesda (MD): AABB; 2008. Reacting to reactions: blood systems changes blood donor eligibility criteria; pp. 23–4. [Google Scholar]
- 10.Price Thomas H., Chair . Standards for Blood Banks and Transfusion Services Standards for blood banks and transfusion services. 25. Blood Bank/Transfusion Services Standards Program Unit, AABB; Bethesda (MD): 2008. [Google Scholar]
- 11.Senn LY, Karlson KE. Methodologic and actual error of plasma volume. Surgery. 1958;44:1095–105. [PubMed] [Google Scholar]
- 12.Nadler SB, Hildago JU, Bloch T. Prediction of blood volume in normal adults. Surgery. 1962;51:224–32. [PubMed] [Google Scholar]
- 13.Newman BH. Vasovagal reactions in high school students: findings relative to race, risk factor synergism, female sex, and non-high school participants. Transfusion. 2002;42:1557–60. doi: 10.1046/j.1537-2995.2002.00238.x. [DOI] [PubMed] [Google Scholar]
- 14.Connor JD, Lipton KS. Association Bulletin #08-04: strategies to reduce adverse reactions and injuries in blood donors. Bethesda (MD): AABB; 2008. [Google Scholar]
- 15.Hanson SA, France CR. Social support attenuates presyncopal reactions to blood donation. Transfusion. 2009;49:843–50. doi: 10.1111/j.1537-2995.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 16.France CR, Menitove JE. Mitigating adverse reactions in youthful donors. Transfusion. 2008;48:1774–6. doi: 10.1111/j.1537-2995.2008.01882.x. [DOI] [PubMed] [Google Scholar]
- 17.France CR, France JL, Roussos M, Ditto B. Mild reactions to blood donation predict a decreased likelihood of donor return. Transfus Apher Sci. 2004;30:17–22. doi: 10.1016/j.transci.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Hanson SA, France CR. Predonation water ingestion attenuates negative reactions to blood donation. Transfusion. 2004;44:924–8. doi: 10.1111/j.1537-2995.2004.03426.x. [DOI] [PubMed] [Google Scholar]
- 19.Benjamin RJ, Dy BA, Kennedy JM, Notari EP, IV, Eder AF. The relative safety of automated two-unit red blood cell procedures and manual whole-blood collection in young donors. Transfusion. 2009;49:1874–83. doi: 10.1111/j.1537-2995.2009.02237.x. [DOI] [PubMed] [Google Scholar]
- 20.Holme S, Elfath MD, Heaton A, Whitley P, McNeil D. Prediction of red cell and blood volumes distribution by various nomograms: do current nomograms overestimate? Transfusion. 2008;48:910–6. doi: 10.1111/j.1537-2995.2007.01619.x. [DOI] [PubMed] [Google Scholar]
- 21.European Commission. Commission directive 2004/33/EC. Official Journal of the European Union 2004. 2004 Mar 22; Annex III. [Google Scholar]
- 22.Council of Europe. Guide to the preparation, use and quality assurance of blood components. 13. Strasbourg (France): Council of Europe Publishing; 2007. pp. 67–70. [Google Scholar]


