Abstract
Background
The target-specific oral anticoagulant agents (TSOACs) do not require routine laboratory monitoring. However, laboratory measurement may be desirable in special situations and populations.
Objectives
This study’s objective is to systematically review and summarize current evidence regarding laboratory measurement of the anticoagulant activity of dabigatran, rivaroxaban, and apixaban.
Methods
We searched PubMed and Web of Science for studies that reported a relationship between drug levels of dabigatran, rivaroxaban, and apixaban and coagulation assay results. Study quality was evaluated using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2).
Results
We identified 17 eligible studies for dabigatran, 15 for rivaroxaban, and 4 for apixaban. For dabigatran, a normal thrombin time excludes clinically relevant drug concentrations. The activated partial thromboplastin time (APTT) and prothrombin time (PT) are less sensitive and may be normal at trough drug levels. The dilute thrombin time (R2 0.92–0.99) and ecarin-based assays (R2 0.92–1.00) show excellent linearity across on-therapy drug concentrations and may be used for drug quantification. In terms of rivaroxaban and apixaban, anti-Xa activity is linear (R2 0.89–1.00) over a wide range of drug levels and may be used for drug quantification. Undetectable anti-Xa activity likely excludes clinically relevant drug concentrations. The PT is less sensitive (especially for apixaban); a normal PT may not exclude clinically relevant levels. The APTT demonstrates insufficient sensitivity and linearity for quantification.
Conclusions
Dabigatran, rivaroxaban, and apixaban exhibit variable effects on coagulation assays. Understanding these effects facilitates interpretation of test results in TSOAC-treated patients. More information on the relationship between drug levels and clinical outcomes is needed.
Keywords: apixaban, dabigatran, laboratory, monitoring, rivaroxaban
Dabigatran etexilate, an oral prodrug of the direct thrombin inhibitor dabigatran and the oral direct inhibitors of factor Xa, rivaroxaban and apixaban, are approved in the United States, Europe, and Canada to prevent stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). They are also variably licensed for treatment of venous thromboembolism (VTE) and prevention of VTE after major orthopedic surgery (MOS) in certain jurisdictions. We refer to these agents collectively as target-specific oral anticoagulant agents (TSOACs) in this article. Synonymous terms preferred by other authors include direct-acting oral anticoagulant agents (DOACs) and new, novel, or nonvitamin K antagonist oral anticoagulant agents (NOACs) (1).
Unlike warfarin and other vitamin K antagonists (VKAs), the TSOACs are administered in fixed doses and do not require routine laboratory monitoring (2–4). However, measurement of their anticoagulant activity may be desirable in special clinical settings such as bleeding; the preoperative state; breakthrough thrombosis; suspected overdose, non-compliance, or drug interactions; and populations, including those with extremes in body weight and in the elderly and patients with renal insufficiency in whom there is a risk of drug accumulation. Assessment of anticoagulant effect may also be important in AF patients presenting with acute ischemic stroke prior to administration of thrombolytic therapy (5).
Numerous studies on use of coagulation assays for measurement of TSOAC activity have been published recently, though a systematic review has not been undertaken. The objective of our analysis was to summarize current evidence regarding laboratory measurement of the TSOAC anticoagulant activity and to provide evidence-based guidance to practicing cardiologists on the interpretation of coagulation tests in TSOAC-treated patients.
Methods
LITERATURE SEARCH
We performed a systematic review of the literature to examine current evidence for laboratory measurement of the TSOACs. A search of PubMed and Web of Science from inception through December 1, 2013, was undertaken separately for dabigatran, rivaroxaban, and apixaban using the following keywords: “Name of drug” AND ((laboratory measurement) OR laboratory monitoring)).
STUDY SELECTION
Articles were examined, first by title and abstract, then by review of the complete paper as indicated. Additional articles were sought by reviewing bibliographies. Liquid chromatography/tandem mass spectrometry (LC-MS/MS) is the reference method for measurement of the plasma concentration of the TSOACs (6). Studies that reported the relationship between drug (or active metabolite) levels in human plasma, as measured directly using LC-MS/MS or indirectly using LC-MS/MS-validated calibration standards and one or more clinical coagulation assays were eligible for inclusion. We excluded animal studies, abstracts only, and non-English language publications.
DATA EXTRACTON
We extracted key characteristics from eligible studies and recorded them in an evidence table. These included: author, year of publication, setting, TSOAC (i.e., dabigatran, rivaroxaban, apixaban), reference method for measurement of drug levels, range of drug concentrations studied, test material (i.e., ex vivo patient plasma, ex vivo healthy control plasma, spiked normal plasma), dose (for studies using ex vivo plasma only), indication (for studies using ex vivo patient plasma only), number of samples (for studies using individual [i.e., unpooled] plasma only), coagulation assays and reagents, and descriptors of the relationship between drug level and coagulation assay (e.g., R2 values, range of linearity, etc.).
QUALITY ASSESSMENT
Study quality was evaluated using Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), a standardized tool for quality assessment of studies of diagnostic accuracy. The tool comprises 4 domains: patient selection, index test, reference standard, and flow and timing. Risk of bias is assessed across all domains; the first 3 domains are also assessed with respect to applicability to clinical practice (7).
Results
DABIGATRAN
Dabigatran etexilate, an oral non-peptide prodrug, is rapidly converted to the active drug dabigatran by ubiquitous esterases. Dabigtran directly inhibits both free and clot-bound thrombin. It has relatively poor bioavailability (~6.5%) and is eliminated predominantly by the kidneys (80%). In individuals with normal renal function, the half-life of dabigatran is 12 to14 hours. Prolonged clearance and bioaccumulation are observed in patients with renal insufficiency (8). In patients with nonvalvular AF and normal kidney function, the dose is 150 mg twice daily, which is reduced in patients with renal insufficiency.
Peak levels of dabigatran occur 2 to 3 hours after ingestion. Steady-state peak and trough concentrations in patients with AF and normal renal function taking dabigatran 150 mg twice daily are shown in Table 1 (8). Substantial interindividual variability in drug exposure is observed. In the PETRO (Prevention of Embolic and Thrombotic Events in Patients With Persistent Atrial Fibrillation) study, the range (5th to 95th percentile) in peak and trough concentrations in patients taking 150 mg twice daily was 64 to 443 ng/ml and 31 to 225 ng/ml, respectively (9,10).
TABLE 1.
Expected Steady-state Peak and Trough Concentrations of Dabigatran, Rivaroxaban, and Apixaban Derived from Pharmacokinetic Studies.
| Drug | Dose | Study Population | Peak (ng/ml) | Trough (ng/ml) | References |
|---|---|---|---|---|---|
| Dabigatran | 150 mg BID | Patients with AF | 184* | 90* | 9,10 |
| Rivaroxaba | 20 mg QD | Patients with AF | 274† | 30† | 33 |
| Apixaban | 5 mg BID | Healthy male volunteers | 129‡ | 50‡ | 52 |
Only standard doses approved for atrial fibrillation are shown.
median;
median estimated by mathematical modeling;
mean
AF = atrial fibrillation; BID = twice daily; QD = once daily.
STUDY SELECTION
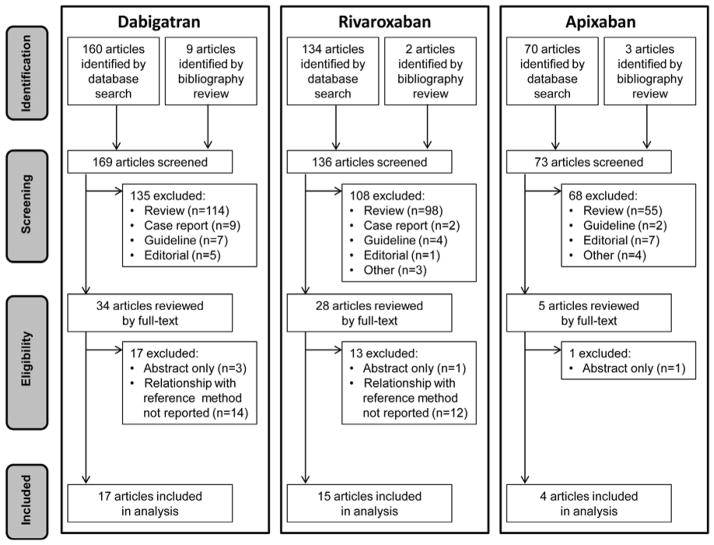
Our literature search yielded 160 articles. Nine additional references were identified from bibliographies. One hundred fifty-two articles were excluded: 135 did not report an original research study, 14 did not report drug levels measured by LC-MS/MS, and 3 were published as abstracts only. The remaining 17 articles (11–27) met eligibility criteria (Figure 1). Eligible studies were collectively conducted in 9 different countries across a range of dabigatran concentrations from 0 to 1886 ng/ml. Only 4 studies used ex vivo plasma from dabigatran-treated patients; the remainder involved ex vivo healthy volunteer plasma or normal plasma spiked in vitro with dabigatran as the test material (Table 2).
FIGURE 1. PRISMA Diagram.
This PRISMA flow diagram illustrates dabigatran, rivaroxaban, and apixaban literature searches
TABLE 2.
Characteristics of Eligible Dabigatran Studies
| Reference | Setting | Range of Drug Concentration (ng/ml) | Test Material | Indication (n) | Dose (mg) | Coagulation Test | R2 |
|---|---|---|---|---|---|---|---|
| 10 | Germany | NR | Ex vivo patient plasma | MOS (287) | 12.5–300 BID; 150–300 QD | APTT ECT |
NR NR |
| 11 | Germany | NR | Ex vivo healthy volunteer plasma | NA (80) | 10–400 SD; 50–400 TID | APTT ECT PT/INR TT |
0.85 0.92–0.96 0.83–0.85 0.60–0.86 |
| 12 | Germany | NR | Ex vivo healthy volunteer plasma | NA (35) | 150 BID | APTT ECT |
NR NR |
| 13 | Sweden | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | APTT PT/INR |
NR NR |
| 14 | USA | 0–500 | Spiked normal pooled plasma | NA (NA) | NA | APTT Dilute TT TT |
0.95* 0.98 NR |
| 15 | USA, UK, Canada | 25–500 | Spiked normal pooled plasma | NA (NA) | NA | APTT PT/INR TT |
0.96 0.99 NR |
| 16 | Belgium | 4.7–943 | Spiked normal pooled plasma | NA (NA) | NA | APTT Dilute PT Dilute TT ECA ECT PiCT PT/INR TT |
0.96–0.99 NR 0.99 0.99 1.00 NR NR NR |
| 17 | Austria | 0–480 | Spiked normal pooled plasma | NA (NA) | NA | APTT PT/INR |
NR NR |
| 18 | Germany | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | APTT ECT PT/INR |
NR NR NR |
| 19 | New Zealand | 30–960 | Spiked normal pooled plasma | NA (NA) | NA | Dilute TT | NR |
| 20 | Germany | 0–1886 | Spiked normal pooled plasma | NA (NA) | NA | Dilute TT | 0.99 |
| 21 | Germany | 50–1400 | Spiked healthy volunteer plasma | NA (4) | NA | POC-INR PT/INR |
NR NR |
| 22 | Sweden | <0.5–586 | Ex vivo patient plasma | AF (70) | NR | APTT Dilute TT ECA PT/INR |
0.58 0.97 0.96 0.48 |
| 23 | USA | 0–500 | Ex vivo patient plasma Spiked normal pooled plasma |
AF (NR) VTE (NR) NA (NA) |
150 BID NA |
Dilute TT ECA |
0.96–0.97 0.99 |
| 24 | USA | 18–487 | Ex vivo patient plasma | AF (29) VTE (6) |
NR | ACT APTT Dilute TT ECA ECT PT/INR TT |
0.71 0.74–0.82 0.92 0.94–0.99 0.98 0.60–0.86 0.75–0.97 |
| 25 | Sweden | 0–985.5 | Spiked normal pooled plasma | NA (NA) | NA | PT/INR | NR |
| 26 | Finland | 120–300 | Spiked normal pooled plasma | NA (NA) | NA | APTT PT/INR TT |
NR NR NR |
Relationship described by second order polynomial regression.
ACT = activated clotting time; APTT = activated partial thromboplastin time; ECA = ecarin chromogenic assay; ECT = ecarin clotting time; INR = international normalized ratio; MOS = major orthopedic surgery; NA = not applicable; NR = not reported; PiCT = prothrombinase-induced clotting time; POC = point of care; PT = prothrombin time; SD = single dose; TT = thrombin time; VTE - venous thromboembolism; other abbreviations as in Table 1.
APTT
Twelve eligible studies reported a relationship between the activated partial thromboplastin time (APTT) and dabigatran levels. Three used ex vivo patient plasma, 2 ex vivo healthy volunteer plasma, and 7 normal plasma spiked with dabigatran in vitro (Table 2). Dabigatran prolonged the APTT in a concentration-dependent manner in both ex vivo and in vitro studies. The dose response was linear up to a concentration of 200 to 300 ng/ml, then flattened out at higher drug levels (17,28). This curvilinear relationship did not permit quantitative assessment of dabigatran levels, particularly at higher concentrations.
Commercial APTT reagents differ widely in their sensitivity to dabigatran. The APTT of plasma spiked with dabigatran 120 ng/ml ranged from 26.0 to 91.9 seconds in a cross-validation study of 9 different APTT methods (27). These findings suggest that coagulation laboratories should perform dose-response studies using calibration standards to determine the sensitivity of their particular APTT method to dabigatran and communicate the results to clinicians. The least sensitive reagents required a dabigatran concentration of ~400 ng/ml to produce a 2-fold prolongation in the APTT over control (19). The APTT may not be prolonged in the presence of typical on-therapy trough levels (Table 1), particularly if a relatively insensitive reagent is used. In a study of ex vivo plasma from patients taking dabigatran 150 mg twice daily, 18% of subjects had a normal APTT at trough (25). In another study of patients with AF, some samples had an APTT within the normal range despite dabigatran concentrations as high as 60 ng/ml (23).
PT/INR
Eleven studies reported a relationship between dabigatran levels and the prothrombin time/international normalized ratio (PT/INR). Eight used spiked plasma, 2 ex vivo patient plasma, and 1 ex vivo plasma from healthy volunteers (Table 2). Dabigatran prolonged the PT in a concentration-dependent manner, as defined by an exponential (i.e., nonlinear) relationship (17). The PT/INR was less sensitive to dabigatran than the APTT. In an ex vivo study of patients with AF, an INR of 1.2 or greater was only observed with dabigatran concentrations in excess of 400 ng/ml (23).
As with APTT, commercial PT reagents differ in their sensitivity to dabigatran. In a survey of 71 coagulation laboratories, the PT of plasma spiked to a concentration of 300 ng/ml ranged from 15.7 to 50.2 seconds, depending on the reagent (27). Prothrombin time may be measured using two assay types. The Quick method is influenced by the entire extrinsic and common pathways of coagulation whereas the Owren method is affected only by factors II, VII, and X. In studies of spiked plasma, Quick PT reagents were more sensitive to dabigatran than Owren reagents (14,27).
Point-of-care devices are available for measuring the INR (POC-INR) in whole blood in VKA-treated patients. In one study assessing the relationship between dabigatran levels and a single POC-INR system, the POC system yielded INR values 2- to 4-fold higher than those obtained using a laboratory PT/INR method. Dabigatran concentrations greater than 500 ng/ml were beyond the POC system’s limit of detection (22).
TT
Six studies reported a relationship between thrombin time (TT) and dabigatran levels (Table 2). The TT (in unmodified form) was inordinately sensitive in both ex vivo and in vitro studies. Depending on the reagent, dabigatran concentrations of as little as 25 and up to 150 ng/ml exceeded the limits of detection (15–17,28).
DILUTE TT
In the dilute TT assay, the excessive sensitivity of the TT is overcome by diluting the plasma sample (21). Seven studies reported a relationship between the dilute TT and dabigatran concentration (Table 2). Two studies used an in-house modification of the TT (15,20); 5 employed the HEMOCLOT Thrombin Inhibitor assay (HYPHEN-BioMed, Neuvillesur-Oise, France), a commercially available dilute TT test (17,21,23–25). Dabigatran prolonged the assay in a concentration-dependent manner. The relationship showed a high degree of linearity with R2 values ranging between 0.92 and 0.99 in both in vitro and ex vivo studies. The lower limit of detection according to the manufacturer is 50 ng/ml (21). Two studies determined the assay to be less accurate and more variable at concentrations below 50 to 100 ng/ml (15,21). The dilute TT is currently not widely available; in a recent survey in Australia and New Zealand, only 9 of 592 laboratories reported using it (29).
ECARIN-BASED ASSAYS
Ecarin is a snake venom that cleaves prothrombin to form meizothrombin, an unstable intermediate of thrombin. Dabigatran inhibits the thrombin-like activity of meizothrombin (24). Two assays use ecarin as an activator: the ecarin clotting time (ECT) and ecarin chromogenic assay (ECA).
Six studies reported a relationship between ECT and dabigatran levels (Table 2). Both in vitro and ex vivo studies demonstrated a high degree of linearity with R2 values ranging between 0.92 and 1.00. Loss of linearity was observed in 2 studies at dabigatran levels in excess of 470 to 500 ng/ml (17,19). A relationship between ECA and drug levels was reported in 4 studies (Table 2). The relationship was linear with R2 values of 0.94 to 0.99 in in vitro and ex vivo samples. One study identified greater variability at dabigatran concentrations <50 ng/ml (23). The ECT and ECA are hampered by lack of standardization, variability in sensitivity to dabigatran among different lots of ecarin, and limited availability (17,20).
OTHER ASSAYS
Relationships between dabigatran level and the dilute PT, prothrombinase-induced clotting time (PiCT), and activated clotting time (ACT) were each reported in a single study. Both the dilute PT and PiCT evinced a complex nonlinear dose-response curve (17). As with heparin, the ACT proved insensitive to lower concentrations of dabigatran. In an ex vivo study, the ACT was normal in 40% of trough samples despite on-therapy dabigatran levels (25).
RIVAROXABAN
Rivaroxaban is an oral inhibitor of free and clot-associated factor Xa through reversible, competitive interactions with its active site (30). Bioavailability following oral administration is dose dependent (80% to 100% following a 10 mg dose; 66% following a 20 mg dose). It is highly bound to plasma proteins (>90%) (31); plasma levels peak 2 to 4 hours following oral administration (32,33). Partially excreted by the kidneys (36%), rivaroxaban has a half-life of 6 to 13 hours depending on dose and age (31–35). Table 1 shows expected peak and trough plasma concentrations in patients with AF treated with 20 mg daily (36).
STUDY SELECTION
Our literature search yielded 134 unique rivaroxaban articles. Two additional references were identified from bibliographies. We excluded 121 articles: 108 did not report an original research study, 12 did not report a relationship between a coagulation assay and drug levels measured by LC-MS/MS, and 1 was published as an abstract only. The remaining 15 articles (27,37–50) met eligibility criteria (Figure 1). Rivaroxaban concentrations in eligible studies ranged from 0 to >1000 ng/ml. Four studies used ex vivo plasma from rivaroxaban-treated patients, one incorporated ex vivo plasma from healthy controls, and the remainder used normal plasma spiked in vitro with rivaroxaban (Table 3).
TABLE 3.
Characteristics of Eligible Rivaroxaban Studies
| Reference | Setting | Range of Drug Concentration (ng/ml) | Test material | Indication (n) | Dose (mg) | Coagulation Test | R2 |
|---|---|---|---|---|---|---|---|
| 36 | USA, Canada | <10 – >1000 | Spiked normal pooled plasma | NA (NA) | NA | PT/INR | NR |
| 37 | France | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa APTT Dilute PT DiluteRVVT PiCT POC-INR PT/INR |
NR 0.90–0.98 0.97–0.99 0.99 NR 1.00 0.99–1.00 |
| 38 | France | NR | Ex vivo patient plasma | MOS (166) | 10 QD | Anti-Xa APTT PT/INR |
0.97–1.00 0.75 0.47 |
| 39 | Germany | 25–900 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa | NR |
| 40 | Sweden | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa APTT PT/INR |
NR NR NR |
| 41 | Italy | 100–700 | Spiked normal pooled plasma | NA (NA) | NA | PT/INR | NR |
| 42 | Switzerland | 40–150 | Ex vivo healthy volunteer plasma Spiked normal pooled plasma |
NA (20) NA (NA) |
10 QD NA |
Anti-Xa | 0.98–0.99 |
| 43 | Germany | 1.9–283.0 | Ex vivo patient plasma | MOS (80) | 10 QD | Anti-Xa | 1.00 |
| 44 | Netherlands | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa APTT PT/INR |
NR 1.00 1.00* |
| 45 | Europe, North America | 20–662 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa | NR |
| 46 | Europe, North America | 19–643 | Spiked normal pooled plasma | NA (NA) | NA | PT/INR | NR |
| 47 | Netherlands | 0–800 | Spiked normal pooled plasma | NA (NA) | NA | PT/INR | 1.00 |
| 48 | Belgium | NR | Ex vivo patient plasma | AF, VTE (52) | NR | Anti-Xa PT/INR |
0.95 0.58–0.66 |
| 26 | Europe | 60–305 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa APTT PT/INR |
NR NR NR |
| 49 | France | NR | Ex vivo patient plasma | MOS (41) | 10 QD | Anti-Xa PT/INR |
0.99 NR |
PT
We found 11 studies evaluating the effect of rivaroxaban on PT (Table 3). In general, rivaroxaban prolonged the PT in a concentration-dependent, linear fashion in plasma spiked with rivaroxaban and in plasma from patients receiving rivaroxaban for approved indications. On-therapy rivaroxaban concentrations showed a modest effect on the PT. Typical trough (41 to 60 ng/ml) and peak (219 to 305 ng/ml) concentrations increased PT by 6% to 19% and 50% to 135%, respectively (27,38,42,47). Assay sensitivity varied significantly among thromboplastin reagents. Inter-assay variability was reduced by use of an international sensitivity index (ISI) specific for rivaroxaban, but not by conversion to an INR used for monitoring VKA therapy (38,42). These observations suggest that coagulation laboratories should perform dose-response studies using calibration standards to determine the sensitivity of their particular PT method to rivaroxaban and communicate the results to clinicians.
APTT
Five studies evaluated the effect of rivaroxaban on APTT (Table 3). While rivaroxaban prolonged APTT in a dose-dependent manner, the overall relationship between rivaroxaban concentration and APTT prolongation was nonlinear with studies reporting conflicting data regarding the concentration ranges over which nonlinearity was most pronounced (27,41,45). Similar to PT results, there was significant variability among reagents and among individual laboratories in a multicenter study (27,45). Hillarp et al. reported that the APTT assay was insensitive at the lowest drug level studied (25 ng/ml) (41).
ANTI-Xa ACTIVITY
Ten studies assessed the effect of rivaroxaban on anti-Xa activity (Table 3). In general, the studies showed a linear, concentration-dependent relationship between rivaroxaban concentration and anti-Xa activity over a wide range of concentrations (e.g.. 20–660 ng/ml) when measured using a standard curve generated with rivaroxaban calibrators and controls with R2 values ranging from 0.95 to 1.00 (39,43,44,49,50). The correlation was less robust at concentrations <100 ng/mL (49). However, Samama and colleagues demonstrated that low rivaroxaban concentrations could be measured with a modified anti-Xa test using less diluted samples (46). Investigators found a greater degree of assay imprecision at higher rivaroxaban concentrations (800 ng/ml) in one study (45). In a multicenter study, both intra- and interlaboratory precision were satisfactory except at the lower limit of detection (20 ng/ml); use of a centrally distributed reagent reduced interlaboratory variability (46). Mathematical modeling also decreased interassay variability resulting from different sensitivities of individual reagents to rivaroxaban (40). When commercial anti-Xa assays were used with unfractionated or low-molecular-weight heparin (LMWH) calibrators (rather than rivaroxaban calibrators), the relationship remained linear up to a rivaroxaban concentration of 500 ng/ml (38,41).
OTHER ASSAYS
The relationship between rivaroxaban concentration and the dilute PT, dilute Russell viper venom time (dRVVT), and PiCT was evaluated in a single study (38). Researchers uncovered a linear, dose-dependent relationship between rivaroxaban and the dilute PT. Rivaroxaban increased the dRVVT ratio (expressed as ratio vs. baseline) in a concentration-dependent, but nonlinear manner. At low concentrations of rivaroxaban (<200 ng/ml), there was a paradoxical shortening of PiCT, whereas PiCT was prolonged in a concentration-dependent fashion at higher concentrations.
APIXABAN
Like rivaroxaban, apixaban is a small, orally available, direct inhibitor of coagulation factor Xa (51). It has 50% bioavailability and, in healthy volunteers, reaches its maximum plasma concentration approximately 3 hours after ingestion. Apixaban is highly protein-bound in plasma and concomitant food intake has little impact on its pharmacokinetics (52). Metabolized through multiple routes, apixaban is less dependent on renal clearance than dabigatran and rivaroxaban. In persons with normal renal function, apixaban has a half-life of approximately 12 hours (53). As measured by LC-MS/MS, the expected steady-state concentrations of apixaban have been published by Frost et al. (52) and are shown in Table 1.
STUDY SELECTION
Our literature search for apixaban yielded 70 articles; 3 additional references were identified from bibliographies. Sixty-nine articles were excluded: 68 did not report an original research study and 1 was published as an abstract only. The remaining 4 articles (37,54–56) met eligibility criteria (Figure 1). Eligible studies collectively evaluated apixaban across a range of concentrations from 0 to 2500 ng/ml (Table 4).
TABLE 4.
Characteristics of Eligible Apixaban Studies
| Reference | Setting | Range of Drug Concentration (ng/ml) | Test Material | Indication (n) | Dose (mg) | Coagulation test | R2 |
|---|---|---|---|---|---|---|---|
| 36 | USA, Canada | <10 – >1000 | Ex vivo patient plasma Spiked normal pooled plasma |
VTE (348) NA (NA) |
5–10 BID, 20 QD NA |
Anti-Xa PT/INR |
0.88–0.89 0.36 |
| 53 | Global | 1–933 | Ex vivo patient plasma | ACS (1691) | 5–20 QD | Anti-Xa | 0.97 |
| 54 | USA | 0–2500 |
Ex vivo healthy volunteer plasma Spiked healthy volunteer plasma |
NA (39) NA (21) |
20 SD NA |
PT/INR | 0.41 |
| 55 | France | 0–1000 | Spiked normal pooled plasma | NA (NA) | NA | Anti-Xa APTT PT/INR |
NR NR NR |
ACS = acute coronary syndrome; other abbreviations as in Table 2.
PT/INR
We found 3 studies that reported the relationship between the PT and apixaban levels. One study used both spiked normal plasma and ex vivo plasma from apixaban-treated patients (37), another used spiked plasma as well as ex vivo plasma from healthy volunteers taking apixaban (55), and the third study included only spiked normal plasma samples (56) (Table 4). For the 2 studies that used ex vivo plasma, the relationship was linear in one (37) and curvilinear in the other (55). Correlation was modest with R2 values of 0.36 and 0.41, respectively. Across in vitro and ex vivo samples and for a variety of reagents, the PT was inadequately sensitive to apixaban, not only below, but also above the expected trough concentration of 50 ng/ml.
APTT
Only one study compared apixaban concentrations to APTT (Table 3), which was measured using 10 different APTT reagents in normal plasma samples spiked with 10 different concentrations of apixaban. The sensitivity of the APTT was unacceptably low; all assays yielded a ratio of 1.1 times control when the spiked apixaban concentration was 100 ng/ml (i.e., twice the expected trough concentration).
ANTI-Xa ACTIVITY
Three studies compared anti-Xa activity measurements to the plasma concentration of apixaban (Table 4). In 2 of the studies (37,54), ex vivo patient samples were used while the third study (56) included only spiked samples of normal plasma. In general, the relationship was linear at all apixaban concentrations, with R2 values ranging from 0.89 to 0.97. Available evidence suggests that an anti-Xa assay calibrated with LMWH standards will also correlate linearly with apixaban concentrations (37,54).
STUDY QUALITY
Assessment of study quality using QUADAS-2 criteria (7) highlighted several recurrent methodologic concerns among eligible studies (Table 5). Many studies used in vitro samples or ex vivo samples from healthy controls rather than ex vivo patient samples. Concern about the applicability of these studies to clinical practice within the patient selection domain was judged to be high. Some studies examined assays not widely available to clinicians (e.g., ECA, ECT, dilute TT). Concern regarding applicability of these studies to clinical practice across the index test domain was judged to be high. Because data correlating plasma TSOAC levels and clinical outcomes are scarce, concern about the applicability of the reference standard (plasma drug concentration) to clinical practice was judged to be high for all eligible studies.
TABLE 5.
Study Quality Assessment Using QUADAS-2 Criteria
| Reference | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard | |
| Dabigatran Studies | |||||||
| 10 | L | U | U | L | L | H | H |
| 11 | NA | U | U | L | H | H | H |
| 12 | NA | U | U | L | H | H | H |
| 13 | NA | U | L | L | H | L | H |
| 14 | NA | U | L | L | H | H | H |
| 15 | NA | L | L | L | H | L | H |
| 16 | NA | U | L | L | H | H | H |
| 17 | NA | U | L | L | H | L | H |
| 18 | NA | U | L | L | H | H | H |
| 19 | NA | U | L | L | H | H | H |
| 20 | NA | U | L | L | H | H | H |
| 21 | NA | U | L | L | H | L | H |
| 22 | U | U | U | L | L | H | H |
| 23 | U | U | U | L | L | H | H |
| 24 | U | U | U | L | L | H | H |
| 25 | NA | U | L | L | H | L | H |
| 26 | NA | U | L | L | H | L | H |
| Rivaroxaban Studies | |||||||
| 26 | NA | U | U | L | H | L | H |
| 36 | NA | U | U | L | L | L | H |
| 37 | NA | U | L | L | H | H | H |
| 38 | U | U | U | L | L | L | H |
| 39 | NA | U | L | L | H | L | H |
| 40 | NA | U | L | L | H | L | H |
| 41 | NA | U | L | L | H | L | H |
| 42 | NA | L | L | L | H | L | H |
| 43 | U | U | U | L | L | L | H |
| 44 | NA | U | L | L | H | L | H |
| 45 | NA | L | L | L | H | L | H |
| 46 | NA | L | L | L | H | L | H |
| 47 | NA | U | L | L | H | L | H |
| 48 | U | U | U | L | L | L | H |
| 49 | U | U | U | L | L | L | H |
| Apixaban Studies | |||||||
| 36 | L | U | U | L | L | L | H |
| 53 | L | U | U | L | H | L | H |
| 54 | NA | U | U | L | H | L | H |
| 55 | NA | L | L | L | H | L | H |
H = high; L = low; U = unclear; other abbreviations as in Table 2.
Discussion
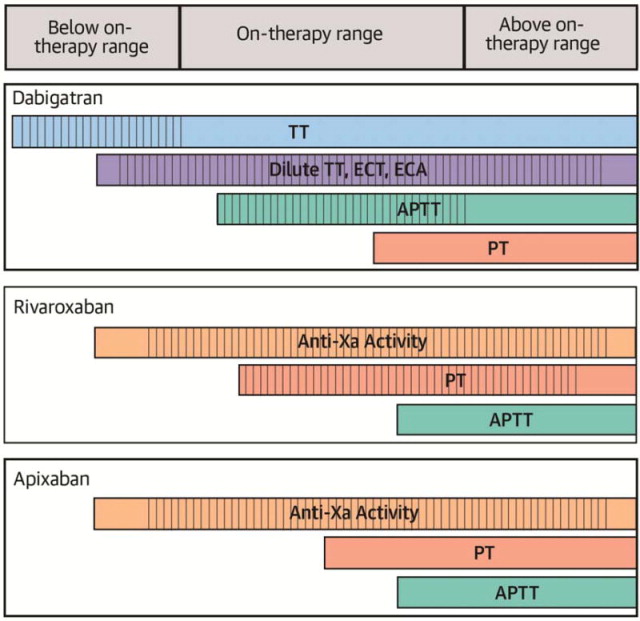
This systematic review sought to examine evidence for laboratory measurement of the anticoagulant activity of dabigatran, rivaroxaban, and apixaban. Although data on the relationship between plasma TSOAC levels and clinical outcomes are beginning to emerge (57), there is, as yet, no evidence that routine monitoring or dose titration will improve outcomes. Nevertheless, measurement may be useful in three circumstances: 1) to determine if very high levels are present (in the case of suspected excess effect; e.g., due to overdose or bioaccumulation); 2) to determine if drug is present in typical on-therapy ranges (e.g., in the case of suspected therapeutic failure); and 3) to determine if any clinically relevant drug effect is present (e.g., in the case of bleeding or planned invasive procedures). An ideal assay would thus show adequate linearity, sensitivity, and reproducibility to enable quantification across a broad range of drug levels. Apart from LC-MS/MS, a test generally restricted to select reference laboratories, no single coagulation assay meets these idealized standards. Therefore, it is important for clinicians to be aware of how coagulation tests perform at TSOAC concentrations below, within, and above typical on-therapy ranges (Central Illustration).
CENTRAL ILLUSTRATION. Sensitivity and Linearity of Coagulation Assays to Below, Within, and Above Typical On-therapy Concentrations of Dabigatran, Rivaroxaban, and Apixaban.
Red bars and vertical hatching correspond to the approximate range of detectability (i.e., sensitivity) and linearity, respectively, of each assay to below, within, and above typical ontherapy concentrations of dabigatran, rivaroxaban, and apixaban. Ranges are approximations and may vary based on choice of reagent. Typical on-therapy drug levels are shown in Table 1.
APTT = activated partial thromboplastin time; ECA = ecarin chromogenic assay; ECT = ecarin clotting time; PT = prothrombin time; TT = thrombin time.
DABIGATRAN
The effect of dabigatran on various coagulation assays is summarized in the Central Illustration. The TT is exquisitely sensitive to dabigatran. A normal TT excludes the presence of clinically relevant drug levels; however, the assay is too sensitive for quantification within and above the on-therapy range. The dilute TT, ECT, and ECA show a high degree of linearity at drug levels >50 ng/ml and are thus useful for quantification across the entire on-therapy range. They may be unreliable at concentrations below this threshold. The APTT is relatively insensitive to dabigatran; a normal APTT may not exclude clinically relevant below or on-therapy drug levels. The curvilinear response of APTT at higher drug levels does not permit accurate quantification. The PT has even poorer sensitivity and may be normal within much of the on-therapy range.
RIVAROXABAN
Anti-Xa activity measured using chromogenic substrates and rivaroxaban or heparin/LMWH calibrators correlates linearly with rivaroxaban over a wide range of concentrations (20 to 660 ng/ml) (Central Illustration). When rivaroxaban calibrators are used, anti-Xa assays can provide a quantitative measure of rivaroxaban concentration. A negative anti-Xa assay likely excludes clinically relevant rivaroxaban levels. Although rivaroxaban prolongs the PT, assay results vary markedly with different thromboplastin reagents. A normal PT does not rule out the presence of clinically significant below or within on-therapy rivaroxaban concentrations; however, a prolonged PT qualitatively indicates the drug’s presence. The APTT is not suitable for measuring rivaroxaban due to the nonlinear relationship with rivaroxaban concentration, poor sensitivity, and significant variability between reagents.
APIXABAN
Although both the PT and APTT may be prolonged in the presence of apixaban, neither is sufficiently sensitive to exclude the presence of clinically relevant on-therapy drug concentrations (Central Illustration). Anti-Xa activity measurements demonstrate a strong linear correlation with apixaban concentration; the absence of detectable anti-Xa activity (whether the standard curve is established with apixaban or LMWH) likely excludes the presence of physiologically important apixaban activity.
SUGGESTIONS AND COMPARISON WITH GUIDANCE DOCUMENTS
Recommendations for laboratory measurement of the TSOACs differ by drug and clinical objective. The findings of our systematic review support the suggestions summarized in Table 6. These suggestions align with recommendations provided in drug labels and published guidance documents, with two notable exceptions. First, we found strong evidence from studies of ex vivo patient samples that a normal APTT does not definitively exclude on-therapy dabigatran concentrations (23,25). This observation is at variance with guidelines from the American Society of Hematology (ASH) and the British Committee for Standards in Haematology (BCSH), which state that a normal APTT is likely to exclude therapeutic intensity dabigatran (58) or contribution of dabigatran to bleeding (59). Second, we found that a normal PT does not exclude clinically relevant rivaroxaban levels. The BCSH statement, in contrast, comments that a normal PT ratio with most reagents excludes therapeutic intensity rivaroxaban (58). The ASH and BCSH statements were published in 2011 and 2012, respectively. Discrepancies between our findings and these statements may reflect availability of new information since their publication regarding a wider variety of test reagents and their sensitivity to the TSOACs.
TABLE 6.
Suggestions for Laboratory Measurement of Target-specific Oral Anticoagulant Agents
| Drug | Clinical Objective | |||||
|---|---|---|---|---|---|---|
| Determine Whether Clinically Relevant Below On-therapy Drug Levels are Present | Estimate Drug Levels Within On-therapy Range | Determine Whether Above On-therapy Drug Levels are Present | ||||
| Suggested Test | Interpretation | Suggested Test | Interpretation | Suggested Test | Interpretation | |
| Dabigatran | TT | A normal TT likely excludes clinically relevant drug levels | Dilute TT, ECA, ECT | - | APTT, Dilute TT, ECA, ECT | A normal APTT likely excludes excess drug levels. Only dilute TT, ECA, and ECT are suitable for quantitation. |
| Rivaroxaban | Anti-Xa | Normal anti-Xa activity likely excludes clinically relevant drug levels | Anti-Xa | - | Anti-Xa, PT | A normal PT likely excludes excess drug levels. Only anti-Xa is suitable for quantitation. |
| Apixaban | Anti-Xa | Normal anti-Xa activity likely excludes clinically relevant drug levels | Anti-Xa | - | Anti-Xa | - |
LIMITATIONS
Quality assessment highlighted several key limitations of eligible studies (Table 5). First, on-therapy ranges (Table 1) were derived from pharmacokinetic analyses. We resisted the term “therapeutic range” because data on how these ranges correlate with clinical outcomes are sparse, though they are beginning to emerge (57). Second, many eligible studies used either ex vivo or spiked plasma samples from healthy controls rather than ex vivo samples from TSOAC-treated patients. Reassuringly, results obtained with patient samples generally aligned with those from healthy controls. Third, we identified only 4 eligible apixaban studies, just 2 of which used ex vivo patient samples. Because apixaban was the most recent TSOAC to receive regulatory approval, we expect additional studies of its laboratory measurement will be forthcoming. Such studies are also needed for TSOACs not yet approved in North America and Europe (e.g., edoxaban).
CONCLUSIONS
A relatively large number of published studies have assessed the relationship between coagulation tests and levels of dabigatran, rivaroxaban, and apixaban. Each drug produces unique effects on coagulation assays. Our systematic review provides guidance to the clinician on how to use and interpret coagulation test results in TSOAC-treated patients. Further studies are needed to define the relationship between drug levels, coagulation test results, and clinical outcomes.
PERSPECTIVES.
Competency In Medical Knowledge 1
The prothrombin time (PT) and activated partial thromboplastin time (APTT) do not show sufficient sensitivity or linearity for quantification of dabigatran, rivaroxaban, or apixaban. A normal PT and/or APTT may not exclude clinically relevant anticoagulant effects of these drugs.
Competency in Medical Knowledge 2
A normal thrombin time likely excludes clinically relevant plasma levels of the direct thrombin inhibitor, dabigatran. The dilute thrombin time and ecarin-based assays may be used to measure dabigatran activity.
Competency In Medical Knowledge 3
An anti-Xa assay using drug-specific calibrators may be used to measure the activity of the factor Xa inhibitors rivaroxaban and apixaban.
Competency In Patient Care
The target-specific oral anticoagulants dabigatran, rivaroxaban and apixaban do not require laboratory monitoring of coagulation during routine clinical use, but measurement of their anticoagulant effect may be desirable in certain circumstances.
Translational Outlook
Development of laboratory assays for measurement of the anticoagulant activity of these target-specific oral anticoagulants is a high priority. Ideally, these assays should be sufficiently sensitive to detect all clinically relevant drug concentrations, show linearity across a wide range of concentrations to permit quantification, and be reproducible and simple to perform at the point of care.
Acknowledgments
Financial support: This work was supported by HL112903 (National Heart Lung and Blood Institute, Bethesda, MD) to AC.
ABBREVIATIONS AND ACRONYMS
- ACT
activated clotting time
- AF
atrial fibrillation
- APTT
activated partial thromboplastin time
- LC-MS/MS
liquid chromatography/tandem mass spectrometry
- PT/INR
prothrombin time/international normalized ratio
- TSOAC
target-specific oral anticoagulant agent
- TT
thrombin time
- VKA
vitamin K antagonist
Footnotes
Relationships with industry:
AC has served as a consultant for Baxter, Bayer, and Genzyme; has served on an advisory board for Daiichi Sankyo and Genzyme; and has received research support from Diagnostica Stago. MAC discloses having sat on an advisory board and or working as a paid consultant for Boehringer Ingelheim, Portola, Viropharm and AKP America. He holds a Career Investigator award from the Heart and Stroke Foundation of Ontario, and the Leo Pharma Chair in Thromboembolism Research at McMaster University. His institution has received funding for research projects from Leo Pharma. He has received funding for presentations from Bayer, Celgene, Shire and CSL Behring. DG has served as a consultant for Boehringer Ingelheim, Bristol Meyers Squibb, CSL Behring, Daiichi Sankyo, Pfizer, and Roche. DMS has no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Husted S, De Caterina R, Andreotti F, et al. Non-vitamin K antagonist oral anticoagulants (NOACs): No longer new or novel. Thromb Haemost. 2014;111:781–2. doi: 10.1160/TH14-03-0228. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 3.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 4.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 5.Tripodi A. The laboratory and the direct oral anticoagulants. Blood. 2013;121:4032–5. doi: 10.1182/blood-2012-12-453076. [DOI] [PubMed] [Google Scholar]
- 6.Eby C. Novel anticoagulants and laboratory testing. Int J Lab Med. 2013;35:262–8. doi: 10.1111/ijlh.12065. [DOI] [PubMed] [Google Scholar]
- 7.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gross PL, Weitz JI. New antithrombotic drugs. Clin Pharmacol Ther. 2009;86:139–46. doi: 10.1038/clpt.2009.98. [DOI] [PubMed] [Google Scholar]
- 9.Ezekowitz MD, Reilly PA, Nehmiz G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study) Am J Cardiol. 2007;100:1419–26. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 10.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate--a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 11.Liesenfeld KH, Schäfer HG, Trocóniz IF, et al. Effects of the direct thrombin inhibitor dabigatran on ex vivo coagulation time in orthopaedic surgery patients: a population model analysis. Br J Clin Pharmacol. 2006;62:527–37. doi: 10.1111/j.1365-2125.2006.02667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stangier J, Stähle H, Rathgen K, et al. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. doi: 10.2165/00003088-200847010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lindahl TL, Baghaei F, Blixter IF, et al. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost. 2011;105:371–8. doi: 10.1160/TH10-06-0342. [DOI] [PubMed] [Google Scholar]
- 15.Avecilla ST, Ferrell C, Chandler WL, et al. Plasma-diluted thrombin time to measure dabigatran concentrations during dabigatran etexilate therapy. Am J Clin Pathol. 2012;137:572–4. doi: 10.1309/AJCPAU7OQM0SRPZQ. [DOI] [PubMed] [Google Scholar]
- 16.Dager WE, Gosselin RC, Kitchen S, et al. Dabigatran effects on the international normalized ratio, activated partial thromboplastin time, thrombin time, and fibrinogen: a multicenter, in vitro study. Ann Pharmacother. 2012;46:1627–36. doi: 10.1345/aph.1R179. [DOI] [PubMed] [Google Scholar]
- 17.Douxfils J, Mullier F, Robert S, et al. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–97. doi: 10.1160/TH11-11-0804. [DOI] [PubMed] [Google Scholar]
- 18.Halbmayer WM, Weigel G, Quehenberger P, et al. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med. 2012;50:1601–5. doi: 10.1515/cclm-2011-0888. [DOI] [PubMed] [Google Scholar]
- 19.Harenberg J, Giese C, Marx S, et al. Determination of dabigatran in human plasma samples. Semin Thromb Hemost. 2012;38:16–22. doi: 10.1055/s-0031-1300947. [DOI] [PubMed] [Google Scholar]
- 20.Jones SD, Eaddy NS, Chan GT. Dabigatran: laboratory monitoring. Pathology. 2012;44:578–80. doi: 10.1097/PAT.0b013e32835833f4. [DOI] [PubMed] [Google Scholar]
- 21.Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–43. doi: 10.1097/MBC.0b013e32834f1b0c. [DOI] [PubMed] [Google Scholar]
- 22.van Ryn J, Baruch L, Clemens A. Interpretation of point-of-care INR results in patients treated with dabigatran. Am J Med. 2012;125:417–20. doi: 10.1016/j.amjmed.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Antovic JP, Skeppholm M, Eintrei J, et al. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. 2013;69:1875–81. doi: 10.1007/s00228-013-1550-4. [DOI] [PubMed] [Google Scholar]
- 24.Gosselin RC, Dwyre DM, Dager WE. Measuring dabigatran concentrations using a chromogenic ecarin clotting time assay. Ann Pharmacother. 2013;47:1635–40. doi: 10.1177/1060028013509074. [DOI] [PubMed] [Google Scholar]
- 25.Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11:1493–502. doi: 10.1111/jth.12308. [DOI] [PubMed] [Google Scholar]
- 26.He S, Wallèn H, Bark N, et al. In vitro studies using a global hemostasis assay to examine the anticoagulant effects in plasma by the direct thrombin inhibitors: dabigatran and argatroban. J Thromb Thrombolysis. 2013;35:131–9. doi: 10.1007/s11239-012-0791-x. [DOI] [PubMed] [Google Scholar]
- 27.Helin TA, Pakkanen A, Lassila R, et al. Laboratory assessment of novel oral anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem. 2013;59:807–14. doi: 10.1373/clinchem.2012.198788. [DOI] [PubMed] [Google Scholar]
- 28.Hapgood G, Butler J, Malan E, et al. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost. 2013;110:308–15. doi: 10.1160/TH13-04-0301. [DOI] [PubMed] [Google Scholar]
- 29.Favaloro EJ, Bonar R, Butler J, et al. Laboratory testing for the new oral anticoagulants: a review of current practice. Pathology. 2013;45:435–7. doi: 10.1097/PAT.0b013e328360f02d. [DOI] [PubMed] [Google Scholar]
- 30.Perzborn E, Strassburger J, Wilmen A, et al. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939--an oral, direct Factor Xa inhibitor. J Thromb Haemost. 2005;3:514–21. doi: 10.1111/j.1538-7836.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed 17 August 2013];Xarelto Summary of Product Characteristics. Available at: http://www.xarelto.com/html/downloads/2013-07_SPC_June2013_F2_low.pdf.
- 32.Kubitza D, Becka M, Voith B, et al. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21. doi: 10.1016/j.clpt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Kubitza D, Becka M, Wensing G, et al. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873–80. doi: 10.1007/s00228-005-0043-5. [DOI] [PubMed] [Google Scholar]
- 34.Ageno W, Gallus AS, Wittkowsky A, et al. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e44S–88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinz C, Schwarz T, Kubitza D, et al. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. 2009;37:1056–64. doi: 10.1124/dmd.108.025569. [DOI] [PubMed] [Google Scholar]
- 36.Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–86. doi: 10.2165/11595320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Barrett YC, Wang Z, Frost C, et al. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263–71. doi: 10.1160/TH10-05-0328. [DOI] [PubMed] [Google Scholar]
- 38.Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban--an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–25. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 39.Freyburger G, Macouillard G, Labrouche S, et al. Coagulation parameters in patients receiving dabigatran etexilate or rivaroxaban: two observational studies in patients undergoing total hip or total knee replacement. Thromb Res. 2011;127:457–65. doi: 10.1016/j.thromres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Harenberg J, Kramer R, Giese C, et al. Determination of rivaroxaban by different factor Xa specific chromogenic substrate assays: reduction of interassay variability. J Thromb Thrombolysis. 2011;32:267–71. doi: 10.1007/s11239-011-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–9. doi: 10.1111/j.1538-7836.2010.04098.x. [DOI] [PubMed] [Google Scholar]
- 42.Tripodi A, Chantarangkul V, Guinet C, et al. The International Normalized Ratio calibrated for rivaroxaban has the potential to normalize prothrombin time results for rivaroxaban-treated patients: results of an in vitro study. J Thromb Haemost. 2011;9:226–8. doi: 10.1111/j.1538-7836.2010.04106.x. [DOI] [PubMed] [Google Scholar]
- 43.Asmis LM, Alberio L, Angelillo-Scherrer A, et al. Rivaroxaban: Quantification by anti-FXa assay and influence on coagulation tests: a study in 9 Swiss laboratories. Thromb Res. 2012;129:492–8. doi: 10.1016/j.thromres.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 44.Mani H, Rohde G, Stratmann G, et al. Accurate determination of rivaroxaban levels requires different calibrator sets but not addition of antithrombin. Thromb Haemost. 2012;108:191–8. doi: 10.1160/TH11-12-0832. [DOI] [PubMed] [Google Scholar]
- 45.Molenaar PJ, Dinkelaar J, Leyte A. Measuring Rivaroxaban in a clinical laboratory setting, using common coagulation assays, Xa inhibition and thrombin generation. Clin Chem Lab Med. 2012;50:1799–807. doi: 10.1515/cclm-2012-0055. [DOI] [PubMed] [Google Scholar]
- 46.Samama MM, Contant G, Spiro TE, et al. Evaluation of the anti-factor Xa chromogenic assay for the measurement of rivaroxaban plasma concentrations using calibrators and controls. Thromb Haemost. 2012;107:379–87. doi: 10.1160/TH11-06-0391. [DOI] [PubMed] [Google Scholar]
- 47.Samama MM, Contant G, Spiro TE, et al. Evaluation of the Prothrombin Time for Measuring Rivaroxaban Plasma Concentrations Using Calibrators and Controls: Results of a Multicenter Field Trial. Clin Appl Thromb Hemost. 2012;18:150–8. doi: 10.1177/1076029611426282. [DOI] [PubMed] [Google Scholar]
- 48.Dinkelaar J, Molenaar PJ, Ninivaggi M, et al. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for Rivaroxaban. J Thromb Haemost. 2013;11:1111–8. doi: 10.1111/jth.12236. [DOI] [PubMed] [Google Scholar]
- 49.Douxfils J, Tamigniau A, Chatelain B, et al. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC-MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110:723–31. doi: 10.1160/TH13-04-0274. [DOI] [PubMed] [Google Scholar]
- 50.Samama MM, Guinet C, Le Flem L, et al. Measurement of dabigatran and rivaroxaban in primary prevention of venous thromboembolism in 106 patients, who have undergone major orthopedic surgery: an observational study. J Thromb Thrombolysis. 2013;35:140–6. doi: 10.1007/s11239-012-0803-x. [DOI] [PubMed] [Google Scholar]
- 51.Scaglione F. New oral anticoagulants: comparative pharmacology with vitamin K antagonists. Clin Pharmacokinet. 2013;52:69–82. doi: 10.1007/s40262-012-0030-9. [DOI] [PubMed] [Google Scholar]
- 52.Frost C, Nepal S, Wang J, et al. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76:776–86. doi: 10.1111/bcp.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. [Accessed 30 December 2013];Eliquis Prescribing Information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/202155s000lbl.pdf.
- 54.Becker RC, Yang H, Barrett Y, et al. Chromogenic laboratory assays to measure the factor Xa-inhibiting properties of apixaban--an oral, direct and selective factor Xa inhibitor. J Thromb Thrombolysis. 2011;32:183–7. doi: 10.1007/s11239-011-0591-8. [DOI] [PubMed] [Google Scholar]
- 55.Barrett YC, Wang Z, Knabb RM. A novel prothrombin time assay for assessing the anticoagulant activity of oral factor Xa inhibitors. Clin Appl Thromb Hemost. 2013;19:522–8. doi: 10.1177/1076029612441859. [DOI] [PubMed] [Google Scholar]
- 56.Gouin-Thibault I, Flaujac C, Delavenne X, et al. Assessment of apixaban plasma levels by laboratory tests: suitability of three anti-Xa assays. A multicentre French GEHT study. Thromb Haemost. 2014;111:240–8. doi: 10.1160/TH13-06-0470. [DOI] [PubMed] [Google Scholar]
- 57.Reilly PA, Lehr T, Haertter S, et al. The Effect of Dabigatran Plasma Concentrations and Patient Characteristics on the Frequency of Ischemic Stroke and Major Bleeding in Atrial Fibrillation Patients in the RE-LY Trial. J Am Coll Cardiol. 2014;63:321–8. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- 58.Baglin T, Keeling D, Kitchen S. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: Guidance from the Britsh Committee for Standards in Haematology. Br J Haematol. 2012;159:427–9. doi: 10.1111/bjh.12052. [DOI] [PubMed] [Google Scholar]
- 59.Cushman M, Lim W, Zakai NA. [Accessed 9 January 2014];American Society of Hematology 2011 Clinical Practice Guide on Anticoagulant Dosing and Management of Anticoagulant-Associated Bleeding Complications in Adults. http://www.hematology.org/Practice/Guidelines/2934.aspx.